Summary
This study examined whether toll-like receptors 2 (TLR2) contribute to the rapid kindling epileptogenesis. A TLR2 agonist, lipoteichoic acid (LTA), LTA antibody (LTA-A) or normal saline (control) were administered daily over three consecutive days, unilaterally into ventral hippocampus of adult male Wistar rats. Thirty minutes after the last injection, the animals were subjected to rapid kindling procedure. The ictogenesis was gauged by comparing afterdischarge threshold (ADT) and afterdischarge duration (ADD) before the treatments, after the treatments prior to kindling, and 24 h after kindling. Kindling progression and retention were analyzed using video recording. The results showed that before kindling, LTA produced an ADT reduction. Neither LTA, nor LTA-A affected baseline ADD. On kindling progression, LTA accelerated occurrence of generalized seizures while LTA-A delayed this effect. The treatment with LTA-A reduced the number of secondary generalized complex partial seizures. Twenty four hours after kindling, the rats of both saline and LTA groups showed the increased hippocampal excitability as compared with pre-kindling parameters. Administration of LTA-A prevented kindling-induced increase of hippocampal excitability. The immunostaining revealed that LTA-A attenuated the inflammatory response produced by seizures. These findings suggest that the activation of TLR2 in the hippocampus may facilitate limbic epileptogenesis.
Keywords: Epilepsy, Toll-like receptor 2, Neuroinflammation, Tumor necrosis factor-alpha
Introduction
Clinical and experimental evidence implicate brain inflammation in mechanisms of epilepsy1. Studies of refractory epilepsy in humans show increased brain expression of pro-inflammatory cytokines (IL-1β, IL-6), stress signals and increased circulating levels of inflammatory mediators2. Genetic and pharmacological studies suggests that the increased neuroinflammatory signaling induced by the activation of Toll-like receptors (TLR) contributes to epilepsy3. TLR are important components of innate immunity that recognize pathogens and molecules related to cell damage under stress conditions including epilepsy4. Ten members in the TLR family in humans and twelve members in rodents have been discovered5. TLR recognize pathogen-associated molecular pattern (PAMP) of microbial origin5. TLRs 1, 2, 4, 5, and 6 bind to components of microbial cell walls, such as lipopolysaccharide (LPS, the endotoxin of Gram-negative bacteria), lipoproteins from the cell membrane, flagellin, and lipoteichoic acid (LTA)6. LTA is an amphiphilic, negatively charged glycolipid anchored in the membrane of Gram-positive bacteria, and plays an important role in related infections and post-infectious sequelae7. Previous studies have shown that the expression of TLR2 is increased following kainic acid-induced seizures and studies of TLR2-knock out mice reveal that TLR2 signaling promotes glia activation and contributes to neuronal death following kainic acid exposure in vitro8. Moreover, in the absence of pathogens that produce infection, as it occurs in the aseptic inflammation process, TLR inflammatory response is mediated by endogenous damage-associated molecular pattern (DAMP) molecules including high-mobility group box 1 (HMGB1), S100 calcium binding protein B (S100B), and heat shock proteins (HSP). In the central nervous system (CNS), HMGB1 is secreted by injured and necrotic cells, acting as a proinflammatory molecule binding its respective receptors (TLR2 and TLR4) expressed by glia5.
While TLR3 and TLR4 have been extensively examined in epilepsy9, the role of TLR2 signaling in the pathophysiology of the epileptic process has not been fully explored. We investigated the modulatory effects of a TLR2 agonist (LTA), mimicking a Gram-positive infection, and its antibody (LTA-A) on seizures in an animal model of limbic epilepsy, hippocampal rapid kindling.
Methods
Animals
Fifty day old male Wistar rats were used at the onset of experiments and maintained under standard vivarium conditions (temperature 20–26 °C, humidity 30–70%, 12 h light–dark cycles, food and water ad libitum). All experiments adhered to NIH standards and guidelines were approved by the UCLA Animal Research Committee.
Surgery and Afterdischarge properties
Under isoflurane anesthesia, rats were stereotaxically implanted with a cannula guide-electrode (internal diameter 0.39 mm, PlasticsOne, Roanoke, VA) on the CA1 area of left ventral hippocampus (4.8 mm posterior and 5.3 mm lateral to bregma, 6.5 mm ventral)10. A tripolar electrode for EEG recording (PlasticsOne) was placed 3 mm anterior to bregma with the ground connected to a screw on the nasal bone. The implantation site was identified by histology using Nissl staining.
Seven-days after surgery, the animals were connected to the DS8000 electrical stimulator via DSI100 stimulus isolators (World Precision Instruments, Sarasota, FL) and to the MP100/EEG100B acquisition system (BIOPAC, Santa Barbara, CA). The afterdischarge threshold (ADT), defined as a response of at least 2 seconds long at the end of the stimulation (Fig. 1A), was detected by the application of trains of electrical stimulations (series of 10 seconds train duration, 20 Hz, 1 ms pulse duration, square-wave monophasic stimuli, starting 0.1 mA, at 0.1 mA increments, applied every 10 minutes). The afterdischarge duration (ADD) was also recorded, and measured off-line.
Figure 1.
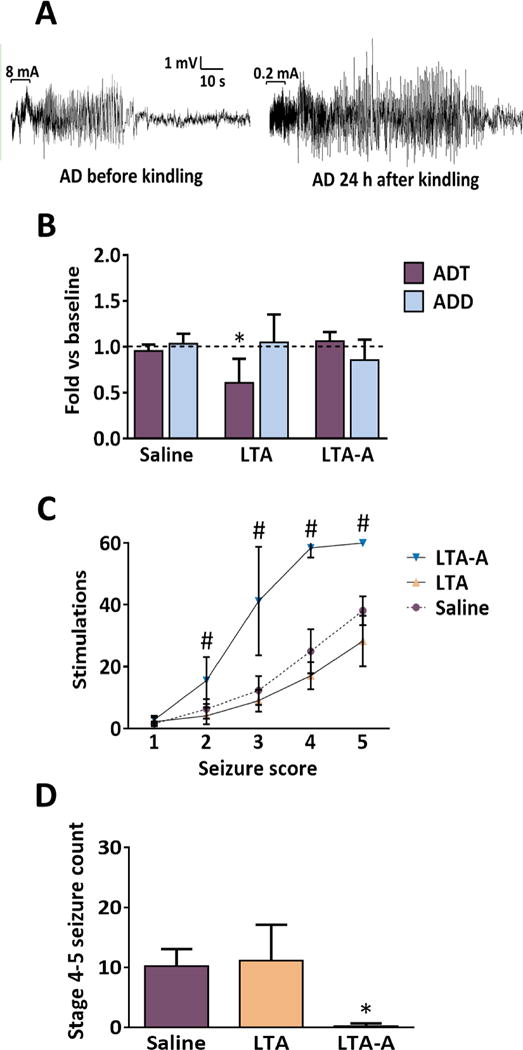
A. Sample EEG recordings obtained before (left) and 24 hours after (right) kindling from a saline treated rat in response to threshold stimulation. Horizontal brackets on the first part of each recording show the stimulus train and the numbers on the top of the brackets indicate the applied current. B. Only the rats of LTA group showed a decreased ADT as compared with the baseline values, LTA-A and saline groups. Neither LTA, nor LTA-A affected the ADD before rapid kindling as compared with baseline. Data are presented as Mean ± SD and are expressed as fold-changes vs. pre-kindling values. For statistical comparisons, absolute values were used. *p<0.05 vs. respective saline group (One way ANOVA followed by Tukey’s test). C. The LTA-A delayed manifestation of the Racine’s stages excluding focal seizures, #p<0.001 vs the rest of the groups. D. Treatment with LTA-A significantly reduced the number of stage 4–5 seizures during rapid kindling. However, the intracerebral administration of LTA did not affect the number of the same behavioral seizures, *p<0.001 vs Saline group. Data are presented as Mean ± SD. (One way ANOVA followed by Tukey’s test).
Drugs and treatments
Thirty minutes after the afterdischarge evaluation, the animals were randomly assigned to one of three treatment groups: normal saline (vehicle, 1 μl, n=9); LTA (Sigma, St. Louis, MO; 50 μg in 1 μl of saline vehicle, n=9), LTA-A (Thermo Fisher Scientific, Waltham, MA; 0.1 μg in 1 μl of saline vehicle, n=9). Concentrations were chosen considering previous in vitro studies showing the neuroinflammatory activation of astrocytes by LTA7 and the activation of sensitized monocytes by LTA-A11. We used concentrations approximately ten-fold lower than those reported as systemic pro-inflammatory to ensure brain specific effects. The drugs were administered at a rate of 0.2 μl/min, using a 2-μl Hamilton microsyringe mounted onto microinfusion pump model sp310i (WPI, Sarasota, FL). The animals received three injections at 24-hour intervals. Thirty minutes after the last injection, ADT and ADD values were detected for the second time to assess the effect of the treatments before performing rapid kindling.
Rapid Kindling
Ten minutes after the ADT detection, the animals received 60 trains delivered every 5 min, using the same parameters previously described, starting 50 μA above the ADT. The rats were continuously video-recorded during the entire procedure. Behavioral seizure responses to each stimulation and the number of stimulations required to reach each behavioral phase were quantified blindly off-line using the Racine’s scale12. For statistical analyses, when the rats skipped one of the consecutive seizure stages, the current value necessary to reach the subsequent stage was designated to the omitted phase (i.e., from stage 2 to stage 4 avoiding stage 3; the same result for stages 3 and 4 was assigned); and when the animals did not display any of the five-stage score, 60 was assigned. The ADT and ADD were gauged once more 24 h after kindling.
TNFα immunohistochemistry
The expression of TNFα has been shown to be upregulated by seizures13. We analyzed TNFα expression upon LTA-A administration and kindling-induced seizures in a separate group of rats. Thirty minutes after the last ADT/ADD detection, rats were anesthetized with pentobarbital (100 mg/kg, i.p.) and perfused with saline followed by 4% phosphate-buffered paraformaldehyde. Brains were post-fixed for 2 h and cryoprotected in 30% sucrose. Immunofluorescence was performed in 40-micrometer thick coronal sections of the hippocampus (1–2 mm anterior to implantation site), cut on the Leica sliding microtome, using primary goat polyclonal antibody against TNFα (1:200, AF-510-NA R&D SYSTEMS, Minneapolis, MN) and donkey anti-goat secondary antibody, Alexa Fluor® 488 conjugate (1:500, Thermo Fisher Scientific). Sections were examined using the Leica microscope equipped with a Micropublisher 5 digital camera (QImaging, Barnaby, BC, Canada). Counting of TNFα positive cells was performed bilaterally in the pyramidal layer of the subiculum-CA3 region of the dorsal hippocampus. The average of the number of injured cells was obtained from six randomly selected sections per animal.
Statistical analysis
Data were analyzed using the Prism 6.0 software (GraphPad Software, San Diego, CA). Each experimental group passed the D’Agostino-Pearson normality test. Statistical analysis was carried out using one-way ANOVA, followed by Tukey’s post-hoc test. Data are presented as Mean ± Standard Deviation of Mean (SD). A confidence level of 95% was accepted as significant.
Results
Effect of LTA and LTA-A on ADT/ADD on baseline hippocampal excitability
Thirty minutes after the completion of the 3-days treatment (before kindling procedure), the animals of the LTA group showed the decreased ADT as compared with the baseline values. The LTA-A had no effects on baseline excitability. None of the administered treatments affected ADD (Fig. 1B).
Kindling progression
Intrahippocampal administration of LTA reduced the number of stimulations required to reach the first stage 4–5 seizure. Conversely, treatment with LTA-A both delayed the onset and reduced the number of secondary generalized complex partial seizures. Furthermore, the antibody reduced the number of stage 2 and 3 seizures (Fig. 1C,D).
Kindling retention
Twenty four hours after kindling the rats of both saline and LTA groups showed increased hippocampal excitability as compared with pre-kindling parameters. There were no differences between the animals of saline and LTA groups in terms of both ADT decrease and ADD increase, induced by kindling. At the same time, the LTA-A administration prevented kindling-induced increase of hippocampal excitability, judging by both ADT and ADD (Fig. 2A).
Figure 2.

A. The animals of saline and LTA groups showed increased hippocampal excitability as compared with the baseline values 24 hours after kindling, but the LTA-A group showed a significant suppression of this effect returning to pre-kindling values. The treatment with the LTA and saline resulted in longer-lasting kindling-induced activity compared to baseline data. The LTA-A treated group of animals returned to baseline. Data are presented as Mean ± SD and are expressed as fold-changes vs. prekindling values. *p<0.05 vs. respective saline group (One way ANOVA followed by Tukey’s test). B. The rapid kindling induced a pro-inflammatory response increasing the number of hippocampal cells that express TNFα. However, the intracerebral application of LTA-A significantly reduced this effect. Bars represent the Mean ± SD of the number of TNFα positive cells detected in the ipsilateral or contralateral hippocampus. Data were analyzed with One-way ANOVA for independent groups followed by Tukey’s test. *p<0.001 vs. respective naïve group. Photomicrographs show the comparison of TNFα expression in the hippocampal region CA3 of a naïve animal (C) and saline (D) or LTA-A (E) treated rats that underwent rapid kindling. In kindled animals, the LTA-A treatment reduced the number of TNFα positive cells compared to saline administration. Scale bar = 100 μm.
TNFα immunohistochemistry
The number of TNFα positive cells was significantly higher in the hippocampus of kindled animals as compared with the naïve rats. The intracerebral administration of LTA-A attenuated the inflammatory response produced by kindling (Fig. 2B–E).
Discussion
The major findings of these studies are: (a) the LTA reduced the number of stimulations required to reach generalized seizures, but had no significant effects on the AD parameters; (b) LTA-A protected against the development of seizures, and reduced the neuroinflammatory response induced by kindling. These findings suggest that TLR2 may be involved in regulating limbic epileptogenesis, even in the absence of Gram-positive infection. Thus, the activation of TLR2 in the hippocampus predisposed the animals to kindling, by increasing hippocampal excitability. At the same time, immunological blockade of TLR2 through introducing LTA-A reduced both seizure frequency and severity. On the one hand, even though LTA increased hippocampal excitability, it had no effects on kindling retention. On the other hand, there was a strong consistency between the kindling progression and kindling retention results suggesting a protective effect of LTA-A against kindling-induced seizures. The most plausible explanation for the observed effects is that LTA-A administration could have blocked the activity of intracellular DAMPs, HMGB1 (located in the nucleus) or the HSPs and S100 calcium-binding proteins (found in the cytosol). It has been demonstrated by several studies that those DAMPs are synthesized and released by astrocytes and microglia during seizures14, and their signaling can be manipulated to prevent an exaggerated and detrimental inflammatory response. Admittedly, these suggestions should be further corroborated.
TLR2 and TNFα are closely related proteins expressed after an insult, such as seizures3. TLR2 is a cell surface receptor that binds components of the Gram-positive bacterial cell walls and it is a marker of microglial activation during neuroinflammation8,9, while TNFα is a pro-inflammatory cytokine that is quickly released following inflammatory stimuli15. Our results showed that intracerebral application of LTA-A reduced the TNFα expression in the hippocampus of kindled animals, which confirms the close relationship between this cytokine expression and the activation of TLR2 signaling during the neuroinflammatory process induced by seizures. These results confirm earlier observations, such as increased TNFα expression after pilocarpine SE16. It has been shown that after pilocarpine-induced seizures TLR2 and TNFα transcripts were expressed mainly in microglia/macrophages across the cerebral cortex, hippocampal formation, amygdala, thalamus, and hypothalamus following convulsive activity16. We observed that unilateral administration with LTA-A reduced the number of hippocampal TNFα positive cells in both hemispheres. The involvement of the contralateral hippocampus was secondary, and would not occur if epileptic activity in the primary focus is suppressed. Since in our studies, LTA-A was administered prior to the induction of kindling, it is reasonable to assume that the alleviation of epileptic activity in the site of primary stimulation also prevented the spread of this activity to the contralateral side. This suggestion was corroborated by the finding that pretreatment with LTA-A reduced TNFα expression both ipsi- and contralaterally to the stimulation. Other studies have shown that unilateral modulation of epileptic activity inhibits kindling seizures17.
In conclusion, our results suggest the involvement of TLR2 in kindling epileptogenesis and kindling-induced increase of seizure susceptibility. We also demonstrated that the exogenous inhibition of TLR2 signaling reduced hippocampal neuroinflammation associated with seizures.
Acknowledgments
This research was supported by research grant R01NS065783 from the National Institutes of Health to AM. JSMM was supported by postdoctoral fellowships 237168 and 264551 from National Council of Science and Technology (CONACYT, Mexico).
Footnotes
Disclosure of Conflicts of Interest
The authors report no conflicts of interest.
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Vezzani A, Friedman A, Dingledine R. The role of inflammation in epileptogenesis. Neuropharmacology. 2013;69:16–24. doi: 10.1016/j.neuropharm.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravizza T, Gagliardi B, Noé F, et al. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis. 2008;29:142–160. doi: 10.1016/j.nbd.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Vezzani A, Aronica E, Mazarati A, et al. Epilepsy and brain inflammation. Exp Neurol. 2011;244:11–21. doi: 10.1016/j.expneurol.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 4.Vezzani A, Maroso M, Balosso S, et al. IL-1 receptor/Toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures. Brain Behav Immun. 2011;25:1281–1289. doi: 10.1016/j.bbi.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 5.Takeda K, Akira S. Toll-like receptors. In: Coligan JE, editor. Current protocols in immunology. 14.12. Vol. 77. Hoboken, NJ: Wiley; 2007. pp. 14.12.1–14.12.13. [DOI] [PubMed] [Google Scholar]
- 6.Christmas P. Toll-Like Receptors: Sensors that Detect Infection. Nat Educ. 2010;3:85. [Google Scholar]
- 7.Boveri M, Kinsner A, Berezowski V, et al. Highly purified lipoteichoic acid from gram-positive bacteria induces in vitro blood-brain barrier disruption through glia activation: role of pro-inflammatory cytokines and nitric oxide. Neuroscience. 2006;137:1193–1209. doi: 10.1016/j.neuroscience.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Hong J, Cho I-H, Kwak K, II, et al. Microglial Toll-like receptor 2 contributes to kainic acid-induced glial activation and hippocampal neuronal cell death. J Biol Chem. 2010;285:39447–39457. doi: 10.1074/jbc.M110.132522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin N, Tabatabaie O, Falsaperla R, et al. Epilepsy and innate immune system: A possible immunogenic predisposition and related therapeutic implications. Hum Vaccin Immunother. 2015;11:2021–2029. doi: 10.1080/21645515.2015.1034921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paxinos G, Watson C. The Rat brain in stereotaxic coordinates. San Diego, CA: Academic Press; 1986. [Google Scholar]
- 11.Mancuso G, Tomasello F, Ofek I, et al. Anti-lipoteichoic acid antibodies enhance release of cytokines by monocytes sensitized with lipoteichoic acid. Infect Immun. 1994;62:1470–1473. doi: 10.1128/iai.62.4.1470-1473.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 13.Lehtimäki KA, Peltola J, Koskikallio E, et al. Expression of cytokines and cytokine receptors in the rat brain after kainic acid-induced seizures. Brain Res Mol Brain Res. 2003;110:253–260. doi: 10.1016/s0169-328x(02)00654-x. [DOI] [PubMed] [Google Scholar]
- 14.Vénéreau E, Ceriotti C, Bianchi ME. DAMPs from Cell Death to New Life. Front Immunol. 2015;6:422. doi: 10.3389/fimmu.2015.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nadeau S, Rivest S. Regulation of the gene encoding tumor necrosis factor alpha (TNF-alpha) in the rat brain and pituitary in response in different models of systemic immune challenge. J Neuropathol Exp Neurol. 1999;58:61–77. doi: 10.1097/00005072-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Turrin N, Rivest S. Innate immune reaction in response to seizures: implications for the neuropathology associated with epilepsy. Neurobiol Dis. 2004;16:321–334. doi: 10.1016/j.nbd.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Mazarati A, Lundström L, Sollenberg U, et al. Regulation of kindling epileptogenesis by hippocampal galanin type 1 and type 2 receptors: The effects of subtype-selective agonists and the role of G-protein-mediated signaling. J Pharmacol Exp Ther. 2006;318:700–708. doi: 10.1124/jpet.106.104703. [DOI] [PubMed] [Google Scholar]


