Abstract
Objective
High-frequency oscillations (HFOs) are a type of brain activity that is recorded from brain regions capable of generating seizures. Due to the close association of HFOs with epileptogenic tissue and ictogenesis, understanding their cellular and network mechanisms could provide valuable information about the organization of epileptogenic networks and how seizures emerge from the abnormal activity of these networks.
Methods
In this review we summarize the most recent advances in the field of HFOs and provide a critical evaluation of new observations within the context of already established knowledge.
Results
Recent improvements in recording technology and the introduction of optogenetics into epilepsy research has intensified experimental work on HFOs. Using advanced computer models, new cellular substrates of epileptic HFOs were identified and the role of specific neuronal subtypes in HFO genesis determined. Traditionally, the pathogenesis of HFOs was explored mainly in patients with temporal lobe epilepsy and in animal models mimicking this condition. HFOs have also been reported to occur in other epileptic disorders and models such as neocortical epilepsy, genetically-determined epilepsies and infantile spasms, which further support the significance of HFOs in the pathophysiology of epilepsy.
Significance
It is increasingly recognized that HFOs are generated by multiple mechanisms at both cellular and network level. Future studies on HFOs combining novel high-resolution in vivo imaging techniques and precise control of neuronal behavior using optogenetics or chemogenetics will provide evidence about the causal role of HFOs in seizures and epileptogenesis. Detailed understanding of the pathophysiology of HFOs will propel better HFO classification and increase their information yield for clinical and diagnostic purposes.
Keywords: high-frequency oscillations, epilepsy, ripples, fast ripples, ictogenesis, epileptogenesis, seizures, interneurons, computer models
Introduction
The identification of high-frequency oscillations (HFOs) in epileptogenic tissue is one of the major discoveries in epilepsy research over the last two decades, attracting the attention of clinical and experimental epileptologists worldwide. HFOs refer to distinct types of brain activity occurring in a frequency band ranging from 80 Hz to 600 Hz. HFOs are classified as waveforms with frequencies faster than gamma activity (30–80 Hz), and are somewhat arbitrarily separated into ripples (80–250 Hz) and fast ripples (250–600 Hz).1; 2
The presence of HFOs between seizures, at seizure onset and during seizures suggests an inherent relationship between the cellular and network mechanisms of seizures and HFOs. Characterizing the underlying mechanisms of seizures has been notoriously difficult due to technological limitations and the vast spatial and temporal scales involved; however, HFOs occur on a much smaller and experimentally tractable scale, down to 1 mm3.3 As recording technology has improved over the past two decades, much research has focused on the underlying mechanisms of HFOs, both those associated with normal brain processes4 as well as with epileptic processes.1 Understanding the mechanisms of so-called ‘epileptic’ or ‘pathological’ HFOs, should lead to a better understanding of the functional organization of epileptogenic tissue and of the abnormal dynamics of epileptic neuronal networks.
In clinical epileptology and particularly in epilepsy surgery, the identification of a close spatial association between the location of HFOs and the epileptogenic zone and/or the seizure onset zone has led to the incorporation of measurements of HFO properties into presurgical examinations (see the review by Frauscher et al. in this Epilepsia issue). Many epilepsy surgery centers are now equipped with devices and analytical approaches that allow recording of wide-band signals and extraction of relevant information about the spatiotemporal properties of HFOs to better delineate the epileptogenic zone, and are currently performing clinical trials to determine if this information will improve the outcomes of epilepsy surgery. In addition, understanding spatial and temporal relationships of HFOs over long time scales offers the unique opportunity to use them as a clinical marker of epileptogenesis.
Our knowledge of HFO mechanisms has been substantially advanced after the first High-Frequency Oscillation Workshop held in Montreal, Qc, Canada in 2011.1 The main aim of our review is to provide the latest insights into the mechanisms of HFOs and to explore possible future research directions to address the unresolved aspects of HFOs which were presented and discussed during 2nd International Workshop on High Frequency Oscillations in Epilepsy held in Freiburg, Germany in 2016. Our review will focus on the following topics: 1) What are the cellular and network mechanisms involved in the genesis of physiological and pathological HFOs; 2) What is the role of interneurons in the genesis of pathologic HFOs; 3) What is known about the causal role of HFOs in ictogenesis; and 4) What progress has been made in characterizing HFOs in different experimental models of epilepsy.
Cellular mechanisms of epileptic HFOs
After the initial descriptions of HFOs occurring under physiological conditions5; 6, it has later become clear that HFOs are increased in epileptic tissue.3; 7 However, this situation poses an important problem: how can one distinguish between physiological and pathological HFOs?8 One intriguing initial finding was that, in both human conditions and animal models, it appeared that higher frequency HFOs (> 250 Hz), i.e. fast ripples, were specific to epileptic tissue. These findings provided an important start to the search for HFO mechanisms and for the characterization of their role in epilepsy
The past two decades have uncovered several key aspects of HFO mechanisms. One clear result is that HFOs are generated by multiple mechanisms such as synchronized inhibitory postsynaptic potentials with sparse pyramidal cell firing6 or principal cell action potentials.9–11 It is now considered that pathological HFOs, whether they be ripples or fast ripples, reflect mainly principal cell action potentials (Fig. 1).9; 12–15 Synchronization of fast firing within the population of interconnected neurons leads to the formation of an episode of high–frequency population spikes, which is extracellularly recorded as an HFO event (Fig. 1A, B). This mechanism requires synchronization on a millisecond time scale, which is achieved via fast synaptic transmission or non-synaptic mechanisms like gap-junction coupling16 or ephaptic interactions - a synchronizing mechanism that depends on specific geometric organization and tight cellular arrangement.17
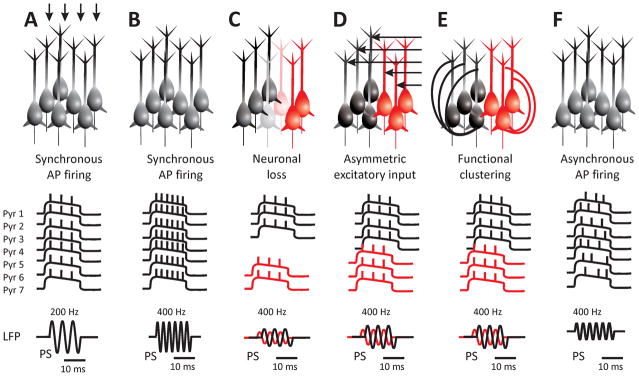
Figure 1. Cellular and network mechanisms of pathological HFOs.
A: HFOs are generated by synchronous action potential firing of principal cells. An individual cycle of an oscillation is a population spike. The frequency of the oscillation is determined by the frequency of the cellular firing – so called ‘pure‘ HFOs. B: Fast ripples, i.e. HFOs with a frequency of up to 600 Hz can be generated, if the cells fire action potentials at the same frequency. Physiological mechanisms underlying the genesis of action potentials limit the rate of cellular firing. Therefore, this mechanism is least probable. C: Out-of-phase firing between two neuronal populations can result in a doubling of the frequency of the extracellularly recorded ‘emergent’ type of HFOs. In this scenario, the out-of-phase firing is due to cell loss. D: Asymmetric excitatory input or polychronicity due to various lengths of axons can result in functional clustering and out-of-phase firing. E: Morphological changes, axonal growth and sprouting may result in functional clustering due to the presence of hub neurons. F: Asynchronous firing within an active neuronal population may result in random co-incidental firing and the occurrence of fast ripples.
The mechanisms establishing the frequency of the oscillation vary. Frequency can be determined purely from the cellular behavior, i.e. from the action potential firing rate of the principal cells. These are called ‘pure’ HFOs.14 However, individual pyramidal cells cannot fire fast enough to produce synchronized oscillations up to 600 Hz, i.e. fast ripples. Even in epileptic neurons the rate of action potential firing is often limited to <300 Hz.14 The currently accepted explanation of fast ripple generation - with frequencies beyond the physiological limits of neuronal firing - is the existence of different subpopulations of synchronized neurons that have a phase delay (Fig. 1C–D).1; 18 Fast ripples may thus represent the net frequency of neuronal populations each oscillating with a lower frequency, and thus they represent an ‘emergent’ HFO.14; 19; 20 Several network mechanisms that are responsible for out-of-phase firing, were proposed based on experimental evidence or results obtained from computer modeling. Reduced spike time-variability13, uncorrelated firing, delayed activation, disconnected neural populations or complex network connectivity patterns with a high level of clustering due to the presence of hub neurons (Fig. 1E) can all contribute to out-of-phase activation. In temporal lobe epilepsy, the rate of fast ripples in the hippocampus correlates positively with the severity of cell loss;21 it has been suggested that the loss of neurons can contribute to out-of-phase firing (Fig. 1C), probably by decreasing the synchronizing effect of ephaptic interactions.13; 21
Research into the precise mechanisms has been limited by recording technology. Even within the very small scale of epileptic fast ripples in the hippocampus, there are thousands of cells involved, far beyond the resolution provided by modern microelectrode arrays. Recent advances in imaging techniques, such as voltage sensitive dyes and calcium imaging, have allowed recordings from many cells simultaneously, but they are still unable to function at the fast time scale necessary for HFOs. Thus, computational models have been necessary to guide research into HFO mechanisms.22 Great care must be taken in both the development and interpretation of computational models to assure they are physiologically grounded and to validate their findings whenever possible. It is also important to note that any modeling result will be limited by the phenomena included within the model itself. When performed properly, modeling predictions can then guide future experiments as technology advances. Several models have used this methodological approach to assess the mechanisms involved in the generation of ripples and fast ripples that could not be tested directly with current technology. Various models of ripples have been proposed and validated experimentally.1 Fast ripples, with their apparent increased specificity to epileptic tissue, have garnered great interest in investigating mechanisms they might share with seizures. However, fast ripples have been very challenging to explain, even in silico.
Previous modelling work had identified three potential mechanisms of fast ripples: gap junction networks23, desynchronous groups of firing cells13; 14 or activated, but weakly synchronized pyramidal cells.12 Other studies have described how physiological HFOs could transition to epileptic HFOs with pathologies such as loss of inhibition, or increased coupling from gap junctions, or recurrent synapses and increased synaptic activity.24; 25 A recent detailed model, designed to mimic how both pyramidal action potentials and inhibitory postsynaptic potentials (IPSP) appear on an intracranial recording electrode, demonstrated several important predictions about HFOs.26 First, at frequencies below 250 Hz, both epileptic and physiological processes can produce HFOs with identical peak frequencies, suggesting that they cannot be distinguished by frequency alone. However, the conditions that produce epileptic ripple oscillations (disrupted inhibitory coupling) often produce brief instances of fast ripples that emerge spontaneously. Second, they demonstrated mathematically that it is impossible for IPSPs to generate oscillations > 250 Hz due to their morphology.
Another important modelling finding is the growing evidence from several groups that fast ripple oscillations are a generic feature of highly active, desynchronized networks.12; 26 These in silico observations are corroborated by the experimental evidence in chronic models of epilepsy and in human epileptic tissue.14; 27; 28 These diverse mechanisms lead to a similar conclusion about the relationship between epileptic spikes and epileptic HFOs: large groups of hyper-excitable, hyper-synchronized pyramidal cells produce spikes, but if the connectivity of cells is disrupted, then cells would fire out of phase and produce fast ripples.
The findings that have been reviewed above should explain how the neuronal network can synchronize and produce HFOs. However, recent work has shown that HFOs can be generated even in the absence of any network connectivity.26 These results were recently proven analytically15, and though they may seem counterintuitive to the neuroscience and HFO community, they are a well-known phenomenon in complex dynamics. Essentially, a synchronous discharge from an ensemble of cells (which results in a local field potential) does not require any network synchrony or coupling at all, only that the neurons be actively firing at similar frequencies (Fig. 1F). The superposition of many neurons firing at a certain frequency, even if they are randomly distributed in phase, will be an oscillation at that frequency. Thus, HFOs may simply represent a marker of highly activated neurons, regardless of the underlying structure or mechanism. A similar phenomenon was proposed based on observations in vitro in low-calcium or high-potassium models of seizures, where intense neuronal activity may lead to co-incidental firing among cells, which manifests as high-frequency activity in the ripple band.20
An interesting result from recent in vitro studies demonstrates the ambiguous behavior of neuronal networks underlying the genesis of HFO under various experimental conditions. For instance, the same neuronal network can have different IPSP and EPSP dynamics during ripple-like activity, depending on extracellular concentration levels of Ca2+.29 These authors reported that under 3 mM Ca2+, the frequency of the HFO was 126 ± 13 Hz and correlated with coordinated firing of putative pyramidal cells and interneurons; instead, under 1 mM Ca2+, the frequency was slightly higher (200–300 Hz), with an important increase in firing of cells.29 Similarly, Alvarado-Rojas et al. reported two types of neuronal behaviour correlated with similar-appearing HFOs in the human subiculum tissue maintained in vitro.27 In this case, ripple-like HFOs (100–250 Hz) were produced by either strong rhythmic inhibitory postsynaptic potentials or strong synaptic depolarization with pyramidal cell bursting. Thus, in agreement with the modelling predictions, the frequency of the HFO may not be a valid method to determine the underlying mechanism or epileptogenicity. Similarly, microelectrode recordings from human tissue have shown that putative pyramidal cells and interneurons have complex behaviors, wherein some cells become more or less active, and often do not appear to synchronize during HFO occurrence.27; 30 Therefore, cell activity is not necessarily synchronized, but heterogeneous firing is a common behaviour for pathological HFOs. These findings in human networks support the results from in vitro and computational models.
It is well documented that ripples can be either physiologic or pathologic. However, fast ripples were at first thought to be reliable markers of epileptic tissue. It is now clear that the activity in fast ripple band can accompany cognitive processes in humans.31 Given the broad range of pathologies, species, and brain regions that produce HFOs2; 32, it is likely that HFOs are a generic phenomenon of neural networks that have some complex relationship with epileptic processes. While their value as a potential biomarker is very strong, it is clear that simply focusing on the peak frequency of HFOs may not be sufficient to determine whether they are pathological.
The role of interneurons in HFOs
The role of individual interneuronal subtypes has been described in detail in the context of physiological gamma oscillations and sharp-wave ripples.4; 33 These oscillations are driven by interneuronal activity and can facilitate temporal coding, fast processing and flexible routing of neuronal activity, which are necessary for cognition.34 Such interneuronal activity makes principal cells generate a series of fast inhibitory postsynaptic potentials that are extracellularly recorded as physiological HFOs (Fig. 2A). Surprisingly, the role of interneurons or individual interneuronal subtypes in pathological HFOs is not so well defined. HFOs often occur superimposed on interictal epileptiform discharges, which display very complex interneuronal activity and have been extensively evaluated by Karlocai et al. in various in vitro models35; these investigators found that while dendritic interneurons and cholecystokinin-positive basket cells increase their firing rate, perisomatic inhibition fails due to depolarization block of parvalbumin-positive neurons. Unfortunately, in their study they did not examine the phase relationship with superimposed HFOs. Recently, Morris et al. evaluated the role of interneurons in the CA3 region during pathological ripples using brain slices with fluorescently labeled interneurons perfused with high-potassium artificial cerebrospinal fluid.36 Approximately 42% of interneurons increased firing during individual cycles of ripple oscillations, suggesting that GABAergic signaling is preserved on a cycle-by-cycle basis during ripples (Fig. 2B).
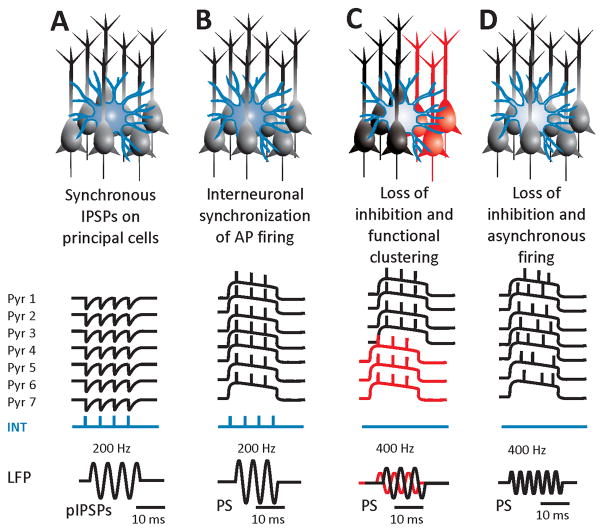
Figure 2. The role of interneurons in HFOs.
A: HFOs may represent extracellularly recorded, synchronous, inhibitory, postsynaptic potentials on the membranes of principal cells. This mechanism underlies physiological sharp-wave ripples. Pathologically, it may be involved in low-amplitude fast activity ictal onset but usually with frequencies lower than ripples. B: In epileptic ripples, the interneuronal activity and inhibitory postsynaptic potentials control the action potential firing of the active epileptic population, which manifests as pathological ripples. This mechanism is dependent on intact peri-somatic inhibition maintained by basket cells. C: Loss of inhibition may play a role in the pathogenesis of fast ripples. The absence of rhythmogenic fast inhibition may result in functional clustering or asynchronous neuronal firing and the generation of fast ripples (D).
While inhibition may be important for ripples, it seems that fast ripples do not depend on intact fast inhibition. Computational studies revealed that reduction of GABAergic connections from basket cells on pyramidal neurons26, or moderate reduction in GABAergic conductance with a simultaneous moderate increase in NMDA conductance12 favored the increased probability of the emergence of fast ripples from ripple activity (Fig. 2C, D). Further support to these experimental observations comes from a model of temporal lobe epilepsy induced by intrahippocampal injection of tetanus toxin.37 Tetanus toxin leads to complete abolition of fast inhibitory transmission around the site of injection by blocking neurotransmitter release, yet the hippocampus was still able to generate fast ripples.38 The evidence obtained from all these studies supports the hypothesis that fast ripple generation does not require intact inhibition and that fast ripples may mark regions with eroded inhibition; this point may also explain the presence of fast ripples at the onset of some types of focal seizures such as those presenting with hypersynchronous onset (see below).
HFOs in ictogenesis
HFOs are involved in seizure genesis prior to1; 20; 39; 40, and during41; 42 the seizure onset. The precise role of HFOs appears to depend on the type of seizure onset pattern.42 Several distinct seizure onset patterns have been reported43 and two of these patterns display clear association with HFOs. The low voltage fast (LVF) seizure onset pattern consists of low-voltage beta and gamma oscillations > 12 Hz44, can be seen in both limbic and neocortical seizures, and can occur during both seizure genesis and spread.40; 43 Inhibitory interneurons play a central role in orchestrating LVF onset.45–47 Intracellular recordings from slices of entorhinal cortex48; 49, and the guinea pig whole brain preparation45 have shown that at LVF onset, principal neurons generate robust IPSPs48 and few action potentials. These events are followed by excitation associated with a dramatic increase in firing rate in principal cells. In support of the fundamental role played by inhibitory interneurons in promoting LVF seizure genesis, optogenetic activation of parvalbumin- or somatostatin inhibitory cells in the mouse entorhinal cortex slice bathed in 4-aminopyridine triggered LVF onset seizures.46 LVF activity is typically associated with ripples band.40–42. The likelihood that the superimposed pathological ripple activity is also generated by inhibitory interneurons is corroborated but optogenetic experiments, which demonstrated that light stimulation of parvalbumin interneurons triggers LVF seizures associated with ripples.42
The hypersynchronous (HYP) onset pattern consists of sharply contoured high voltage ictal discharges that occur at a frequency of <2 Hz.44 HYP onset seizures appear to begin exclusively in limbic structures43, and often in the setting of mesial temporal sclerosis.50; 51. Typically, fast ripples precede or accompany HYP discharges.39; 40. From the cellular perspective, HYP onset involves increased principal neuron firing and a progressive weakening of inhibition. Intracellular recordings from principal neurons in the perirhinal cortex during application of 4-aminopyridine have shown that principal neurons generate action potential bursts during the HYP discharge as well as that the post-burst hyperpolarization (presumably caused by activation of post-synaptic GABAA receptors) progressively decreases in amplitude. This phenomenon is accompanied by a gradual positive shift in the reversal potential of the post-burst hyperpolarizations along with an increase in the associated transient elevations in [K+]o.52 In such a scenario, superimposed epileptic fast ripples are assumed to mirror action potential firing of principal cells.3; 9; 53
Studies of human seizures using devices capable of resolving multiunit and single unit activity have revealed that within brain regions demonstrating the EEG signature of a seizure, two territories can be defined based on characteristic multiunit firing patterns. The relatively small ictal core is defined by high amplitude EEG discharges corresponding to synchronized, intense multiunit firing bursts, occurring in the context of paroxysmal depolarizing shifts. In the penumbra, dominated by surround inhibition54; 55, firing is sparse and heterogeneous, unrelated to the ongoing EEG rhythm.56; 57 This distinction may provide a useful framework for interpreting HFO activity detected during seizures.58
Trains of high-gamma band phase-locked to the dominant rhythm of ictal discharges can pinpoint the location of the ictal core.58 The high-gamma band activity is characterized by a delayed onset, several seconds after the start of the seizure, and sustained activity through the remainder of the seizure. In this type of seizure onset, mathematical modelling suggests that the superimposed high-gamma reflect a slightly jittered but otherwise synchronous summation of strong postsynaptic currents generated by paroxysmal depolarization shifts on the membrane of intensely firing principal cells.59 In the surrounding penumbra, pyramidal cell firing is not sufficiently synchronized to generate a high gamma signature, resulting in an absence of ictal high-gamma activity in this region. Thus, the ictal core can be effectively distinguished from the penumbra by the presence of sustained trains of ictal high-gamma activity. This potentially useful EEG interpretation method has been shown to be an effective predictor of surgical outcome, with a trend toward superior performance compared to a predictor based on the seizure onset zone.30
Recently, the relationship between HFOs and the DC shift during the seizure onset in patients with implanted electrodes was explored.60 This study has demonstrated that the seizure onset was accompanied by a negative DC shift and co-localized with the presence of HFOs (from both ripple and fast ripple bands). The DC shift onset slightly preceded the onset of HFOs, and both electrographic phenomena were more spatially limited compared to conventionally defined seizure onset zones.
HFOs in epileptogenesis
The first study of HFOs during the development of epilepsy was performed in the intrahippocampal kainic acid rat model.61 In this study, microelectrode recordings showed the appearance of ripple- and fast ripple-frequency HFOs in the dentate gyrus ipsilateral to the injection in rats that subsequently developed spontaneous seizures, whereas HFOs were not found in those kainic acid treated animals that did not develop epilepsy. In addition, an earlier appearance of HFOs after status epilepticus correlated with a shorter latency and earlier appearance of spontaneous seizures as well as with a higher rate of seizures per month. These results support the hypothesis that the neuronal disturbances associated with pathological HFOs play a role in epileptogenesis. Surprisingly, not so many studies have examined this predictive role of HFOs, which - if confirmed in humans - could substantially impact the clinical approach to patients at risk of developing epilepsy after brain trauma, stroke or other insults.
It has also been reported in this animal model of temporal lobe epilepsy that following the initial pilocarpine-induced status epilepticus, there is a shift in the occurrence of both interictal spikes and HFOs between the hippocampus and the entorhinal cortex at the transition from the latent to the chronic period.62 In addition, in this study, it was found that fast ripples occurring outside of spikes had higher rates in the entorhinal cortex than in the hippocampus during the latent phase while the opposite occurred during the epileptic phase.62 This transition may thus reflect the dynamic activity and progressive pathological reorganization of limbic neuronal networks during epileptogenesis.
Once epilepsy develops, HFOs may also be used as a marker of disease activity. Both experimental and clinical studies have shown that the incidence of HFOs correlates with seizure frequency and introduction or withdrawal of antiepileptic drugs can affect both seizure occurrence and HFO rate.63; 64 Elucidation of the long-term dynamics of HFOs is a challenge that needs to be resolved as well as clarification of whether HFOs can be used prospectively as a screening technique to predict epilepsy and whether they are a better marker of disease activity and epilepsy remission than the traditional hallmark, i.e., interictal spikes. Further work is also necessary to establish the response of HFOs to antiepileptic drugs. In general, information about the response of HFOs to antiepileptic drugs is currently lacking, but ongoing studies are examining this clinically important topic. For instance, in the pilocarpine model, seizures and HFO rates decreased following the application of levetiracetam64 or lacosamide.65 The response to antiepileptic drugs has the potential to be used as a diagnostic test to differentiate between pathological HFOs and physiological ones.
HFOs in models of epilepsy
HFOs in the kainic acid and pilocarpine model of temporal lobe epilepsy
Much of our understanding of pathological HFOs derives from studies carried out in the intra-hippocampal kainic acid model of human temporal lobe epilepsy.7; 66 Most of these data have been summarized in previous reviews.1; 67 More recently, investigators used a unilateral supra-hippocampal injection of kainic acid in mice that produced cell loss in the ipsilateral CA3, CA1, and hilar regions, as well granule cell dispersion and mossy fiber sprouting within two weeks after the injection.68 The contralateral hippocampus, however, was relatively unaffected. Overall the histopathological changes seen in these experiments along with the appearance of seizures from the injected hippocampus reproduced several aspects of human TLE. Notably, silicon probes recorded pathological HFOs (200–600 Hz) as early as four days after status epilepticus. During the development of epilepsy, pathological HFOs in the CA1 and DG occurred in stable bursts, but the duration and spectral frequencies evolved from relatively brief bursts of low spectral frequency (~200 Hz) to longer duration bursts that contained higher frequencies between 400 and 450 Hz. The changes in duration and spectral power of pathological HFOs could correspond with progressive neuronal disturbances associated with epileptogenesis.
The role of HFOs in ictogenesis and epileptogenesis has been further explored in the pilocarpine model of temporal lobe epilepsy. Pilocarpine-treated epileptic rats present with recurrent, spontaneous seizures that are characterized (even in the same animal) by LVF or HYP onset patterns.41; 69 As already stated in a previous section, these in vivo experiments have demonstrated that LVF onset seizures have a prevalence of ripples preceding the seizure, while in HYP onset seizures ripples predominate. Therefore, these findings suggest that different cellular or network mechanisms are responsible for initiating these two seizure onset patterns in the pilocarpine model of temporal lobe epilepsy
Development of epilepsy after traumatic brain injury
There are several models of traumatic brain injury that can be associated with varying degrees of focal or diffuse damage. Focal injuries typically include contusion, lacerations, intracranial hematoma, while diffuse ones are usually in the form of widespread axonal, neuronal or microvascular damage.70 A well characterized model of post-traumatic epilepsy, is the fluid percussion injury (FPI) rat model 71. Most of the histological studies in the FPI model were carried out within two months post-injury and have reported neurodegeneration, neurogenesis, astrocytosis, microgliosis, axonal and myelin injury, axonal sprouting, vascular damage and angiogenesis within and surrounding the injured cortex and the underlying hippocampus and thalamus.
Electrophysiological recordings obtained immediately after traumatic brain injury (i.e. <24 hr post-injury) or later (<7 days), either in hippocampal slices maintained in vitro or in in vivo preparations, have shown hippocampal hyperexcitability and early seizures, while at > 7 days after injury, similar recordings confirmed increased excitability and spontaneous seizures; these latter findings are the defining characteristics of post-traumatic epilepsy.72; 73 In FPI rats, a microelectrode and cortical screws recorded pathological HFOs (100–600 Hz) in cortical areas adjacent to or within the injury core during the first two weeks following injury.74 Moreover, pathological HFOs have been reported to occur in almost 60% of FPI rats, but in none of the control rats. In FPI rats that had completed long-term monitoring, pathological HFOs were found in only those rats that later developed post-traumatic seizures, but were not observed in rats that did not develop late seizures.74
A new phenomenon was also observed by Bragin et al. in this study.74 They found that a complex of repetitive HFOs superimposed on arcuate-shaped 10–16 Hz activity. These repetitive HFOs reflected population spikes with hypersynchronous multi-unit firing, and were only found in those FPI rats that later developed seizures. There were clear differences between repetitive HFOs and sleep spindles, both of which were found in FPI rats, as well as in spike-and-wave discharges observed in some rodent strains. The appearance of repetitive HFOs could indicate disturbances in thalamo-cortical circuits and their presence in cortical screw recordings only in FPI rats that develop late seizures make them a candidate for a non-invasive biomarker of post-traumatic epileptogenesis.
HFOs in the tetanus toxin model of temporal and neocortical epilepsy
The tetanus toxin model is a well-established model of temporal lobe epilepsy in which injection of a minute dose of tetanus toxin into the CA3 region of the hippocampus cause a chronic epileptic condition.37; 38; 75 Approximately one week after tetanus toxin injection the animals develop spontaneous and repeated seizures, which closely resemble complex partial seizures in humans, and can become secondarily generalized. In the tetanus toxin model, HFOs are observed between seizures, at their onset, and during their course. While ripples are generated in both hippocampi, fast ripples occur predominantly or exclusively in the injected hippocampus.37 Studies in the kainate model of epilepsy and in humans suggested that cell loss could be one of the key mechanisms involved in the pathogenesis of fast ripples.13; 21 However, experimental evidence obtained from the tetanus toxin model has shown that fast ripples can be generated even in the absence of obvious hippocampal sclerosis and major cell loss.37
Tetanus toxin injected into the neocortex is currently considered a model of neocortical epilepsy. When injected into the motor cortex, it manifests with partial motor status resembling epilepsia partialis continua, in which frequent interictal discharges are interspersed with seizures. It has been reported that in this model the neocortex also generates HFOs in the 70–160 Hz band, but no study has examined the presence of fast ripples in this model or explored the cellular mechanisms.76
HFOs and model of infantile spasms
Infantile spasms are a specific type of seizure in the developing brain associated with pathognomonic spasms, severe cognitive decline, intellectually disability, distinct EEG patterns known as hypsarrhythmia, and poor response to antiepileptic drugs. Chronic injection of the voltage-gated sodium channel antagonist, tetrodotoxin during the early postnatal period (postnatal days 10–12), is one model of infantile spasms. Seizures in this experimental model physically mimic the spasms and produce hypsarrhythmia on EEG. HFOs with a frequency up to 600 Hz occur at the onset of seizures and during the interictal period in this experimental condition.77; 78 From the spatial perspective, HFOs were distributed over large areas of the neocortex of both hemispheres, but with amplitude predominance in the neocortex, contralateral to the tetrodotoxin injection site. An increasing intensity in HFOs was associated with an increased probability of transition to infantile spasms. These observations are in agreement with clinical studies, which demonstrated the presence of HFOs during both spasms and interictal EEG in infants diagnosed with West syndrome.79
Future directions of HFOs research
This review shows the complexity of the “problem” that reflects how HFOs (and associated time-frequency features) are likely generated by multiple, possibly not exclusive, mechanisms at both cellular and network level. To fully elucidate the relationship between these observations and the presumptive underlying mechanisms, further studies are needed combining physiologically-based models with experimental in vivo or in vitro techniques able to specifically target model-predicted key factors in small-scale networks. Future studies should also resolve unanswered questions about pathological HFOs and bring direct evidence firmly demonstrating whether cellular, and network mechanisms underlying pathological HFOs play a causal role in ictogenesis and whether HFOs are capable of inducing permanent changes in brain structure and function that leads to the development of spontaneous seizures.
In recent years we have observed dramatic advances in the development and application of optogenetic or chemogenetic techniques that allow neuron-specific and precise spatiotemporal control of neuronal activity, and optical labelling that allows monitoring thousands of cells simultaneously. Immediately after their discovery, it was predicted that these sophisticated techniques could determine the exact role of individual neuronal subtypes in HFOs, particularly in fast ripples. However, to date there have only been limited findings from these studies.
Much of the knowledge about the role of HFOs in animal models of epilepsy has been obtained from those presenting with a hippocampal origin, and less attention has been paid to extra-hippocampal structures such as the neocortex. Whether similar pathophysiological principles are involved in HFOs of neocortical origin needs to be clarified. This work, in combination with studies examining the causal role of HFOs in epileptogenesis, is important for establishing future directions of HFO research.
Identifying the various HFO generating mechanisms may lead to assigning specific pathophysiological interpretations to distinct HFO patterns detected in clinical recordings, accessible with currently available EEG acquisition systems. This will enable expanding HFO classification beyond the traditional distinction between ripples and fast ripples, to include the fine details of the clinical, EEG, and pathophysiological context. Although technical barriers to clinical use of HFOs still need to be addressed, a systematic approach to clinical HFO interpretation will help to establish their role as an adjunct to traditional EEG interpretation.
Key points.
This review examines recent insights into the cellular and network mechanisms of HFOs.
HFOs are likely generated by multiple, possibly not exclusive, mechanisms occurring at the cellular and network level.
Interneurons play a complex role in epileptic HFOs, and their involvement is dependent on the subtype of HFOs
Cellular mechanisms and features of HFOs associated with seizure initiation are determined by the nature of the seizure onset type.
Epileptic HFOs are widely abundant phenomenon which can be observed in various types of epilepsies ranging from temporal lobe epilepsy, post-traumatic epilepsy to epilepsies occurring during development.
Acknowledgments
The original work reviewed here was supported by the grants from DFG JA1725/3-1, the Ministry of Health of the Czech Republic (AZV 15-29835A), the Czech Science Foundation GACR 14-02634S, the Neuron Fund for Support of Science 001/2012, the Canadian Institutes for Health Research (Grants 8109 and 74609,MA), CURE (MA), the Savoy Foundation (MA), and National Institutes of Health R01-NS094399 (WS). We would like to thank Dr. Shannon Weiss for his comments and valuable feedback. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Footnotes
Conflict of interests
MA received a grant for pre-clinical studies from UCB PHARMA. The remaining authors have no conflicts of interest.
References
- 1.Jefferys JG, Menendez de la PL, Wendling F, et al. Mechanisms of physiological and epileptic HFO generation. Prog Neurobiol. 2012;98:250–264. doi: 10.1016/j.pneurobio.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zijlmans M, Jiruska P, Zelmann R, et al. High-frequency oscillations as a new biomarker in epilepsy. Ann Neurol. 2012;71:169–178. doi: 10.1002/ana.22548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bragin A, Mody I, Wilson CL, et al. Local generation of fast ripples in epileptic brain. J Neurosci. 2002;22:2012–2021. doi: 10.1523/JNEUROSCI.22-05-02012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buzsaki G. Hippocampal Sharp Wave-Ripple: A Cognitive Biomarker for Episodic Memory and Planning. Hippocampus. 2015;25:1073–1188. doi: 10.1002/hipo.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buzsaki G, Horvath Z, Urioste R, et al. High-frequency network oscillation in the hippocampus. Science. 1992;256:1025–1027. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- 6.Ylinen A, Bragin A, Nadasdy Z, et al. Sharp Wave-Associated High-Frequency Oscillation (200-Hz) in the Intact Hippocampus - Network and Intracellular Mechanisms. J Neurosci. 1995;15:30–46. doi: 10.1523/JNEUROSCI.15-01-00030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bragin A, Engel J, Jr, Wilson CL, et al. Hippocampal and entorhinal cortex high-frequency oscillations (100--500 Hz) in human epileptic brain and in kainic acid--treated rats with chronic seizures. Epilepsia. 1999;40:127–137. doi: 10.1111/j.1528-1157.1999.tb02065.x. [DOI] [PubMed] [Google Scholar]
- 8.Engel J, Jr, Bragin A, Staba R, et al. High-frequency oscillations: what is normal and what is not? Epilepsia. 2009;50:598–604. doi: 10.1111/j.1528-1167.2008.01917.x. [DOI] [PubMed] [Google Scholar]
- 9.Bragin A, Benassi SK, Kheiri F, et al. Further evidence that pathologic high-frequency oscillations are bursts of population spikes derived from recordings of identified cells in dentate gyrus. Epilepsia. 2011;52:45–52. doi: 10.1111/j.1528-1167.2010.02896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Antuono M, de GP, Kano T, et al. Ripple activity in the dentate gyrus of dishinibited hippocampus-entorhinal cortex slices. J Neurosci Res. 2005;80:92–103. doi: 10.1002/jnr.20440. [DOI] [PubMed] [Google Scholar]
- 11.Traub RD, Contreras D, Cunningham MO, et al. Single-column thalamocortical network model exhibiting gamma oscillations, sleep spindles, and epileptogenic bursts. J Neurophys. 2005;93:2194–2232. doi: 10.1152/jn.00983.2004. [DOI] [PubMed] [Google Scholar]
- 12.Demont-Guignard S, Benquet P, Gerber U, et al. Distinct hyperexcitability mechanisms underlie fast ripples and epileptic spikes. Ann Neurol. 2012;71:342–352. doi: 10.1002/ana.22610. [DOI] [PubMed] [Google Scholar]
- 13.Foffani G, Uzcategui YG, Gal B, et al. Reduced spike-timing reliability correlates with the emergence of fast ripples in the rat epileptic hippocampus. Neuron. 2007;55:930–941. doi: 10.1016/j.neuron.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 14.Ibarz JM, Foffani G, Cid E, et al. Emergent dynamics of fast ripples in the epileptic hippocampus. J Neurosci. 2010;30:16249–16261. doi: 10.1523/JNEUROSCI.3357-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gliske SV, Stacey WC, Lim E, et al. Emergence of Narrowband High Frequency Oscillations from Asynchronous, Uncoupled Neural Firing. Int J Neural Systems. 2017:27. doi: 10.1142/S0129065716500490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Draguhn A, Traub RD, Schmitz D, et al. Electrical coupling underlies high-frequency oscillations in the hippocampus in vitro. Nature. 1998;394:189–192. doi: 10.1038/28184. [DOI] [PubMed] [Google Scholar]
- 17.Jefferys JG. Nonsynaptic modulation of neuronal activity in the brain: electric currents and extracellular ions. Physiol Rev. 1995;75:689–723. doi: 10.1152/physrev.1995.75.4.689. [DOI] [PubMed] [Google Scholar]
- 18.Jiruska P, de Curtis M, Jefferys JG, et al. Synchronization and desynchronization in epilepsy: controversies and hypotheses. J Physiol. 2013;591:787–797. doi: 10.1113/jphysiol.2012.239590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bikson M, Fox JE, Jefferys JG. Neuronal aggregate formation underlies spatiotemporal dynamics of nonsynaptic seizure initiation. J Neurophys. 2003;89:2330–2333. doi: 10.1152/jn.00764.2002. [DOI] [PubMed] [Google Scholar]
- 20.Jiruska P, Csicsvari J, Powell AD, et al. High-frequency network activity, global increase in neuronal activity, and synchrony expansion precede epileptic seizures in vitro. J Neurosci. 2010;30:5690–5701. doi: 10.1523/JNEUROSCI.0535-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Staba RJ, Frighetto L, Behnke EJ, et al. Increased fast ripple to ripple ratios correlate with reduced hippocampal volumes and neuron loss in temporal lobe epilepsy patients. Epilepsia. 2007;48:2130–2138. doi: 10.1111/j.1528-1167.2007.01225.x. [DOI] [PubMed] [Google Scholar]
- 22.Wendling F, Benquet P, Bartolomei F, et al. Computational models of epileptiform activity. J Neurosci Met. 2016;260:233–251. doi: 10.1016/j.jneumeth.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 23.Roopun AK, Simonotto JD, Pierce ML, et al. A nonsynaptic mechanism underlying interictal discharges in human epileptic neocortex. PNAS. 2010;107:338–343. doi: 10.1073/pnas.0912652107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stacey WC, Lazarewicz MT, Litt B. Synaptic Noise and Physiological Coupling Generate High-Frequency Oscillations in a Hippocampal Computational Model. J Neurophys. 2009;102:2342–2357. doi: 10.1152/jn.00397.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stacey WC, Krieger A, Litt B. Network recruitment to coherent oscillations in a hippocampal computer model. J Neurophys. 2011;105:1464–1481. doi: 10.1152/jn.00643.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fink CG, Gliske S, Catoni N, et al. Network Mechanisms Generating Abnormal and Normal Hippocampal High-Frequency Oscillations: A Computational Analysis. eNeuro. 2015:2. doi: 10.1523/ENEURO.0024-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alvarado-Rojas C, Huberfeld G, Baulac M, et al. Different Mechanisms of Ripple-like Oscillations in the Human Epileptic Subiculum. Ann Neurol. 2015;77:281–290. doi: 10.1002/ana.24324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valero M, Cid E, Averkin RG, et al. Determinants of different deep and superficial CA1 pyramidal cell dynamics during sharp-wave ripples. Nat Neurosci. 2015;18:1281. doi: 10.1038/nn.4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aivar P, Valero M, Bellistri E, et al. Extracellular Calcium Controls the Expression of Two Different Forms of Ripple-Like Hippocampal Oscillations. J Neurosci. 2014;34:2989–3004. doi: 10.1523/JNEUROSCI.2826-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiss SA, Lemesiou A, Connors R, et al. Seizure localization using ictal phase-locked high gamma A retrospective surgical outcome study. Neurology. 2015;84:2320–2328. doi: 10.1212/WNL.0000000000001656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kucewicz MT, Cimbalnik J, Matsumoto JY, et al. High frequency oscillations are associated with cognitive processing in human recognition memory. Brain. 2014;137:2231–2244. doi: 10.1093/brain/awu149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacobs J, LeVan P, Chatillon CE, et al. High frequency oscillations in intracranial EEGs mark epileptogenicity rather than lesion type. Brain. 2009;132:1022–1037. doi: 10.1093/brain/awn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fries P, Nikolic D, Singer W. The gamma cycle. Trends Neurosci. 2007;30:309–316. doi: 10.1016/j.tins.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 35.Karlocai MR, Kohus Z, Kali S, et al. Physiological sharp wave-ripples and interictal events in vitro: what’s the difference? Brain. 2014;137:463–485. doi: 10.1093/brain/awt348. [DOI] [PubMed] [Google Scholar]
- 36.Morris G, Jiruska P, Jefferys JG, et al. A New Approach of Modified Submerged Patch Clamp Recording Reveals Interneuronal Dynamics during Epileptiform Oscillations. Front Neurosci. 2016;10:519. doi: 10.3389/fnins.2016.00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiruska P, Finnerty GT, Powell AD, et al. Epileptic high-frequency network activity in a model of non-lesional temporal lobe epilepsy. Brain. 2010;133:1380–1390. doi: 10.1093/brain/awq070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferecsko AS, Jiruska P, Foss L, et al. Structural and functional substrates of tetanus toxin in an animal model of temporal lobe epilepsy. Brain Struct Funct. 2015;220:1013–1029. doi: 10.1007/s00429-013-0697-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bragin A, Azizyan A, Almajano J, et al. Analysis of chronic seizure onsets after intrahippocampal kainic acid injection in freely moving rats. Epilepsia. 2005;46:1592–1598. doi: 10.1111/j.1528-1167.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- 40.Weiss SA, Alvarado-Rojas C, Bragin A, et al. Ictal onset patterns of local field potentials, high frequency oscillations, and unit activity in human mesial temporal lobe epilepsy. Epilepsia. 2016;57:111–121. doi: 10.1111/epi.13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levesque M, Salami P, Gotman J, et al. Two seizure-onset types reveal specific patterns of high-frequency oscillations in a model of temporal lobe epilepsy. J Neurosci. 2012;32:13264–13272. doi: 10.1523/JNEUROSCI.5086-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shiri Z, Manseau F, Levesque M, et al. Activation of Specific Neuronal Networks Leads to Different Seizure Onset Types. Ann Neurol. 2016;79:354–365. doi: 10.1002/ana.24570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perucca P, Dubeau F, Gotman J. Intracranial electroencephalographic seizure-onset patterns: effect of underlying pathology. Brain. 2014;137:183–196. doi: 10.1093/brain/awt299. [DOI] [PubMed] [Google Scholar]
- 44.Spencer SS, Guimaraes P, Katz A, et al. Morphological Patterns of Seizures Recorded Intracranially. Epilepsia. 1992;33:537–545. doi: 10.1111/j.1528-1157.1992.tb01706.x. [DOI] [PubMed] [Google Scholar]
- 45.Gnatkovsky V, Librizzi L, Trombin F, et al. Fast Activity at Seizure Onset Is Mediated by Inhibitory Circuits in the Entorhinal Cortex In Vitro. Ann Neurol. 2008;64:674–686. doi: 10.1002/ana.21519. [DOI] [PubMed] [Google Scholar]
- 46.Shiri Z, Manseau F, Levesque M, et al. Interneuron Activity Leads to Initiation of Low-Voltage Fast-Onset Seizures. Ann Neurol. 2015;77:541–546. doi: 10.1002/ana.24342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yekhlef L, Breschi GL, Lagostena L, et al. Selective activation of parvalbumin- or somatostatin-expressing interneurons triggers epileptic seizurelike activity in mouse medial entorhinal cortex. J Neurophys. 2015;113:1616–1630. doi: 10.1152/jn.00841.2014. [DOI] [PubMed] [Google Scholar]
- 48.Uva L, Breschi GL, Gnatkovsky V, et al. Synchronous Inhibitory Potentials Precede Seizure-Like Events in Acute Models of Focal Limbic Seizures. J Neurosci. 2015;35:3048–3055. doi: 10.1523/JNEUROSCI.3692-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopantsev V, Avoli M. Participation of GABA(A)-mediated inhibition in ictallike discharges in the rat entorhinal cortex. J Neurophys. 1998;79:352–360. doi: 10.1152/jn.1998.79.1.352. [DOI] [PubMed] [Google Scholar]
- 50.Memarian N, Madsen SK, Macey PM, et al. Ictal Depth EEG and MRI Structural Evidence for Two Different Epileptogenic Networks in Mesial Temporal Lobe Epilepsy. Plos One. 2015:10. doi: 10.1371/journal.pone.0123588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ogren JA, Bragin A, Wilson CL, et al. Three-dimensional hippocampal atrophy maps distinguish two common temporal lobe seizure-onset patterns. Epilepsia. 2009;50:1361–1370. doi: 10.1111/j.1528-1167.2008.01881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kohling R, D’Antuono M, Benini R, et al. Hypersynchronous ictal onset in the perirhinal cortex results from dynamic weakening in inhibition. Neurobiol Dis. 2016;87:1–10. doi: 10.1016/j.nbd.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bragin A, Wilson CL, Engel J., Jr Chronic epileptogenesis requires development of a network of pathologically interconnected neuron clusters: a hypothesis. Epilepsia. 2000;41(Suppl 6):S144–S152. doi: 10.1111/j.1528-1157.2000.tb01573.x. [DOI] [PubMed] [Google Scholar]
- 54.Trevelyan AJ, Baldeweg T, van Drongelen W, et al. The source of afterdischarge activity in neocortical tonic-clonic epilepsy. J Neurosci. 2007;27:13513–13519. doi: 10.1523/JNEUROSCI.3005-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trevelyan AJ, Sussillo D, Watson BO, et al. Modular propagation of epileptiform activity: evidence for an inhibitory veto in neocortex. J Neurosci. 2006;26:12447–12455. doi: 10.1523/JNEUROSCI.2787-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schevon CA, Weiss SA, McKhann G, Jr, et al. Evidence of an inhibitory restraint of seizure activity in humans. Nat Commun. 2012;3:1060. doi: 10.1038/ncomms2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Truccolo W, Donoghue JA, Hochberg LR, et al. Single-neuron dynamics in human focal epilepsy. Nat Neurosci. 2011;14:635–641. doi: 10.1038/nn.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weiss SA, Banks GP, McKhann GM, Jr, et al. Ictal high frequency oscillations distinguish two types of seizure territories in humans. Brain. 2013;136:3796–3808. doi: 10.1093/brain/awt276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eissa TL, Tryba AK, Marcuccilli CJ, et al. Multiscale Aspects of Generation of High-Gamma Activity during Seizures in Human Neocortex. eNeuro. 2016:3. doi: 10.1523/ENEURO.0141-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kanazawa K, Matsumoto R, Imamura H, et al. Intracranially recorded ictal direct current shifts may precede high frequency oscillations in human epilepsy. Clin Neurophys. 2015;126:47–59. doi: 10.1016/j.clinph.2014.05.028. [DOI] [PubMed] [Google Scholar]
- 61.Bragin A, Wilson CL, Almajano J, et al. High-frequency oscillations after status epilepticus: epileptogenesis and seizure genesis. Epilepsia. 2004;45:1017–1023. doi: 10.1111/j.0013-9580.2004.17004.x. [DOI] [PubMed] [Google Scholar]
- 62.Salami P, Levesque M, Benini R, et al. Dynamics of interictal spikes and high-frequency oscillations during epileptogenesis in temporal lobe epilepsy. Neurobiol Dis. 2014;67:97–106. doi: 10.1016/j.nbd.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zijlmans M, Jacobs J, Zelmann R, et al. High-frequency oscillations mirror disease activity in patients with epilepsy. Neurology. 2009;72:979–986. doi: 10.1212/01.wnl.0000344402.20334.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levesque M, Behr C, Avoli M. The anti-ictogenic effects of levetiracetam are mirrored by interictal spiking and high-frequency oscillation changes in a model of temporal lobe epilepsy. Seizure. 2015;25:18–25. doi: 10.1016/j.seizure.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Behr C, Levesque M, Ragsdale D, et al. Lacosamide modulates interictal spiking and high-frequency oscillations in a model of mesial temporal lobe epilepsy. Epilepsy Res. 2015;115:8–16. doi: 10.1016/j.eplepsyres.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bragin A, Engel J, Jr, Wilson CL, et al. Electrophysiologic analysis of a chronic seizure model after unilateral hippocampal KA injection. Epilepsia. 1999;40:1210–1221. doi: 10.1111/j.1528-1157.1999.tb00849.x. [DOI] [PubMed] [Google Scholar]
- 67.Bragin A, Engel J, Jr, Staba RJ. High-frequency oscillations in epileptic brain. Curr Opin Neurol. 2010;23:151–156. doi: 10.1097/WCO.0b013e3283373ac8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jones RT, Barth AM, Ormiston LD, et al. Evolution of temporal and spectral dynamics of pathologic high-frequency oscillations (pHFOs) during epileptogenesis. Epilepsia. 2015;56:1879–1889. doi: 10.1111/epi.13218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Levesque M, Bortel A, Gotman J, et al. High-frequency (80–500 Hz) oscillations and epileptogenesis in temporal lobe epilepsy. Neurobiology of Disease. 2011;42:231–241. doi: 10.1016/j.nbd.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O’Connor WT, Smyth A, Gilchrist MD. Animal models of traumatic brain injury: A critical evaluation. Pharmac Ther. 2011;130:106–113. doi: 10.1016/j.pharmthera.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 71.Pitkanen A, Immonen RJ, Grohn OHJ, et al. From traumatic brain injury to posttraumatic epilepsy: What animal models tell us about the process and treatment options. Epilepsia. 2009;50:21–29. doi: 10.1111/j.1528-1167.2008.02007.x. [DOI] [PubMed] [Google Scholar]
- 72.D’Ambrosio R, Fairbanks JP, Fender JS, et al. Post-traumatic epilepsy following fluid percussion injury in the rat. Brain. 2004;127:304–314. doi: 10.1093/brain/awh038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kharatishvili I, Nissinen JP, McIntosh TK, et al. A model of posttraumatic epilepsy induced by lateral fluid-percussion brain injury in rats. Neuroscience. 2006;140:685–697. doi: 10.1016/j.neuroscience.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 74.Bragin A, Li L, Almajano J, et al. Pathologic electrographic changes after experimental traumatic brain injury. Epilepsia. 2016;57:735–745. doi: 10.1111/epi.13359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jefferys JGR, Jiruska P. The Tetanus Toxin Model of Temporal Lobe Epilepsy. Encyclopedia of Basic Epilepsy Research. 2009;1–3:804–807. [Google Scholar]
- 76.Wykes RC, Heeroma JH, Mantoan L, et al. Optogenetic and potassium channel gene therapy in a rodent model of focal neocortical epilepsy. Sci Transl Med. 2012;4:161ra152. doi: 10.1126/scitranslmed.3004190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Frost JD, Lee CL, Hrachovy RA, et al. High frequency EEG activity associated with ictal events in an animal model of infantile spasms. Epilepsia. 2011;52:53–62. doi: 10.1111/j.1528-1167.2010.02887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Frost JD, Lee CL, Le JT, et al. Interictal high frequency oscillations in an animal model of infantile spasms. Neurobiol Dis. 2012;46:377–388. doi: 10.1016/j.nbd.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kobayashi K, Akiyama T, Oka M, et al. A storm of fast (40–150Hz) oscillations during hypsarrhythmia in West syndrome. Ann Neurol. 2015;77:58–67. doi: 10.1002/ana.24299. [DOI] [PubMed] [Google Scholar]