Abstract
Objective
We have previously found that the transcription factor PPARγ contributes to the mechanism of action of the ketogenic diet (KD), an established treatment for pediatric refractory epilepsy. We have found that the KD increases brain PPARγ and that inhibition or genetic loss of PPARγ prevents the antiseizure effects of the KD on (1) acutely induced seizures in non-epileptic mice and (2) spontaneous recurrent seizures in epileptic mice. Here, we tested the hypothesis that adjuvant treatment of KD-treated mice with a PPARγ agonist, pioglitazone, would result in an additive effect.
Methods
Acute seizures were induced in three groups of C57Bl/6 mice by inhalation exposure to flurothyl gas. In Group 1, mice were weaned onto either a standard diet or KD comprised of a fat:carbohydrate/protein ratio of either 6:1, 3:1 or 1:1 for two weeks. In Group 2, vehicle or pioglitazone (0.1, 1, 10, 80 mg/kg) was administered four hours prior to flurothyl exposure. In Group 3, vehicle or increasing doses of pioglitazone were administered to KD-treated mice four hours prior to flurothyl exposure. Latency times to clonic seizures and generalized tonic-clonic (GTC) seizures were recorded and isobolographic analysis was used to determine combinatorial interactions.
Results
Neither KD treatment nor pioglitazone alone or in combination affected clonic seizures. However, the latency to GTC seizures was dose-dependently and significantly increased by both KD (∼57%, p<0.05) and pioglitazone (∼28%, p<0.05). Co-administration of an ineffective 1:1 KD and pioglitazone resulted in ∼47-55% (p<0.05) increase in latency to GTC. Isobolographic analysis indicated a synergistic interaction of the KD and pioglitazone.
Significance
These results suggest co-administration may enable reduction of the KD ratio without loss of seizure protection. Such adjuvant treatment could improve quality of life and limit adverse effects of a classic KD or high dose pioglitazone.
Keywords: Epilepsy, in vivo, PPAR, PPARgamma, peroxisome proliferator activated receptor, metabolism
1. Introduction
The ketogenic diet (KD) is an anti-seizure therapy primarily used in pediatric, refractory epilepsy. In this difficult to treat population, many retrospective and prospective studies have consistently demonstrated that the KD is an effective treatment in about 50% of the patients1. The classic KD consists of strict consumption of a diet of high fat and low carbohydrate/protein in a 4:1 ratio. Palatability and stringency contribute to tolerability and non-compliance in some patients. For others a less strict 3:1 or 2:1 KD or alternative medium chain triglyceride diet, modified Atkins diet or low glycemic index diet may provide sufficient seizure control2.
Though it predates most conventional anti-seizure drugs the mechanism of action of the KD is still not entirely understood3. Recently, we identified the peroxisome proliferator activated receptor gamma (PPARγ) as a significant contributor to the anti-seizure mechanism of the KD4. PPARγ is a nuclear transcription factor most noted as a master regulator of adipogenesis, lipid metabolism and insulin sensitivity; but, PPARγ also increases transcription of anti-inflammatory, anti-oxidant and mitochondrial genes5. For this reason PPARγ agonists, in particular the Type II Diabetes drug pioglitazone, are currently under investigation in preclinical and clinical studies for treatment of neurodegenerative diseases such as Alzheimer's, Parkinson's and Amyotrophic Lateral Sclerosis6.
In our previous study, we demonstrated the anti-seizure efficacy of pioglitazone in reducing spontaneous recurrent seizures in a genetic model of epilepsy4. Here, we hypothesized that co-administering pioglitazone with the KD would have additive effects and may allow reduction of the KD ratio without sacrificing seizure protection.
2. Materials and Methods
2.1. Animals
All mice were housed in the Animal Resource Facilities at Creighton University School of Medicine in a temperature (25°C) and humidity (50-60%) controlled and pathogen-free environment. Mice were given food and water ad libitum and kept on a 12-hour light/dark cycle. Male and female C57Bl/6 mice were purchased from Envigo (Indianapolis, IN) and used to establish an in house breeding colony. All procedures involving animals were in accordance with National Institutes of Health guidelines, the EU Directive 2010/63/EU and were approved by the Institutional Animal Care and Use Committees at Creighton University School of Medicine.
2.2. Dietary and pharmacological treatments
On P21, 110 male mice were randomly assigned to one of three experimental groups. Group 1 mice were randomly weaned onto either a standard diet (SD) or one of three KD diets with ratios of 1:1, 3:1 or 6.3:1 (fat to carbohydrates plus proteins see Table 1 for nutritional content; Bio-Serv F3666, Frenchtown, NJ, U.S.A.) for 13-18 days. Group 2 mice were weaned onto an SD for 13-29 days. On the experimental day, four hours prior to flurothyl-exposure, Group 2 mice were intraperitoneal-injected (i.p.) with vehicle (1% carboxymethylcellulose in saline) or vehicle-pioglitazone (0.1, 1, 10, 80 mg/kg)7. Group 3 mice were randomly weaned onto either SD or a 1:1 KD for 12-16 days. On the experimental day, four hours prior to flurothyl-exposure, Group 3 mice were injected i.p. with vehicle (1% carboxymethylcellulose in saline) or pioglitazone (0.1, 1, 10, 80 mg/kg in 1% carboxymethylcellulose in saline).
Table 1.
Dietary Constituents.
| INGREDIENT | SD | KD 1:1 | KD 3:1 | KD 6:1 | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| %weight | %kcal | %weight | %kcal | %weight | %kcal | %weight | %kcal | |
|
|
||||||||
| Fat | 6.2 | 18 | 43.5 | 69.2 | 65.1 | 87 | 75.1 | 93.4 |
| Carbohydrate | 44.2 | 58 | 25.8 | 18.3 | 10.1 | 6 | 3.2 | 1.8 |
| Protein | 18.6 | 24 | 17.8 | 12.6 | 11.7 | 7 | 8.6 | 4.7 |
2.3. Blood β-hydroxybutyrate and glucose measurements
β-hydroxybutyrate and glucose levels were measured prior to dietary assignment and one day before flurothyl testing from blood samples collected from the tail vein of each mouse using a test strip system and reader (Precision Xtra Advance Diabetes Management System with Precision Xtra blood ketone test strips and blood glucose test strips; Abbott Diabetes Care Inc., Alameda, CA, U.S.A.) as previously performed4,8,9.
2.4. Flurothyl-induced Seizures
All experiments were performed in a fume hood as previously described (Simeone et al., 2016b). Mice were acclimated 1 h before testing. Mice were individually placed in a 2.7 L airtight glass chamber. A 10% solution (in 95% ethanol) of flurothyl (bis-2,2,2-trifluoroethyl ether; Sigma-Aldrich, St. Louis, MO, U.S.A.) was delivered by a syringe pump (KD Scientific, Holliston, MA, U.S.A.) at a constant rate of 0.05 ml/min and allowed to drip onto a Whatman grade 1 filter paper until the mouse reached a generalized tonic-clonic seizure with loss of posture10,11. Seizure latency was measured from the first drop of flurothyl onto the filter paper to the onset of the first clonic seizure and first generalized tonic-clonic (GTC) seizure. A clonic seizure was recognized when forelimb clonus, usually bilateral but sometimes unilateral, with or without hindlimb clonus occurred for greater than 5 seconds. The number of clonic seizures before the GTC seizure was recorded. Once a GTC seizure developed, the mouse was promptly removed from the chamber and allowed to recover.
2.5. Isobolographic Analysis
Additive, subadditive or synergistic effects of treatment combinations were detected with isobolographic analysis methods as previously described by Grabovsky and Tallarida12. Briefly, simple, linear isoboles only occur for drugs with similar maximal efficacies, but for drugs with different efficacies isoboles must be scaled accordingly. Within each treatment group data were normalized to the peak effect and dose-response curves were generated and fit with the equation
| (1) |
where E is the effect, Emax is the maximum effect for a specific drug, ED50 is the dose eliciting a half-maximal effect, [agonist] is the drug dose (note: for KD ratios the numerator or fat content is used as the dose) and h is the Hill coefficient. The peak effect of pioglitazone (designated as Drug B below) was half of the peak response of the KD (designated as Drug A), therefore, for our purposes here, the effect elicited by the 6:1 KD was assumed to be the maximum response possible to which all combination effects were normalized. Using the calculated KD and pioglitazone ED50s and hill coefficients, dose effects of treatment combinations in fixed ratios were simulated with the equation
| (2) |
where B is the dose of pioglitazone (b) after taking into account the difference in efficacies and hill coefficients (b′), B50 is the experimentally-derived ED50 of pioglitazone (0.8315 mg/kg), EBmax is the maximum effect of pioglitazone relative to the KD maximum effect (50%), ABmax is the maximum effect of the KD (100%), A50 is the experimentally-derived ED50 of KD (2.338), a is the dose of KD and p and q are the pioglitazone and KD hill coefficients, respectively. The isobole was constructed for treatment combinations resulting in responses 50% of the KD maximum effect and were anchored to the intercepts by the experimental observations of equieffective treatment doses alone (KD: 0, 2.338; pioglitazone: 80, 0). The isobole data points were fit with a two-phase decay equation. All graphs and fits were generated with Prism6 software (Graphpad Software Inc., La Jolla, CA, U.S.A.).
2.6. Reagents and Statistics
Unless otherwise specified, all reagents were purchased from Sigma-Aldrich. Statistical significance was determined with Prism6 software using a one-way or two-way ANOVA with a Dunnett's post hoc test.
3. Results
3.1. The Ketogenic Diet Dose-dependently raises flurothyl-induced GTC seizure threshold
The KD has been demonstrated to have consistent effects across laboratories on seizure threshold in the flurothyl model of acutely provoked generalized clonic and tonic-clonic seizures4,13,14; thus, this model was chosen to test our hypothesis. Before performing co-administration studies, it was necessary to determine both effective and ineffective KD ratios against flurothyl-induced clonic and GTC seizures. The parameters we examined included latency to the first clonic seizure, number of clonic seizures prior to the onset of the GTC seizure, total duration in clonus prior to GTC seizure and the latency between the first clonic seizure and the GTC seizure. We observed that no matter the ratio, the KD did not affect the latency to the first clonic seizure, the number of clonic seizures or the total duration spent in clonus (Fig 1a-c). The KD did have a dose-dependent effect on the latency to the GTC seizure (Fig. 1d). A 1:1 KD did not influence latency to GTC. However, in mice treated with a 6:1 KD GTC was significantly increased by 57.2 ± 18.3% (16.9 ± 2 v. 10.8 ± 1.1 min., p = 0.029). Weight and blood glucose and β-hydroxybutyrate also demonstrated expected dose-dependent changes with increasing ratios (Fig. 2).
Figure 1. Ketogenic diet (KD) dose-dependently increases latencies to generalized tonic-clonic (GTC) seizures.
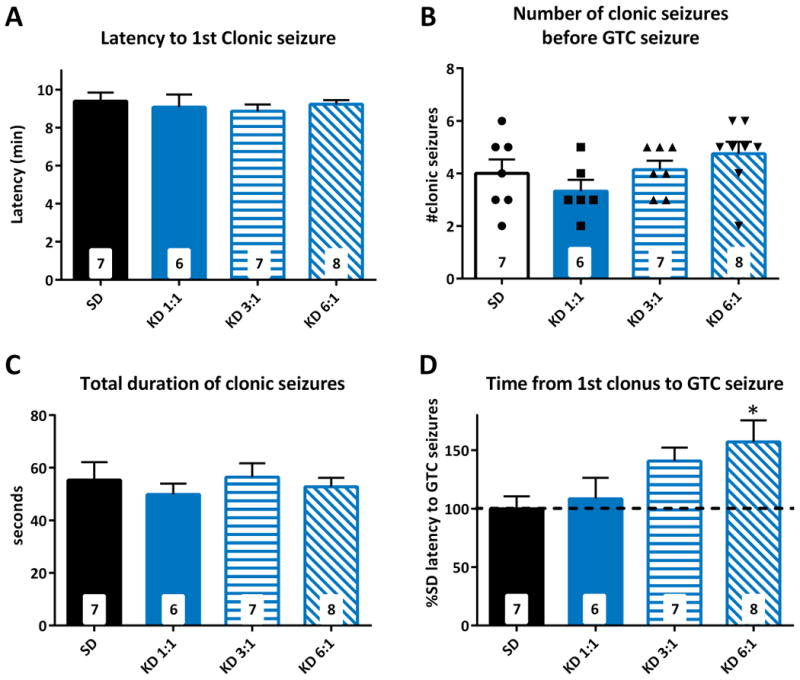
Increasing KD ratios had no effect on latency to clonic seizures (A), number of clonic seizures (B) or total duration of clonic seizures (C). (D) Latencies to GTC seizures were increased by a 3:1 KD and 6:1 KD. Significance determined by an one-way ANOVA followed by Dunnett's multiple comparisons test, *p < 0.05 (n = 6 – 8 mice/group; specific n for each group are in the inset of the corresponding bar).
Figure 2. Ketogenic diet (KD) dose-dependently increases ketone bodies and decrease glucose and weight.
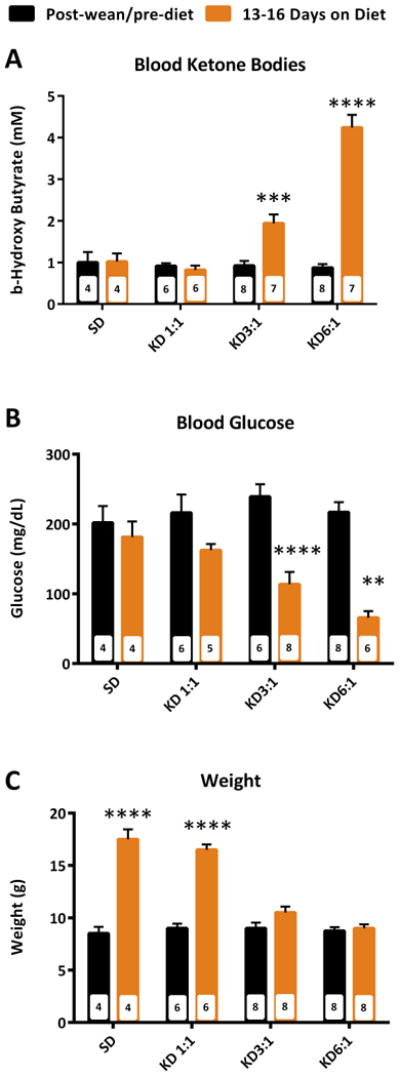
A 3:1 KD and 6:1 KD significantly increased blood β-hydroxybutyrate (A), decreased blood glucose (B) and decreased body weight. Significance determined by a two-way ANOVA followed by Tukey's multiple comparisons test, $p < 0.05 vs. SD, $$p < 0.01 vs. SD, $$$p < 0.001 vs. SD, &p < 0.05 vs. 1:1KD, &&p < 0.01 vs. 1:1KD, &&&p < 0.001 vs. 1:1KD, ###p < 0.001 vs. 3:1KD, ***p < 0.001 vs. within group (n = 4 – 8 mice/group; specific n for each group are in the inset of the corresponding bar).
3.2. Pioglitazone Dose-dependently raises flurothyl-induced GTC seizure threshold
Next we determined the dose response of pioglitazone against flurothyl-induced clonic and GTC seizures. Similar to the KD, pioglitazone failed to affect the latency to the first clonic seizure, the number of clonic seizures or the total duration spent in clonus (Fig 3a-c). Pioglitazone did have a dose-dependent effect on the latency to the GTC seizure with a peak increase of 27.5 ± 1.8% with the 80 mg/kg dose compared to vehicle control (16 ± 0.2 v. 12.6 ± 0.4 min., p = 0.017) (Fig. 3d).
Figure 3. Pioglitazone dose-dependently increases latencies to generalized tonic-clonic (GTC) seizures.
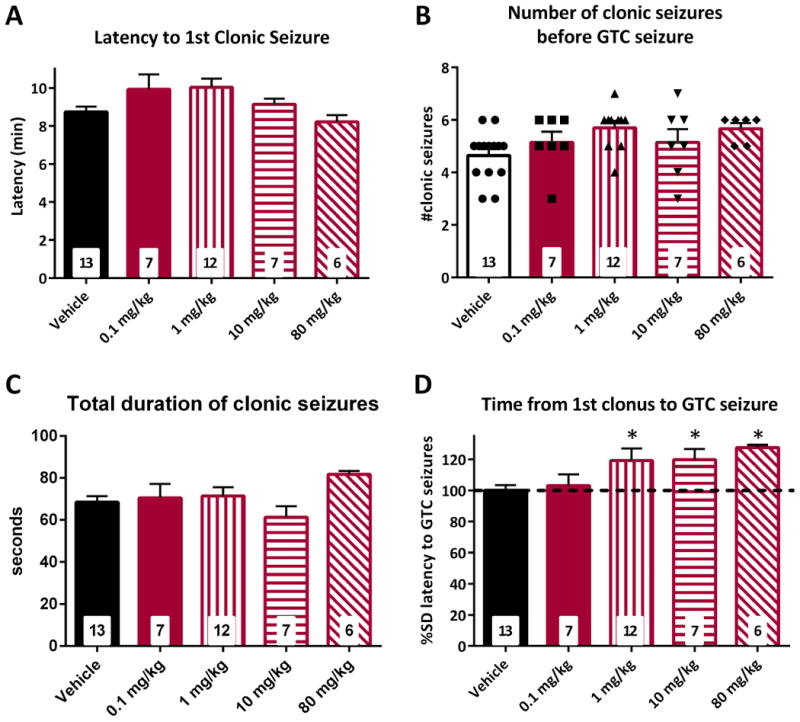
Pioglitazone had no effect on latency to clonic seizures (A), number of clonic seizures (B) or total duration of clonic seizures (C). (D) Latencies to GTC seizures were increased by a 1, 10, and 80 mg/kg. Significance determined by an one-way ANOVA followed by Dunnett's multiple comparisons test, *p < 0.05 (n = 6 – 13 mice/group; specific n for each group are in the inset of the corresponding bar).
3.3. Adjuvant treatment of 1:1 KD with pioglitazone synergistically raises flurothyl-induced GTC seizure threshold
Our previous study indicated that the KD and pioglitazone share a mechanism of action via the transcription factor PPARγ4. Therefore, we hypothesized that co-administration of a KD and pioglitazone would have additive effects. We tested this by co-administering increasing dosages of pioglitazone in a new cohort of mice treated with the ineffective 1:1 KD. We found that adjuvant treatment did not affect the latency to the first clonic seizure, the number of clonic seizures or the total duration spent in clonus relative to SD (Fig 4a-c). The addition of pioglitazone had a greater than additive effect on delaying the onset of GTC seizures (Fig. 4d). Adjuvant treatment of a 1:1 KD with a previously ineffective 0.1 mg/kg dose of pioglitazone resulted in a 46.5 ± 9.9% increase in GTC seizure latency compared to the vehicle-SD mice (17.8 ± 1.2 v. 12.2 ± 0.7 min., p = 0.044). This was similar to the mean effect of a 3:1 KD alone. Co-administration of 10 mg/kg pioglitazone with the 1:1 KD delayed GTC onset by 55.2 ± 14.7% compared to the vehicle-SD mice (18.9 ± 1.8 min., p = 0.009) equivalent to the mean protection given by a 6:1 KD alone (Fig. 1D).
Figure 4. Adjuvant treatment of a 1:1 ketogenic diet (KD) with pioglitazone increases latencies to generalized tonic-clonic (GTC) seizures.
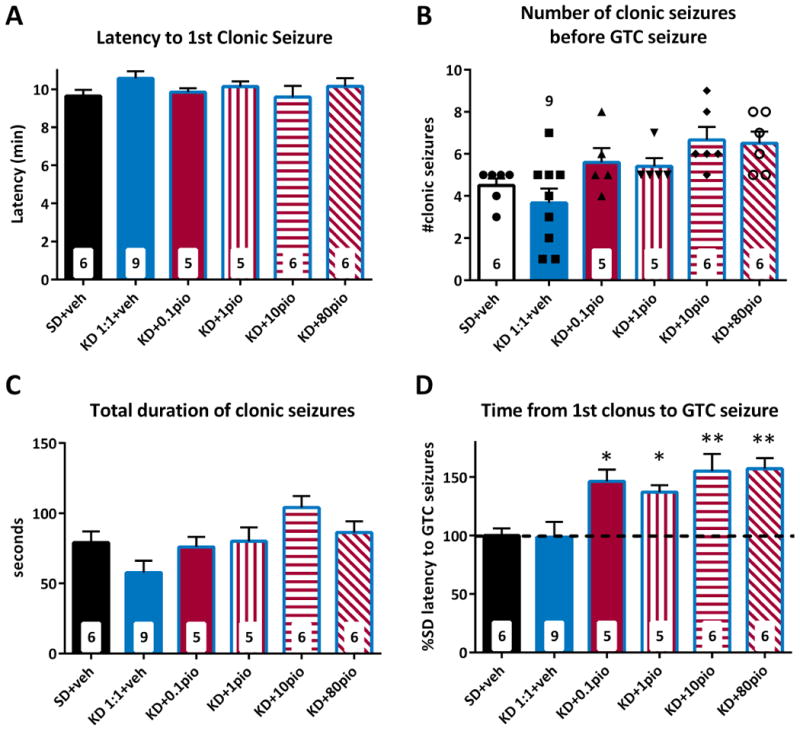
Treatment with increasing doses of pioglitazone and a fixed 1:1 KD had no effect on latency to clonic seizures (A), but the highest doses of 10 and 80 mg/kg of pioglitazone did significantlyincrease the number of clonic seizures compared to the 1:1 KD control group (B). This was accompanied by an increase of the total duration of clonic seizures in the 10 mg/kg group (C). (D) Latencies to GTC seizures were increased by every dose of pioglitazone combined with 1:1 KD suggesting an increase in potency and efficacy. Significance determined by an one-way ANOVA followed by Dunnett's multiple comparisons test, *p < 0.05, **p < 0.01 (n = 5 – 9 mice/group; specific n for each group are in the inset of the corresponding bar).
To determine the type of interaction of the KD and pioglitazone we used isobolographic analysis. The isobole provides a method to detect additive, subadditive or synergistic effects of treatment combinations12. As we have observed, the KD and pioglitazone have different efficacies with pioglitazone only achieving about 50% of the maximal response to a 6:1 KD. Therefore, we used an isobolographic method that focuses on the effect scale which accounts for the different efficacies and potencies of treatments in order to calculate theoretical additivity to which experimental data was compared12. We began by constructing dose-response curves for the experimental KD and pioglitazone effects and calculated the ED50s (2.338:1 and 0.535 mg/kg, respectively) and hill coefficients (3.775 and 0.832, respectively; Fig. 5A, B). Co-administration of pioglitazone with the 1:1 KD caused a 23-fold shift to the left of the pioglitazone ED50 (0.0237 mg/kg) and decreased the hill coefficient to 0.437 (Fig. 5B). Using the ED50s and hill coefficients from the experimental data and the method of Grabovsky and Tallarida12 (see methods), combinations of KD and pioglitazone in fixed ratios were simulated. Ratios favoring the KD reduced the pioglitazone ED50 and increased hill coefficients to near KD values, whereas ratios favoring pioglitazone shifted the curves to the right and decreased hill coefficients (Fig. 5C). Moreover, even though pioglitazone alone has half-maximal efficacy compared to the KD, every simulated combination achieved maximal efficacy because the KD could be increased to its ED50 even if the pioglitazone doses were unrealistically high.
Figure 5. Ketogenic diet (KD) and pioglitazone act synergistically to increase the latency to flurothyl-induced generalize tonic colonic seizures.
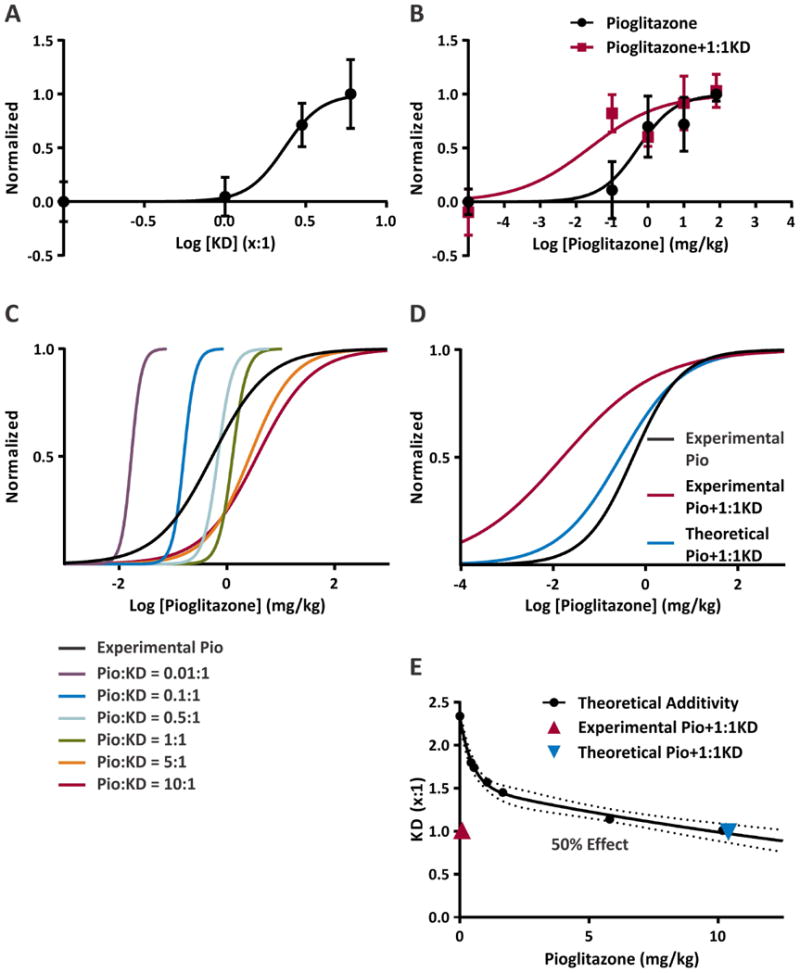
(A) Dose-response curve for KD ratios. X equals the fat component in the KD and serves as the drug dose in the graph. (ED50 = 2.338:1, h = 3.775). (B) Dose-response curve for pioglitazone (black) and combination treatment with pioglitazone and an 1:1 KD (red) (ED50 = 0.5352:1, h = 0.8315; ED50 = 0.0237:1, h = 0.4373, respectively). (C) Simulated dose-response curves for fixed combinations taking into account the effect of the different efficacies of KD and pioglitazone on the combinatorial total effect. (D) Comparison of simulated dose-response of combination treatment with pioglitazone and an 1:1 KD (blue; ED50 = 0.2833:1, h = 0.6382) with the replotted fits of the experimental data in panel B. (E) Isobolograph of the predicted additivity of doses of pioglitazone and KD resulting in a 50% maximum effect. The x- and y-ordinate intersections are from experimental data, 2.338 for KD and 80 mg/kg pioglitazone. The curvature of the isobole reflects the combination of treatments with different efficacies, potencies and hill coefficients. The experimental data (red) falls well below the isobole indicating a synergistic interaction of pioglitazone and a 1:1 KD. The hashed lines represent the 95% confidence interval of the isobole fit.
Next, a theoretical dose-response curve for increasing pioglitazone doses with a fixed 1:1 KD was generated. Unlike our experimental observations (Fig. 4D, 5B), we found that the pioglitazone ED50 only decreased to 0.283 mg/kg with a hill coefficient of 0.638 (Fig. 5D) and that the peak efficacy (53% of KD maximum) was similar to pioglitazone alone (Fig. 3D). From these simulations, we were able to construct an effect scale isobole that plots the treatment dose combinations resulting in 50% of the maximum effect observed with a 6:1 KD (Fig.5E). Therefore, the intersection with the y-ordinate is the ED50 of KD, whereas a high dose of pioglitazone (in this case our experimental dose 80 mg/kg) is required to achieve the same response and is the intersection of the x-ordinate. The curvature of the isobole results from taking into account the different efficacies, potencies and hill coefficients. Any combination that lands on the line is additive, above the line is sub-additive and below the line is synergistic. To achieve half-maximal efficacy, we find that our simulated pioglitazone + 1:1 KD combination falls on the additivity line at 10.4 mg/kg pioglitazone, whereas the experimental data is well below the line at 0.0237 mg/kg. These data suggest that the combination of a KD and pioglitazone has synergistic effects.
4. Discussion
Regulation of the nuclear transcription factor PPARγ contributes to the antiseizure efficacy of the KD4. Here, we demonstrate that the KD and the PPARγ agonist pioglitazone delay the onset of flurothyl-induced GTC seizures. Moreover, we demonstrate for the first time that adjuvant treatment results in a greater than additive effect, indicating a synergistic interaction between the KD and pioglitazone.
The thiazolidinedione pioglitazone is a FDA-approved drug indicated for use in Type II Diabetes Mellitus. It is a potent and highly selective agonist for the transcription factor PPARγ which regulates hundreds of genes involved in glucose and lipid metabolism, anti-inflammation, anti-oxidation and mitochondrial function. PPARγ is expressed in adipose tissue, skeletal muscle, liver and brain5. Activation of PPARγ is nutritionally regulated by natural ligands including polyunsaturated, saturated and oxidized fatty acids5. Recently, it was found that the KD increases the PPARγ2 splice variant in brain of normal and epileptic mice4. Pioglitazone administration also increased the PPARγ2 splice variant in brain. Furthermore, a PPARγ antagonist or genetic loss of PPARγ attenuated the antiseizure effect of the KD, and pioglitazone provided seizure protection4.
In the current study, based on the putative shared PPARγ mechanism of the KD and pioglitazone, we hypothesized that adjuvant treatment with pioglitazone may be additive and allow reduction of the KD ratio. To our surprise, the lowest ratio of KD (1:1) and lowest dose of pioglitazone (0.1 mg/kg) that alone had no effect on GTC seizures, acted synergistically when combined and provided an effect equivalent to a 3:1 KD. At this time the reason for this interaction is unknown.
One potential explanation of synergism is direct action of either the KD or pioglitazone on other effectors simultaneous to additive action on PPARγ. This could include different targets in the same or related pathways, or targets involved in brain penetration or metabolism. The greater efficacy of the KD compared to pioglitazone observed in our study supports the possibility of an “off” target mechanism. The saturated and unsaturated fatty acids provided by the KD are most likely the ligands that activate PPARγ, but they may also modulate ion channels and neurotransmitter receptors important for dampening cellular hyperexcitability37. Also, ketone bodies generated during a KD have been implicated in the antiseizure effects of the KD via diverse mechanisms including, but not limited to, increased ATP generation, inhibition of the mitochondrial membrane transition pore, opening KATP channels, inhibiting vesicular glutamate transporters, increasing adenosine and activating A1 receptors3,9,23-27,36. However, the lack of increase of ketone bodies in mice treated with a 1:1 KD (Fig. 2a) suggests that the actions of ketone bodies do not contribute to the synergism. Pioglitazone also has a PPARγ-independent target in the mitochondrial protein mitoNEET that mediates neuroprotective effects38.
Alternatively, crosstalk between protective pathways can result in synergism. PPARγ activity is inhibited by many post-translational modifications such as ERK/MEK phosphorylation5. The KD has been shown to modify many of these signaling pathways, therefore, it is possible that the 1:1 KD may relieve inhibition of PPARγ and prime it for activation by pioglitazone.
It could also be that KD treatment increases pioglitazone accumulation in brain by increasing penetration or decreasing metabolism. Brain penetration of pioglitazone is severely limited by P-glycoprotein29. Inhibition of the mammalian target of rapamycin (mTOR) reduces P-glycoprotein expression in epilepsy models30 and the KD inhibits mTOR31; thus, though speculative, it is possible that the 1:1 KD reduces P-glycoprotein via inhibition of the mTOR pathway, thereby allowing more pioglitazone into the brain30,31. Alternatively, both pioglitazone and KD inhibit relevant cytochrome P450s, which metabolize pioglitazone or KD biochemical constituents. Pioglitazone is metabolized by CYP2C8 and CYP3A432, but currently there is no evidence that the KD regulates these enzymes. On the other hand, one of the metabolic pathways of the putative biochemical effectors of the KD, acetone and polyunsaturated fatty acids, involve CYP1- and CYP2-enzymes which are inhibited by pioglitazone33-35; thus, KD constituents could increase with adjuvant treatment with pioglitazone.
The potential mechanisms of synergism become more complicated in the case of transcription factors as the many genes under their control may be differentially regulated depending on the nature of the bound agonist (full or partial) which determines the degree of conformational change and ability of transcription factors to interact with particular corepressors and coactivators39-41. For example, the full agonist thiazolidinediones, including pioglitazone, exert beneficial insulin-sensitizing effects as well as detrimental side effects such as weight gain, whereas several partial agonists or so-called selective PPARgamma modulators (SPPARMs) have been shown to retain anti-diabetic effects while eliminating weight gain via attenuated and selective gene regulatory activity in comparison to full agonists42,43. The PPARγ ligand binding pocket is large (1300 angstroms3) which allows structural promiscuity for a wide variety of endogenous or natural agonists (i.e. saturated and unsaturated fatty acids, eicosanoids, oxidized lipids, nitroalkenes), synthetic agonists (e.g. thiazolidinediones or TZDs) and synthetic anatagonists40,41,44,45. It was recently determined that the saturated fatty acid decanoic acid, a primary constituent of the medium chain triglyceride ketogenic diet, is a ligand for PPARγ at physiologically relevant concentrations46,47. In vivo treatment suggests that decanoic acid is a partial agonist as it improves glucose sensitivity and lipid profiles without weight gain in diabetic mice47. Other fatty acids and oxidized lipids (e.g., DHA) implicated in the classic KD have likewise been found to be PPARγ partial agonists44,48. Functionally, co-treatment with full and partial agonists may increase expression of gene sets important for seizure control. One potentially important gene is the antioxidant catalase, which we and others have identified as upregulated by decanoic acid or the KD via PPARγ46 (personal unpublished observations). Structurally, partial and full agonists bind to different sites within the PPARγ βινδινγ pocket44,48 raising the possibility that cooperativity may occur between sites; thus, enhancing the transcriptional activity of PPARγ similar to the interactions of partial and full agonists on ligand gated receptors49,50. However, whether such an action occurs is unknown at this time. All of these possibilities pose pertinent questions worthy of future studies into the mechanisms of action of the KD and pioglitazone. Effects on longevity are also of interest as the KD has been demonstrated to increase lifespan in a mouse model of chronic epilepsy and SUDEP, and PPARγ2 is a determinant of longevity8,28.
Pioglitazone reaches a peak at 4hrs post-administration and approximately 18% of the serum concentration can be found in the cerebrospinal fluid of rats15. Moreover, pioglitazone orally administered to mice in chow at a dose of 4 mg/kg/day (assuming consumption by a 20 g mouse of 4 g/day of 20.4 mg pioglitazone/kg chow) results in a brain concentration of 1 nM/g wet brain16. In the present study, 4hr pretreatment of pioglitazone dose-dependently provided seizure protection with an ED50 of 0.5 mg/kg indicating that the doses required to achieve an antiseizure effect most likely resulted in significant accumulation in brain tissue.
Interestingly, none of the treatments affected the characteristics of the clonic seizures. The progression from generalized clonic seizures to tonic seizures involves the propagation of seizure activity from the low threshold forebrain circuit to the high threshold brainstem circuit10,11. This suggests that the KD and pioglitazone delay propagation to or raise the seizure threshold of the brainstem even higher in this model of acute seizures. In contrast, Araki et al.10 found that high doses of ethosuximide, clonazepam and diazepam greatly prolonged the latency of flurothyl-induced clonic seizures in Mongolian gerbils suggesting that these ion channel modulators increase the forebrain seizure threshold.
Previously, Bough et al.17 found that higher KD ratios increased pentylenetetrazole seizure thresholds in rats plateauing with a 6:1 ratio. Similarly, our study demonstrates that KD effects have a clear dependence on the fat:carbohydrate/protein ratio with a 6:1 ratio providing the greatest increase in seizure threshold against acute flurothyl seizures. Given the unavoidable heterogeneity of patients included in human studies, it is not surprising that KD ratio-dependent antiseizure efficacy is less understood. It is frequently reported that lower KD ratios are as effective as higher ratios; however, a minority of patients do experience improved seizure control when switched from a lower ratio to the classic 4:1 KD18. Additionally, on an individual basis, after initiating treatment with higher ratios, KD ratios can be lowered without reduction of efficacy18. Lower KD ratios with equivalent seizure control is preferred as patients are more compliant (i.e., the KD is more palatable) and experience fewer or less severe adverse effects such as weight loss, delayed growth, gastrointestinal intolerance, etc. This is exemplified in our study as the mice on a 3:1 and 6:1 KD have minimal weight gain throughout the course of the two-week treatment, but mice on the 1:1 KD experienced normal weight gain.
Pioglitazone dosing for monotherapy of Type II Diabetes is in the range of 15-45 mg/day. For a 100 kg adult, this translates into a dosage of 0.15-0.45 mg/kg/day similar to the low to ED50 doses (0.1 – 0.5 mg/kg) used in this study. The most common adverse events in humans at these doses include fluid retention, weight gain, edema, abdominal pain, diarrhea and nausea19. Less common severe adverse effects include cardiac failure and bladder cancer which are associated with pre-existing conditions or combination with insulin and high cumulative doses of pioglitazone, respectively20. The potential for cardiac failure has recently earned pioglitazone a black box warning by the FDA and it is not recommended in patients with symptomatic heart failure. Nevertheless, a Phase II study in Alzheimer's disease, and its previous indication for diabetes, has demonstrated that pioglitazone is safe and well tolerated19. Therefore, similar to the KD and any drug for that matter, the lowest dose that retains beneficial effects and limits adverse effects is preferred. Information on pioglitazone use in children is lacking; however, a modeling study of pioglitazone dosing for use in septic pediatric patients suggests doses of about 0.3-2 mg/kg/day inversely related to weight21. Recently, this estimate was used for the pioglitazone dosing rationale and successful treatment of a child with refractory nephrotic syndrome for at least 35 weeks without adverse effects attributable to pioglitazone22. This is an encouraging case-study, but additional preclinical studies in appropriate pediatric models are needed to determine the potential safety issues of pioglitazone and KD combination treatment before larger clinical studies can be pursued.
In conclusion, the synergistic protective effects of the KD and pioglitazone in the flurothyl model of acute seizures suggest that combining pioglitazone with a KD will allow drastic reduction in the KD ratio without compromising antiseizure efficacy, thereby possibly limiting adverse effects and improving compliance and quality of life of patients and families. Future pre-clinical studies are required to determine the efficacy and toxicities (if any) of long-term combination treatment in models of chronic epilepsy. Hopefully, a future prospective clinical trial will enable validation of the potential repurposing of pioglitazone as adjuvant treatment with the KD for pediatric refractory epilepsy, and possibly other diseases treated with the KD.
Key Point Box.
KD increases latency to provoked GTC seizures in a fat:carbohydrate-protein ratio dependent manner.
Pioglitazone dose-dependently increases latency to provoked GTC seizures.
Adjuvant treatment of low ratio (1:1) KD with low dose pioglitazone act synergistically to increase latency to provoked GTC to a degree similar to a 6:1 KD.
Acknowledgments
This work was supported by Citizens United for Research in Epilepsy Foundation (TAS), NIH NS072179 (KAS) and NIH NS085389 (TAS). The project described was also supported by the National Center for Research Resources grant G20RR024001. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. Please note, the last author has published under the names K Dorenbos, KA Fenoglio, KA Fenoglio-Simeone and KA Simeone.
Footnotes
Disclosure of Conflicts of Interest: We wish to draw the attention of the Editor to the following facts which may be considered as potential conflicts of interest. Drs. Timothy A. Simeone and Kristina A. Simeone have a patent pending for use of pioglitazone in combination with the KD to treat pediatric epilepsy. The remaining authors have no conflicts of interest.
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Bergin AM. Ketogenic diet in established epilepsy indications. In: Masino SA, editor. Ketogenic diet and metabolic therapies: expanded roles in health and disease. New York: Oxford University press; 2017. pp. 40–49. [Google Scholar]
- 2.Neal E. “Alternative” ketogenic diets. In: Masino SA, editor. Ketogenic diet and metabolic therapies: expanded roles in health and disease. New York: Oxford University press; 2017. pp. 5–15. [Google Scholar]
- 3.Gano LB, Patel M, Rho JM. Ketogenic diets, mitochondria, and neurological diseases. J Lipid Res. 2014;55:2211–2228. doi: 10.1194/jlr.R048975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simeone TA, Matthews SA, Samson KK, et al. Regulation of brain PPARgamma2 contributes to ketogenic diet anti-seizure efficacy. Exp Neurol. 2017;287:54–64. doi: 10.1016/j.expneurol.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simeone TA. Ketogenic Diet and PPARgamma. In: Masino SA, editor. Ketogenic diet and metabolic therapies: expanded roles in health and disease. New York: Oxford University press; 2017. pp. 167–185. [Google Scholar]
- 6.Agarwal S, Yadav A, Chaturvedi RK. Peroxisome proliferator-activated receptors (PPARs) as therapeutic target in neurodegenerative disorders. Biochem Biophys Res Commun. 2017;483:1166–1177. doi: 10.1016/j.bbrc.2016.08.043. [DOI] [PubMed] [Google Scholar]
- 7.Adabi Mohazab R, Javadi-Paydar M, Delfan B, et al. Possible involvement of PPAR-gamma receptor and nitric oxide pathway in the anticonvulsant effect of acute pioglitazone on pentylenetetrazole-induced seizures in mice. Epilepsy Res. 2012;101:28–35. doi: 10.1016/j.eplepsyres.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Simeone KA, Matthews SA, Rho JM, et al. Ketogenic diet treatment increases longevity in Kcna1-null mice, a model of sudden unexpected death in epilepsy. Epilepsia. 2016;57:e178–82. doi: 10.1111/epi.13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim DY, Simeone KA, Simeone TA, et al. Ketone bodies mediate antiseizure effects through mitochondrial permeability transition. Ann Neurol. 2015;78:77–87. doi: 10.1002/ana.24424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Araki H, Kobayashi Y, Hashimoto Y, et al. Characteristics of flurothyl-induced seizures and the effect of antiepileptic drugs on flurothyl-induced seizures in Mongolian gerbils. Pharmacol Biochem Behav. 2002;74:141–7. doi: 10.1016/s0091-3057(02)00965-6. [DOI] [PubMed] [Google Scholar]
- 11.Ferland RJ, Applegate CD. Decreased brainstem seizure thresholds and facilitated seizure propagation in mice exposed to repeated flurothyl-induced generalized forebrain seizures. Epilepsy Res. 1998;30:49–62. doi: 10.1016/s0920-1211(97)00093-4. [DOI] [PubMed] [Google Scholar]
- 12.Grabovsky Y, Tallarida RJ. Isobolographic analysis for combinations of a full and partial agonist: curved isoboles. J Pharmacol Exp Ther. 2004;310:981–986. doi: 10.1124/jpet.104.067264. [DOI] [PubMed] [Google Scholar]
- 13.Rho JM, Kim DW, Robbins CA, et al. Age-dependent differences in flurothyl seizure sensitivity in mice treated with a ketogenic diet. Epilepsy Res. 1999;37:233–240. doi: 10.1016/s0920-1211(99)00068-6. [DOI] [PubMed] [Google Scholar]
- 14.Dutton SB, Sawyer NT, Kalume F, et al. Protective effect of the ketogenic diet in Scn1a mutant mice. Epilepsia. 2011;52:2050–2056. doi: 10.1111/j.1528-1167.2011.03211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maeshiba Y, Kiyota Y, Yamashita K, et al. Disposition of the new antidiabetic agent pioglitazone in rats, dogs, and monkeys. Arzneimittelforschung. 1997;47:29–35. [PubMed] [Google Scholar]
- 16.Grommes C, Karlo JC, Caprariello A, et al. The PPARγ agonist pioglitazone crosses the blood-brain barrier and reduces tumor growth in a human xenograft model. Cancer Chemother Pharmacol. 2013;71:929–936. doi: 10.1007/s00280-013-2084-2. [DOI] [PubMed] [Google Scholar]
- 17.Bough KJ, Yao SG, Eagles DA. Higher ketogenic diet ratios confer protection from seizures without neurotoxicity. Epilepsy Res. 2000;38:15–25. doi: 10.1016/s0920-1211(99)00077-7. [DOI] [PubMed] [Google Scholar]
- 18.Wirrell EC. Ketogenic ratio, calories, and fluids: do they matter? Epilepsia. 2008;49(Suppl 8):17–19. doi: 10.1111/j.1528-1167.2008.01825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galimberti D, Scarpini E. Pioglitazone for the treatment of Alzheimer's disease. Expert Opin Investig Drugs. 2017;26:97–101. doi: 10.1080/13543784.2017.1265504. [DOI] [PubMed] [Google Scholar]
- 20.Sinha B, Ghosal S. Pioglitazone--do we really need it to manage type 2 diabetes? Diabetes Metab Syndr. 2013;7:52–55. doi: 10.1016/j.dsx.2013.02.033. [DOI] [PubMed] [Google Scholar]
- 21.Sherwin CM, Ding L, Kaplan J, et al. Optimal study design for pioglitazone in septic pediatric patients. J Pharmacokinet Pharmacodyn. 2011;38:433–47. doi: 10.1007/s10928-011-9202-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agrawal S, Chanley MA, Westbrook D, et al. Pioglitazone Enhances the Beneficial Effects of Glucocorticoids in Experimental Nephrotic Syndrome. Sci Rep. 2016;6:24392. doi: 10.1038/srep24392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim DY, Abdelwahab MG, Lee SH, et al. Ketones prevent oxidative impairment of hippocampal synaptic integrity through KATP channels. PLoS One. 2015;10:e0119316. doi: 10.1371/journal.pone.0119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim DY, Vallejo J, Rho JM. Ketones prevent synaptic dysfunction induced by mitochondrial respiratory complex inhibitors. J Neurochem. 2010;114:130–141. doi: 10.1111/j.1471-4159.2010.06728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bough KJ, Wetherington J, Hassel B, et al. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol. 2006;60:223–235. doi: 10.1002/ana.20899. [DOI] [PubMed] [Google Scholar]
- 26.Lusardi TA, Akula KK, Coffman SQ, et al. Ketogenic diet prevents epileptogenesis and disease progression in adult mice and rats. Neuropharmacology. 2015;99:500–509. doi: 10.1016/j.neuropharm.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masino SA, Li T, Theofilas P, et al. A ketogenic diet suppresses seizures in mice through adenosine A1 receptors. J Clin Invest. 2011;121:2679–2683. doi: 10.1172/JCI57813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Argmann C, Dobrin R, Heikkinen S, et al. Ppargamma2 is a key driver of longevity in the mouse. PLoS Genet. 2009;5:e1000752. doi: 10.1371/journal.pgen.1000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang KL, Pee HN, Yang S, et al. Influence of drug transporters and stereoselectivity on the brain penetration of pioglitazone as a potential medicine against Alzheimer's disease. Sci Rep. 2015;5:9000. doi: 10.1038/srep09000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chi X, Huang C, Li R, et al. Inhibition of mTOR Pathway by Rapamycin Decreases P-glycoprotein Expression and Spontaneous Seizures in Pharmacoresistant Epilepsy. J Mol Neurosci. 2017;61:553–562. doi: 10.1007/s12031-017-0897-x. [DOI] [PubMed] [Google Scholar]
- 31.McDaniel SS, Rensing NR, Thio LL, et al. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia. 2011;52:e7–11. doi: 10.1111/j.1528-1167.2011.02981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaakkola T, Laitila J, Neuvonen PJ, et al. Pioglitazone is metabolised by CYP2C8 and CYP3A4 in vitro: potential for interactions with CYP2C8 inhibitors. Basic Clin Pharmacol Toxicol. 2006;99:44–51. doi: 10.1111/j.1742-7843.2006.pto_437.x. [DOI] [PubMed] [Google Scholar]
- 33.Arnold C, Konkel A, Fischer R, et al. Cytochrome P450-dependent metabolism of omega-6 and omega-3 long-chain polyunsaturated fatty acids. Pharmacol Rep. 2010;62:536–47. doi: 10.1016/s1734-1140(10)70311-x. [DOI] [PubMed] [Google Scholar]
- 34.Sahi J, Black CB, Hamilton GA, et al. Comparative effects of thiazolidinediones on in vitro P450 enzyme induction and inhibition. Drug Metab Dispos. 2003;31:439–446. doi: 10.1124/dmd.31.4.439. [DOI] [PubMed] [Google Scholar]
- 35.Palmer M. Combination treatment of epilepsy with ketogenic diet and concurrent pharmacological inhibition of cytochrome P450 2E1. Med Hypotheses. 2013;80:481–485. doi: 10.1016/j.mehy.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 36.Simeone TA, Simeone KA, Rho JM. Ketone Bodies as Anti-Seizure Agents. Neurochem Res. doi: 10.1007/s11064-017-2253-5. Epub April 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Antollini SS, Barrantes FJ. Fatty Acid Regulation of Voltage- and Ligand-Gated Ion Channel Function. Front Physiol. 2016;7:573. doi: 10.3389/fphys.2016.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yonutas HM, Sullivan PG. Targeting PPAR isoforms following CNS injury. Curr Drug Targets. 2013;14:733–742. doi: 10.2174/1389450111314070003. [DOI] [PubMed] [Google Scholar]
- 39.Ahmadian M, Suh JM, Hah N, et al. PPARγ signaling and metabolism: the good, the bad and the future. Nat Med. 2013;19:557–566. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kroker AJ, Bruning JB. Review of the Structural and Dynamic Mechanisms of PPARγ Partial Agonism. PPAR Res. 2015;816856 doi: 10.1155/2015/816856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sauer S. Ligands for the Nuclear Peroxisome Proliferator-Activated Receptor Gamma. Trends Pharmacol Sci. 2015;36:688–704. doi: 10.1016/j.tips.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 42.Carmona MC, Louche K, Lefebvre B, et al. S26948: a new specific peroxisome proliferator activated receptor gamma modulator with potent antidiabetes and antiatherogenic effects. Diabetes. 2007;56:2797–2808. doi: 10.2337/db06-1734. [DOI] [PubMed] [Google Scholar]
- 43.Tan Y, Muise ES, Dai H, et al. Novel transcriptome profiling analyses demonstrate that selective peroxisome proliferator-activated receptor γ (PPARγ) modulators display attenuated and selective gene regulatory activity in comparison with PPARγ full agonists. Mol Pharmacol. 2012;82:68–79. doi: 10.1124/mol.111.076679. [DOI] [PubMed] [Google Scholar]
- 44.Itoh T, Fairall L, Amin K, et al. Structural basis for the activation of PPARgamma by oxidized fatty acids. Nat Struct Mol Biol. 2008;15:924–931. doi: 10.1038/nsmb.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fong WH, Tsai HD, Chen YC, et al. Anti-apoptotic actions of PPAR-gamma against ischemic stroke. Mol Neurobiol. 2010;41:180–186. doi: 10.1007/s12035-010-8103-y. [DOI] [PubMed] [Google Scholar]
- 46.Hughes SD, Kanabus M, Anderson G, et al. The ketogenic diet component decanoic acid increases mitochondrial citrate synthase and complex I activity in neuronal cells. J Neurochem. 2014;129:426–433. doi: 10.1111/jnc.12646. [DOI] [PubMed] [Google Scholar]
- 47.Malapaka RR, Khoo S, Zhang J, et al. Identification and mechanism of 10-carbon fatty acid as modulating ligand of peroxisome proliferator-activated receptors. J Biol Chem. 2012;287:183–195. doi: 10.1074/jbc.M111.294785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muralikumar S, Vetrivel U, Narayanasamy A, et al. Probing the intermolecular interactions of PPARγ-LBD with polyunsaturated fatty acids and their anti-inflammatory metabolites to infer most potential binding moieties. Lipids Health Dis. 2017;16:17. doi: 10.1186/s12944-016-0404-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simeone TA, Wilcox KS, White HS. Subunit selectivity of topiramate modulation of heteromeric GABA(A) receptors. Neuropharmacology. 2006;50:845–857. doi: 10.1016/j.neuropharm.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 50.Simeone TA, Wilcox KS, White HS. Topiramate modulation of β(1)- and β(3)-homomeric GABA(A) receptors. Pharmacol Res. 2011;64:44–52. doi: 10.1016/j.phrs.2011.03.004. [DOI] [PubMed] [Google Scholar]


