Summary
Objective
The mutant γ-aminobutyric acid type A (GABAA) receptor γ2(Q390X) subunit, (Q351X in the mature peptide) has been associated with the epileptic encephalopathy, Dravet syndrome, and the epilepsy syndrome genetic epilepsy with febrile seizures plus (GEFS+). The mutation generates a premature stop codon that results in translation of a stable truncated and misfolded γ2 subunit that accumulates in neurons, forms intracellular aggregates, disrupts incorporation of γ2 subunits into GABAA receptors and affects trafficking of partnering α and β subunits. Heterozygous Gabrg2+/Q390X knock-in (KI) mice had reduced cortical inhibition, spike wave discharges on EEG, a lower seizure threshold to the convulsant drug pentylenetetrazol (PTZ) and spontaneous generalized tonic-clonic seizures. In this proof of principal study, we attempted to rescue these deficits in KI mice using a γ2 subunit gene (GABRG2) replacement therapy.
Methods
We introduced the GABRG2 allele by crossing Gabrg2+/Q390X KI mice with bacterial artificial chromosome (BAC) transgenic mice overexpressing HA (hemagglutinin) tagged human γ2HA subunits and compared GABAA receptor subunit expression by western blot and immunohistochemical staining, seizure threshold by monitoring mouse behavior after PTZ-injection, and thalamocortical inhibition and network oscillation by slice recording.
Results
Compared to KI mice, adult mice carrying both mutant allele and transgene had increased wild-type γ2 and partnering α1 and β2/3 subunits, increased miniature inhibitory postsynaptic current (mIPSC) amplitudes recorded from layer VI cortical neurons, reduced thalamocortical network oscillations, and higher PTZ seizure threshold.
Significance
Based on these results we suggest that seizures in a genetic epilepsy syndrome caused by epilepsy mutant γ2(Q390X) subunits with dominant negative effects could be rescued potentially by overexpression of wild-type γ2 subunits.
Keywords: GABAA receptors, genetic epilepsies, GABRG2(Q390X) mutation, gene-replacement therapy, Dravet syndrome, epileptic encephalopathy
Introduction
Epilepsy is a common neurological disorder affecting more than 65 million people worldwide 1. Some of the epilepsies, including the severe epileptic encephalopathy Dravet syndrome, are poorly responsive to conventional treatments 2–5. Many antiepileptic drugs (AEDs) have undesirable side effects, and while some current therapies provide symptomatic treatment, none of them interfere with the epileptogenesis process 6. Alternative strategies that target the underlying genetic causes of epilepsy could be promising in the future.
Many epilepsy-associated mutations in individuals and families with epilepsy have been shown to alter or even disrupt function of ion channels such as γ-aminobutyric acid (GABA) type A (GABAA) receptor channels 7; 8. GABAA receptors are the major receptor mediating fast inhibitory neurotransmission and controlling network excitability in the CNS. Among currently known epilepsy-associated mutations identified in GABAA receptor subunit genes (GABRs), about one half of them are found in GABRG2, which encodes the γ2 subunit 9. Epilepsy-associated mutant γ2 subunits exhibit a wide range of effects on the function and biogenesis of GABAA receptors in vitro and in vivo, from subtle alteration in current kinetic properties to loss of function and dominant negative effects 10. Mice carrying the GABRG2 mutation (R82Q) have reduced cortical inhibition, spontaneous spike-wave discharges and thermal-induced seizures, mimicking seizure phenotypes identified in patients 8; 11–13.
The GABRG2(Q390X) mutation, one of the most severe epilepsy-associated GABRG2 mutations, is associated with genetic epilepsy with febrile seizures plus (GEFS+) and was identified in a patient with Dravet syndrome 14. In vitro mutant γ2(Q390X) subunits severely disrupted the trafficking and function of mutant γ2(Q390X) subunits and also affected the trafficking of partnering α and β subunits and wild-type γ2 subunits 15. Mutant γ2(Q390X) subunits were slowly degraded and formed intracellular aggregates 16. Recently we found that heterozygous Gabrg2+/Q390X KI mice showed decreased expression of wild-type γ2 subunits, aggregation of mutant subunits, reduced cortical inhibition, and behavioral and electrographic seizures, which were much more severe than in heterozygous Gabrg2+/− knock-out mice17,18. Thus, it is interesting to ask whether the epilepsy phenotype in Gabrg2+/Q390X mice expressing the mutant subunits could be improved/rescued by CNS expression of functional wild-type γ2 subunits.
Here we explored whether gene-replacement could rescue the seizure phenotype of Gabrg2+/Q390X KI mice. To compensate for the reduced inhibition caused by mutant γ2(Q390X) subunits, we introduced hemagglutinin (HA) tagged γ2HA subunits into Gabrg2+/Q390X KI mice using Tg(hGABRG2HA) bacterial artificial chromosome (BAC) transgenic mice 19. We found that expressing the BAC transgene in the KI mice not only restored the total amount of wild-type γ2 subunits, but also increased the expression of partnering α and β subunits in the somatosensory cortex, reversed the reduction of miniature inhibitory postsynaptic currents (mIPSCs) and pentylenetetrazol (PTZ) seizure threshold, and reduced the intensified thalamocortical oscillations, demonstrating a rescue of the seizure phenotype.
Materials and Methods
Mice
All animal experiments were approved by the Institutional Animal Care and Use Committee of Vanderbilt University. Tg(hGABRG2HA) transgenic mice containing the GABRG2HA gene and endogenous hGABRG2 promoter were generated in C57BL/6J mice by pronucleus injection in the Vanderbilt Transgenic/ES Cell Shared Resource Facility 19. Four different transgenic mouse lines were generated, but only one of them expressed transgene protein. Thus, we used this mouse line in our study. Gabrg2+/Q390X KI mice were generated in a C57BL/6J;129svJ mixed background and were backcrossed into the C57BL/6J background for more than 8 generations. Tg(hGABRG2HA) transgenic mice were bred with Gabrg2+/Q390X KI mice, generating offspring with four different genotypes, as described in Figure 1A.
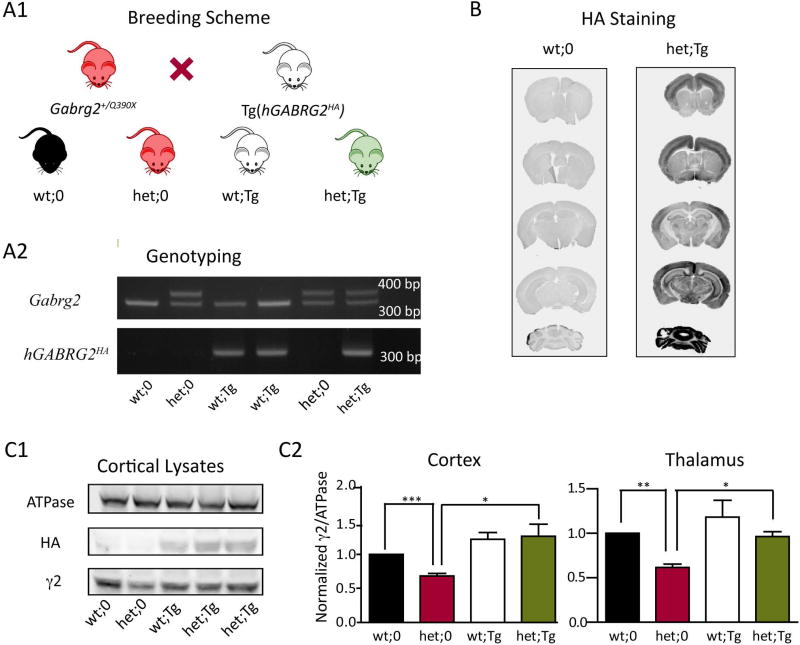
Figure 1. Exogenous γ2HA subunits were introduced in Gabrg2+/Q390X KI mice by crossing them with Tg(hGABRG2HA) mice, and the total amount of full length γ2 subunits in KI mice was restored by the transgene.
A1. The schematic diagram shows the breeding strategy. Heterozygous Gabrg2+/Q390X KI mice were crossed with heterozygous Tg(hGABRG2HA) BAC transgenic mice, generating offspring with four different genotypes: wt;0 denotes Gabrg2+/+ mice; het;0 denotes Gabrg2+/Q390X mice; wt;Tg denotes Gabrg2+/+;Tg(hGABRG2HA) mice; and het;Tg denotes Gabrg2+/Q390X;Tg(hGABRG2HA) mice. A2. PCR and gel electrophoresis were used for genotyping, and the gel presents results from littermates with the four different genotypes. Primers amplifying the endogenous Gabrg2 allele generated one 323 bp band for the wild-type allele and one 405 bp band for the mutant allele. Primers amplifying the transgenic hGABRG2HA allele generated one specific band of 324 bp for the transgene. B. Coronal brain sections from adult wt;0 and het;Tg mice were stained by an anti-HA antibody. C1. Cortex was collected from adult wt;0, het;0, wt;Tg, and het;Tg mice and blotted by anti-ATPase, anti-HA and anti-γ2 subunit antibodies. The anti-γ2 subunit antibody only recognized the full length γ2 subunits (including endogenous γ2 and transgenic γ2HA subunits). C2. Expression levels of wild-type γ2 subunits in cortex and thalamus from adult wt;0, het;0, wt;Tg, and het;Tg mice were plotted. The band intensity of γ2 subunits was normalized to that of ATPase and was further normalized to that of wt;0 littermates (n = 7 for each genotype for cortical samples and n = 4 for each genotype for thalamic samples, mean ± SEM). Differences among littermates were analyzed by one way ANOVA followed by two-tailed paired t test (*** p< 0.001; ** p < 0.01; * p < 0.05).
Mouse tail samples collected at P14 – P21 were extracted using REDExtract-N-Amp tissue PCR kit (Sigma-Aldrich) according to the manufacturer's manual. Mice were genotyped with Tg(hGABRG2HA) forward (5’-TACCCCTACGACGTGCCCGACTACGCC-3’) and reverse (5’ -CACCTCTCCCACTCATAGGCCTGAATG-3’) (324 bp) primers for the transgene; and Gabrg2 forward (5’-ATGGCGATGGAAGTTGACA-3’) and reverse (5’-TGATGTTGCTCATGCCTCTC-3’) primers (323 bp for the wild-type allele and 405 bp for the mutant allele) for the endogenous alleles.
Immunohistochemistry
Adult mice were anesthetized using isoflurane, followed by transcardial perfusion of 20 ml ice-cold 4% paraformaldehyde. Brains were removed and postfixed at 4 °C overnight, then cryoprotected in 30% sucrose at 4 °C for 48 hours. Brain sections of 50 µm thickness were cut using a microtome and stored at −20 °C prior to immunostaining. Slices were incubated in rabbit monoclonal anti-HA antibody (1:500; Cell Signaling, #3724) in PBS with 0.3% Triton X and 4% horse serum at 4 °C for 48 h, followed by 2 h incubation in IRDye800-conjugated donkey anti-rabbit IgG secondary antibody (1:1000; Li-COR). Immunolabeled slices were mounted using glycerol/propyl gallate and scanned with a resolution of 42 µm using the Odyssey imaging system (LI-COR). To study the cellular expression of GABAA receptor subunits, the brains were fixed in 4% paraformaldehyde for 30 min, and maintained in 30% sucrose before sectioning on a cryostat at 30 µm before staining. The rabbit anti-α1 antibody (1:100; Abcam, #ab33299), the mouse anti-β2/3 (1:200 ; Millipore, #MAB341) antibody, and the nuclei staining reagent To-pro-3 (1:500) were used 20. The images were acquired under confocal microscopy with objective of 63X and analyzed by ImageJ.
Western blot
Brains were removed from CO2-euthanized adult mice followed by dissection out of the cortices and thalami. Cortical and thalamic samples were collected in RIPA buffer (25 mM Tris-HCl (pH 7.6), 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) with 1% protease inhibitor (Sigma) and homogenized by sonication. Collected samples were subjected to gel electrophoresis using NuPAGE® (Invitrogen) precast gel and then transferred to PVDF-FL membranes (Millipore). Monoclonal anti-HA antibody (mouse; 1:1000; Covance, # MMS-101P; or rabbit; 1:1000, Cell Signaling, #3724) and polyclonal anti-γ2 antibodies (rabbit, 1:500, Millipore, AB5559) were used to detect the HA-tagged and untagged γ2 subunits, respectively. Anti-sodium potassium ATPase antibody (Abcam) was used as a loading control. After incubation with primary antibodies, IRDye® (LI-COR Biosciences) conjugated secondary antibody was used, and the signals were detected using the Odyssey Infrared Imaging System (LI-COR Biosciences). The integrated intensity value of each specific band was calculated using the Odyssey 3.0 software (LI-COR Biosciences).
PTZ seizure threshold
Male adult mice (2 – 4 month old) received intraperitoneal injection of 55 mg/kg of PTZ, and then were video-monitored for 20 min. The latency to generalized tonic clonic seizures and death were recorded.
Whole cell slice recording
Brain slices were prepared according to published methods 21; 22. Briefly, adult mice (2–6 month old, either gender) were anesthetized with isoflurane and perfused transcardially with ice-cooled dissection solution (4 °C) (mM: 2.5 KCl, 0.5 CaCl2, 10 MgSO4, 1.25 NaH2PO4, 24 NaHCO3, 11 Glucose, 214 Sucrose). Mice were then decapitated, and their brains were removed. Coronal slices (300 µm) were prepared using a LEICA VT-1200S vibratome (Leica Inc) with oxygenated (bubbling with 95% O2/5% CO2) dissection solution. The slices were transferred to an incubation chamber containing oxygenated ACSF (mM: 126 NaCl, 2.5 KCl, 2 CaCl2, 2 MgCl2, 26 NaHCO3, 10 Glucose, pH 7.4). After a 40 min incubation at 35 – 36 °C, the slices were allowed to recover at room temperature for at least 1 hour before experiments.
Slices were viewed with an upright Eclipse FN-1IR-DIC microscope (Nikon) and recordings were obtained and digitized out using a MultiClamp 700B amplifier and Digidata 1440A (Molecular devices Inc.), respectively. Whole cell recordings were made from layer VI pyramidal neurons in the somatosensory cortex. The neurons were selected based on their apical dendrites and location above the white matter, and mIPSCs were isolated pharmacologically by including 10–20 µM NBQX and 1 µM tetrodotoxin in the ACSF (flow rate: 1–1.5 ml per min). The internal pipette solution for recordings 21 contained (mM): 135 CsCl, 10 HEPES, 10 EGTA, 5 QX-314, 5 ATP-Mg (290–295 mOsm, pH = 7.3), and filled glass electrodes had 3–5 MΩ resistances. Access resistances during recording were continuously monitored and confirmed to be less than 20–25 MΩ. The access resistances were compensated by 70% and cell capacitances were also compensated. Recordings with access resistance variations >20% or magnitudes larger than 25 MΩ were discarded. Junction potentials were compensated when electrodes were in ACSF. The chloride ion reversal potential was calculated to be close to 0 mV, and cells were clamped at −60 mV. Data were collected using the Clampex program 10.2 (Molecular devices Inc.), and synaptic currents were filtered at 2 kHz and digitized at 10 KHz. All recordings were performed at room temperature (24 °C) and were made continuously for 20–30 min after membrane rupture.
Thalamocortical oscillations
Horizontal slices (350–400 µm) containing the thalamocortical circuitry were prepared 23 and placed on top of a self-made nylon mesh interface (only one side of the slice contacted the ACSF solution). The network oscillations were recorded using one tungsten electrode (MultiClamp 700B, current-clamp mode) in the ventrobasal nucleus (VBn) of the thalamus and were evoked using one concentric bipolar stimulating electrode in the internal capsule. The multiunit recordings were band-filtered (between 100 Hz and 3 kHz) 24. Each stimulus lasted for 0.1 or 0.3 ms. The experiments were performed at 31 – 32°C.
Data analysis
The whole cell slice recordings were analyzed with Clampfit (Molecular Devices Inc.) using threshold detection (at least 2.5x baseline RMS with no clear synaptic events) 22; 24. Histogram and cumulative graphs were constructed. The network oscillation data were analyzed with both Clampfit (for spike histogram and autocorrelation function) and Matlab to obtain autocorrelograms. Oscillation indices were calculated to compare different groups (both littermates and congenic mice were used) 23; 25. Numerical data were reported as mean ± S.E for all experiments, and statistical differences were determined by the pair wise Student’s t-test/One-Way ANOVA or by the Mantel-Cox method.
Results
Reduced total levels of wild-type γ2 subunits were restored in Gabrg2+/Q390X KI mice by introducing the human GABRG2HA BAC transgene
Previously we generated BAC transgenic mice expressing HA-tagged human γ2 subunits under the control of the endogenous hGABARG2 promoter (Tg(hGABRG2HA) mice) that express γ2HA subunits in neurons 19. The human γ2HA subunits were expressed in mouse brain in a pattern that was similar to that of endogenous γ2 subunits, and the behavior of transgenic mice was indistinguishable from their wild-type littermates. To introduce the transgene to the Gabrg2+/Q390X mice that carried the Dravet syndrome and GEFS+-associated Q390X mutation 17, we crossed the heterozygous KI mice with the transgenic mice. Offspring with four different genotypes were generated: wt;0 (Gabrg2+/+), het;0 (Gabrg2+/Q390X), wt;Tg (Gabrg2+/+;Tg(hGABRG2HA)), and het;Tg (Gabrg2+/Q390X;Tg(hGABRG2HA)) (Figure 1A). Similar numbers of mice from each genotype were born, consistent with Mendelian inheritance (data not shown). Although a reduced birthrate of heterozygous knock-in mice was reported in a mixed C57BL/6J/129Svj background, this was not found in the pure C57BL/6J background17, suggesting different susceptibility of different strains. Overexpressing wild-type γ2 subunits did not affect the birth rate of any of the mice with different genotypes, and thus expression of the transgene could be a potential experimental method for treatment of epilepsy.
We collected brain sections (50 µm) from wt;0 and het;Tg littermate mice and stained the transgenic human γ2HA subunits using an anti-HA antibody. As reported in the transgenic mice, exogenous human γ2HA subunits were expressed across the whole brain of het;Tg mice, including cortex, hippocampus, thalamus, and cerebellum (Figure 1B). To determine whether the total amount of wild-type γ2 subunits was up-regulated by the transgene, we prepared cortical lysates from adult mice and performed western blotting for full length γ2 subunits using an antibody recognizing the M3-M4 loop of both human and mouse γ2 subunits but not recognizing truncated mutant γ2(Q390X) subunits (Figure 1C1). While the total amount of wild-type γ2 subunits was reduced in the cortex of het;0 mice (0.68 ± 0.04, n = 7 for each genotype, p = 0.0001, two-tailed t test), it was restored to control levels in het;Tg mice (1.27 ± 0.18, n = 7 for each genotype, p = 0.01, two-tailed t test) (Figure 1C2, left ). A similar restoration was also observed in thalamic lysates (het;0: 0.68 ± 0.04, het;Tg: 0.96 ± 0.06, n = 4 for each genotype) (wt;0 vs. het;0, p = 0.0019; het;0 vs. het;Tg, p = 0.0262) (Figure 1C2, right). Thus, the total amount of wild-type γ2 subunits in the somatosensory cortex and thalamus, which were reduced in the het;0 mice, were restored to the level in wt;0 mice by introducing the transgene.
The reduction of partnering subunits in the somatosensory cortex of Gabrg2+/Q390X KI mice was reversed by introducing the human GABRG2HA BAC transgene
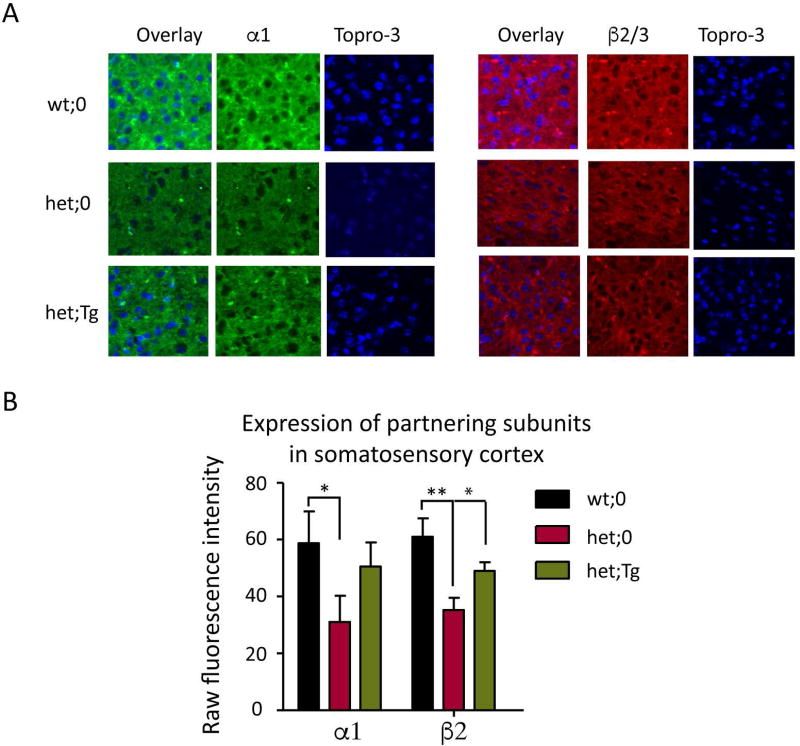
Mutant γ2(Q390X) subunits severely affected the trafficking of partnering α and β subunits15 in vitro and reduced γ2 subunit puncta in somatosensory cortex of Gabrg2+/Q390X KI mice 18. Here we further compared the expression pattern of partnering α1 and β2/3 subunits ex vivo among wt;0, het;0, and het;Tg littermates by immunohistochemistry (Figure 2A). We found that α1 and β2/3 subunit expression was greatly reduced in the somatosensory cortex of KI mice reflecting the dominant negative effects of mutant γ2(Q390X) subunits. The mean raw fluorescence value of α1 subunits in wild-type mice was 58.8 ± 9.2 while the mean value of α1 subunits in mutant mice was 31 ± 9.63 (p = 0.021 het;0 vs wt;0, Figure 2B). Similarly, the mean raw fluorescence value of β2/3 subunits in the wild-type mice was 61± 6.5 while the mean value of the β2/3 subunits in the mutant mice was 35.3± 4.3 (p = 0.003 het;0 vs wt;0, Figure 2B) (n = 12 different brain sections from 3 different mice for each genotype). In the presence of the transgene, the levels of α1 and β2/3 subunits were increased in the somatosensory cortex (50.6 ± 8.4 (p = 0.075 het; Tg vs het;0) for α1 subunits and 49.0 ± 3.1 for β2/3 subunits (p = 0.017 het;Tg vs het;0, Figure 2B), suggesting an alleviation of the dominant negative effects imposed by the mutant γ subunits.
Figure 2. The expression levels of partnering α1 and β2/3 subunits were restored in the somatosensory cortex by the hGABRG2HA transgene.
A. Immunostaining of α1 and β2/3 subunits in somatosensory cortex of wt;0, het;0 and het;Tg mice was performed. Brains from 2 month old wt;0, het;0, and het;Tg mouse littermates were fixed and sectioned on a cryostat at 30 µm. The brain cortices were stained with anti-α1 or anti-β2/3 subunit antibody, and cortical layer VI was visualized. The nuclei were stained with TO-PRO-3 (blue). Images were acquired under confocal microscopy. B. Expression levels from 12 sections from 3 different mice for each genotype were plotted. The total raw fluorescence values of the α1 or β2/3 subunits in somatosensory cortex were quantified by ImageJ. (* p < 0.05; **p < 0.01 vs wt, *p< 0.05 vs het), unpaired t test).
Reduced GABAergic synaptic transmission in Gabrg2+/Q390X KI mice was partially rescued by overexpression of wild-type γ2 subunits
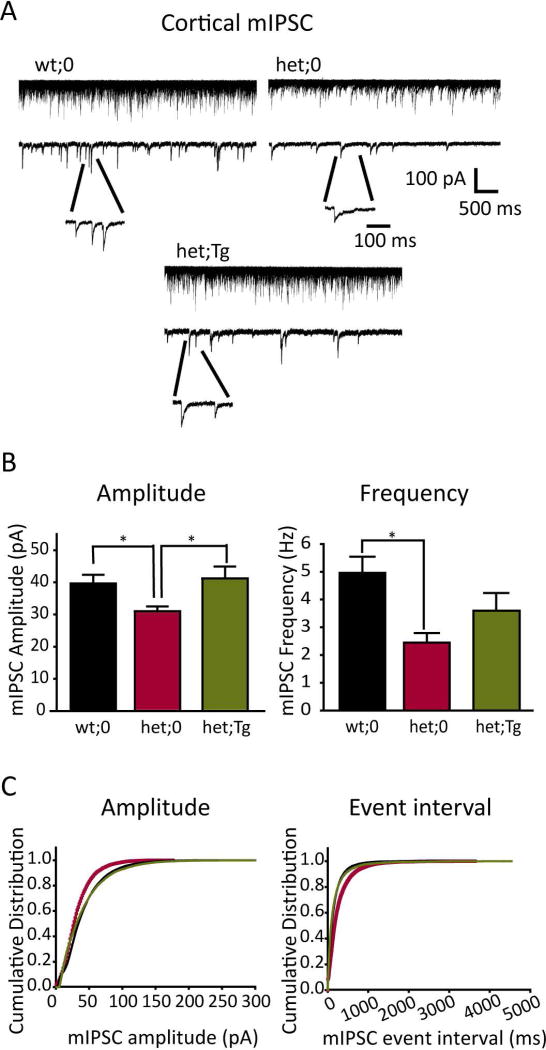
Mutant γ2(Q390X) subunits were stable, but were trapped in the ER with partnering subunits, abolishing receptor function. We wondered whether the overexpressed wild-type γ2 subunits would alleviate or aggravate the defects. To study whether the wild-type transgene could restore the neuronal inhibition mediated by functional GABAA receptors, we characterized inhibitory synaptic transmission in the thalamocortical circuitry, which is involved in the pathogenesis of generalized epilepsy 26; 27. GABAA receptor-mediated mIPSCs were recorded from pyramidal neurons in cortical layer VI (Figure 3A, see methods). All mIPSCs recorded from slices from wild-type littermates exhibited fast rising and slow decaying phases, which were similar to previous reports 28 Compared to those from wt;0 mice (39.65 ± 2.69 pA, 4.96 ± 0.58 Hz, n = 11 cells), mIPSCs recorded in het;0 mice had significantly smaller amplitudes (31.02 ± 1.51 pA, n = 7 cells, t-test p = 0.018) and lower frequencies (2.45 ± 0.34 Hz, n = 7 cells, t-test; p = 0.003) (Figure 3B, C), showing impaired GABAergic neurotransmission. With het;Tg mice expressing exogenous wild-type γ2HA subunits, layer VI pyramidal neurons exhibited mIPSC amplitudes (41.23 ± 3.70 pA, n = 11 cells) that were similar to those in wt;0 mice (t-test p = 0.7) and were larger than those in het;0 mice (t-test, p = 0.02), suggesting increased postsynaptic GABAA receptors. However, mIPSC frequency recorded from het;Tg mice only showed a trend for recovery (3.59 ± 0.64 Hz, n = 11 cells; t-test with het, p = 0.315), compared with those in het;0 mice. Together these data suggested that overexpressing wild-type γ2HA subunits in Gabrg2+/Q390X KI mice could fully rescue the reduced mIPSC amplitude, but not the frequency, in cortical neurons. The partial rescue of mIPSC frequency (Figure 3B) and the incomplete restoration of partnering subunits (Figure 2B) suggested that deficits caused by mutant γ2(Q390X) subunits could not be completely reversed by an addition of wild-type subunits, consistent with our previous findings that this mutation is more than loss-of-function 17 (see discussion below).
Figure 3. Cortical mIPSCs were restored by the hGABRG2HA transgene.
A. Representative mIPSC traces from layer 6 cortical pyramidal neurons were obtained from wt;0, het;0 and het;Tg mice. The top traces represented 50 continuous overlapped sweeps. The lower traces represented a single 5 s trace that has representative mIPSCs time-expanded to show rising and decaying current phases. Scale bars were indicated as labeled. B. The averaged mIPSC amplitudes (left panel) and the averaged mIPSC frequencies (right panel) recorded from slices from wt;0 (black, n = 11 cells), het;0 (red, n = 7 cells) and het;Tg mice (green, n = 11 cells) mice (* p<0.05; t test) were plotted. C. Normalized cumulative curves of mIPSC amplitude (left) and event interval (right) recorded from slices from wt;0 (black), het;0 (red) and het;Tg (green) mice were plotted. The mIPSCs were recorded from the same number of cells (n = 7, randomly chosen) and for the same duration of recording (10 min).
Reduction of PTZ seizure threshold was reversed by overexpressing wild-type γ2HA subunits in Gabrg2+/Q390X KI mice
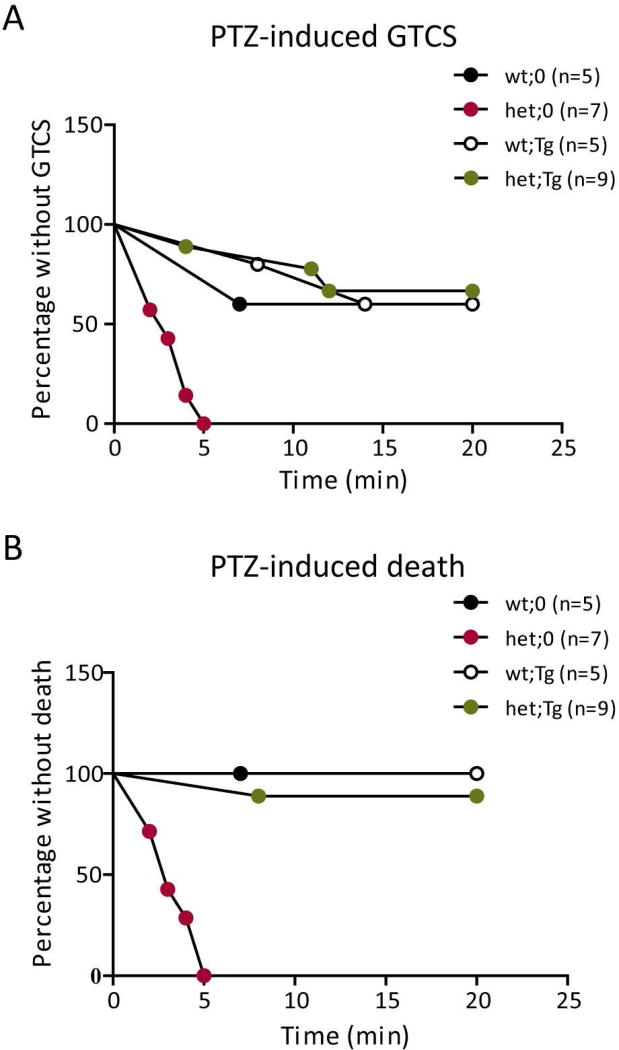
To determine whether the restoration of GABAergic neurotransmission through overexpression of wild-type γ2HA subunits could rescue the seizure phenotype in Gabrg2+/Q390X KI mice, we compared PTZ seizure threshold among the four genotypes, wt;0 (Gabrg2+/+), het;0 (Gabrg2+/Q390X), wt;Tg (Gabrg2+/+; Tg(hGABRG2HA)), and het;Tg (Gabrg2+/Q390X; Tg(hGABRG2HA)). At high doses, PTZ induced generalized tonic-clonic seizures (GTCSs) in rodents 29, one of the seizure phenotypes observed in Gabrg2+/Q390X KI mice. We injected 2–4 month-old mice with 55 mg/kg PTZ i.p. and video monitored the mice after injection to assess development of seizures. Het;0 mice developed seizures soon after injection, showing limb extension, tail-jerks, rearing or jumping, and many of the mice also died during seizures. We plotted the survival curve for PTZ-induced GTCSs (Figure 4A) and death (Figure 4B) among the four genotypes and found that het;0 mice had a lower seizure threshold compared to mice with the other three genotypes. The het;0 mice developed characteristic GTCSs, including clonic jerks and limb extension, and died much sooner and more often than wt;0 littermates. Seizure threshold and PTZ-induced death of het;Tg mice were not different from littermate wt;0 mice (GTCS: wt;0 (n = 5) vs. het;0 (n = 7): p = 0.0009; het;0 (n = 7) vs. het;Tg (n = 9): p = 0.0001; wt;0 (n = 5) vs. het;Tg (n = 9): p = 0.7713. Death: wt;0 (n = 5) vs. het;0 (n = 7): p = 0.0034; het;0 (n = 7) vs. het;Tg (n = 9): p = 0.0004; wt;0 (n = 5) vs. het;Tg (n = 9): p = 0.5050), indicating restored expression of full length γ2 subunits by expression of the transgene restored the seizure threshold of Gabrg2+/Q390X KI mice to that of wild-type mice.
Figure 4. Increased PTZ seizure sensitivity was reversed by the hGABRG2HA transgene.
A, B. Mice were injected i.p. with PTZ (55 mg/kg) to induce seizures. The susceptibility to (A) PTZ-induced GTCS and (B) death were assessed by survival curves. Differences between littermates were analyzed by the Mantel-Cox method. (A. wt;0 vs. het;0: p = 0.0009; het;0 vs. het;Tg: p = 0.0001; wt;0 vs. het;Tg: p = 0.7713. B. wt;0 vs. het;0: p = 0.0034; het;0 vs. het;Tg: p = 0.0004; wt;0 vs. het;Tg: p = 0.5050).
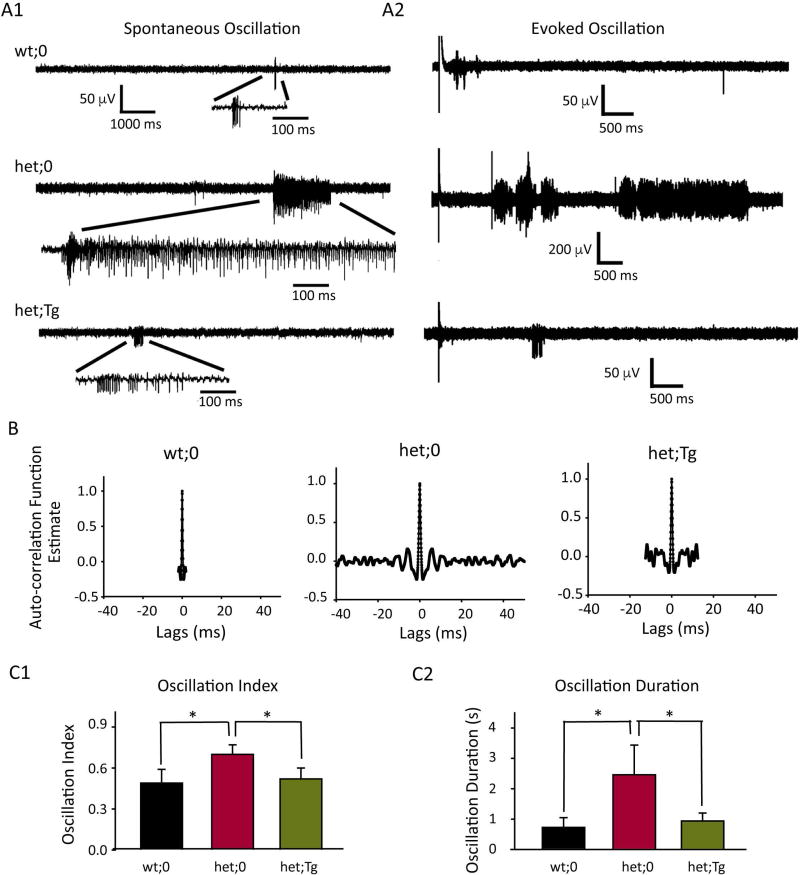
The intensity of thalamocortical network oscillation was reduced by overexpression of wild-type γ2HA subunits in Gabrg2+/Q390X KI mice
Network oscillation in the thalamocortical circuitry plays a significant role in the pathogenesis of generalized epilepsies and strengthened thalamocortical oscillation is a hallmark of generalized epilepsy 21; 23; 27; 30; 31. Although still controversial, it was suggested that hyperexcited cortical neurons can convert thalamocortical synchronous firing into generalized seizures 26. To determine whether the network oscillation or neuronal synchrony in Gabrg2+/Q390X KI mice was increased, we obtained extracellular multiunit recordings from the VBn of thalamus. Both spontaneous (Figure 5A1) and evoked (Figure 5A2) neuronal firing were observed, and rhythmic synchronous firing occasionally occurred spontaneously in horizontal slices from het:0 mice. Spikes with different amplitudes (based on spike-sorting) were identified in het;0 and het:Tg mice (not wt:0 mice), indicating involvement of multiple neurons and the presence of network oscillations. We studied the oscillatory characteristics of network activity of wt;0, het;0 and het;Tg mice by studying autocorrelation of neuron firing and comparing oscillatory indices derived from autocorrelograms of both spontaneous (Figure 5B) and evoked oscillations (autocorrelograms not shown). We found that neuronal synchrony/oscillation was infrequently observed in wt;0 mice, and when it occurred as bursts, its duration was short (oscillatory index: 0.49 ± 0.10; oscillatory duration: 0.72 ± 0.32s; n = 8 slices). In contrast, we found that neuronal synchrony/oscillation was frequently observed in het,0 mice. The neuronal oscillation index in het;0 mice was larger (0.70 ± 0.07, n = 8 slices, t-test p = 0.03) (Figure 5C1), and the duration was longer (2.46 ± 0.98 s, n = 10 slices, t-test p = 0.04) (Figure 5C2) than in wt;0 mice. With wild-type γ2HA subunits introduced into KI mice (het;Tg), spontaneous and evoked oscillations were less frequent (oscillation index 0.52 ± 0.02, n = 10 slices) compared with het;0 mice and were very similar to the firing pattern observed in wt;0 mice (Figure 5C1). Meanwhile, the oscillation duration of het;Tg mice was also shorter (0.94 ± 0.26 s, n = 8 slices) than in het;0 mice (Figure 5C2), suggesting that introducing wild-type γ2HA subunits into het;0 mice rescued mutation-enhanced neuronal synchrony and network oscillation, which can lead to the elevation of seizure threshold.
Figure 5. Spontaneous and evoked thalamocortical oscillations were less intense in het;Tg mice than in het;0 mice.
A. Representative extracellular multiple unit recordings (A1, spontaneous and A2, evoked) from slices from wt;0, het;0 and het;Tg mice were presented. One burst was expanded to show the multiple spikes in the burst. Scale bars were indicated as labeled. B. Autocorrelograms generated from spontaneous (B) and evoked (not shown) burst activity calculated using Clampfit. C. Summaries of averaged oscillatory index (C1) from both spontaneous and evoked burst activity for wt;0 (n = 8 slices), het;0 (n = 8 slices) and het;Tg mice (n = 11 slices) and averaged duration of oscillatory activity (C2) were presented. (* p < 0.05, t-test).
Discussion
Target-specific gene therapy has been explored in a variety of disease models including the genetic neurological disorder Rett syndrome 32–35. Although different genes, including those encoding the Kv1.1 potassium channel 36 and GABAA receptor α1 subunit 37, have been delivered into brain to inhibit the hyperexcitability and seizure development in several epilepsy models, little has been reported for genetic epilepsies, especially those caused by dominant negative mutations. Here we found that target-specific gene replacement therapy could become a future direction to treat severe genetic epilepsies caused by GABRG2 and by extension to other ion channel gene mutations.
In this study we report rescue of reduced PTZ seizure threshold and reduced thalamocortical oscillations in a Gabrg2+/Q390X KI mouse model of Dravet syndrome/GEFS+ by overexpression of wild-type γ2HA subunits. The GABRG2(Q390X) mutation is a severe epilepsy-associated nonsense mutation with loss-of-function and dominant-negative-effects. In vitro, mutant γ2(Q390X) subunits were not only trapped in the endoplasmic reticulum but also prevented the trafficking of wild-type α, β and γ2 subunits 15. In vivo Gabrg2+/Q390X KI mice displayed spontaneous myoclonic jerks and GTCS17. Compared to heterozygous Gabrg2+/− KO mice, KI mice had a larger reduction of wild-type γ2 subunits, a larger decrease of mIPSC amplitude/frequency and more frequent and severe seizures17; 18, likely caused by the dominant negative effects of the mutant subunits. Our study explored the effects of gene-replacement therapy in Gabrg2+/Q390X KI mice by overexpressing the wild-type GABRG2 gene using a BAC transgenic mouse, the Tg(hGABRG2HA) mouse. Similar to other transgenic mice expressing β-actin driven γ2 subunits 38, Tg(hGABRG2HA) mice exhibited normal behavior and life span and had a PTZ seizure threshold that was similar to that of wild-type mice. Although the accumulation of mutant γ2(Q390X) subunits was toxic, the supplementation of wild-type γ2 subunits did not seem to be. We found complete recovery of total γ2 subunit level and mIPSC amplitude, suggesting a restoration of postsynaptic GABAA receptors. However, we found that the mutation-induced decrease of partnering α and β subunits as well as mIPSC frequency in the somatosensory cortex was only partially reversed. GABAA receptors play an important role in establishing inhibitory synapses during development, and loss of postsynaptic GABAA receptors can affect the presynaptic innervation 39. The incomplete recovery of mIPSC frequency and partnering subunits suggested that synaptic deficits might not be fully rescued, which could be caused by the dominant negative effects of mutant γ2(Q390X) subunits, especially during early development. However, the PTZ seizure threshold was still restored to control levels. While we did not compare the severity of spontaneous seizures, which is a more subtle phenotype and highly affected by the genetic background of mice, we found that the intensities of both spontaneous and evoked thalamocortical oscillations, which are hallmarks of generalized epilepsy, was greatly reduced by the transgene. The presence of extra wild-type γ2 subunits could not only complement the loss of function of the hGABRG2 allele, but may also compete with the mutant γ2(Q390X) subunits to reduce the dominant negative effects. As Q390X is the most detrimental epilepsy-associated mutation identified in GABRG2, showing not only loss of function but also dominant negative effects, the same strategy is speculated to also rescue other genetic epilepsies caused by GABRG2 mutations
In this study, however, we have only provided a proof-of-principle. It would be important to determine whether the severity of genetic epilepsy can still be reduced through gene therapy after the epilepsy onset and interesting to test whether gene therapy could be applied in non-genetic epilepsy models. In the future, gene delivery strategies using AAV or lentivirus and small chemical or genetic molecules regulating the gene expression of γ2 subunits will have more promising clinical application. Here we introduced exogenous wild-type γ2 subunits from the beginning of gastrulation, but it would be important to study whether there is an important time window for therapeutic intervention as patients cannot be treated until being diagnosed with epilepsy. In addition, in this study we also overexpressed the transgene across the whole brain. Normal thalamocortical oscillation such as sleep spindles have been suggested to be hijacked in generalized epilepsies 27 and optogenetic inhibition of VBn neurons in thalamus has been shown to inhibit thalamocortical seizures induced by stroke 40. Thus it would be interesting to investigate whether overexpressing wild-type γ2 subunits in a specific brain region is sufficient to attenuate seizures.
Key Points.
The Dravet syndrome-associated GABRG2(Q390X) mutation affected trafficking of GABAA receptor subunits and caused epilepsy in both humans and mice.
Gene-replacement therapy by expressing wild-type GABRG2 in mice carrying the heterozygous Gabrg2(Q390X) mutation increased the wild-type γ2 subunit level.
Restoration of wild-type γ2 subunit expression increased partnering subunit levels and inhibitory neurotransmission in somatosensory cortex
Restoration of wild-type γ2 subunit expression was sufficient to decrease the seizure threshold.
Restoration of wild-type γ2 subunit expression was sufficient to decrease thalamocortical oscillations.
Acknowledgments
This work was supported by NIH R01 NINDS NS051590 to RLM, R01 NINDS NS082635 to JQK, AES Pre-doctoral fellowship to XH, and R21NS096483 to CZ, MJG and RLM. Thanks for Huancheng Dong for his excellent assistance on brain slice preparations.
Footnotes
Author Contribution
Conception and design of the study: X.H., C.Z., R.L.M.; Data collection: X.H., C.Z., J.Q.K., W.S., M.T., K.V.; Data analysis: X.H., C.Z., A.P., J.Q.K., R.L.M.; Manuscript preparation: X.H., C.Z., J.Q.K., R.L.M.; Contributed equally: X.H., C.Z.
Potential Conflicts of Interest
The authors have no potential conflicts of interest to disclosure.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.England MJ, Liverman CT, Schultz AM, et al. Epilepsy Across the Spectrum: Promoting Health and Understanding. National Academies Press (US); Washington DC: 2012. [PubMed] [Google Scholar]
- 2.MacDonald B. The prognosis of epilepsy. Seizure. 2001;10:347–358. doi: 10.1053/seiz.2000.0523. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt D, Sillanpaa M. Evidence-based review on the natural history of the epilepsies. Curr Opin Neurol. 2012;25:159–163. doi: 10.1097/WCO.0b013e3283507e73. [DOI] [PubMed] [Google Scholar]
- 4.Kwan P, Sander JW. The natural history of epilepsy: an epidemiological view. J Neurol Neurosurg Psychiatry. 2004;75:1376–1381. doi: 10.1136/jnnp.2004.045690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas EA, Petrou S. Network-specific mechanisms may explain the paradoxical effects of carbamazepine and phenytoin. Epilepsia. 2013;54:1195–1202. doi: 10.1111/epi.12172. [DOI] [PubMed] [Google Scholar]
- 6.Trinka E, Brigo F. Antiepileptogenesis in humans: disappointing clinical evidence and ways to move forward. Curr Opin Neurol. 2014;27:227–235. doi: 10.1097/WCO.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 7.Wallace RH, Wang DW, Singh R, et al. Febrile seizures and generalized epilepsy associated with a mutation in the Na+-channel beta1 subunit gene SCN1B. Nat Genet. 1998;19:366–370. doi: 10.1038/1252. [DOI] [PubMed] [Google Scholar]
- 8.Wallace RH, Marini C, Petrou S, et al. Mutant GABA(A) receptor gamma2-subunit in childhood absence epilepsy and febrile seizures. Nat Genet. 2001;28:49–52. doi: 10.1038/ng0501-49. [DOI] [PubMed] [Google Scholar]
- 9.Macdonald RL, Kang JQ. Molecular pathology of genetic epilepsies associated with GABAA receptor subunit mutations. Epilepsy Curr. 2009;9:18–23. doi: 10.1111/j.1535-7511.2008.01278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macdonald RL, Kang JQ. mRNA surveillance and endoplasmic reticulum quality control processes alter biogenesis of mutant GABAA receptor subunits associated with genetic epilepsies. Epilepsia. 2012;53(Suppl 9):59–70. doi: 10.1111/epi.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marini C, Harkin LA, Wallace RH, et al. Childhood absence epilepsy and febrile seizures: a family with a GABA(A) receptor mutation. Brain. 2003;126:230–240. doi: 10.1093/brain/awg018. [DOI] [PubMed] [Google Scholar]
- 12.Tan HO, Reid CA, Single FN, et al. Reduced cortical inhibition in a mouse model of familial childhood absence epilepsy. Proc Natl Acad Sci U S A. 2007;104:17536–17541. doi: 10.1073/pnas.0708440104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reid CA, Kim T, Phillips AM, et al. Multiple molecular mechanisms for a single GABAA mutation in epilepsy. Neurology. 2013 doi: 10.1212/WNL.0b013e3182872867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harkin LA, Bowser DN, Dibbens LM, et al. Truncation of the GABA(A)-receptor gamma2 subunit in a family with generalized epilepsy with febrile seizures plus. Am J Hum Genet. 2002;70:530–536. doi: 10.1086/338710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang JQ, Shen W, Macdonald RL. The GABRG2 mutation, Q351X, associated with generalized epilepsy with febrile seizures plus, has both loss of function and dominant-negative suppression. J Neurosci. 2009;29:2845–2856. doi: 10.1523/JNEUROSCI.4772-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang JQ, Shen W, Lee M, et al. Slow degradation and aggregation in vitro of mutant GABAA receptor gamma2(Q351X) subunits associated with epilepsy. J Neurosci. 2010;30:13895–13905. doi: 10.1523/JNEUROSCI.2320-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang JQ, Shen W, Zhou C, et al. The human epilepsy mutation GABRG2(Q390X) causes chronic subunit accumulation and neurodegeneration. Nat Neurosci. 2015;18:988–996. doi: 10.1038/nn.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warner TA, Shen W, Huang X, et al. Differential molecular and behavioural alterations in mouse models of GABRG2 haploinsufficiency versus dominant negative mutations associated with human epilepsy. Hum Mol Genet. 2016 doi: 10.1093/hmg/ddw168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tian M, Macdonald RL. The intronic GABRG2 mutation, IVS6+2T->G, associated with childhood absence epilepsy altered subunit mRNA intron splicing, activated nonsense-mediated decay, and produced a stable truncated gamma2 subunit. J Neurosci. 2012;32:5937–5952. doi: 10.1523/JNEUROSCI.5332-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia G, S PP, Warner TA, et al. Altered GABAA receptor expression in brainstem nuclei and SUDEP in Gabrg2(+/Q390X) mice associated with epileptic encephalopathy. Epilepsy Res. 2016;123:50–54. doi: 10.1016/j.eplepsyres.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schofield CM, Kleiman-Weiner M, Rudolph U, et al. A gain in GABAA receptor synaptic strength in thalamus reduces oscillatory activity and absence seizures. Proc Natl Acad Sci U S A. 2009;106:7630–7635. doi: 10.1073/pnas.0811326106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou C, Lippman JJ, Sun H, et al. Hypoxia-induced neonatal seizures diminish silent synapses and long-term potentiation in hippocampal CA1 neurons. J Neurosci. 2011;31:18211–18222. doi: 10.1523/JNEUROSCI.4838-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sohal VS, Keist R, Rudolph U, et al. Dynamic GABA(A) receptor subtype-specific modulation of the synchrony and duration of thalamic oscillations. J Neurosci. 2003;23:3649–3657. doi: 10.1523/JNEUROSCI.23-09-03649.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huguenard JR, Prince DA. Intrathalamic rhythmicity studied in vitro: nominal T-current modulation causes robust antioscillatory effects. J Neurosci. 1994;14:5485–5502. doi: 10.1523/JNEUROSCI.14-09-05485.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugihara I, Lang EJ, Llinas R. Serotonin modulation of inferior olivary oscillations and synchronicity: a multiple-electrode study in the rat cerebellum. Eur J Neurosci. 1995;7:521–534. doi: 10.1111/j.1460-9568.1995.tb00657.x. [DOI] [PubMed] [Google Scholar]
- 26.McCormick DA, Contreras D. On the cellular and network bases of epileptic seizures. Annu Rev Physiol. 2001;63:815–846. doi: 10.1146/annurev.physiol.63.1.815. [DOI] [PubMed] [Google Scholar]
- 27.Beenhakker MP, Huguenard JR. Neurons that fire together also conspire together: is normal sleep circuitry hijacked to generate epilepsy? Neuron. 2009;62:612–632. doi: 10.1016/j.neuron.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salin PA, Prince DA. Spontaneous GABAA receptor-mediated inhibitory currents in adult rat somatosensory cortex. J Neurophysiol. 1996;75:1573–1588. doi: 10.1152/jn.1996.75.4.1573. [DOI] [PubMed] [Google Scholar]
- 29.Luttjohann A, Fabene PF, van Luijtelaar G. A revised Racine's scale for PTZ-induced seizures in rats. Physiol Behav. 2009;98:579–586. doi: 10.1016/j.physbeh.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Blumenfeld H. Cellular and network mechanisms of spike-wave seizures. Epilepsia. 2005;46(Suppl 9):21–33. doi: 10.1111/j.1528-1167.2005.00311.x. [DOI] [PubMed] [Google Scholar]
- 31.Huntsman MM, Porcello DM, Homanics GE, et al. Reciprocal inhibitory connections and network synchrony in the mammalian thalamus. Science. 1999;283:541–543. doi: 10.1126/science.283.5401.541. [DOI] [PubMed] [Google Scholar]
- 32.Luikenhuis S, Giacometti E, Beard CF, et al. Expression of MeCP2 in postmitotic neurons rescues Rett syndrome in mice. Proc Natl Acad Sci U S A. 2004;101:6033–6038. doi: 10.1073/pnas.0401626101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jugloff DG, Vandamme K, Logan R, et al. Targeted delivery of an Mecp2 transgene to forebrain neurons improves the behavior of female Mecp2-deficient mice. Hum Mol Genet. 2008;17:1386–1396. doi: 10.1093/hmg/ddn026. [DOI] [PubMed] [Google Scholar]
- 34.Guy J, Gan J, Selfridge J, et al. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315:1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akil O, Seal RP, Burke K, et al. Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy. Neuron. 2012;75:283–293. doi: 10.1016/j.neuron.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wykes RC, Heeroma JH, Mantoan L, et al. Optogenetic and potassium channel gene therapy in a rodent model of focal neocortical epilepsy. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3004190. 161ra152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raol YH, Lund IV, Bandyopadhyay S, et al. Enhancing GABA(A) receptor alpha 1 subunit levels in hippocampal dentate gyrus inhibits epilepsy development in an animal model of temporal lobe epilepsy. J Neurosci. 2006;26:11342–11346. doi: 10.1523/JNEUROSCI.3329-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wick MJ, Radcliffe RA, Bowers BJ, et al. Behavioural changes produced by transgenic overexpression of gamma2L and gamma2S subunits of the GABAA receptor. Eur J Neurosci. 2000;12:2634–2638. doi: 10.1046/j.1460-9568.2000.00160.x. [DOI] [PubMed] [Google Scholar]
- 39.Huang ZJ. Activity-dependent development of inhibitory synapses and innervation pattern: role of GABA signalling and beyond. J Physiol. 2009;587:1881–1888. doi: 10.1113/jphysiol.2008.168211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paz JT, Davidson TJ, Frechette ES, et al. Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury. Nat Neurosci. 2013;16:64–70. doi: 10.1038/nn.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]