Abstract
Objective:
Most studies suggest that poor cognitive functioning in adolescence increases risk of alcohol use disorders (AUDs). We seek to clarify the causes of this association.
Method:
In Swedish individuals born from 1972 to 1990 in whom cognitive functioning was assessed by school achievement at age 16 years (males and females, N = 1,796,048) and by IQ at ages 18–20 (males, N = 554,644), we examined the hazard ratio (HR) for AUD ascertained from public registries. We examined and modeled risk of AUD in cousins, full siblings, and monozygotic twin pairs discordant for school achievement and IQ scores.
Results:
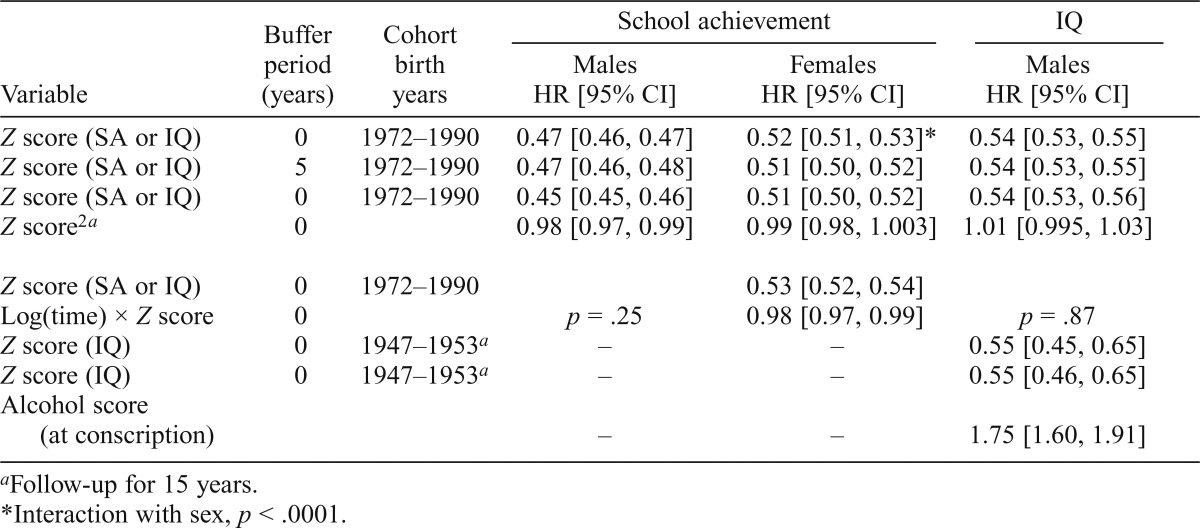
In males and females, HRs for AUD per standard deviation of increasing school achievement equaled 0.47 (95% CI [0.46, 0.47]) and 0.52 (95% CI [0.51, 0.53]), respectively. In males, the HR for AUD per standard deviation of increasing IQ was 0.54 (95% CI [0.53, 0.55]). Excluding onsets of AUD within 5 years of the cognitive evaluation did not weaken the association, nor did controlling for alcohol intake and problems at IQ assessment. The HRs for AUD in relative pairs were higher than those observed in the population but significantly less than unity. We predicted the following HRs for AUD in discordant monozygotic twins for school achievement in males and females and for IQ in males: 0.66 (95% CI [0.62, 0.70]), 0.67 (95% CI [0.62, 0.73]), and 0.72 (95% CI [0.65, 0.79]), respectively.
Conclusions:
Cognitive ability in adolescence, assessed by two different measures, strongly predicts risk of AUD. This association cannot be explained by early symptoms of AUD impairing performance. Co relative analyses suggest that this association arises partly from familial confounding and partly from a causal impact of low cognitive ability on AUD risk.
An inverse relationship between measures of cognitive ability in adolescence and risk of future heavy alcohol use and alcohol use disorder (AUD) has been reported in many studies (Crum et al., 1993; Finn & Hall, 2004; Fothergill & Ensminger, 2006; Hawkins et al., 1992; Huurre et al., 2010; Sjolund et al., 2012) but not all (Crum et al., 2006; Grant et al., 2015; Hatch et al., 2007; Maggs et al., 2008). However, if present, the causal nature of this association is unclear. The association between adolescent cognitive ability and AUD could arise from confounders including low social class, family disruption, and genetic factors that predispose to both cognitive ability and AUD (Bernardi & Radl, 2014; Bouchard, Jr., 1998; Hawkins et al., 1992; Moss et al., 1995; Verhulst et al., 2015). Heavy alcohol consumption in adolescence could result in poor school functioning (Chatterji, 2006; Cook & Moore, 1993) and, via effects on the developing brain, lowered subsequent cognitive ability (Brown et al., 2000; Lisdahl et al., 2013; Luciana et al., 2013). However, lowered adolescent cognitive ability could directly contribute to increased AUD risk.
In this report, we first seek to quantify, in a large general population sample, the prospective association between cognitive ability in adolescence, measured as both school achievement and IQ, and risk of AUD. Second, we evaluate the validity of the three hypotheses articulated above as explanations for the association between school achievement and IQ with AUD—that is, does the school achievement–AUD association arise because of confounders or because of a causal relationship from cognitive ability to AUD or from AUD to cognitive ability. These results have implications for understanding important pathways of risk to AUD and the subsequent ability to plan effective prevention methods. To address these issues, we use longitudinal cohort and corelative designs in Swedish national population samples.
Method
We analyzed information on individuals from Swedish population based registers with national coverage. These registers were linked using each person’s unique identification number replaced by a serial number to preserve confidentiality. We secured ethical approval for this study from the Regional Ethical Review Board of Lund University.
We assessed cognitive ability first using school achievement (i.e., grade point average) for all students in grade nine (usually age 16 years) from 1988 to 2012 contained in the National School Registry. Students had an incentive to perform well because those who scored highly were more likely to gain admission to desirable secondary schools. We standardized grade score for each year and gender and called this variable school achievement.
From 1988 to 1997, scores were expressed on a scale from 1 to 5, and students were assessed by a peer-referencing system. Under this system, grades had minimal inflation over time and were normally distributed. After 1997, scores were expressed on a scale between 10 and 320 using a criterion referenced system and were not standardized across schools.
The second cognitive ability we examined was IQ as measured by four subtests representing logical, spatial, verbal, and technical abilities. This information was contained in the Military Conscription Register for nearly all 18- to 20-year old men in Sweden. During these years, examination was legally required. Only those with serious medical conditions or disabilities were excused (about 4.2%). The global IQ score—a summation of the four subtests—was standardized within each year to give a Gaussian score between 1 and 9.
AUD was defined in three ways, as follows: (a) by ICD codes for main and secondary diagnoses from Swedish medical and Cause of Death registries (the Swedish Hospital Discharge Register, containing all hospitalizations for all Swedish inhabitants from 1973 to 2012, and the Outpatient Care Register, containing information from all outpatient clinics from 2001 to 2012; the Swedish Cause of Death Register, containing information on all deaths in Sweden from 1963 to 2012) for the following diagnoses: ICD-8 and -9: alcohol-related psychiatric disorders (291), alcohol dependence (303), alcohol abuse (305A), alcohol-related polyneuropathy (357F), alcohol-related cardiomyopathy (425F), alcohol-related gastritis (535D), alcoholic fatty liver, alcohol hepatitis, alcoholic cirrhosis, unspecified liver damage caused by alcohol (571A–D), toxic effects of alcohol (980), alcoholism (V79B); ICD 10: alcohol-related psychiatric and behavioral disorders (F10, excluding acute alcohol intoxication: F10.0), rehabilitation of a person with alcohol abuse (Z50.2), guidance and medical advice to a person with alcohol abuse (Z71.4), alcohol-related pseudo Cushing syndrome (E24.4), alcohol-related degeneration of the nervous system and brain (G31.2), alcohol-related polyneuropathy (G62.1), alcohol-related myopathy (G72.1), alcohol-related cardiomyopathy (I42.6), alcohol-related gastritis (K29.2), liver diseases caused by alcohol (K70.0-K70.9), acute pancreatitis caused by alcohol (K85.2), chronic pancreatitis caused by alcohol (K86.0), treatment of pregnant alcoholic woman (O35.4), toxic effects of alcohol (T51.0-T51.9); (b) by Anatomical Therapeutic Chemical (ATC) codes in the Prescribed Drug Register (containing all prescriptions in Sweden picked up by patients from July 2005 to 2012): disulfiram (N07BB01), acamprosate (N07BB03), or naltrexone (N07BB04); or (c) by registrations of individuals in the Swedish Crime Registers (the Swedish Crime Register included national complete data on all convictions from 1973 to 2010, and the Swedish suspicion register included national complete data on all individuals strongly suspected of crime from 1998 to 2010) with at least two convictions of drunk driving (suspicion code 3005, law 1951:649 [paragraphs 4 and 4A]) or drunk in charge of a maritime vessel (suspicion code 3201, law 1994:1009 [chapter 20, paragraphs 4 and 5]).
We ensured that we did not count arrests in the suspicion register that described the same event contained in the conviction register. The validity of our AUD diagnosis was supported by high rates of co ascertainment across our registries (mean odds ratio [OR] = 32.7) (Kendler et al., 2015).
The school achievement database contained all individuals born in Sweden from 1972 to 1990 who had not died or emigrated before age 16 years and were registered in the National School Register. Furthermore, the individual could not have an onset of AUD before their school achievement assessment. The IQ database was built on the school achievement database, but additionally the individual had to have an IQ score in the Conscription Register and could not have an onset of AUD before IQ testing.
Analysis
In the first analysis, we used Cox proportional hazards models to investigate the risk of AUD as a function of school achievement, from year of school achievement registration until end of follow-up (AUD registration, death, emigration, or 2012). As the database contained individuals from the same family, robust standard errors were used to adjust the 95% confidence intervals (CIs). We then performed an exploratory/descriptive analysis examining the rates of AUD in pairs of monozygotic (MZ) twins identified from the Swedish Twin Register discordant for their level of school achievement. The initial question was whether the poorer performing member of these pairs had higher rates of AUD. If this was the case, we would then proceed to a full model that would assess the degree to which the regression results reflect confounding by familial risk factors using a corelative design.
From the Swedish Multi-Generation and Twin Registers, we identified all MZ twin, full-sibling, and cousin pairs. Using stratified Cox proportional hazards models, with a separate stratum for each relative pair, we refitted the analysis. The HR was then adjusted for a range of unmeasured genetic and environmental factors shared within the relative pair. MZ twins share 100% of their genes and a large part of environmental factors, suggesting that the HR for MZ twins is controlled for all possible confounding by genes and shared environment. Full siblings and cousins share, respectively, on average 50% and 12.5% of their genes identical by descent.
In the next step, we combined all four samples (i.e., population, twin, full siblings, and cousins) into one data set in which we performed two analyses. The first allowed all parameters for each sample to be independent (i.e., similar to four separate analyses). In the second, we modeled the association between school achievement and AUD with two parameters: one main effect and one as a linear function of the genetic resemblance (i.e., 0 for the population, 0.125 for cousins, 0.5 for siblings, and 1 for MZ twins). The HR for the second parameter gave an indication of the size of the familial confounding. If the second model fitted the data well, we also obtained an improved estimation of the association among all relatives, especially MZ twins, where the data were sparse.
All analyses were replicated on IQ. To test if the association between IQ and AUD could partly result from early symptoms of AUD on IQ, we used information on males conscripted into military service in 1969 and 1970 because more extensive data, including alcohol consumption and alcohol-related problems, were collected at conscription during these years.
First, using factor analysis we constructed an “alcohol score” based on all questions regarding alcohol consumption and alcohol-related problems. Second, by allowing approximately the same follow up time as for our original sample and including the alcohol score as a control variable in the models, we were able to partly control for early symptoms of AUD. All statistical analyses were performed using SAS Version 9.3 (SAS Institute Inc., Cary, NC).
All procedures contributing to this work complied with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Results
School achievement
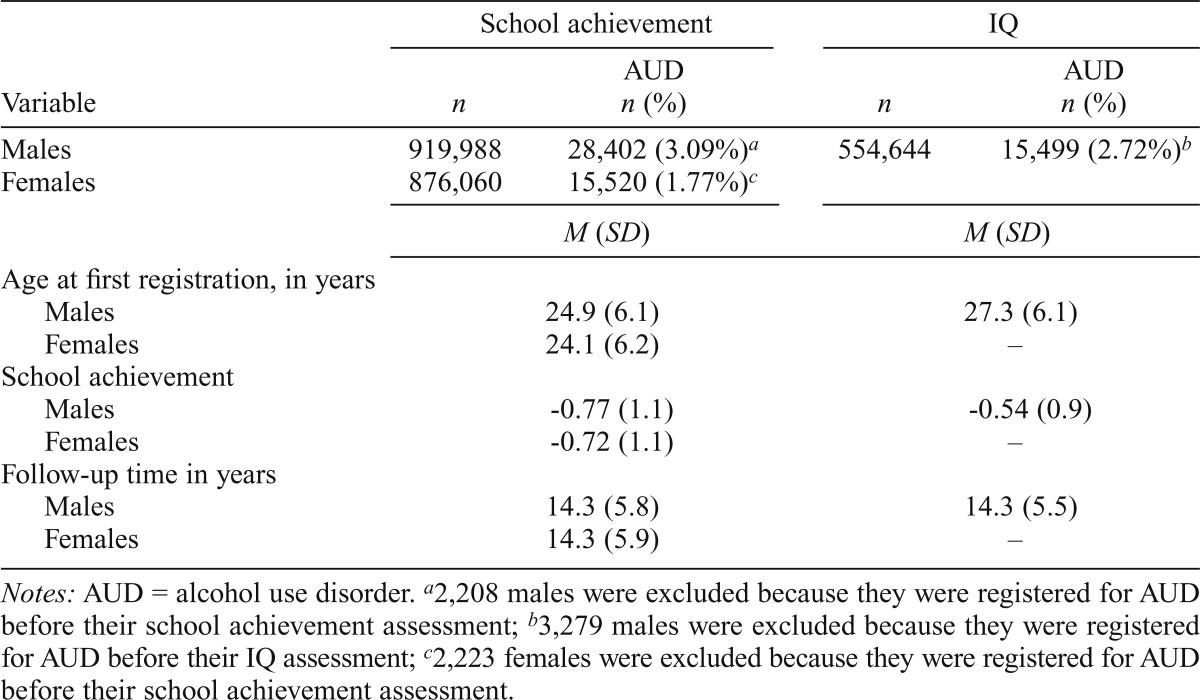
In all males and females born from 1972 to 1990 for whom school achievement scores were available (total N = 1,796,048), 3.1% of males and 1.8% of females had an onset of AUD after the school achievement assessment. As seen in Table 1, the mean age at first AUD registration in this sample was about 25 years, and individuals who later developed AUD had school achievement scores that were, on average, 0.75 standard deviations below the mean. We were able to follow up this cohort for, on average, 14.3 years.
Table 1.
Descriptive statistics for population born in Sweden from 1972 to 1990 and with results available for school achievement and IQ
| School achievement |
IQ |
|||
| Variable | n | AUD n (%) | n | AUD n (%) |
| Males | 919,988 | 28,402 (3.09%)a | 554,644 | 15,499 (2.72%)b |
| Females | 876,060 | 15,520 (1.77%)c | ||
| M (SD) | M (SD) | |
| Age at first registration, in years | ||
| Males | 24.9 (6.1) | 27.3 (6.1) |
| Females | 24.1 (6.2) | – |
| School achievement | ||
| Males | 0.77 (1.1) | -0.54 (0.9) |
| Females | 0.72 (1.1) | – |
| Follow-up time in years | ||
| Males | 14.3 (5.8) | 14.3 (5.5) |
| Females | 14.3 (5.9) | – |
Notes: AUD = alcohol use disorder.
2,208 males were excluded because they were registered for AUD before their school achievement assessment;
3,279 males were excluded because they were registered for AUD before their IQ assessment;
2,223 females were excluded because they were registered for AUD before their school achievement assessment.
In both males and females, the prevalence of AUD was strongly associated with school achievement scores (Figure 1). The curves were monotonic in both sexes, steeper in males than females, and steeper at lower than at higher school achievement scores. A linear model predicted in males an HR of 0.47 (95% CI [0.46, 0.47]) or a reduction of risk of 53% per standard deviation of increasing school achievement (Table 2). The HR in females was slightly weaker (0.52, 95% CI [0.51, 0.53]; 48% reduction in risk per standard deviation) and significantly different from that seen in males. We next added a quadratic function that was very modest but significant in males but not significant in females. Our test for the proportionality assumption was met in the males but failed in the females, as the association was slightly stronger with longer follow-up.
Figure 1.
Observed prevalence of alcohol use disorder (AUD) as a function of the school achievement scores as measured in standard deviation units at age 16 in males and females. Only onsets after school achievement testing are included.
Table 2.
Hazard ratios (HRs) and 95% confidence intervals (CIs) for association between school achievement (SA) and IQ (standardized as Z scores) and risk of alcohol use disorder (AUD)
| Variable | Buffer Period (years) | Cohort Birth years | School achievement |
IQ |
|
| Males HR [95% CI] | Females HR [95% CI] | Males HR [95% CI] | |||
| Z score (SA or IQ) | 0 | 1972–1990 | 0.47 [0.46, 0.47] | 0.52 [0.51, 0.53]* | 0.54 [0.53, 0.55] |
| Z score (SA or IQ) | 5 | 1972–1990 | 0.47 [0.46, 0.48] | 0.51 [0.50, 0.52] | 0.54 [0.53, 0.55] |
| Z score (SA or IQ) | 0 | 1972–1990 | 0.45 [0.45, 0.46] | 0.51 [0.50, 0.52] | 0.54 [0.53, 0.56] |
| Z score2a | 0 | 0.98 [0.97, 0.99] | 0.99 [0.98, 1.003] | 1.01 [0.995, 1.03] | |
| Z score (SA or IQ) | 0 | 1972–1990 | 0.53 [0.52, 0.54] | ||
| Log(time) × Z score | 0 | p = .25 | 0.98 [0.97, 0.99] | p = .87 | |
| Z score (IQ) | 0 | 1947–1953a | – | – | 0.55 [0.45, 0.65] |
| Z score (IQ) Alcohol score | 0 | 1947–1953a | – | – | 0.55 [0.46, 0.65] |
| (at conscription) | – | – | 1.75 [1.60, 1.91] | ||
Follow-up for 15 years.
Interaction with sex, p < .0001.
The association between school achievement and AUD could result from an adverse effect of early symptoms of AUD on school achievement. If true, this would predict a decline in the school achievement–AUD association if we censored onsets of AUD within a few years of the school achievement assessment. We therefore repeated analyses predicting onset of AUD, deleting from the analyses all AUD onsets within 5 years of the school achievement assessment (Table 2). The school achievement–AUD associations were nearly identical to those seen initially in both males and females.
Co-relative analyses
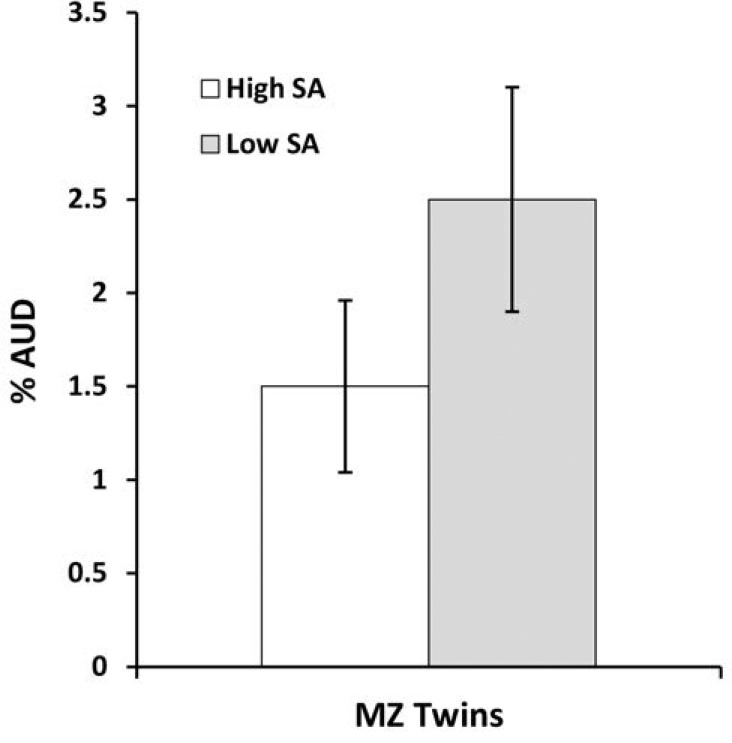
To explore whether a co-relative design might help us understand the causal relationship between school achievement and AUD, we examined the risk of AUD in all MZ twins discordant for their school achievement scores (n = 2,642). As seen in Figure 2, the low-scoring member of the pair had a mean risk of AUD that was 67% higher than their higher scoring co-twin. These results encouraged us to conduct a full co-relative analysis separately by sex.
Figure 2.
The prevalence of alcohol use disorder in monozygotic (MZ) twin pairs discordant for their school achievement (SA) scores IQ.
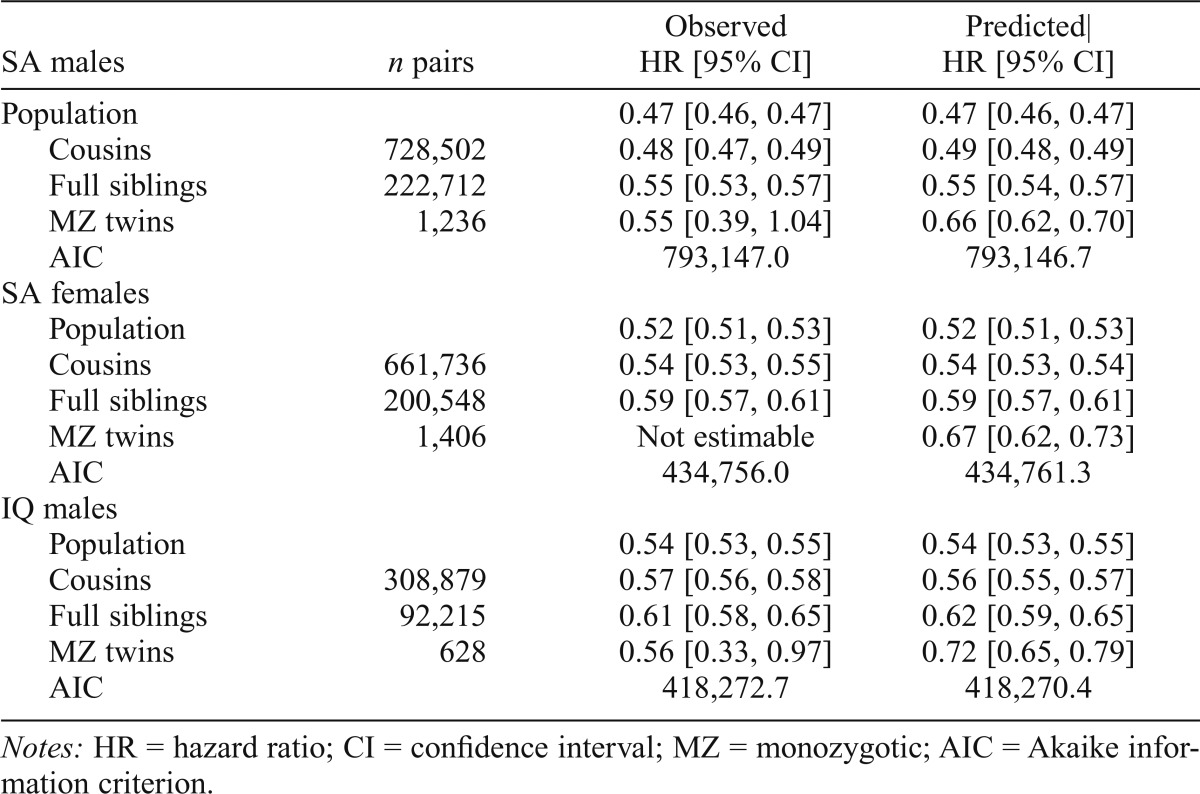
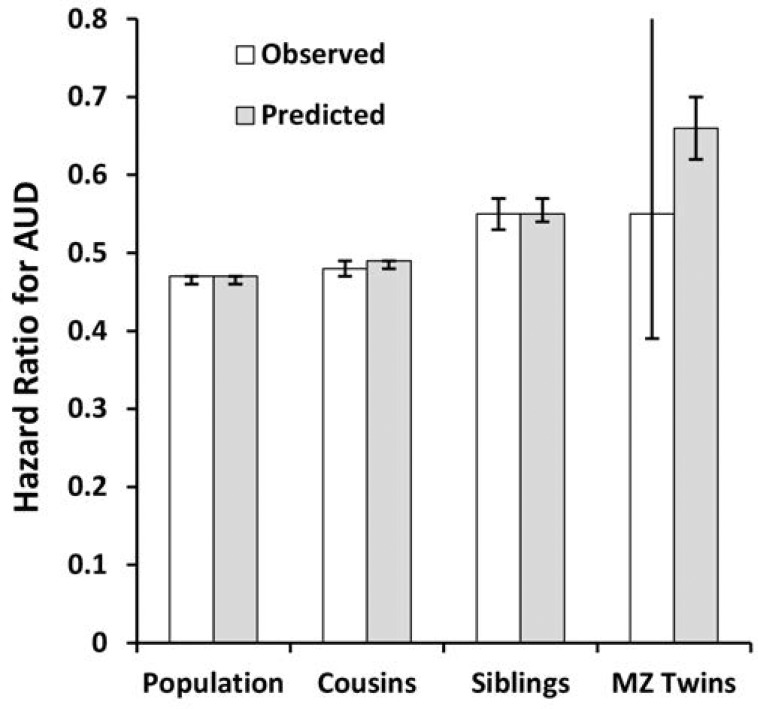
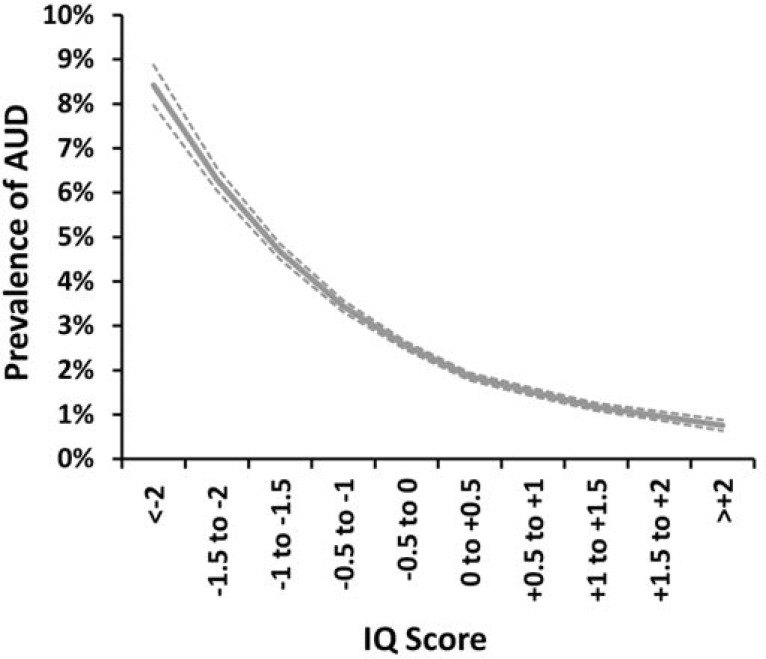
Compared with those for the general population, HRs for school achievement predicting AUD in males were modestly greater (i.e., closer to unity) in discordant first cousin, sibling, and MZ pairs (Table 3, Figure 3a). By comparison with estimates in cousins and siblings, those in MZ twins were known less precisely because of the much smaller sample size. When we fitted our co-relative model to these results, the fit improved. The predicted estimates across the four groups were similar to those observed with the exception of MZ twins. Here the predicted HR (0.66) was higher than that observed (0.55) but well within the CIs of the initial estimate. The pattern was similar for school achievement and AUD in females (Table 3, Figure 3b), with two differences. First, the sample size of discordant MZ pairs was too small to produce reliable estimates taken on their own. Second, the fit of our model was modestly worse than that seen for the raw data.
Table 3.
Observed results of co relative analyses and predicted results from a linear model based on degree of genetic relationship for standardized school achievement (SA) in males and females and IQ in males
| SA males | n pairs | Observed HR [95% CI] | Predicted| HR [95% CI] |
| Population | 0.47 [0.46, 0.47] | 0.47 [0.46, 0.47] | |
| Cousins | 728,502 | 0.48 [0.47, 0.49] | 0.49 [0.48, 0.49] |
| Full siblings | 222,712 | 0.55 [0.53, 0.57] | 0.55 [0.54, 0.57] |
| MZ twins | 1,236 | 0.55 [0.39, 1.04] | 0.66 [0.62, 0.70] |
| AIC | 793,147.0 | 793,146.7 | |
| SA females | |||
| Population | 0.52 [0.51, 0.53] | 0.52 [0.51, 0.53] | |
| Cousins | 661,736 | 0.54 [0.53, 0.55] | 0.54 [0.53, 0.54] |
| Full siblings | 200,548 | 0.59 [0.57, 0.61] | 0.59 [0.57, 0.61] |
| MZ twins | 1,406 | Not estimable | 0.67 [0.62, 0.73] |
| AIC | 434,756.0 | 434,761.3 | |
| IQ males | |||
| Population | 0.54 [0.53, 0.55] | 0.54 [0.53, 0.55] | |
| Cousins | 308,879 | 0.57 [0.56, 0.58] | 0.56 [0.55, 0.57] |
| Full siblings | 92,215 | 0.61 [0.58, 0.65] | 0.62 [0.59, 0.65] |
| MZ twins | 628 | 0.56 [0.33, 0.97] | 0.72 [0.65, 0.79] |
| AIC | 418,272.7 | 418,270.4 |
Notes: HR = hazard ratio; CI = confidence interval; MZ = monozygotic; AIC = Akaike information criterion.
Figure 3a.
The hazard ratio for alcohol use disorder (AUD) in the general population and in cousin, full sibling, and monozygotic (MZ) twin pairs discordant as a function of the standardized school achievement scores in males. The white columns are the observed results and the gray columns reflect those predicted from our co-relative model. See the Methods section for details.
Figure 3b.
The hazard ratio for alcohol use disorder (AUD) in the general population and in cousin, full sibling, and monozygotic (MZ) twin pairs discordant for standardized school achievement scores in females. The white columns are the observed results and the gray columns reflect those predicted from our co-relative model. See the Method section for details.
IQ
In the males born from 1972 to 1990 for whom IQ scores were available (total N = 554,644), 2.7% had an onset of AUD after the IQ assessment. The mean age at first AUD registration in this sample was 27.3 years, and individuals who later developed AUD had IQ scores that were, on average, 0.54 standard deviations below the mean (Table 1). The mean follow-up period was 14.3 years.
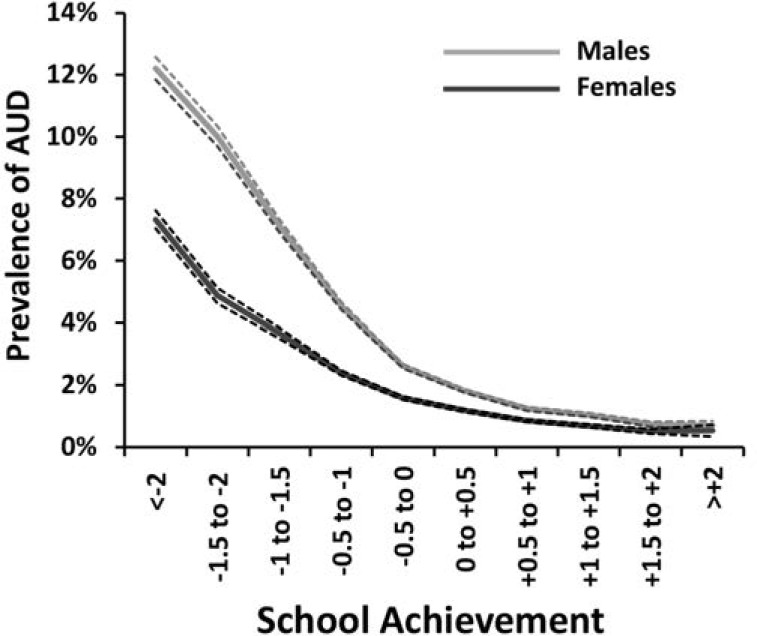
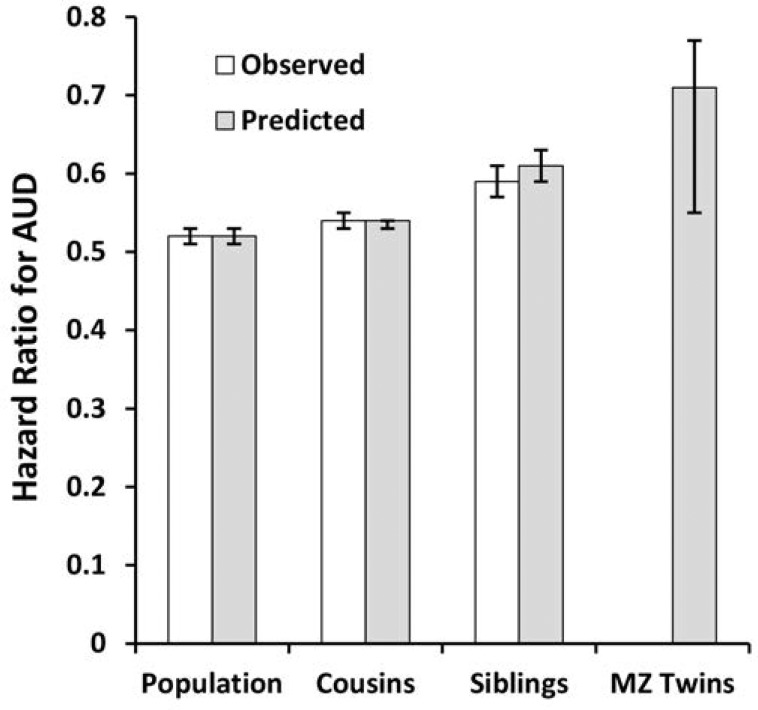
The prevalence of AUD in this sample was strongly associated with IQ (Figure 4). The curve was monotonic and showed a stronger association in the lower than in the higher ranges of IQ. A linear model estimated the HR of 0.54 (95% CI [0.53, 0.55]) equaling a reduction in risk of 46% per standard deviation of increasing IQ. Both a quadratic effect and a departure from the proportionality assumption were tested. Neither was significant.
Figure 4.
Observed prevalence of alcohol use disorder as a function of IQ scores, as measured in standard deviation units, in males assessed at ages 18–20. Only onsets after IQ testing are included.
The association between IQ and AUD could be influenced by an adverse effect of the early symptoms of AUD on IQ. We tested for this effect in two ways. First, we built in a buffer period not counting any AUD onsets within 5 years of IQ testing. This had no effect on the observed HR (Table 3). Second, at the time of the IQ testing, the conscripts completed a self-report questionnaire that included alcohol consumption and alcohol-related problems and was available to us only for conscripts examined in 1969–1970. To make the results of this sample as comparable as possible to our main analyses, we truncated our follow-up of these older individuals at 15 years. As seen in Table 2, we replicated in a different sample the overall association between IQ and AUD (HR = 0.55, 95% CI [0.45, 0.65]). Furthermore, we showed that although the aggregate score of alcohol consumption and problems strongly predicted future AUD risk (HR per standard deviation = 1.75, 95% CI [1.60, 1.91]), its inclusion in the model had no impact on the IQ–AUD association.
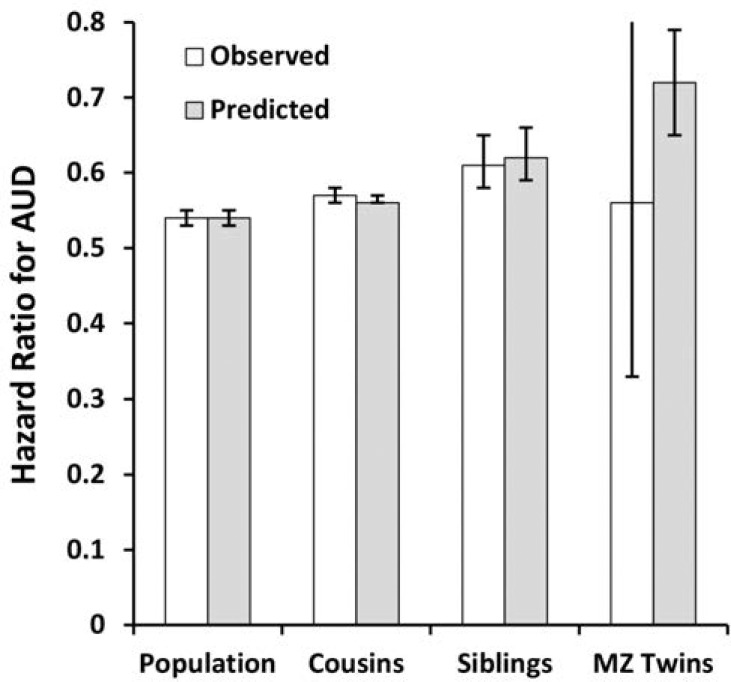
As seen in Table 3 and Figure 5, the co-relative analyses for IQ in males predicting AUD closely resembled those seen for school achievement, including the improved fit of the model by AIC compared with that found for the raw data. The observed HR in discordant MZ twins was known very imprecisely (0.56, 95% CI [0.33, 0.97]) and when estimated by our co-relative model equaled 0.72 (95% CI [0.65, 0.79]), again well within the CIs of the original raw estimate.
Figure 5.
The hazard ratio for alcohol use disorder in the general population and in cousin, full sibling, and monozygotic twin pairs discordant for standardized IQ scores in males. The white columns are the observed results and the gray columns reflect those predicted from our co-relative model. See the Method section for details.
Discussion
The goals of these analyses were to quantify, in a large general population cohort, the prospective association between two measures of cognitive ability assessed in adolescence (school achievement and IQ) and risk of AUD and second to gain insight into the nature of that association. We review these results in turn.
Consistent with prior studies (Crum et al., 1993; Finn & Hall, 2004; Fothergill & Ensminger, 2006; Hawkins et al., 1992; Huurre et al., 2010), we found a robust association between cognitive ability assessed in adolescence and the probability of future AUD registration. Specifically, for each standard deviation of increasing school achievement assessed at age 16 years, risk of AUD fell 53% and 48% in males and females, respectively. The association between IQ assessed at ages 18–20 and risk of subsequent registration for AUD, available only in males, was slightly weaker, with a 46% decrease in risk for every standard deviation of increasing IQ.
Because of differences in measures and statistical approaches, our results are difficult to compare directly with those of prior studies. Of particular relevance to this report is a prior study of the association between IQ and future alcohol-related outcomes in Sweden based on the same conscript sample (examined in 1969–1970) we used above (Sjolund et al., 2012). They reported their results per 1-point change on a 9-point stanine scale and found HRs of 1.21 and 1.29 for alcohol-related hospitalization and death, respectively, for each-1 point decrease in IQ on the stanine scale. In one of the more comparable non-Swedish studies, Huurre et al. (2010) assessed school performance by self-reported grade point average in 16-year-old residents of one Finnish city. The outcome was excessive alcohol intake as assessed by the Alcohol Use Disorders Identification Test (AUDIT; Babor et al., 2001) administered at age 32 years. School performance was significantly lower in those who reported excessive alcohol use at age 32 years in both sexes. Consistent with our findings, their effect size was larger in males than in females.
However, not all studies show this relationship. For example, Crum et al., in the Woodlawn study, examined a range of assessments of cognitive ability in childhood and found few significant associations with future AUD risk (Crum et al., 2006). More divergent are results from the National Child Development Study (Maggs et al., 2008). In that sample, Maggs et al. found that academic ability and test scores assessed at both ages 7 and 11 were significantly and positively associated with scores on the “Cut-down, Annoyed, Guilt, Eye-opener” (CAGE) questionnaire (Mayfield et al., 1974) assessed at age 42 years. The degree to which these divergent results arise from differences in samples, age at assessment, measures of cognitive ability, or alcohol-related outcomes remains to be determined.
Given the strong observed associations between cognitive ability and AUD risk, we then sought to clarify the causal nature of this relationship. We began by evaluating, in two different ways, the hypothesis that the early symptoms of AUD that occur before registration could negatively affect school achievement or IQ. First, we examined whether building a buffer period that would exclude the AUD onsets that occurred within a few years of the school achievement or IQ assessments could affect results. We examined 5-year buffer periods for both school achievement and IQ, and these had no effect on the magnitude of the observed associations with AUD. Second, and more directly, we had good measures of alcohol intake and problems reported at the time of IQ testing in a subset of our male sample. When we controlled for an aggregate index of high intake/problems, the association between IQ and AUD risk did not decrease. These results strongly suggest that the association observed between cognitive ability in adolescence and AUD was not the result of early AUD symptoms directly impairing performance.
Last, we evaluated, using a co-relative design, the hypothesis that the relationship of school achievement and IQ with AUD was influenced by familial confounding and/or true causal effects. Our results, which were consistent across school achievement in males and females and IQ in males, provided support for both hypotheses. When we examined the associations of school achievement and IQ with AUD among pairs of discordant relatives, the association became weaker and the degree of weakening was related to the degree of genetic relationship. This is the expected pattern given partial familial confounding—meaning that part of the association is not causal but results from familial risk factors that predispose to both lowered cognitive ability and risk of AUD. These results are consistent with prior evidence that sons of parents with drug or alcohol use disorders have lower school achievement and IQ scores (Moss et al., 1995), so that part of the school achievement, IQ, and AUD link results from common genetic factors.
However, even within closely related pairs of relatives in our data—especially full siblings and MZ twins—we saw significant associations between cognitive ability and risk of AUD. These findings are therefore consistent with a causal relationship between adolescent cognitive ability and AUD. Unlike standard regression methods, which must specify individual confounders, a strength of the co-relative design is that it controls for all potential confounding variables that are familial, which includes a large proportion of all human traits (Polderman et al., 2015). However, it cannot be ruled out that our findings result from some relatively common environmental experience unique to the individual that strongly predisposes both to lowered cognitive ability and risk of AUD.
Our co-relative analyses included results from MZ twin pairs because, despite their relative rarity, they are particularly informative in that they share all their genetic background and rearing experiences. One consequence of their rarity is that estimates of the associations between our cognitive measures and AUD are known either with low precision or (in our females) cannot be calculated because of the relative rarity of AUD. Partly because of this, we developed a statistical approach that models the expected association with the general population and relative pairs of varying degrees of affinity. In so doing, we used the associations we know with high precision (the general population, cousins, and siblings) to estimate associations that we know less well, especially MZ twins. Importantly, we also tested how well our model fit the observed data.
For two of our three models (school achievement and IQ in males), the model fit very well, and in a third model (school achievement in females) it fitted reasonably well. In aggregate, both the observed results from our co-relative design and those obtained from our co-relative model-fitting supported our conclusions that both familial confounding and causal effects likely contributed substantially to the association between our cognitive measures and AUD.
In males, school achievement was a moderately stronger predictor of AUD risk than IQ. Although substantially correlated, school achievement is not identical to IQ and has some different predictors including work habits, personality, and sociability (Perry, 2003). For example, could the stronger effect we observed for school achievement reflect low commitment to and involvement in school, which has been shown to predict a range of externalizing outcomes including substance abuse and dependence (Hawkins et al., 1992)?
Finally, if school achievement and IQ are causally linked to an increased risk of AUD, our results do not clarify the mediational mechanisms that might be involved. Given the strength of our observed association, an important area for future research will be to further delineate the risk pathways, particularly as this effort may help define points of interventions for high-risk populations. One area of particular interest is the degree to which our broad measures of cognitive ability reflect the executive dysfunctions in planning, abstract conceptualization, conceptual shifting, and psychomotor functioning that have been linked to risk of AUD (Nigg et al., 2004; Sher et al., 2005).
Limitations
These results should be interpreted in the context of five potential methodological limitations. First, our assessments for AUD relied on information from Swedish registries. Compared with epidemiologically based interview studies, this approach has strengths. Our assessments were independent of subject cooperation, covered nearly the entire population, and were not affected by selective recall or social desirability biases. However, our ascertainment methods were surely not complete and contain both false-positive and false-negative diagnoses. Given that the population prevalence of AUD in this sample is much lower than estimates from most epidemiologic surveys (Grant et al., 2015; Kessler et al., 1994), including those from Sweden and Norway (Kessler et al., 1994; Kringlen et al., 2001; Lundin et al., 2015), false-negative diagnoses are more likely than false-positive ones. Importantly, the validity of our registry-based definition of AUD is supported by the high rates of concordance for registration across our difference methods (Kendler et al., 2015).
Second, our results are specific to the ethnic and cultural features of the Swedish population and may not extrapolate to other countries.
Third, because our findings included alcohol problems detected in the Swedish criminal registries, our association of low school achievement and IQ with AUD might not be an effect on risk of AUD but rather on getting caught with alcohol problems. To address this, we repeated our main analyses excluding cases of AUD only ascertained through criminal registries. For females, the association between school achievement and AUD was unchanged. For males, the impact of school achievement and IQ on risk of AUD weakened only slightly, with HRs changing from 0.47 to 0.50 and from 0.54 to 0.56, respectively, suggesting at most a minor bias.
Fourth, over the time of our study, different methods were used for assessing school achievement before and after 1997. To determine if these different methods affected the association with AUD, we fitted an interaction term to our models in males and females. Although the interaction term was significant in both sexes, the differences detected were very small, with the effect of school achievement on AUD risk being slightly stronger for subjects assessed before 1997.
Summary
We examined large samples of Swedish individuals with available school achievement scores at age 16 years and IQ scores at ages 18–20 years and showed that higher school achievement and IQ scores are strongly and inversely associated with risk of AUD. Excluding onsets of AUD within 5 years of school achievement or IQ assessment did not weaken the association, nor did controlling for alcohol intake and problems at IQ assessment. We then modeled risk of AUD in cousins, full siblings, and MZ twin pairs discordant for school achievement and IQ scores. HRs for AUD in these relative pairs were higher than those observed in the population but significantly less than unity. Cognitive ability in adolescence is strongly predictive of risk of AUD. This association cannot be explained by early symptoms of AUD. Co-relative analyses indicate that part of this association results from familial confounding and part from a causal impact of low cognitive ability on AUD risk.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
References
- Babor T., Higgins-Biddle J., Saunders J., Monteiro M. Geneva, Switzerland: World Health Organization, Department of Mental Health and Substance Disorders; 2001. AUDIT: The Alcohol Use Disorders Identification Test: Guidelines for use in primary health care. Retrieved from http://apps.who.int/iris/bitstream/10665/67205/1/WHO_MSD_MSB_01.6a.pdf. [Google Scholar]
- Bernardi F., Radl J. The long-term consequences of parental divorce for children’s educational attainment. Demographic Research. 2014;30:1653–1680. doi:10.4054/DemRes.2014.30.61. [Google Scholar]
- Bouchard T. J., Jr Genetic and environmental influences on adult intelligence and special mental abilities. Human Biology. 1998;70:257–279. [PubMed] [Google Scholar]
- Brown S. A., Tapert S. F., Granholm E., Delis D. C. Neurocognitive functioning of adolescents: Effects of protracted alcohol use. Alcoholism: Clinical and Experimental Research. 2000;24:164–171. doi:10.1111/j.1530-0277.2000.tb04586.x. [PubMed] [Google Scholar]
- Chatterji P. Does alcohol use during high school affect educational attainment? Evidence from the National Education Longitudinal Study. Economics of Education Review. 2006;25:482–497. doi:10.1016/j.econedurev.2005.05.005. [Google Scholar]
- Cook P. J., Moore M. J. Drinking and schooling. Journal of Health Economics. 1993;12:411–429. doi: 10.1016/0167-6296(93)90003-w. doi:10.1016/0167-6296(93)90003-W. [DOI] [PubMed] [Google Scholar]
- Crum R. M., Helzer J. E., Anthony J. C. Level of education and alcohol abuse and dependence in adulthood: A further inquiry. American Journal of Public Health. 1993;83:830–837. doi: 10.2105/ajph.83.6.830. doi:10.2105/AJPH.83.6.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum R. M., Juon H. S., Green K. M., Robertson J., Fothergill K., Ensminger M. Educational achievement and early school behavior as predictors of alcohol use disorders: 35-year follow-up of the Woodlawn Study. Journal of Studies on Alcohol. 2006;67:75–85. doi: 10.15288/jsa.2006.67.75. doi:10.15288/jsa.2006.67.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn P. R., Hall J. Cognitive ability and risk for alcoholism: Short-term memory capacity and intelligence moderate personality risk for alcohol problems. Journal of Abnormal Psychology. 2004;113:569–581. doi: 10.1037/0021-843X.113.4.569. doi:10.1037/0021-843X.113.4.569. [DOI] [PubMed] [Google Scholar]
- Fothergill K. E., Ensminger M. E. Childhood and adolescent antecedents of drug and alcohol problems: A longitudinal study. Drug and Alcohol Dependence. 2006;82:61–76. doi: 10.1016/j.drugalcdep.2005.08.009. doi:10.1016/j.drugalcdep.2005.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant B. F., Goldstein R. B., Saha T. D., Chou S. P., Jung J., Zhang H., Hasin D. S. Epidemiology of DSM-5 alcohol use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757–766. doi: 10.1001/jamapsychiatry.2015.0584. doi:10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch S. L., Jones P. B., Kuh D., Hardy R., Wadsworth M. E., Richards M. Childhood cognitive ability and adult mental health in the British 1946 birth cohort. Social Science and Medicine. 2007;64:2285–2296. doi: 10.1016/j.socscimed.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins J. D., Catalano R. F., Miller J. Y. Risk and protective factors for alcohol and other drug problems in adolescence and early adulthood: Implications for substance abuse prevention. Psychological Bulletin. 1992;112:64–105. doi: 10.1037/0033-2909.112.1.64. doi:10.1037/0033-2909.112.1.64. [DOI] [PubMed] [Google Scholar]
- Huurre T., Lintonen T., Kaprio J., Pelkonen M., Marttunen M., Aro H. Adolescent risk factors for excessive alcohol use at age 32 years. A 16 year prospective follow-up study. Social Psychiatry and Psychiatric Epidemiology. 2010;45:125–134. doi: 10.1007/s00127-009-0048-y. [DOI] [PubMed] [Google Scholar]
- Kendler K. S., Ji J., Edwards A. C., Ohlsson H., Sundquist J., Sun dquist K. An extended Swedish national adoption study of alcohol use disorder. JAMA Psychiatry. 2015;72:211–218. doi: 10.1001/jamapsychiatry.2014.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R. C., McGonagle K. A., Zhao S., Nelson C. B., Hughes M., Eshleman S., Kendler K. S. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Archives of General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kringlen E., Torgersen S., Cramer V. A Norwegian psychiatric epidemiological study. American Journal of Psychiatry. 2001;158:1091–1098. doi: 10.1176/appi.ajp.158.7.1091. [DOI] [PubMed] [Google Scholar]
- Lisdahl K. M., Gilbert E. R., Wright N. E., Shollenbarger S. Dare to delay? The impacts of adolescent alcohol and marijuana use onset on cognition, brain structure, and function. Frontiers of Psychiatry. 2013;4:53. doi: 10.3389/fpsyt.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M., Collins P. F., Muetzel R. L., Lim K. O. Effects of alcohol use initiation on brain structure in typically developing adolescents. American Journal of Drug and Alcohol Abuse. 2013;39:345–355. doi: 10.3109/00952990.2013.837057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin A., Hallgren M., Forsman M., Forsell Y. Comparison of DSM-5 classifications of alcohol use disorders with those of DSM-IV DSM-III-R, and ICD-10 in a general population sample in Sweden. Journal of Studies on Alcohol and Drugs. 2015;76:773–780. doi: 10.15288/jsad.2015.76.773. doi:10.15288/jsad.2015.76.773. [DOI] [PubMed] [Google Scholar]
- Maggs J. L., Patrick M. E., Feinstein L. Childhood and adolescent predictors of alcohol use and problems in adolescence and adulthood in the National Child Development Study. Addiction. 2008;103(Supplement 1):7–22. doi: 10.1111/j.1360-0443.2008.02173.x. [DOI] [PubMed] [Google Scholar]
- Mayfield D., McLeod G., Hall P. The CAGE questionnaire: Validation of a new alcoholism screening instrument. American Journal of Psychiatry. 1974;131:1121–1123. doi: 10.1176/ajp.131.10.1121. [DOI] [PubMed] [Google Scholar]
- Moss H. B., Vanyukov M., Majumder P. P., Kirisci L., Tarter R. E. Prepubertal sons of substance abusers: Influences of parental and familial substance abuse on behavioral disposition, IQ, and school achievement. Addiction Behaviors. 1995;20:345–358. doi: 10.1016/0306-4603(94)00077-c. doi:10.1016/0306-4603(94)00077-C. [DOI] [PubMed] [Google Scholar]
- Nigg J. T., Glass J. M., Wong M. M., Poon E., Jester J. M., Fitzgerald H. E., Zucker R. A. Neuropsychological executive functioning in children at elevated risk for alcoholism: Findings in early adolescence. Journal ofAbnormal Psychology. 2004;113:302–314. doi: 10.1037/0021-843X.113.2.302. [DOI] [PubMed] [Google Scholar]
- Perry S. R. Big five personality traits and work drive as predictors of adolescent academic performance (Unpublished doctoral dissertation) University of Tennessee; Knoxville, Knoxville, TN: 2003. [Google Scholar]
- Polderman T. J., Benyamin B., de Leeuw , C. A., Sullivan P. F., van B. A., Visscher P. M., Posthuma D. Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nature Genetics. 2015;47:702–709. doi: 10.1038/ng.3285. [DOI] [PubMed] [Google Scholar]
- Sher K. J., Grekin E. R., Williams N. A. The development of alcohol use disorders. Annual Review of Clinical Psychology. 2005;1:493–523. doi: 10.1146/annurev.clinpsy.1.102803.144107. [DOI] [PubMed] [Google Scholar]
- Sjolund S., Allebeck P., Hemmingsson T. Intelligence quotient (IQ) in adolescence and later risk of alcohol-related hospital admissions and deaths—37-year follow-up of Swedish conscripts. Addiction. 2012;107:89–97. doi: 10.1111/j.1360-0443.2011.03544.x. [DOI] [PubMed] [Google Scholar]
- Verhulst B., Neale M. C., Kendler K. S. The heritability of alcohol use disorders: A meta-analysis of twin and adoption studies. Psychological Medicine. 2015;45:1061–1072. doi: 10.1017/S0033291714002165. [DOI] [PMC free article] [PubMed] [Google Scholar]