Abstract
Objective:
Unhealthy alcohol use is a crucial driver of HIV in sub-Saharan Africa, and interventions are needed. The goal of this study was to assess whether assessment itself (assessment reactivity) causes declines in alcohol use in a research study in persons with HIV in Uganda.
Method:
Study participants were adult patients of the Immune Suppression Syndrome (ISS) Clinic in Mbarara, Uganda, who were new to HIV care and reported any alcohol consumption in the prior year. Participants were randomized to (a) a study cohort, with structured interviews, breath alcohol analysis tests, and blood draws conducted quarterly, or (b) a minimally assessed arm that engaged in these procedures only once, at 6 months after baseline. The main outcome was unhealthy drinking at 6 months, defined as Alcohol Use Disorders Identification Test-Consumption [AUDIT-C] positive (≥3 for women, ≥4 for men) or phosphatidylethanol (PEth; an alcohol biomarker) level ≥ 50 ng/ml. We also examined this outcome stratified by gender.
Results:
We examined 175 and 139 persons in the quarterly assessed versus minimally assessed arms, respectively. Overall, 54.8% were male, the median age was 30 (interquartile range: 25–36), and 58.0% initiated anti-retroviral therapy at 6 months. Nearly equal proportions (53.7% and 51.1% in the study quarterly assessed vs. minimally assessed arm, respectively) engaged in unhealthy drinking in the 3 months before the 6-month study visit (p = .64), and we found no evidence of interaction by gender (p = .36).
Conclusions:
We found no evidence of assessment reactivity in a study that included quarterly study visits. Assessment is not sufficient to act as an intervention itself in this population with high levels of unhealthy drinking. Interventions are needed to decrease alcohol consumption in this population.
Unhealthy alcohol use is a crucial driver of HIV, especially in sub-Saharan Africa (SSA) and particularly in Uganda where heavy alcohol consumption and HIV are both common. SSA continues to be disproportionately affected by the HIV/AIDS epidemic, home to more than 70% of the cases (UNAIDS, 2013), with the majority of infected persons living in rural areas. Alcohol use plays a key role in HIV transmission, adherence to anti-retroviral therapy (ART), and mortality (Braithwaite et al., 2014; Hahn et al., 2011; Schneider et al., 2012). Yet little has been done to intervene on unhealthy drinking in HIV positive persons in rural, resource-limited settings. HIV clinic patients in Uganda frequently receive advice on drinking (Bajunirwe et al., 2014; Sundararajan et al., 2015), but few systematic interventions have been attempted.
Screening and behavioral counseling to reduce unhealthy alcohol consumption have shown promising results in reducing alcohol consumption in those who are not alcohol dependent (Jonas et al., 2012). Such interventions could have an important impact on HIV because most persons with unhealthy alcohol use are not alcohol dependent. The separate effects of screening versus counseling in these interventions have not been examined. Reductions in alcohol use may occur from solely responding to alcohol consumption questions. This effect—assessment reactivity—can have effect sizes for reductions in alcohol use that are similar to those of brief interventions (Heather, 2014; McCambridge, 2009; Walters et al., 2009). A plausible mechanism for this phenomenon is that questioning about alcohol use may result in increased self-awareness and self-reflection that can lead to behavior change (Moos, 2008; Walters et al., 2009). A recent large randomized trial of brief interventions conducted in a primary care setting proposed that assessment reactivity may have been the reason for the lack of effect (Kaner et al., 2013). Thus, it is important to assess the existence and extent of assessment reactivity when considering appropriate alcohol interventions for persons with HIV in SSA, because cost is a key determinant of the usefulness of such interventions. If assessments themselves reduce unhealthy alcohol consumption, then routine alcohol screening could be an extremely cost-effective intervention for settings with limited resources.
Several studies conducted in the United States, the United Kingdom, and New Zealand have used randomized designs to directly examine the effect of study assessment on alcohol use; their findings have been mixed (Clifford et al., 2007; Donovan et al., 2012; Magill et al., 2012; Maisto et al., 2007; McCambridge, 2009; McCambridge & Day, 2008; Walters et al., 2009). Assessments occurred in person, over the telephone, or via the Internet; participants included either college students (Magill et al., 2012; McCambridge, 2009; McCambridge & Day, 2008; Walters et al., 2009) or outpatients in clinical settings such as hospital emergency departments or substance abuse clinics (Clifford & Davis, 2012; Clifford et al., 2007; Donovan et al., 2012; Maisto et al., 2007; McCambridge, 2009; McCambridge & Day, 2008; Walters et al., 2009).
A number of these studies showed a positive effect of assessment on alcohol use (Clifford et al., 2007; Kypri et al., 2007; McCambridge & Day, 2008; Worden et al., 2008). Assessment before alcohol treatment was cited as a reason for change in drinking in a qualitative study (Orford et al., 2006). Similar pre-treatment changes in drinking were seen in alcohol-dependent women (Epstein et al., 2005), as well as in adolescent drinkers (Kaminer et al., 2008). In contrast, several randomized studies found no difference in drinking between assessed and minimally assessed study arms, for example, among college students (Fazzino et al., 2016; Magill et al., 2012) or hazardous drinkers in hospital emergency departments (Cherpitel et al., 2010; Daeppen et al., 2007).
A reduction in drinking frequency before intervention has been attributed to assessment reactivity in studies of female drinkers (Epstein et al., 2005; Magill et al., 2012; Worden et al., 2008, 2015), and Magill et al. found that women had a reduced number of drinking days following exposure to assessments when compared with men (Magill et al., 2012). The authors speculate that women may have a greater tendency for self-exploration in the context of an interpersonal interaction (e.g., questions about alcohol use), which may ultimately lead to behavior change (Magill et al., 2012).
Although it is important to know whether alcohol screening can reduce alcohol use in SSA, and trials have been or are being conducted to examine the efficacy of interventions to reduce alcohol use in persons with HIV in SSA (Papas et al., 2011; Parry et al., 2014), we are unaware of any previous study of assessment reactivity in SSA. Previous studies of assessment reactivity have relied on self-reported behavior; however, there is evidence of a high degree of socially desirable reporting on alcohol use among persons in HIV care in Uganda (Bajunirwe et al., 2014; Hahn et al., 2012, 2016). Thus, objective measures of alcohol consumption, such as the alcohol biomarker phosphatidylethanol (PEth), are needed to examine this issue.
To better understand the effects of assessment reactivity in a population of HIV-infected adults in SSA, we conducted a randomized study of unhealthy alcohol consumption at 6 months after enrollment in a research study, comparing (a) a quarterly assessed arm with 60-minute interviews conducted at baseline and 3 months with (b) a minimally assessed arm. In both arms, PEth was used to augment self-reported unhealthy drinking. The primary goal of this study was to assess whether unhealthy alcohol use declines after research assessments in a study in persons with HIV in rural Uganda. A secondary goal was to examine whether there was Study Arm x Gender interaction.
Method
BREATH Study
Data for this study were collected as part of a mixed methods, prospective cohort study of HIV positive alcohol consumers to quantify changes in alcohol consumption in their first year of HIV care. The study was called the Biomarker Research on Ethanol Among Those with HIV (BREATH) Study (Asiimwe et al., 2015; Hahn et al., 2016). Study subjects were patients of the Immune Suppression Syndrome (ISS) Clinic in Mbarara, Uganda, who were new to HIV care. Eligibility criteria included HIV positive adults (≥18 years of age), newly enrolled into HIV care, lived within 60 km of the Mbarara ISS Clinic, fluent in Runyankole or English, and prior-year alcohol use as follows. All patients were screened for alcohol use by clinic counselors as part of routine care using the Alcohol Use Disorders Identification Test-Consumption (AUDIT-C; Bush et al., 1998) or the full 10-item AUDIT (Babor et al., 2001) (from June 2013 onward) at their first clinic visit. Those who reported any prior-year alcohol use were referred to the study for further eligibility screening and written informed consent. Counselors also referred patients they suspected to have consumed alcohol in the prior year even if they reported otherwise (n = 2).
Study subjects
Mbarara Regional Referral Hospital ISS Clinic served as the study site. Mbarara ISS Clinic serves as the regional referral HIV clinic for the entire Southwestern region of Uganda.
Randomization
Participants were randomly assigned to participate in either the main study cohort or the minimally assessed comparison arm. Randomization occurred via computergenerated randomization lists, in blocks of 20, with assignments written in sealed envelopes for each study ID that were opened after completing study enrollment for each participant. The goal was to enroll an equal number of participants in each arm. However, we varied our randomization ratios over time to initially favor adequate recruitment of quarterly assessed participants for the purposes of the larger study, and then later to ensure enough minimally assessed patients. Thus, the ratios of quarterly to minimally assessed participants were 3:2 for Blocks 1–10, 4:1 for Blocks 11–15, and 1:9 for Blocks 16–19. The Mbarara ISS Clinic staff were blinded to the participants’ randomization. Clinic counselors often provide advice or instructions to patients about alcohol use if they suspected a problem, but the nature and content of the advice given were neither structured nor systematic.
Study procedures
As noted above, all participants received an AUDIT or AUDIT-C at their initial clinic visit administered by the clinic counselors as part of the clinic intake form. After enrollment, collection of tracking information, and randomization, the quarterly assessed arm received an interviewer-administered structured interview along with breath alcohol concentration testing and phlebotomy at baseline and quarterly thereafter (Asiimwe et al., 2015; Hahn et al., 2016). Additional assessments in the quarterly assessed arm included T-helper cell (CD4) count and viral load tests at baseline as markers of HIV disease progression. Results of these tests were given to these participants as they became available. A subset of participants in the quarterly assessed arm (n = 49) was invited to participate in a semi-structured qualitative interview (Sundararajan et al., 2015). In comparison, the minimally assessed arm received the main study procedures (interview, breath alcohol concentration test, and phlebotomy) only at 6 months after baseline, after which they exited the study. Participants in the minimally assessed arm were informed of the breath alcohol concentration test and phlebotomy during informed consent at baseline, but these were not performed until the 6-month visit. All study procedures were approved by the University of California San Francisco Committee on Human Research, the Mbarara University of Science and Technology Institutional Ethical Review Committee, and the Uganda National Council for Science and Technology. All participants gave written informed consent to participate in the study.
Study interviews
The structured interview was interviewer-administered. The interviews included demographics (baseline only), health status, alcohol consumption, symptoms of depression, social support (baseline only), spirituality/religiosity (baseline only), alcohol expectancies (baseline only), and most recent sexual event. Alcohol consumption questions included questions on lifetime drinking, peer norms and drinking locations and companions, and current volume of drinking (using local beverages and their usual container sizes), expenditures, symptoms of intoxication, and time spent drinking, as well as the AUDIT-C, modified to assess current (prior 3 months) unhealthy drinking. The baseline interview took a median of 64 minutes to complete (interquartile range [IQR]: 51–83), and the 3-month interview took a median of 43 minutes to complete (IQR: 35–56).
Laboratory measures
Measurements of T-helper (CD4) cells (Pan-leukogate method [Beckman Coulter, Fullerton, CA]), HIV viral load (Versant HIV-1 RNA 3.0 Assay, bDNA [Bayer, Tarrytown, NY]), and PEth were conducted following the schedule described above. The PEth testing was performed from dried blood spots by the United States Drug Testing Laboratory in Des Plaines, IL, using Agilent 6460 liquid chromatography-tandem mass spectrometry following extraction into methanol (Jones et al., 2011). The lower limit of quantitation was 8 ng/ml, and the most common PEth homologue (16:0/18:1) was detected.
Outcomes
The main outcome was unhealthy alcohol use at 6 months. This was assessed using a combined measure of self-report or PEth: AUDIT-C positive (i.e., a score of ≥3 for women and ≥4 for men) or PEth ≥ 50 ng/ml. We used this PEth cutoff because it was highly sensitive (93%) and reasonably specific (83%) for detecting average daily drinking of at least two drinks per day in a study of 222 patients with liver disease (Scott H. Stewart, personal communication). We also examined PEth levels to see if continuous levels of the biomarker differed by study arm, and we examined the results using self-report (AUDIT-C positive, yes vs. no).
Covariates
We included participant gender, age, marital status, religion, education, household assets, months since HIV diagnosis, and unhealthy alcohol reported to clinic counselors at initial clinic visit (AUDIT-C) as potential confounders. We calculated a household asset index using principal components analysis to group households based on ownership of durable goods, housing quality, and available energy sources. We grouped the bottom 40% as low, the middle 40% as middle, and the top 20% as high. We also included the number of clinic visits in the prior 6 months and any ART use in the prior 6 months, both extracted from the clinic electronic medical records, and CD4 cell counts at 6 months.
Analysis
To describe study participants, we calculated frequency distributions for categorical variables as well as medians and IQRs for continuous variables. To assess whether the two randomization groups were similar, we also conducted chi-square tests for categorical variables or Wilcoxon rank sum tests for continuous variables of interest. To examine differences in alcohol use between the two groups at 6 months, we conducted a chi-square test for unhealthy alcohol use (yes/no) and other alcohol use variables as well as the Wilcoxon rank sum test for continuous PEth levels. We conducted multivariable logistic regression to assess differences in unhealthy alcohol use by study arm, adjusted for any covariates that were associated with study randomization arm in bivariate analyses at p < .15. Last, we examined the interaction of gender and study arm, using the likelihood ratio test to determine whether there was significant (using a cutoff of p < .15) interaction. Because 27 participants were missing AUDIT-C scores from the clinic database, we also ran the regression using data imputed with multiple imputations using chained equations.
Sample size
The sample size for this study was determined to obtain precise estimates of drinking across 1 year in HIV care, the main aim of the BREATH Study (Hahn et al., 2016). For that analysis, we calculated that we would need to enroll 212 persons into the quarterly assessed arm; expecting 15% loss to follow-up, we would have 180 persons for analysis to estimate unhealthy drinking with a 95% confidence interval (CI) that was at most 15% wide. Our goal in this substudy was to enroll an equal number (212 persons) into the minimally assessed arm. Thus, with 85% retention, we would have 80% power (at a significance level of .05) to detect a difference of 15% or more in unhealthy drinking at follow-up, assuming the proportion with unhealthy drinking in the quarterly assessed arm at follow-up was 50%. Under the actual recruitment conditions, the minimum detectable difference was increased to 16.5%.
Sensitivity analyses
As part of the study interview, participants were asked if they had taken part in another research study in the past 3 months. We conducted a sensitivity analysis, excluding minimally assessed participants who reported participating in another research study in the past 3 months from the multivariable regression model of unhealthy alcohol use, to assess whether this affected our results. We also conducted a second sensitivity analysis to determine if the change from use of the AUDIT-C to the full AUDIT in 2013 at the initial clinic visit affected our results, by excluding participants from the multivariable model who initiated care at the clinic following this change.
Results
Recruitment and retention
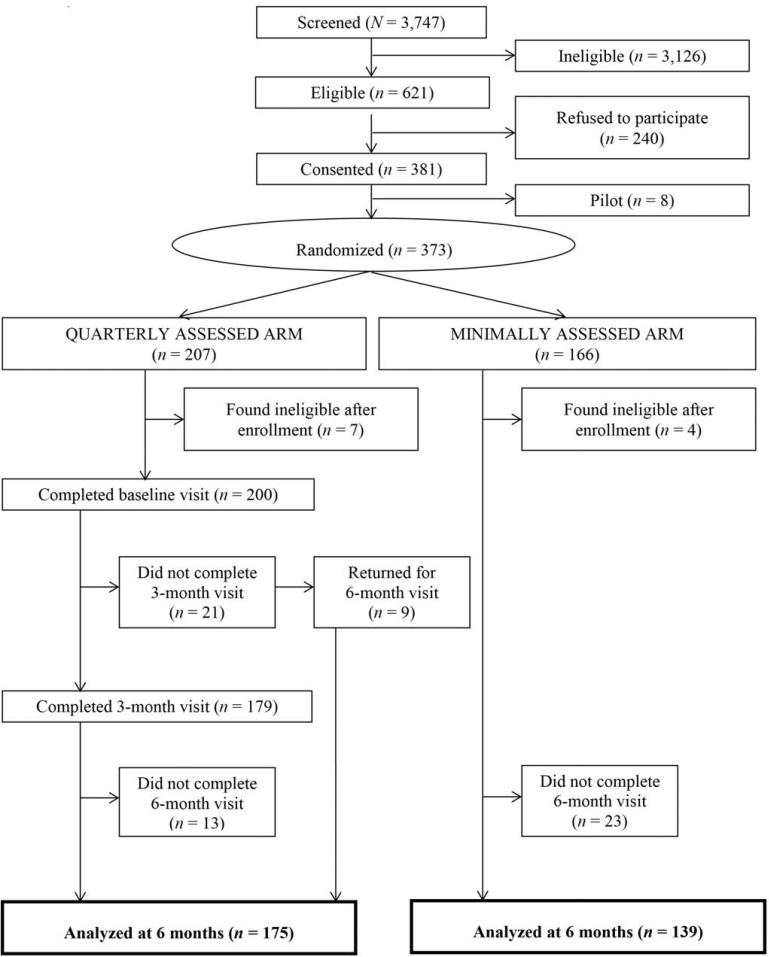
Of the 3,747 new patients screened by Mbarara ISS Clinic counselors between July 1, 2011, and July 31, 2013, 621 were eligible for the BREATH Study (Figure 1). Sixty-one percent (n = 381) gave written consent to participate in the study. Reasons for declining consent included (in hierarchical order): lacking time to participate (n = 60), feeling too weak (n = 29), fearing the blood draw (n = 21), needing permission from a husband or someone else (n = 11), other reasons (n = 51), and no reason given (n = 68). The proportion of women who enrolled in the study was similar to those who refused (45% vs. 47%, respectively, p = .59). The first eight participants enrolled were pilot participants who were not randomized; they are excluded from these analyses. Of the remaining 373 participants, 207 were randomized to the quarterly assessed arm and 166 were randomized to the minimally assessed arm. Following randomization, seven quarterly assessed arm participants and four minimally assessed arm participants were found to be ineligible and were disenrolled. Twenty-five participants in the quarterly assessed arm failed to complete their 6-month visit, leaving 175 in the quarterly assessed arm for analysis. Of these, 49 also participated in semi-structured qualitative interviews at baseline. Twenty-three participants in the minimally assessed arm did not complete their 6-month visit, leaving 139 in the minimally assessed arm for analysis.
Figure 1.
Study flow diagram for BREATH participants included in this analysis
Participant characteristics
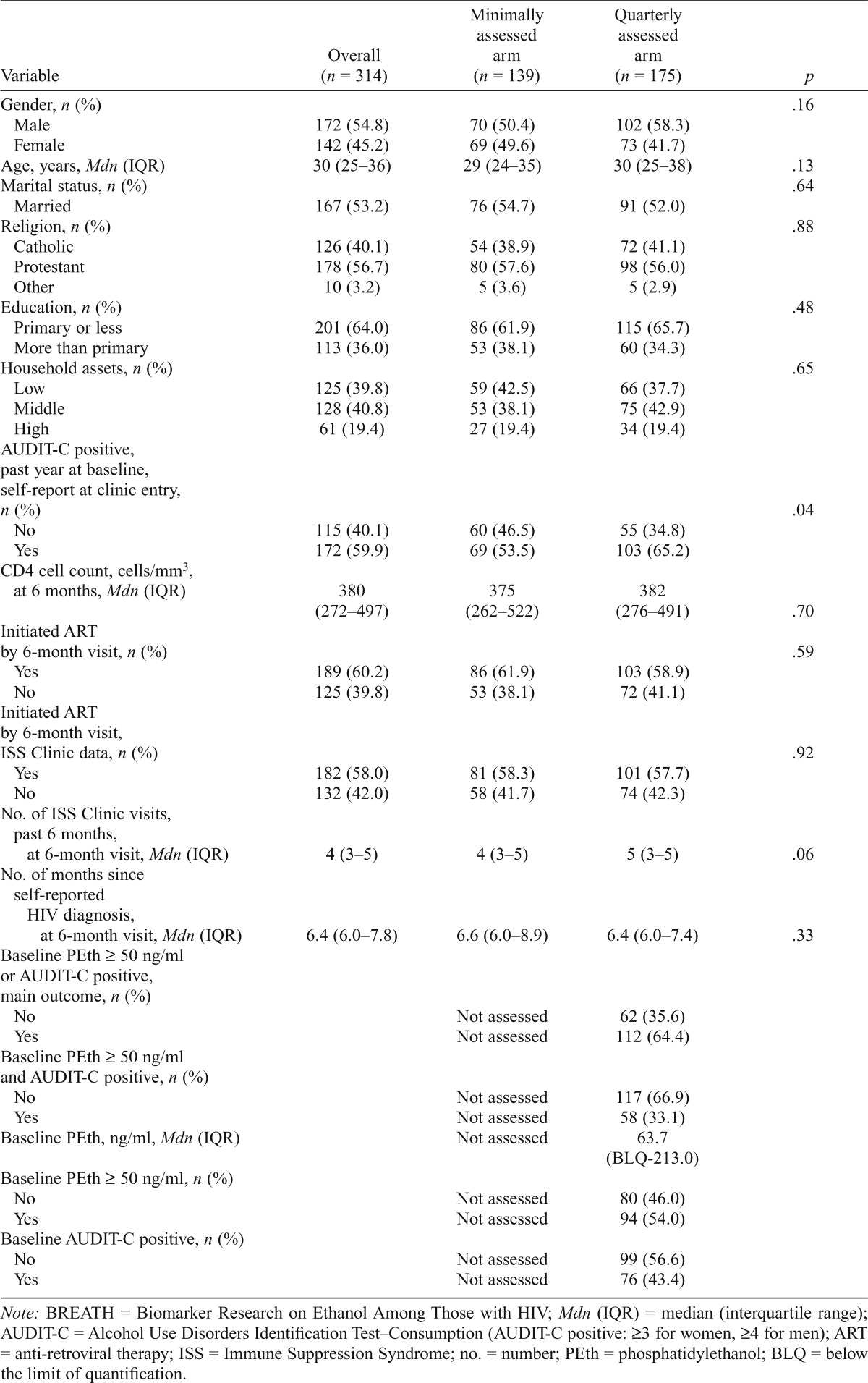
Of those in this analysis, 55% were male, the median age was 30 (IQR: 25–36), and 60% were AUDIT-C positive via clinic screening (Table 1). At 6 months, the median CD4 cell count was 380 (IQR: 272–497), and 60% had initiated ART. Participant age, AUDIT-C at clinic enrollment, and the number of prior ISS Clinic visits at 6 months differed somewhat by randomization arm (with p < .15).
Table 1.
Characteristics of BREATH Study participants who have a 6-month study interview, overall and by study arm (n = 314)
| Variable | Overall (n = 314) | Minimally Assessed arm (n = 139) | Quarterly Assessed arm (n = 175) | P |
| Gender, n (%) | .16 | |||
| Male | 172 (54.8) | 70 (50.4) | 102 (58.3) | |
| Female | 142 (45.2) | 69 (49.6) | 73 (41.7) | |
| Age, years, Mdn (IQR) | 30 (25–36) | 29 (24–35) | 30 (25–38) | .13 |
| Marital status, n (%) | .64 | |||
| Married | 167 (53.2) | 76 (54.7) | 91 (52.0) | |
| Religion, n (%) | .88 | |||
| Catholic | 126 (40.1) | 54 (38.9) | 72 (41.1) | |
| Protestant | 178 (56.7) | 80 (57.6) | 98 (56.0) | |
| Other | 10 (3.2) | 5 (3.6) | 5 (2.9) | |
| Education, n (%) | .48 | |||
| Primary or less | 201 (64.0) | 86 (61.9) | 115 (65.7) | |
| More than primary | 113 (36.0) | 53 (38.1) | 60 (34.3) | |
| Household assets, n (%) | .65 | |||
| Low | 125 (39.8) | 59 (42.5) | 66 (37.7) | |
| Middle | 128 (40.8) | 53 (38.1) | 75 (42.9) | |
| High | 61 (19.4) | 27 (19.4) | 34 (19.4) | |
| AUDIT-C positive, past year at baseline, self-report at clinic entry, n (%) | .04 | |||
| No | 115 (40.1) | 60 (46.5) | 55 (34.8) | |
| Yes | 172 (59.9) | 69 (53.5) | 103 (65.2) | |
| CD4 cell count, cells/mm3, at 6 months, Mdn (IQR) | 380 (272–497) | 375 (262–522) | 382 (276–491) | .70 |
| Initiated ART by 6-month visit, n (%) | .59 | |||
| Yes | 189 (60.2) | 86 (61.9) | 103 (58.9) | |
| No | 125 (39.8) | 53 (38.1) | 72 (41.1) | |
| Initiated ART by 6-month visit, ISS Clinic data, n (%) | .92 | |||
| Yes | 182 (58.0) | 81 (58.3) | 101 (57.7) | |
| No | 132 (42.0) | 58 (41.7) | 74 (42.3) | |
| No. of ISS Clinic visits, past 6 months, at 6-month visit, Mdn (IQR) | 4 (3–5) | 4 (3–5) | 5 (3–5) | .06 |
| No. of months since self-reported HIV diagnosis, at 6-month visit, Mdn (IQR) | 6.4 (6.0–7.8) | 6.6 (6.0–8.9) | 6.4 (6.0–7.4) | .33 |
| Baseline PEth ≥ 50 ng/ml or AUDIT-C positive, main outcome, n (%) | ||||
| No | Not assessed | 62 (35.6) | ||
| Yes | Not assessed | 112 (64.4) | ||
| Baseline PEth ≥ 50 ng/ml and AUDIT-C positive, n (%) | ||||
| No | Not assessed | 117 (66.9) | ||
| Yes | Not assessed | 58 (33.1) | ||
| Baseline PEth, ng/ml, Mdn (IQR) | Not assessed | 63.7 (BLQ-213.0) | ||
| Baseline PEth ≥ 50 ng/ml, n (%) | ||||
| No | Not assessed | 80 (46.0) | ||
| Yes | Not assessed | 94 (54.0) | ||
| Baseline AUDIT-C positive, n (%) | ||||
| No | Not assessed | 99 (56.6) | ||
| Yes | Not assessed | 76 (43.4) |
Note: BREATH = Biomarker Research on Ethanol Among Those with HIV; Mdn (IQR) = median (interquartile range); AUDIT-C = Alcohol Use Disorders Identification Test-Consumption (AUDIT-C positive: ≥3 for women, ≥4 for men); ART = anti-retroviral therapy; ISS = Immune Suppression Syndrome; no. = number; PEth = phosphatidylethanol; BLQ = below the limit of quantification.
Assessment reactivity
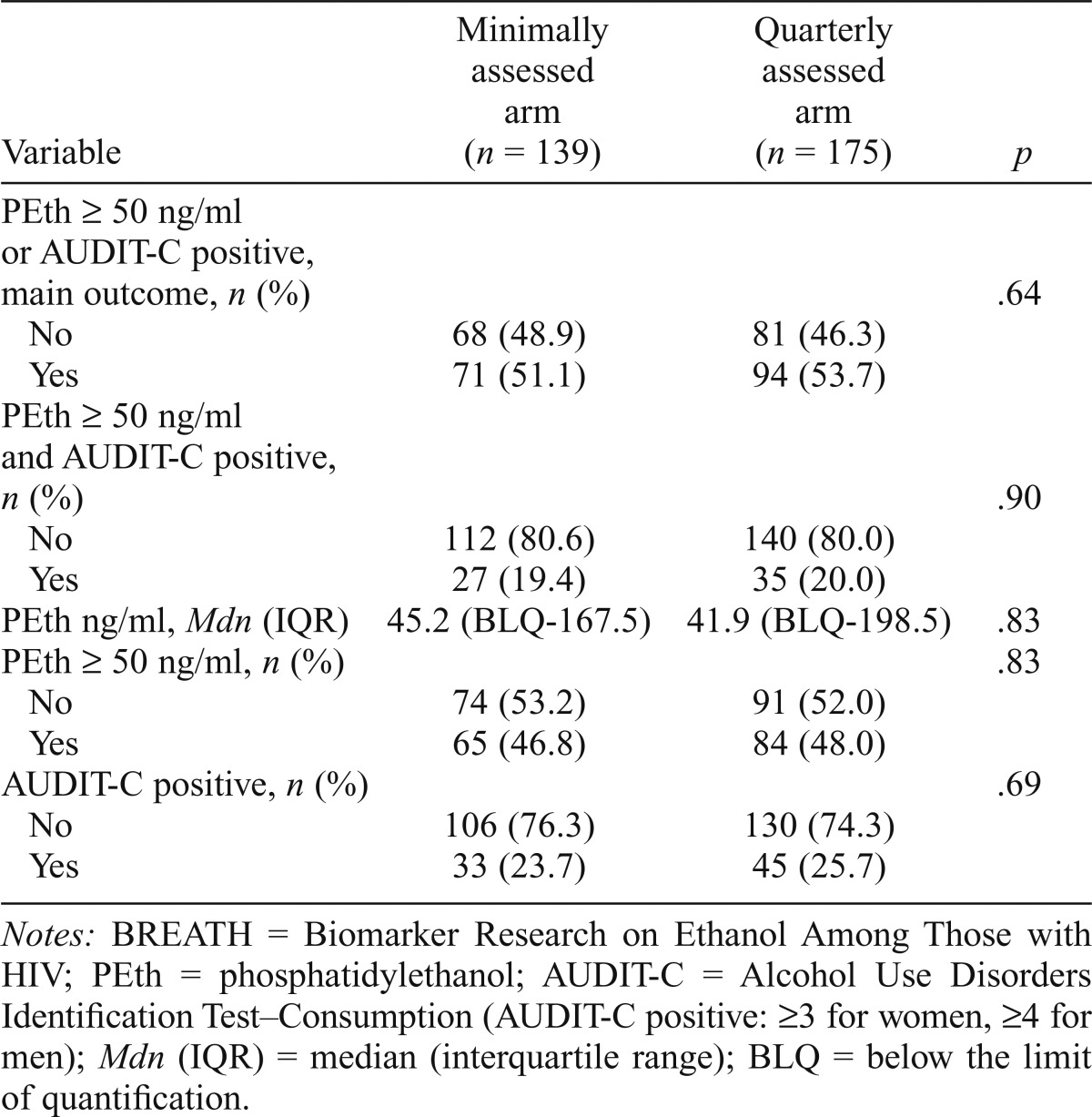
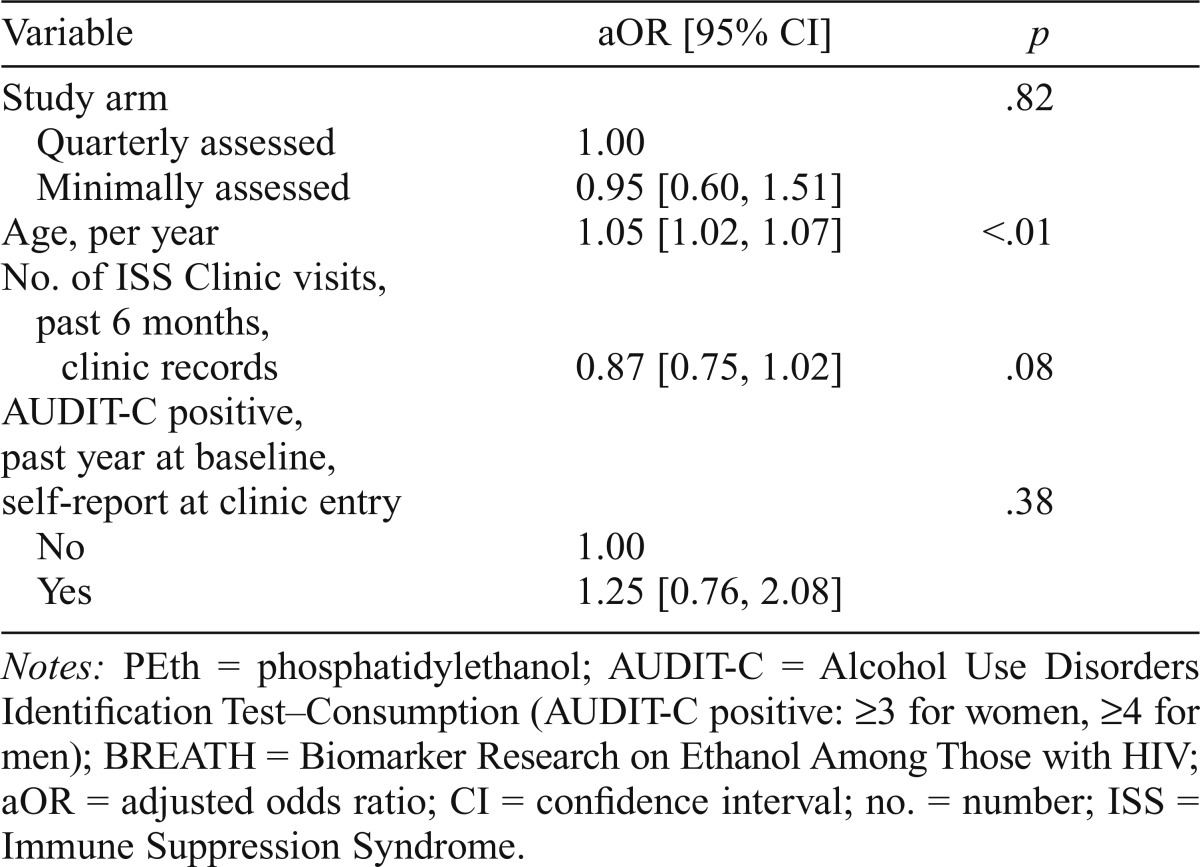
We found no significant difference in unhealthy alcohol consumption between the two study arms at 6 months. Nearly equal proportions in the quarterly assessed arm versus the minimally assessed arm (53.7% vs. 51.1%) engaged in unhealthy drinking in the 3 months before the 6-month study visit (chi-square p = .64). In addition, the PEth levels were very similar, as were the proportions of participants who were AUDIT-C positive, those with PEth ≥ 50 ng/ml, and those who were AUDIT-C positive and with PEth ≥ 50 ng/ml (Table 2). The odds of unhealthy alcohol use did not differ significantly by study arm after we controlled for potential confounders (adjusted odds ratio = 0.95, 95% CI [0.60, 1.51], multiple imputation results) (Table 3). Within the quarterly assessed arm, there was no difference in unhealthy alcohol use between the 49 who participated in the additional qualitative interview compared with the 126 who did not (59% vs. 52%, p = .37; data not shown).
Table 2.
Alcohol use 6 months after enrollment in the BREATH Study, by study arm (n = 314)
| Variable | Minimally Assessed arm (n = 139) | Quarterly Assessed arm (n = 175) | P |
| PEth ≥ 50 ng/ml or AUDIT-C positive, main outcome, n (%) | .64 | ||
| No | 68 (48.9) | 81 (46.3) | |
| Yes | 71 (51.1) | 94 (53.7) | |
| PEth ≥ 50 ng/ml and AUDIT-C positive, n (%) | .90 | ||
| No | 112 (80.6) | 140 (80.0) | |
| Yes | 27 (19.4) | 35 (20.0) | |
| PEth ng/ml, Mdn (IQR) | 45.2 (BLQ-167.5) | 41.9 (BLQ-198.5) | .83 |
| PEth ≥ 50 ng/ml, n (%) | .83 | ||
| No | 74 (53.2) | 91 (52.0) | |
| Yes | 65 (46.8) | 84 (48.0) | |
| AUDIT-C positive, n (%) | .69 | ||
| No | 106 (76.3) | 130 (74.3) | |
| Yes | 33 (23.7) | 45 (25.7) |
Notes: BREATH = Biomarker Research on Ethanol Among Those with HIV; PEth = phosphatidylethanol; AUDIT-C = Alcohol Use Disorders Identification Test-Consumption (AUDIT-C positive: ≥3 for women, ≥4 for men); Mdn (IQR) = median (interquartile range); BLQ = below the limit of quantification.
Table 3.
Multivariable logistic regression for unhealthy alcohol use (PEth ≥ 50 ng/ml or AUDIT-C positive) 6 months after enrollment, using multiple imputation, BREATH Study (n = 314)
| Variable | aOR [95% CI] | P |
| Study arm | .82 | |
| Quarterly assessed | 1.00 | |
| Minimally assessed | 0.95 [0.60, 1.51] | |
| Age, per year | 1.05 [1.02, 1.07] | <.01 |
| No. of ISS Clinic visits, past 6 months, clinic records | 0.87 [0.75, 1.02] | .08 |
| AUDIT-C positive, past year at baseline, self-report at clinic entry | .38 | |
| No | 1.00 | |
| Yes | 1.25 [0.76, 2.08] |
Notes: PEth = phosphatidylethanol; AUDIT-C = Alcohol Use Disorders Identification Test-Consumption (AUDIT-C positive: ≥3 for women, ≥4 for men); BREATH = Biomarker Research on Ethanol Among Those with HIV; aOR = adjusted odds ratio; CI = confidence interval; no. = number; ISS = Immune Suppression Syndrome.
The interaction between gender and study arm was not statistically significant (p = .36). Among women, the adjusted odds ratio for unhealthy drinking for the minimally assessed arm versus the quarterly assessed arm was 1.35 (95% CI [0.66, 2.77]). For men, the adjusted odds ratio for minimally versus quarterly assessed arm was 0.85 (95% CI [0.44, 1.67]). The multivariable regression results using multiple imputation were very similar to those obtained using listwise deletion (data not shown).
The introduction of the full AUDIT screening at the clinic did not affect our study results. When we excluded participants who were administered the full 10-item AUDIT at clinic entry (n = 47; i.e., 3 quarterly assessed and 44 minimally assessed) rather than the 3-item AUDIT-C that was previously administered, our results remained unchanged, with no statistically significant difference between the quarterly assessed arm and the minimally assessed arm (p = .59). Similar results were obtained when we excluded participants from the minimally assessed arm (n = 6) who reported concurrent participation in other research studies.
Discussion
To our knowledge, this is the first randomized study in SSA to evaluate the effect of study assessment on alcohol use. We found no evidence of assessment reactivity resulting from study interviews in persons with HIV in Uganda. This is consistent with previous findings of no difference between study and minimally assessed or control groups with the outcome of reduced drinking or risk reduction (Cherpitel et al., 2010; Fazzino et al., 2016; Magill et al., 2012) but differs from other studies that did find evidence of assessment reactivity on alcohol use (Clifford et al., 2007; Kypri et al., 2007; McCambridge & Day, 2008; Worden et al., 2008). We also found no evidence of differences in assessment reactivity by gender, contrary to previous findings (Magill et al., 2012). The implications of these findings are twofold. First, they imply that screening alone will not be sufficient to reduce alcohol use in this population. Second, these results suggest that future studies to test alcohol interventions in this population will not need to include a minimally assessed control arm.
Given the stigma associated with drinking in this study setting and the advice given by clinic staff to stop drinking, it is surprising that additional assessments did not have an effect on reducing alcohol use. Previous studies have shown that patients may think their clinicians view their drinking unfavorably or in a stigmatized light. For example, Papas and colleagues (2012) reported that participants were hesitant to disclose their drinking to their HIV clinician, and Morris et al. (2006) described patients being in “fear of being denied ART” if they disclosed their drinking to their clinicians. The recent study by Sundarajan et al. (2015) described the general perception by clinicians that “alcohol and ART do not mix,” and this information was often communicated to patients. Several factors may have contributed to our unexpected findings. The study participants were recently diagnosed with HIV and were in their first year of care, with many initiating ART within the same period. Perhaps the overwhelming nature of these major life events, coupled with other assessments received as part of HIV care, dominated their self-reflection and thus diminished the impact of alcohol-related assessments on behavior change. Similar to this hypothesis is one suggesting that heavy drinkers may be less reactive to assessments because they have integrated nonstandard norms in their behavior and respond accordingly to negative feedback as a coping mechanism for their drinking problems (Nye et al., 1999). Because our study participants admitted to drinking at clinic enrollment, in an environment where under-report is prevalent and stigma is high, the above suggestions about the behavior of heavy drinkers, or self-admitted drinkers, may apply.
Strengths and weaknesses of the study
This is the first study in SSA to evaluate the effect of study assessment on alcohol use using a randomized design. This study is also novel in its use of the biomarker PEth to measure alcohol levels in participants concurrent with selfreport. We had good follow-up of participants in both study arms (≥86%). We studied an important population of HIV positive drinkers in a resource-limited setting where HIV is prevalent and alcohol consumption is high. Our findings have the potential to guide interventions.
Although our goal was to assess the impact of the research interview assessing alcohol use on subsequent alcohol use, it is a limitation of the study that the quarterly assessed arm also underwent breath alcohol analysis testing, underwent specimen collection, and received CD4 count and HIV viral load results and that a subset participated in qualitative interviews. Thus, had we seen a difference in 6-month unhealthy alcohol use, we would not have been able to definitively attribute it to the alcohol assessment alone. Another limitation of the study is that participants may have received research assessments as part of other studies, or they may have received counseling to reduce alcohol use while they were in the study. However, this would affect our results only if these were administered differentially by study arm. The clinic staff were blinded to the patients’ study arms; therefore, we do not believe this to be a bias. In addition, we were not able to independently assess behavior change resulting from study participation (Hawthorne Effect) because all of our participants were enrolled in the study. However, we have previously reported that there were no reductions in unhealthy alcohol use measured by a combination of PEth and self-report across the first year of HIV care in this cohort; therefore, we suspect little overall study effect (Hahn et al., 2016). All assessments in our study were interviewer-administered; as such, our results may not be generalizable to other types of assessment, including self-assessments. Randomization ratios varied over the study period to ensure we reached recruitment goals for the main quarterly assessed study arm. We do not know if this affected our results. As such, any temporal trends in the clinic may have affected our results.
This study was presented as an observational study to participants, and we do not believe that they perceived it otherwise as an intervention. We had a relatively high number of patients decline participation in the study. Although their reasons for declining are not completely clear to us, the study research assistants felt that, because patients were new to care and perhaps recently diagnosed with HIV, many were not ready to participate in a study at the time of recruitment. We did not believe that this affected the generalizability of our findings.
The purpose of this study was to focus on the potential for screening for alcohol use to affect subsequent unhealthy alcohol use. Thus, we did not focus on treatments that are targeted to alcohol dependence, such as 12-step groups, and pharmacological treatment with disulfiram (Antabuse), which exist in Uganda but are rare (Kalema & Vanderplass-chen, 2015).
In summary, we found no evidence of assessment reactivity resulting from study interviews. The high level of unhealthy drinking in the first year of HIV care suggests that interventions will be needed to decrease alcohol consumption in this population; however, assessment alone will not be sufficient to act as an intervention itself. In addition, future trials of further interventions in this setting need not include a minimally assessed arm.
Acknowledgments
We thank the study participants for their time, the Mbarara ISS Clinic staff and the Mbarara Regional Referral Hospital for their support and cooperation, and the BREATH Study team for their hard work.
References
- Asiimwe S. B., Fatch R., Emenyonu N. I., Muyindike W. R., Kekibiina A., Santos G. M., Hahn J. A. Comparison of traditional and novel self-report measures to an alcohol biomarker for quantifying alcohol consumption among HIV-infected adults in sub-Saharan Africa. Alcoholism: Clinical and Experimental Research. 2015;39:1518–1527. doi: 10.1111/acer.12781. doi:10.1111/acer.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babor T., Higgins-Biddle J., Saunders J. B., Monteiro M. 2nd ed. Geneva, Switzerland: World Health Organization; 2001. AUDIT: The Alcohol Use Disorders Identification Test. Guidelines for use in primary care. [Google Scholar]
- Bajunirwe F., Haberer J. E., Boum Y., II, Hunt P., Mocello R., Martin J. N., Hahn J. A. Comparison of self-reported alcohol consumption to phosphatidylethanol measurement among HIV-infected patients initiating antiretroviral treatment in southwestern Uganda. PLOS ONE. 2014;9(12):e113152. doi: 10.1371/journal.pone.0113152. doi:10.1371/journal.pone.0113152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite R. S., Nucifora K. A., Kessler J., Toohey C., Mentor S. M., Uhler L. M., Bryant K. Impact of interventions targeting unhealthy alcohol use in Kenya on HIV transmission and AIDS-related deaths. Alcoholism: Clinical and Experimental Research. 2014;38:1059–1067. doi: 10.1111/acer.12332. doi:10.1111/acer.12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K., Kivlahan D. R., McDonell M. B., Fihn S. D., Bradley K. A. The AUDIT alcohol consumption questions (AUDIT-C): An effective brief screening test for problem drinking. Archives of Internal Medicine. 1998;158:1789–1795. doi: 10.1001/archinte.158.16.1789. doi:10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- Cherpitel C. J., Korcha R. A., Moskalewicz J., Swiatkiewicz G., Ye Y., Bond J. Screening, brief intervention, and referral to treatment (SBIRT): 12-month outcomes of a randomized controlled clinical trial in a Polish emergency department. Alcoholism: Clinical and Experimental Research. 2010;34:1922–1928. doi: 10.1111/j.1530-0277.2010.01281.x. doi:10.1111/j.1530-0277.2010.01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford P. R., Davis C. M. Alcohol treatment research assessment exposure: A critical review of the literature. Psychology of Addictive Behaviors. 2012;26:773–781. doi: 10.1037/a0029747. doi:10.1037/a0029747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford P. R., Maisto S. A., Davis C. M. Alcohol treatment research assessment exposure subject reactivity effects: Part I. Alcohol use and related consequences. Journal of Studies on Alcohol and Drugs. 2007;68:519–528. doi: 10.15288/jsad.2007.68.519. doi:10.15288/jsad.2007.68.519. [DOI] [PubMed] [Google Scholar]
- Daeppen J. B., Gaume J., Bady P., Yersin B., Calmes J. M., Givel J. C., Gmel G. Brief alcohol intervention and alcohol assessment do not influence alcohol use in injured patients treated in the emergency department: A randomized controlled clinical trial. Addiction. 2007;102:1224–1233. doi: 10.1111/j.1360-0443.2007.01869.x. doi:10.1111/j.1360-0443.2007.01869.x. [DOI] [PubMed] [Google Scholar]
- Donovan D. M., Bogenschutz M. P., Perl H., Forcehimes A., Adinoff B., Mandler R., Walker R. Study design to examine the potential role of assessment reactivity in the Screening, Motivational Assessment, Referral, and Treatment in Emergency Departments (SMART-ED) protocol. Addiction Science & Clinical Practice. 2012;7:16. doi: 10.1186/1940-0640-7-16. doi:10.1186/1940-0640-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E. E., Drapkin M. L., Yusko D. A., Cook S. M., McCrady B. S., Jensen N. K. Is alcohol assessment therapeutic? Pretreatment change in drinking among alcohol-dependent women. Journal ofStudies onAlcohol. 2005;66:369–378. doi: 10.15288/jsa.2005.66.369. doi:10.15288/jsa.2005.66.369. [DOI] [PubMed] [Google Scholar]
- Fazzino T. L., Rose G. L., Helzer J. E. An experimental test of assessment reactivity within a web-based brief alcohol intervention study for college students. Addictive Behaviors. 2016;52:66–74. doi: 10.1016/j.addbeh.2015.08.011. doi:10.1016/j.addbeh.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn J. A., Emenyonu N. I., Fatch R., Muyindike W. R., Kekibiina A., Carrico A. W., Shiboski S. Declining and rebounding unhealthy alcohol consumption during the first year of HIV care in rural Uganda, using phosphatidylethanol to augment self-report. Addiction. 2016;111:272–279. doi: 10.1111/add.13173. doi:10.1111/add.13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn J. A., Fatch R., Kabami J., Mayanja B., Emenyonu N. I., Martin J., Bangsberg D. R. Self-report of alcohol use increases when specimens for alcohol biomarkers are collected in persons with HIV in Uganda. Journal of Acquired Immune Deficiency Syndromes. 2012;61:e63–e64. doi: 10.1097/QAI.0b013e318267c0f1. doi:10.1097/QAI.0b013e318267c0f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn J. A., Woolf-King S. E., Muyindike W. Adding fuel to the fire: Alcohols effect on the HIV epidemic in sub-Saharan Africa. Current HIV/AIDS Reports. 2011;8:172–180. doi: 10.1007/s11904-011-0088-2. doi:10.1007/s11904-011-0088-2. [DOI] [PubMed] [Google Scholar]
- Heather N. Interpreting null findings from trials of alcohol brief interventions. Frontiers in Psychiatry. 2014:85. doi: 10.3389/fpsyt.2014.00085. doi:10.3389/fpsyt.2014.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas D. E., Garbutt J. C., Amick H. R., Brown J. M., Brownley K. A., Council C. L., Harris R. P. Behavioral counseling after screening for alcohol misuse in primary care: A systematic review and meta-analysis for the U.S. Preventive Services Task Force. Annals of Internal Medicine. 2012;157:645–654. doi: 10.7326/0003-4819-157-9-201211060-00544. doi:10.7326/0003-4819-157-9-201211060-00544. [DOI] [PubMed] [Google Scholar]
- Jones J., Jones M., Plate C., Lewis D. The detection of 1-palmi-toyl-2-oleoyl-sn-glycero-3-phosphoethanol in human dried blood spots. Analytical Methods. 2011;3:1101–1106. doi:10.1039/c0ay00636j. [Google Scholar]
- Kalema D., Vanderplasschen W. Features and challenges of alcohol abuse treatment in Uganda. African Journal ofDrug and Alcohol Studies. 2015;14 Retrieved from http://www.ajol.info/index.php/ajdas/article/view/133133/122758. [Google Scholar]
- Kaminer Y., Burleson J. A., Burke R. Can assessment reactivity predict treatment outcome among adolescents with alcohol and other substance use disorders? Substance Abuse. 2008;29:63–69. doi: 10.1080/08897070802093262. doi:10.1080/08897070802093262. [DOI] [PubMed] [Google Scholar]
- Kaner E., Bland M., Cassidy P., Coulton S., Dale V, Deluca P., Drummond C. Effectiveness of screening and brief alcohol intervention in primary care (SIPS trial): Pragmatic cluster randomised controlled trial. BMJ. 2013;346:e8501. doi: 10.1136/bmj.e8501. doi:10.1136/bmj.e8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kypri K., Langley J. D., Saunders J. B., Cashell-Smith M. L. Assessment may conceal therapeutic benefit: Findings from a randomized controlled trial for hazardous drinking. Addiction. 2007;102:62–70. doi: 10.1111/j.1360-0443.2006.01632.x. doi:10.1111/j.1360-0443.2006.01632.x. [DOI] [PubMed] [Google Scholar]
- Magill M., Kahler C. W., Monti P., Barnett N. P. Do research assessments make college students more reactive to alcohol events? Psychology of Addictive Behaviors. 2012;26:338–344. doi: 10.1037/a0025571. doi:10.1037/a0025571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisto S. A., Clifford P. R., Davis C. M. Alcohol treatment research assessment exposure subject reactivity effects: Part II. Treatment engagement and involvement. Journal ofStudies on Alcohol and Drugs. 2007;68:529–533. doi: 10.15288/jsad.2007.68.529. doi:10.15288/jsad.2007.68.529. [DOI] [PubMed] [Google Scholar]
- McCambridge J. [Commentary] Research assessments: Instruments of bias and brief interventions of the future? Addiction. 2009;104:1311–1312. doi: 10.1111/j.1360-0443.2009.02684.x. doi:10.1111/j.1360-0443.2009.02684.x. [DOI] [PubMed] [Google Scholar]
- McCambridge J., Day M. Randomized controlled trial of the effects of completing the Alcohol Use Disorders Identification Test questionnaire on self-reported hazardous drinking. Addiction. 2008;103:241–248. doi: 10.1111/j.1360-0443.2007.02080.x. doi:10.1111/j.1360-0443.2007.02080.x. [DOI] [PubMed] [Google Scholar]
- Moos R. H. Context and mechanisms of reactivity to assessment and treatment. Addiction. 2008;103:249–250. doi: 10.1111/j.1360-0443.2007.02123.x. doi:10.1111/j.1360-0443.2007.02123.x. [DOI] [PubMed] [Google Scholar]
- Morris C., Levine B., Goodridge G., Luo N., Ashley J. Three-country assessment of alcohol-HIV related policy and programmematic responses in Africa. African Journal of Drug and Alcohol Studies. 2006;5:170–184. [Google Scholar]
- Nye E. C., Agostinelli G., Smith J. E. Enhancing alcohol problem recognition: A self-regulation model for the effects of self-focusing and normative information. Journal ofStudies on Alcohol. 1999;60:685–693. doi: 10.15288/jsa.1999.60.685. doi:10.15288/jsa.1999.60.685. [DOI] [PubMed] [Google Scholar]
- Orford J., Hodgson R., Copello A., John B., Smith M., Black R., Slegg G. the UKATT Research Team. The clients’ perspective on change during treatment for an alcohol problem: Qualitative analysis of follow-up interviews in the UK Alcohol Treatment Trial. Addiction. 2006;101:60–68. doi: 10.1111/j.1360-0443.2005.01291.x. doi:10.1111/j.1360-0443.2005.01291.x. [DOI] [PubMed] [Google Scholar]
- Papas R. K., Gakinya B. N., Baliddawa J. B., Martino S., Bryant K. J., Meslin E. M., Sidle J. E. Ethical issues in a stage 1 cognitive-behavioral therapy feasibility study and trial to reduce alcohol use among HIV-infected outpatients in western Kenya. Journal of Empirical Research on Human Research Ethics: An International Journal. 2012;7:29–37. doi: 10.1525/jer.2012.7.3.29. doi:10.1525/jer.2012.7.3.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papas R. K., Sidle J. E., Gakinya B. N., Baliddawa J. B., Martino S., Mwaniki M. M., Maisto S. A. Treatment outcomes of a stage 1 cognitive-behavioral trial to reduce alcohol use among HIV-infected outpatients in western Kenya. Addiction. 2011;106:2156–2166. doi: 10.1111/j.1360-0443.2011.03518.x. doi:10.1111/j.1360-0443.2011.03518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry C. D. H., Morojele N. K., Myers B. J., Kekwaletswe C. T., Manda S. O. M., Sorsdahl K., Shuper P. A. Efficacy of an alcohol-focused intervention for improving adherence to antiretroviral therapy (ART) and HIV treatment outcomes - A randomised controlled trial protocol. BMC Infectious Diseases. 2014;14:500. doi: 10.1186/1471-2334-14-500. doi:10.1186/1471-2334-14-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M., Chersich M., Neuman M., Parry C. Alcohol consumption and HIV/AIDS: The neglected interface. Addiction. 2012;107:1369–1371. doi: 10.1111/j.1360-0443.2012.03824.x. doi:10.1111/j.1360-0443.2012.03824.x. [DOI] [PubMed] [Google Scholar]
- Sundararajan R., Wyatt M. A., Woolf-King S., Pisarski E. E., Emenyonu N., Muyindike W. R., Ware N. C. Qualitative study of changes in alcohol use among HIV-infected adults entering care and treatment for HIV/AIDS in rural southwest Uganda. AIDS and Behavior. 2015;19:732–741. doi: 10.1007/s10461-014-0918-5. doi:10.1007/s10461-014-0918-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS. Global Report: UNAIDS report on the global AIDS epidemic 2013. 2013 Retrieved from http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Global_Report_2013_en_1.pdf.
- Walters S. T., Vader A. M., Harris T. R., Jouriles E. N. Reactivity to alcohol assessment measures: An experimental test. Addiction. 2009;104:1305–1310. doi: 10.1111/j.1360-0443.2009.02632.x. doi:10.1111/j.1360-0443.2009.02632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worden B. L., Epstein E. E., McCrady B. S. Pretreatment assessment-related reductions in drinking among women with alcohol use disorders. Substance Use & Misuse. 2015;50:215–225. doi: 10.3109/10826084.2014.962662. doi:10.3109/10826084.2014.962662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worden B. L., McCrady B. S., Epstein E. E. Assessment reactivity to follow-up in a study of women’s treatment for alcohol dependence. Addictive Behaviors. 2008;33:831–835. doi: 10.1016/j.addbeh.2007.12.004. doi:10.1016/j.addbeh.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]