Abstract
Spore-forming bacilli are being explored for the production and preservation of food for many centuries. The inherent ability of production of large number of secretory proteins, enzymes, antimicrobial compounds, vitamins, and carotenoids specifies the importance of bacilli in food chain. Additionally, Bacillus spp. are gaining interest in human health related functional food research coupled with their enhanced tolerance and survivability under hostile environment of gastrointestinal tract. Besides, bacilli are more stable during processing and storage of food and pharmaceutical preparations, making them more suitable candidate for health promoting formulations. Further, Bacillus strains also possess biotherapeutic potential which is connected with their ability to interact with the internal milieu of the host by producing variety of antimicrobial peptides and small extracellular effector molecules. Nonetheless, with proposed scientific evidences, commercial probiotic supplements, and functional foods comprising of Bacillus spp. had not gained much credential in general population, since the debate over probiotic vs pathogen tag of Bacillus in the research and production terrains is confusing consumers. Hence, it’s important to clearly understand the phenotypic and genotypic characteristics of selective beneficial Bacillus spp. and their substantiation with those having GRAS status, to reach a consensus over the same. This review highlights the probiotic candidature of spore forming Bacillus spp. and presents an overview of the proposed health benefits, including application in food and pharmaceutical industry. Moreover, the growing need to evaluate the safety of individual Bacillus strains as well as species on a case by case basis and necessity of more profound analysis for the selection and identification of Bacillus probiotic candidates are also taken into consideration.
Keywords: spore formers, Bacillus, beneficial microbes, probiotics, intestinal microbiota, human health, mechanism of action
Introduction
The interest in the field of beneficial microbes has emerged multiple folds since its inception by the Russian Noble laureate, Elie Metchnikoff. The term Probiotics, taken as an un-challenged synonym to beneficial microbes, has gained popularity over the years and has found application in several general health and clinical scenarios. Probiotics are live microorganisms, which when administered in adequate amounts confer health benefits to the host (FAO/WHO, 2002). Probiotic formulations are being developed and standardized for both human and animal consumption. Different dairy/functional foods/dietary supplements and pharma formulations harbor probiotic strains, intended for various health benefits in humans. Probiotics have also found application in animal feed for prevention of gastrointestinal infections, with extensive use in the poultry and aquaculture industries (Hong et al., 2005). The consumer awareness, search for alternate, safe and cost-effective treatments, and concern of developing antibiotic resistance has compelled researchers to find an alternate to ongoing therapeutic regimes, mainly dependent over antibiotics. Among the large number of suggested options, probiotic therapy seems to be the most viable one, with long history of consumption and assured safety. LAB and Bifidobacterium spp. are the two globally recognized groups of bacteria that are being consumed for their potential health benefits. Other preferred bacteria include strains of Enterococcus, Streptococcus and Bacillus spp. (Majeed et al., 2016); along with few strains of Saccharomyces spp. Several reference probiotic strains have been shown to play a potential role in management of several clinical scenarios viz. diarrhea, inflammatory bowel disease, obesity, type 2 diabetes, cardiovascular disease, cancer etc. (Mallappa et al., 2012; Panwar et al., 2014, 2016; Ranji et al., 2015). It has been clearly understood that the gut inhabitants and their proper balance is the foremost criteria that determines healthy status, particularly in terms of metabolic disorders (Wang et al., 2015). Research updates from several in vivo (Ellekilde et al., 2014) and human clinical trials (Shimizu et al., 2013; Scott et al., 2015) supports the hypothesis that any strategies targeting the re-customization of the gut inhabitants can help in reverting back to normal healthy phenotype.
Besides the commonly explored strains, bacterial spore formers, mostly of the genus Bacillus do carry probiotic attributes. The value of non-spore former LAB for the maintenance of human and animal health has been acknowledged both scientifically in terms of published research data and commercially in form of the availability of probiotic products. However, in comparison to LAB, bacterial spore formers have not gained high popularity, particularly in terms of research interest (Figure 1). Several Bacillus strains have been screened for their potential probiotic functionalities, in several in vitro and in vivo models. Besides qualifying the mandatory bench marks for a candidate probiotic; Bacillus spp. offers higher acid tolerance and better stability during heat processing and low temperature storage (Bader et al., 2012). Additionally, they have also been shown to possess pathogen exclusion, anti-oxidant, antimicrobial, immuno-modulatory (Lefevre et al., 2015; Shobharani et al., 2015; Ripert et al., 2016) and food fermentation (Terlabie et al., 2006) abilities.
FIGURE 1.
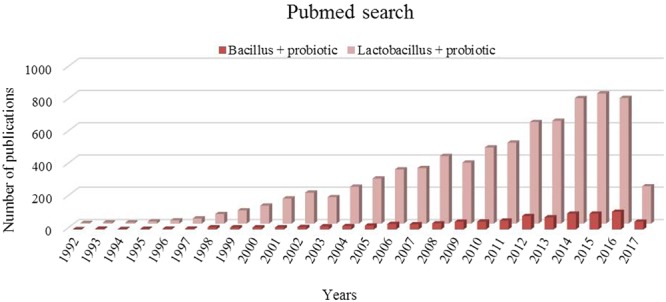
Pubmed trends for key words “Bacillus+ probiotic” and “Lactobacillus + probiotic” for last 25 years.
Furthermore, scientific reports supported with evidence of safe use and long history of consumption supports the candidature of spore formers as potential probiotics and as functional food supplements due to their significant capacity of production of extracellular enzymes. Bacillus spp. has been used for production of food grade amylase, glucoamylase, protease, pectinase and cellulase in varying food stuffs (Ghani et al., 2013; Ouattara et al., 2017). Different species of Bacillus has also been used for the production of additional nutraceuticals including vitamins (e.g., riboflavin, cobalamin, inositol) and carotenoids for the synthesis of several health supplements for human consumption (Mohammed et al., 2014; Tanaka et al., 2014; Takano, 2016). Nevertheless, despite above benefits, these strains have not gained much importance and attention in current functional food industry due to their relatedness with few human pathogens. Few of the members of Bacillus spp. particularly, B. cereus, B. weihenstephanensis, B. anthracis, and B. thuringiensis species are known to produce various toxins, including ematic or enterotoxin (Cereulide), Bipartite exotoxins: protective antigen- lethal factor (PA-LF) and PA-edema factor (PA-EF), Cry and Cyt. Among them, Cereulide produced by B. cereus and B. weihenstephanensis is a major cause of food borne intoxications; while PA-LF and PA-EF are B. anthracis generated toxins associated with deadly illness in humans and animals.
Thereby, to understand the nature of beneficial spore former probiotic strains, their probiotic potential and safety concerns are important; not only because of their complexity and behavior in human GIT, but also due to their allochthonous (free living) nature, questioning their ability to colonize in the human gut (Hong et al., 2005). Though, the members of Bacillus genus have been consumed in the form of fermented foods since long time (Tamang et al., 2016), concerns regarding their safety are also raised. This review highlights the probiotic candidature of spore forming Bacillus spp. and presents an overview of the proposed health benefits, including application in food and pharmaceutical industry. The associated safety and licensing issues that influence the use of Bacillus spp. for commercial development has been summarized, together with evidence showing the growing need to evaluate the safety of individual Bacillus strains as well as species on a case by case basis.
Niche and Availability of Bacillus Probiotics
Bacillus signifies a Gram-positive, rod shaped, spore-forming, aerobic or facultative anaerobic bacterium. In general, the genus Bacillus is designated as a group of soil inhabitants. However, Bacillus spp. can be isolated from varied sources including air, water, human and animal gut, and also from vegetables and food (Alou et al., 2015; Kotb, 2015). Nevertheless, Bacillus spp. represents the most heterogeneous group in terms of phenotypic and genotypic characters. Some distinct species have also been recognized as opportunistic pathogen or toxin producer in human or animal hosts. The genus Bacillus is closely related to Lactobacillus spp., the distinguished candidate probiotic. Both share the same class, Bacilli under the phylum Firmicutes (Figure 2).
FIGURE 2.
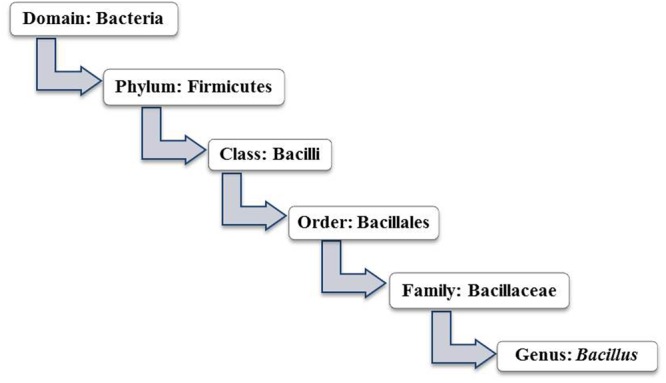
Taxonomy of genus Bacillus (Source: Bergey’s manual of systematic bacteriology: volume 3: The Firmicutes).
Looking toward the probiotic prospective, it is proclaimed that the candidate probiotic should be isolated from the gut of the target population, which helps them to thrive well within the gut. However, elementary attributes of native flora for survivability are not essential for spore-former(s). Bacillus spores can survive in extreme acidity of stomach, and tolerate bile salts and other hostile conditions of GIT. Besides, bacilli are more stable during processing and storage of food and pharmaceutical preparations, which make them more suitable ingredient for health promoting formulations. Thereby, in addition to human sources, Bacillus strains with probiotic attributes are also isolated from fermented or unfermented food sources (Adewumi et al., 2014; Rao et al., 2015) and are commercialized in the form of diverse range of health supplements (Table 1).
Table 1.
Examples of probiotic supplements containing Bacillus spp. available in global market.
| Product | Country | Components |
|---|---|---|
| Nutrition essentials Probiotic | United States | Bacillus coagulans 15B and fructooligosaccharide |
| NutriCommit | United States | Bacillus subtilis, Bacillus coagulans |
| Flora3 | United States | Bacillus coagulans, Saccharomyces boulardii, and FOS |
| LifeinUTM | Europe | Bacillus subtilis CU1 |
| THORNE | United States | Bacillus coagulans |
| Sunny Green Cleansing Green | United States | Bacillus coagulans |
| Just Thrive | United States | Bacillus indicus HU36, Bacillus coagulans, Bacillus clausii, Bacillus subtilis HU58 |
| Vital Probiotics | United States | Bacillus subtilis, L. rhamnosus, L. casei, Bifidobacterium longum, L. acidophilus, L. plantarum, Bifidobacterium breve |
| MegaSporeBiotic | United Kingdom | Bacillus indicus, Bacillus subtilis, Bacillus coagulans |
| Bacillus licheniformis, Bacillus clausii | ||
| Bio-Kult | United Kingdom | Bacillus subtilis PXN 21, Lactobacillus, Bifidobacterium, and Streptococcus strains |
| Enterogermina® | Europe | B. clausii |
| BioPlus 2B® | Denmark | B. subtilis CH201/DSM5749 and B. licheniformis CH200/DSM5749 |
| GanedenBC30 | United States | B. coagulans |
| AnabanTM | Europe | B. subtilis |
| Biosporin® | Europe | B. subtilis, B. licheniformis |
Spore-Formers In Human Gut
It is believed that Bacillus spp. is not a natural inhabitant of gut. They get colonized in to the intestinal tract after consumption of vegetables or raw food materials contaminated with soil microflora. Moreover, ingestion of fermented cereals or beans, such as Iru and Natto also make their way in to the intestine. Findings from in vitro studies claim that vegetative cells and spores of B. cereus can well defend the GIT stress and adhere to the intestinal epithelium. However commensal gut microbiota possesses inhibitory activity against them (Berthold-Pluta et al., 2015). A study by Tam et al. (2006) demonstrated that spores of Bacillus spp. could readily be recovered in the range of 103-108cfu/g of human feces. The 16S rRNA gene phylogenetic analysis of isolates demonstrated the presence of 10 different Bacillus species in examined fecal samples of 30 volunteers (Tam et al., 2006). Hoyles et al. (2012) further studied the diversity of Bacillus spp. and related spore-former bacteria in human feces and documented that the majority of recovered isolates belonged to Bacillaceae family. Two species, B. clausii and B. licheniformis were recovered most frequently. To find out the stage of Bacillus in human gut, Casula and Cutting (2002) targeted a genetically engineered chimeric gene, ftsH-lacZ, which is selectively and strongly expressed in the vegetative cells of Bacillus subtilis and reported their presence throughout the GIT. They stated that the spores germinated in significant numbers in the jejunum and ileum, suggesting their colonization into the small intestine. Recently, Ghelardi et al. (2015) documented that the orally administered Bacillus spp. follows transient colonization in to the intestine. Nevertheless, the impact of allochthonous Bacillus strains on the profile of fecal flora of the host during transition period has been proven significant. Nyangale et al. (2014) observed that after 28 day treatment of Bacillus coagulans in elderly subjects, baseline populations of Faecalibacterium prausnitzii, Clostridium lituseburense and Bacillus spp. were significantly higher, relative to the placebo group. Likewise, a study by Adami and Cavazzoni (1999) in piglet model has also shown that the feeding of Bacillus coagulans CNCM I-1061 increased aerobic and anaerobic sporeformers, decreased lactococci, enterococci, anaerobic cocci, and fecal coliforms in the treatment group.
Spore-Formers In Food Chain
The consumption of vegetative cells and spores of Bacillus spp. by human beings is frequent through fermented foods and raw vegetables. A diverse range of Bacillus species are found to be associated to the natural fermentation of soy, locust been, maize, rice and many more substrates. For example, Natto (Japan), Gari (Africa) TapaiUbi (Malaysia), Douchi (China), Rabadi (India, Pakistan), Soibum (India), Ugba (Nigeria) etc. are among the popular functional foods naturally harboring the blend of Bacillus spp. and LAB. These fermented products exhibit unique sensory attributes, probably due to the activity of extracellular carbohydrate and protein degrading enzymes of Bacillus spp. origin (Table 2). The palatability and health promoting characteristic of these locally produced supplements has also attracted the attention of global market. A diverse range of LAB and Bacillus spp. isolated from such indigenous foods has been studied and are being used for the commercial preparations of functional products (Angmo et al., 2016; Von Mollendorff et al., 2016). Respectively, the strains of B. subtilis, B. subtilis var. natto, B. clausii, B. licheniformis, and B. coagulans etc. are being utilized to improve the quality and demand of functional foods globally (Beaumont, 2002; Chantawannakul et al., 2002; Inatsu et al., 2006).
Table 2.
List of Bacillus species used for production of enzymes.
| Class of enzyme | Name of enzymes | Enzyme producing Bacillus species |
|---|---|---|
| Carbohydrate degrading | α-Amylase, β-Amylase, Arabinase, Cellulase, Chitinase, Chitosanase, Dextranase, Galactanase, | B. coagulans, B. subtilis, B. licheniformis, B. amyloliquifaciens, B. cereus, B. megaterium, B. caldolyticus, B. polymyxa, B. pumilus, B. circulans, B. firmus, B. brevis, B. macerans, B. stearothermophilus |
| β-1,3-glucanase, β-1,6-glucanase, Isoamylase, Lichenase, Levansucrase, Maltase, Mannanase, Pactate lysase, Phosphomannase, Pullulanase, Xylanase, Glucose isomerase | ||
| Proteases | Aminopeptidase, Esterase, metal proteases, Serine proteases | B. subtilis, B. cereus, B. licheniformis, B. amyloliquifaciens, B. megaterium, B. polymyxa, B. thermoproteolyticus, B. thuringiensis, B. pumilus |
| Lipase | Phospholipase C, Thiaminase | B. licheniformis, B. cereus, B. anthracis, B. thuringiensis, B. thiaminolyticus |
| Nucleases | Doxyribonuclease, Ribonucleases, 3-nucleotidases, 5-nucleotidases | B. amyloliquifaciens, B. subtilis, B. cereus, B. megaterium, B. pumilus |
| Phosphatases | Alkaline phosphatase | B. amyloliquifaciens, B. subtilis, B. cereus |
| Other | β-lactamase, | B. subtilis, B. cereus, B. anthracis, B. licheniformis, B. megaterium |
| Endo-N-acetylglucosaminidase, | ||
| Exo-N-acetylglucosaminidase | ||
| Endo-N-acetymuraminidase, | ||
| Exo-N-acetylglucosaminidase |
Bacillus strains are also getting recognition as potential probiotics which could promote human health by direct consumption of high concentrations of viable number of cells (Abdhul et al., 2015; Manhar et al., 2016). Several Bacillus probiotic strains have been approved by regulatory agencies of different countries for human use and are popular as general health promoting pharmaceutical formulations in global market (Table 1). So far commercial products of Bacillus composing functional foods are not popular in nutraceuticals market because the debate over probiotic vs. pathogen tag of Bacillus spp. is persisting in the research and production terrains. Hence, it’s important to clearly understand the phenotypic and genotypic characteristics of selective Bacillus spp. and their substantiation with those having GRAS status, to reach a consensus over the same.
Genus Bacillus: Probiotic Or Pathogen
Bacillus spp. has received considerable taxonomic attention because of its economic as well as medical importance. The genus Bacillus has undergone considerable taxonomic changes over time. The number of species allocated to this genus increased to 318 in the “List of prokaryotic names with standing in nomenclature”1. With the advent of molecular taxonomy, Ash et al. (1991) separated 51 distinct Bacillus species into five phylogenetic clusters. In this review, we are looking over the significance and approach of Bacillus probiotics in current scenario. Thus, it will also be interesting to take note of the evolutionary connections of probiotic Bacillus species/strains with other members of the genera. According to Ash et al. (1991), group 1 of Bacillus forms the largest cluster of 28 species. Evolutionary distance tree showed specific relation between species, for example, there were three distinct clades of closely related species. First clade included B. subtilis, B. atrophaeus, B. amyloliquifaciens, B. lautus, B. lentimorhus, B. licheniformis, B. popilhe, and B. pumilus. Second and third clades comprised of B. anthracis, B. cereus, B. meduso, B. mycoides, B. thuringiensis, B. maroccanus, B. simplex, and B. psychrosaccharolyticus. However, all remaining members of the group did not show any significant relationship with them. Group 2, 3, 4, and 5 consisted of 7, 10, 2, and 3 species respectively. Two species, B. alcalophilus and B. aneurinolyticus remained ungrouped and formed a separate line of descent. Interestingly, the available data points out that both the probiotic Bacillus species (e.g., B. subtilis, B. coagulance, B. licheniformis, and B. megaterium) and potential human pathogens (B. anthracis and B. cereus) fall into group 1; however, both the forms are separated into distinct clans. Therefore, more deep analysis of the taxonomic positions of these species is needed to reach a definite conclusion.
In this context, Xu and Cote (2003) made an effort to determine the phylogenic relationship among 40 Bacillus species using nucleotide sequences of the 16S rDNA and the 16S–23S internal transcribed spacer (ITS). In this study, comparative sequence analysis of the 16S-23S ITS sequences, revealed 10 distinct phylogenetic clusters. Twenty-six evaluated Bacillus species were separated in seven groups; with groups II, V, VI, and X comprising of seven, two, nine and five Bacillus species, respectively (Figure 3). Bacillus subtilis, the type species fall in Group VI along with B. amyloliquefaciens, B. atrophaeus, and B. mojavensis. B. coagulans was placed in group I; B. maroccanus and Bacillus simplex in Group II; and B. anthracis, B. cereus, B. mycoides, and B. thuringiensis in Group X. This study revealed that two major pathogenic spp. of Bacillus (B. anthracis and B. cereus) fall into the Xth group, which is apparently unrelated to the Bacillus spp. used as human and animal probiotics. Interestingly, B. cereus exhibits virulence in a strain specific manner. The pathogenic characteristic depends over strain and variety specific production of several extracellular factors viz. (phospholipase, cereulide (emetic toxin), enterotoxin Hbl, non-haemolytic toxin (Nhe), haemolysin IV) having role in cellular membrane disruption and induction of necrotic enterocolitis cytotoxin (Berthold-Pluta et al., 2015). Toxicity of virulent forms of B. cereus had been linked to the expression of plcR gene, that codes for most of the extracellular virulence factors (Hbl, Nhe, cytK) and proteins (Ramarao and Lereclus, 2006). Recently, data obtained from draft genome assemblies of 25 B. cereus strains clearly indicated sub-division of B. cereus into seven phylogenetic groups. Frequent horizontal transfer of pathogenicity factors among B. cereus has been proposed as an important factor determining the distribution of Bacillus spp. among pathogenic, non-pathogenic and probiotic species. The enterotoxin operons viz. nheABC and hblCDAB are chromosomally located and are abundant within B. cereus, which along with possibility of horizontal transfer raises safety concerns (Bohm et al., 2015).
FIGURE 3.
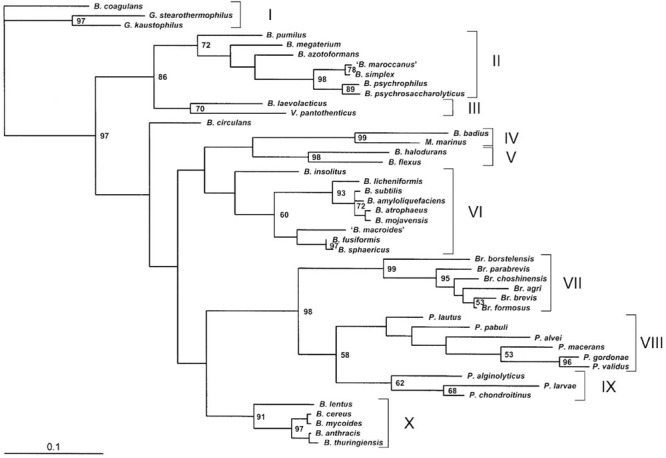
Phylogenetic relationships of 46 Bacillus species. The bar represents the unit length of the number of nucleotide substitutions per site (adopted from Xu and Cote, 2003).
These studies have revealed the phylogenetic relations among important species of Bacillus genus and are also indicating toward the necessity of more profound process for the selection and identification of Bacillus probiotic candidates. In this way, the complete genome sequencing and comparative analysis of various close relative Bacillus species of different groups has been provided by different research groups (Rey et al., 2004; Kolsto et al., 2009; Earl et al., 2012; Jeong et al., 2016; Krawczyk et al., 2016; Fan et al., 2017). The data of deeply sequenced Bacillus species from different sources can be utilized for the accurate identification and characterization of candidate probiotic strains. This may include characterization using advanced molecular techniques (such as PFGE, 16S rDNA sequencing, AFLP and MLST etc.).
Bacillus: Probiotic Attributes In Gut
Bacillus spp. are preferentially aerobic to facultative aerobic (Hoffman et al., 1995), germination and outgrowth of spores in the intestine seem difficult to envisage because the mammalian intestine is an anaerobic environment. In order to qualify as a potential probiotic candidate, the Bacillus strains must possess the primary requirement of GIT stress tolerance, besides having good adhesion and bio-therapeutic properties (Thakur et al., 2016). Survival under the GIT stress represents another challenge for safe transit and localization in the gut. Bacilli are normally considered soil organisms however, number of them including B. subtilis have been reported in feces and ileal biopsies of volunteers (Fakhry et al., 2008). In this regard, Hong et al. (2009) reinforced a growing view that B. subtilis and probably other species had adapted to life within the human GIT, including ability to form biofilm, sporulate anaerobically and produce antimicrobials, and should be considered gut commensals, rather than solely soil microorganisms.
Earlier, Hyronimus et al. (2000) evaluated thirteen spore forming lactic acid bacilli for their resistance to acid and bile salt. B. laevolacticus DSM 6475 and B. racemicus IAM 12395 were found to tolerate pH 2.5 for up to six hours. Also, B. racemilacticus and B. coagulans strains were reported to be tolerant to bile concentrations over 0.3% (w/v). This study indicated high acid tolerance of Bacillus spp., however, the same was questionable for bile tolerance for few strains. In a recent study, twenty healthy European subjects were administered orally with Enterogermina® containing spores of four strains of B. clausii, which could survive transit through the human GIT, during which germination, out-growth and multiplication could happen (Ghelardi et al., 2015). Similar to non-spore forming Lactobacillus spp., the survival rate of spore forming bacilli is also strain specific. Additionally, food matrix also plays an important role in survival of probiotics during simulated gastric juice conditions (Karu and Sumeri, 2016).
Different Bacillus strains have been reported to display antimicrobial, anti-oxidative and immune-modulatory activity in the host. The elements behind beneficial attributes of Bacillus spp. probiotics are explored in various studies, wherein these activities were found connected to their ability to produce antimicrobial peptides, small extracellular effector molecules and their ability to interact with host with the help of adhesion and attachment features (Khochamit et al., 2015). The antagonistic activity of Bacillus spp. has been explored against large number of pathogens. A study by Pinchuk et al. (2001) also reported the anti H. pylori activity of tested probiotic B. subtilis strains, which was attributed to the secretion of aninocoumacin A antibiotic. The antagonistic activity of aninocoumacin A was also documented against enteric E. faecium and Shigella flexneri. An interesting communication by Ripert et al. (2016) revealed that the probiotic B. clausii O/C strain protected vero and Caco-2 cells from the cytotoxic effect of Clostridium difficili and B. cereus toxins. This activity was mediated by serine protease(s) of B. clausii. On the other hand, exclusion of pathogen by the inhibition of bacterial biofilm is another potential attribute proposed for Bacillus strains. In this context, Gobi et al. (2016) demonstrated that the cell free extracts of B. licheniformis Dahb1 decreased the microbial adhesion to hydrocarbon, exopolysaccharide and biofilm metabolic activity, and decreased biofilm formation by the virulent V. parahaemolyticus Dahv2, in Asian catfish.
Recently, a bacteriocin producing strain of probiotic Bacillus coagulans had been isolated from traditional fermented fish of Manipur, India. The purified bacteriocin of low molecular weight displayed broad spectrum of antimicrobial activity against food borne and related clinically relevant pathogens, besides having lower cytotoxicity (Abdhul et al., 2015). Earlier, Joseph et al. (2013) also isolated and characterized bacteriocin producing strain of B. subtilis and displayed high level of antimicrobial activity of partially purified bacteriocin against foot ulcer bacterial pathogens, with highest recorded against Klebsiella spp. Such bacteriocin producing strains of Bacillus spp. have potential to be introduced as food biopreservative and as an antimicrobial in human and animal infections. In spite of pathogenic status of B. cereus, this species has also been explored for probiotic attributes. For example, B. cereus var. vietnami which used in BiosubtlyDL was found to produce bacteriocins like activity against different Bacillus species and it was shown to persist the mouse GIT up to 18 days. However, it was also found to produce enterotoxins (Duc et al., 2004). In addition, B. cereus var. toyoi, B. cereus YB-2, B. cereus G19 and B. cereus BC-01 were studied for their beneficial effect on livestock including rabbit, piglets and sea cucumber (Trocino et al., 2005; Altmeyer et al., 2014; Li et al., 2015).
Health Benefits of Probiotic Spore Formers
In the food sector, the global trend is to incorporate probiotics into food matrix in order to provide some health-promoting component(s) beyond its traditional nutrients (Butel, 2014). Lactobacillus and Bifidobacterium spp. have earned many health claims, including their immune-modulation (Shida and Nomoto, 2013; Maneerat et al., 2014), anti-mutagenic (Sah et al., 2014), lowering of plasma triglycerides (Guo et al., 2013; London et al., 2014) and the potential to modulate host physiology via direct and indirect means (Di Caro et al., 2005; Fujiya et al., 2007). On contrary, there are few published reports dealing with the health benefits of probiotic spore formers (Figure 1). Efficacy of Bacillus spp. as probiotic has been screened in several in vitro and in vivo animal models and a few have also been validated in human clinical trials. The available data from these studies has presented the beneficial effects of different strains of Bacillus spp. for human health. For example, several researchers have recognized the preventive role of Bacillus probiotic in gut physiology impairment conditions (Lopetuso et al., 2016; Zhang et al., 2016). The amelioration of dysbiosis and gut inflammation by probiotic Bacillus strains was established by the ability of balancing gut flora toward beneficial microbial population and associated anti-inflammatory agents which helped to recover intestinal mucosa from illness generated injuries. Recently, an in vivo study by Haldar and Gandhi (2016) revealed that the oral administration of skim milk containing Bacillus coagulans B37 and Bacillus pumilus B9 decrease coliform counts in feces of treatment groups. Besides, the beneficial effect of B. coagulans on the gut metabolism is also evaluated by Lee et al. (2016), who reported that feeding of B. coagulans along with soya pulp to cholic acid fed rats, suppressed the production of secondary bile acid, improved intestinal permeability and reduced the bactericidal effect of bile acid which supported the growth of beneficial microbiota into the intestine. An exopolysaccharide (EPS) producing strain of B. cereus SZ-1 delayed DNA damage, increased cell survival and glutathione and catalase expression in H2O2 induced PC12 cells. The purified EPS from B. cereus could be useful for preventing oxidative DNA damage and cellular oxidation in pharmaceutical and food products (Zheng et al., 2016). The protective effect of Bacillus probiotics against toxins has also been reported by Ripert et al. (2016). In this interesting communication, authors perceived involvement of secreted serine proteases from B. clausii in normalization of toxin produced by Clostridium difficili and B. cereus. The purified component also exhibited antitoxic potential on Vero cell line treated with cell culture supernatants of both pathogens. Similarly, an antimicrobial peptide, antilisterial Subtilosin A derived from Bacillus tequilensis FR9 was also reported to exhibit pathogen invasion protection ability in HCT-116 human colon carcinoma cell line (Rani et al., 2016). In contrast, the bioavailability of nutraceuticals has seen to be increased in the presence of Bacillus strains. For example, Dudonné et al. (2015) has shown increased microbial degradation products of native cranberry phenolic compounds in B. subtilis CU1 supplemented rats.
The positive effect of Bacillus spp. to distant cells, beyond GIT has also been established by several researchers. In this context, Foligné et al. (2012) studied the effect of probiotic Bacillus subtilis PB6 on cytokine release profile of human immunocompetent peripheral blood mononuclear cells (PBMC). Strain PB6 induced substantial levels of IL-10 but very low levels of IL-12, TNFα, and IFNγ on human PBMC. In an interesting study, Abhari et al. (2016) demonstrated that oral administration of B. coagulans and inulin combination could improve the biochemical and clinical parameters of rheumatoid arthritis in rat model. Briefly, pre-treatment with the symbiotic diet significantly inhibited the fibrinogen, serum amyloid A and TNFα production, and significantly inhibited the development of paw swelling induced by complete Freund’s adjuvant. The immune-modulatory potential of probiotic B. cereus for livestock has also been investigated by few researchers. In an animal study, dietary supplementation of B. cereus var. toyoi to sows and piglets resulted in its recovery from feces, however, interestingly probiotic was detected in piglet feces before its dietary intake, indicating a secondary route of its intake besides diet. Dietary intake of B. cereus var. toyoi reduced the incidence of liquid feces and diarrhea (Taras et al., 2005). Additionally, the positive effect of B. cereus var. toyoi on secretion of interferon gamma and IL-4 and natural killer receptor 2D (NKG2D) expression in intraepithelial CD5+ γδ T cells of piglets has been suggested by few workers (Schierack et al., 2007; Altmeyer et al., 2014). Additionally, components secreted by potential probiotic Bacillus strains also possess anti-cancer activity. In an important analysis by Lee et al. (2012), surfactin like compound of Bacillus subtilis CSY191 could inhibit the growth of MCF-7 human breast cancer cells in a dose-dependent manner. Furthermore, the health benefits of Bacillus probiotic strains have also been proven in human subjects of different health and age groups. Some of the important clinical trials demonstrating the impact of spore-former probiotic Bacillus strains have been highlighted in Table 3.
Table 3.
Clinical trials of probiotic Bacillus strains representing health benefits on human subjects.
| Strain | Study | Outcomes | Reference |
|---|---|---|---|
| B. coagulans Unique IS-2 | Phase II clinical study upon 28 patients with acute diarrhea | – Mean values for duration of diarrhea decreased | Sudha and Bhonagiri, 2012 |
| – Frequency of defecation was decreased | |||
| – Abdominal pain decreased | |||
| – Consistency of stool improved in treatment group | |||
| B. subtilis 3 and B. licheniformis 31 | Single-center, randomized, double-blinded, placebo-controlled clinical trial on 574 patients suffering from antibiotic-associated diarrhoea (AAD) | – The incidence of AAD and adverse effects related to the use of antibiotics in treatment group were significantly decreased | Horosheva et al., 2014 |
| B. coagulans (Colinox) | Monocentric double-blind, placebo-controlled parallel group study on 52 adult subjects suffering from IBS | – Significant reduction of the bloating, discomfort and pain in Colinox group compared to placebo group | Urgesi et al., 2014 |
| B. coagulans GBI-30 and 6086 | Double-blind placebo-controlled trial on 17 HIV-1 infected persons | – The probiotic was safe and well tolerated | Yang O.O. et al., 2014 |
| – Appeared to improve chronic gastrointestinal symptoms | |||
| B. subtilis CU1 | Randomized, double-blind, placebo-controlled, | – Fecal and salivary secretory IgA concentrations significantly increased compared to the placebo | Lefevre et al., 2015 |
| parallel-arms study on 100 healthy subjects aged 60–74 | – Frequency of respiratory infections in the probiotc group decreased | ||
| B. coagulans GBI-30 and 6086 | Double-blind, placebo-controlled crossover design on 36 healthy volunteers aged 65–80 years | – Significantly increased populations of Faecalibacterium prausnitzii and | Nyangale et al., 2015 |
| – Peripheral blood mononuclear cells (PBMCs) showed increase in the anti-inflammatory cytokine IL-10 after stimulation with LPS | |||
| B. clausii | Double-blinded, placebo-controlled, randomized trial in 244 preterm neonates | – No significant difference in the incidence of late-onset sepsis (LOS), however, full feeds were achieved significantly faster in the probiotic group | Tewari et al., 2015 |
| B. coagulans MTCC 5856 | Double blind placebo controlled multi-centered trial in 36 diarrhea predominant IBS patients | – Decrease in the clinical symptoms like bloating, vomiting, diarrhea, abdominal pain and stool frequency | Majeed et al., 2016 |
| – Disease severity decreased and the quality of life increased | |||
| B. coagulans GBI-30 and 6086 with casein protein | Placebo and diet-controlled study in 29 healthy male subjects | – Significantly increased post exercises perceived recovery and decreased muscle soreness | Jager et al., 2016 |
| – Showed a trend toward reduced muscle damage |
Besides having direct effect over host health, strains of Bacillus are currently also being employed for protective and therapeutic effect against several systemic clinical syndromes particularly, metabolic disorders. Several studies have established that natural products involving Bacillus spp. can be an alternate, safe and cost effective therapy for the management of metabolic syndromes. In one such study, solid state fermentation of soybean with B. amyloliquefaciens resulted in production of 1-Deoxynojirimycin, a potent alpha-glucosidase inhibitor (Cai et al., 2017). On similar lines, 13 weeks of dietary intervention with B. licheniformis 67 fermented soybean paste significantly prevented obesity related parameters in diet induced obese C57BL/6J mice (Choi et al., 2016). Fermented soybean fed group displayed lower values for blood glucose, insulin, serum and hepatic lipid profile, and body weight compared with high fat diet control group. The anti-obesity activity was attributed to the production of poly gamma glutamic acid by selective strains. The anti-diabetic functionality of B. licheniformis fermented soybean had also been attributed to the reduced accumulation of beta amyloid in brain hippocampus, preventing beta amyloid mediated insulin resistance and beta cell death. The study displayed glucose homeostaisis effects of fermented soybean in diabetic rats with experimental Alzheimer’s type dementia (Yang H.J. et al., 2014). Similarly, Purified exopolysaccharide from B. subtilis suppressed cardiovascular disease related parameters in streptozoticin induced diabetic rats (Ghoneim et al., 2016). In this study, the therapeutic effect of EPS was recorded due to reduced blood glucose, troponin, total serum cholesterol, LDL, and VLDL as well as suppression of ICAM and VCAM expression. Earlier, Zouari et al. (2015) also explored the anti-diabetic and anti-lipidemic properties of biosurfactant produced by B. subtilis SPB1 strain in alloxan induced diabetic rats. Upon oral administration, the biosurfactant reduced the plasma alpha-amylase activity and rendered protection to pancreatic beta cells. Besides displaying hyperglycemic effects, biosurfactant administration regulated serum lipid profile by promoting HDL-cholesterol and delaying the absorption of LDL-cholesterol and triglycerides. Aforesaid studies clearly support the rich bio-therapeutic potential of spore forming Bacillus strains, which further needs validation in human clinical trials. In light of the above reports, it can be stated that the candidate probiotic Bacillus strains themselves or their metabolites could also be considered as a potential candidate for management of metabolic disorder.
Probable Mechanism of Action
Several mechanistic studies have attempted to underline the probable mechanism of action of candidate probiotic Bacillus strains. The mechanisms by which spore forming probiotics (SFP) could enhance health of the host include stimulation of immune system, synthesis of different antimicrobials, like bacteriocins, enzymes and modulation of the composition of gut microbiota (Figure 4). The mechanism behind establishment of gut homeostasis involves promotion of growth of other beneficial microbes and suppression of pathogen and pathogen induced inflammatory response of intestinal mucosa. Microbial interference therapy depends on production of antimicrobial(s) by different probiotic strains. Bacillus spp. are known to produce several antimicrobial substances, e.g., bacteriocins, bacteriocins like inhibitory substances (e.g., Subtilin and Coagulin) and antibiotics (e.g., Surfactin and Bacilysin). B. subtilis var. natto has also been shown to inhibit the growth of Candida albicans in the intestinal tract, which is attributed to production of antibiotic, Surfactin, having activity against yeast (Ozawa et al., 1979; Nagal et al., 1996). In a recent study, B. subtilis R0179 was shown to have significant inhibitory effect on the growth of Candida spp. proposing it as an alternate therapy against oral candidiasis (Zhao et al., 2016). Also, Biosporin® containing B. subtilis 2335 and B. licheniformis 2336 (Biofarm, Ukraine) had been applied as probiotic supplement for humans and it possess antibacterial activity against Heliobacter pylori (Pinchuk et al., 2001). On the other hand, the positive influence of probiotic Bacillus strains on the growth and composition of commensal and beneficial species on gut could be mediated by the production of extracellular enzymes, vitamins and peptides. The impact of probiotic strains on host physiology is also a major factor behind maintenance of gut homeostasis. A comprehensive study in human subjects using DNA microarray technique observed that the genes involved in inflammation, immune response, defense response, intestinal permeability, cell adhesion, cell growth, cell differentiation, cell signaling, apoptosis, signal transcription, and transduction in intestinal mucosa were modulated upon B. clausii consumption (Di Caro et al., 2005). Surface-associated proteins from vegetative cells and spores of B. cereus might play an important role in interaction between these strains within human GIT (Sanchez et al., 2009). In addition, Fujiya et al. (2007) has also explained the role of quorum sensing molecules (QSMs) secreted by B. subtilis strain JH 642 in preservation of intestinal health. In this study, authors found that quorum sensing pentapeptide, competence and sporulation factor of B. subtilis JH 642 is involved in organic cation transporter mediated activation of p38 and MAPK pathways in Caco2bbe cells. This interaction serves an example of probiotic mediated change in behavior of host and composition of colonic flora.
FIGURE 4.
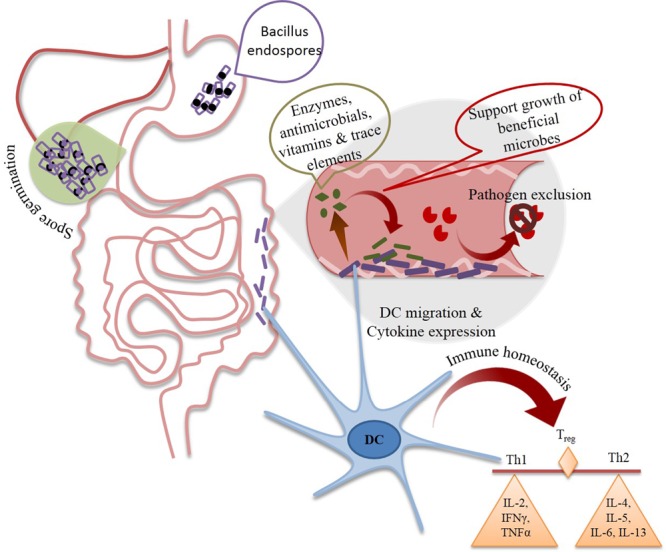
Different possible mechanisms of health benefits of spore forming Bacillus probiotics (SFBP). Daily intake of SFBP is resulted in increased their colonization in gut resulting in increased number of beneficial microbial population and decreased number of pathogenic strains. Moreover, SFBP could proliferate different immune cell for production of anti-inflammatory cytokines to maintain immune homeostasis. DC, Dendritic cells, Treg, regulatory T cells, Th, T helper cell, IL, interleukin, IFNγ, interferon γ, TNFα, tumor necrosis factor α.
The impact of probiotic Bacillus strain has also been reported on distal organs. For instance, the spores of B. clausii (Enterogermina®) were shown to enhance the production of IFN-γ in murine spleen cells, rabbits and mice animal models (Muscettola et al., 1991, 1992; Kosak et al., 1998). Vegetative cells of B. firmus have been shown to stimulate the proliferation of human peripheral blood lymphocytes in vitro (Prokesova et al., 1994). Also, spores of B. subtilis PB6 from AnabanTM had displayed anti-inflammatory effect in mice models that has been observed to be mediated through the modulation of IL-10, TNF-α and IFN-γ expression (Foligné et al., 2012).
Safety of Probiotic Spore Formers
Safety of a food product signifies the absence of any notable adverse health effects upon consumption under defined conditions (Kalliomäki et al., 2001). Probiotics are recognized for their long history of safe use. However, consumption in large amounts under immune compromised state may raise several safety concerns. Among Bacillus spore-formers, B. anthracis and B. cereus are well known pathogens. In case of B. cereus, pathogenicity varies from case to case; with some strains being the carriers for enterotoxin genes (Rowan et al., 2001). The occurrence of Bacillus spp. in food does not always cause foodborne illnesses or food spoilage; some species likewise, B. subtilis are used for preparation of East Asian fermented foods such as natto (Hosoi and Kiuchi, 2003). B. subtilis causes food-borne illnesses, with different symptoms, mainly vomiting (Kramer and Gilbert, 1989). At least one B. subtilis strain carries all three genes required to produce the Hbl enterotoxin normally produced by B. cereus (Rowan et al., 2001). On the other hand, Bacillus subtilis ATCC 6633 is known to produce a wide variety of antibacterial and antifungal compounds (Stein, 2005).
Furthermore, Bacillus spp. are used widely in transformation, whereas the plasmid encodes conjugative or mobile elements (Mullany et al., 2004). The commercial B. cereus IP5832 (Bactisubtil®) was isolated from the stools of patients with diarrhea (Kniehl et al., 2003). The strain was later shown to carry genes coding for endotoxin (Duc et al., 2004). Toyocerin® (Asahi Vet S.A., Tokyo, Japan), containing B. cereus var. toyoi, is licensed in EU for animal feed (reviewed in Cutting, 2011). In market, several probiotic Bacillus subtilis based products like BioGrow®, mixture of B. subtilis and B. licheniformis (Provita Eurotech Ltd, Omagh, UK); BioPlus®2B, mixture of Bacillus spp. (CHR. Hansen, Hoersholm, Denmark, EU approved); and AlCareTM, containing B. licheniformis (Alpha-pharmaInc, Melbourne, VIC, Australia, not licensed in EU) are being used for animal feed and aquaculture.
In South East Asia, different probiotic products containing Bacillus stains, either as single or mixed with other Lactobacillus strains, are used as an alternative to conventional antibiotics. These strains are marketed as antibiotic resistant probiotics. Therefore, there is a risk of transferring antibiotic resistance genes to commensal and pathogens in gut of humans and animals and release of drug resistance genes to the environment through feces (SCAN, 1999, 2003). Enterogermina® contains spores of four strains of B. clausii, which are resistant toward chloramphenicol and tetracyclin. Suspension of these spores (2 × 109cfu/ml) is used as medical supplement along with antibiotics against infantile diarrhoea (Green et al., 1999; Senesi et al., 2001).
Esporafeed® Plus, a feed additive containing B. cereus strain carrying a plasmid borne tetB gene was withdrawn from use in different European countries (SCAN, 1999). Also, due to concern of transfer of erythromycin resistance, AlCareTM was considered unsafe for feeding pigs (SCAN, 2002). In Vietnamese market, several Bacillus spp. products have been applied and licensed for human use such as, Biobaby® (ILdongPharma Co., Ltd., Korea), which contains three different spore forming probiotics, including B. coagulans (incorrectly, Lactobacillus sporogenes, widely used in India and one strain that has been granted as GRAS by FDA in the United States), B. subtilis and C. butyricum. Also, several in vitro and in vivo studies were performed in order to examine the toxicity of different species including B. subtilis var. natto and Bacillus indicus (Hong et al., 2008), B. licheniformis 2336 (Sorokulova et al., 2008), and B. coagulans (Endres et al., 2009). All appear to show no indications of adverse effects indicating the most occurrence of illness associated with Bacillus probiotic strains supplement of humans are result of either opportunistic infections or miss-diagnosis (reviewed in Cutting, 2011). Recently, Zhu et al. (2016) evaluated the safety of 15 commercial probiotic B. cereus products in China, they found that these products represent a potential risk for public health in China because and they recommended stricter safety regulation for these products is needed.
Regulatory/Legislative Status
The “health claims” of commercially available bio-therapeutics are allied to the viability, activity and composition of component microorganisms. Likewise, the entitlements of probiotic products being marketed as functional food, dietary supplement or drug are also elucidated by the state of probiotic strains. Thereby, the quality of commercial probiotic products is an important issue to be considered for regulation. Several researchers have identified discrepancies between labeled and actual contents of commercial probiotic products (Weese and Martin, 2011; Drago et al., 2013). Viability of probiotics, misidentifications at the genus/species level (marking Lactobacillus sporogenes in place of B. coagulans is a common example) and cross contamination by microorganisms are the main non-conformities during the evaluation of the quality of commercial probiotics. Consequently, despite the fact that significant amount of scientific literature is being produced about specific clinical benefits of probiotic microorganisms, there is an increasing demand for the legislative regulation on manufacturing practice, labeling and advertising of probiotic products.
Thereby some countries are developing their regulatory system to warrant the safety and wellbeing of consumers. In Japan, Food for Specified Health Use (FOSHU) system has defined functional food as ‘processed foods containing ingredients that aid specific bodily functions in addition to being nutritious’. The country has also regulation on the prevention of mislabeling of such foods. European countries have Food Products Directive for the regulation of food labeling. Probiotic foods also come under the regulation of this law. According to Directive, the contents in labeling must not be misleading for the purchasers. In UK, Joint Health Claims Initiative (JHCI) defines a health claim as ‘a direct, indirect or implied claim in food labeling, advertising and promotion that consumption of a food carries a specific health benefit or avoids a specific health detriment’. In United States, FDA has approved 12 health claims for foods. In addition, a joint FAO/WHO expert consultation on health and nutritional properties of probiotics in food have given scientific recommendations about the characterization, safety, efficacy, and labeling of the probiotic products. The report of this consultation also stated that the regulatory status of probiotics is not well established on an international basis (FAO/WHO, 2001). Thereby experts of consultation also made a number of recommendations pertaining to regulatory matters which will help to improve the regulatory status of functional food in global market.
Current Global Status
The probiotic market share is valued at the size of USD 36.6 billion in 2015 which will be widened with over 7% CAGR growth from 2016 to 20232. The Asia pacific will make the largest industry participant as they accounted for more than 40% of the global industry. India, China, and Japan are the major contributing factors in this field while both India and China will also see maximum growth in upcoming years3. Growing awareness about health, lifestyle, and increasing issues related to metabolic and digestive disorders are important contributing factors in the hike in probiotic market share. Moreover, the presence of international companies is also increasing the attention of consumers toward this health promoting supplements. If we look by the types of organisms, the probiotic market is categorized into five groups, i.e., Lactobacillus, Bifidobacterium, spore formers, yeast and others. Hence, the spore former probiotics are making a major contribution in global nutraceutical as well as pharmaceutical market.
Challenges Ahead
Probiotics enjoy the GRAS status and are freely consumed globally without any safety concern. Their efficacy and safety has been demonstrated in several in vitro, in vivo and human clinical trials. However, few recent studies have raised concern about their safety and dose in immune-compromised people (Redman et al., 2014). Although Bacillus probiotics have an overall excellent health promoting record, especially in preventing and curing of diarrhoea, giggivitis, H. pylori infection and maintaining homeostasis of intestine (Khodadad et al., 2013; Lefevre et al., 2015; Alkaya et al., 2016; Lopetuso et al., 2016). Their application with certain immune deficient population especially for critically ill, neonates and elderly groups should be evaluated and regulated carefully since reports related to bacteremia in immune-compromised patient treated with spore former and other probiotics has been recorded repetitively (Doron and Snydman, 2015). However, the importance of identification to strain level is also important to detect and eliminate any causal link between probiotics and strains isolated from immune-compromised hosts. Thereby, it is important to keep in mind that clinical trials of these formulations should cover the sufficient ratio of target population including people with low immunity.
Author Contributions
FE, NR, and HP has drafted the manuscript. FE, NR, RG, CS, and HP equally contributed in writing of manuscript. HP did the editing of manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
- GIT
gastrointestinal tract
- GRAS
generally recognized as safe
- ITS
internal transcribed spacer
- LAB
lactic acid bacteria
- PBMC
peripheral blood mononuclear cells
- SFP
spore forming probiotics.
Funding. The authors acknowledge the research fellowship to NR and CS from SERB-Ministry of Food Processing Industries (SERB/MoFPI/026/2015), Government of India.
References
- Abdhul K., Ganesh M., Shanmughapriya S., Vanithamani S., Kanagavel M., Anbarasu K., et al. (2015). Bacteriocinogenic potential of a probiotic strain Bacillus coagulans [BDU3] from Ngari. Int. J. Biol. Macromol. 79 800–806. 10.1016/j.ijbiomac.2015.06.005 [DOI] [PubMed] [Google Scholar]
- Abhari K., Shekarforoush S. S., Hosseinzadeh S., Nazifi S., Sajedianfard J., Eskandari M. H. (2016). The effects of orally administered Bacillus coagulans and inulin on prevention and progression of rheumatoid arthritis in rats. Food Nutr. Res. 60:30876 10.3402/fnr.v60.30876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adami A., Cavazzoni V. (1999). Occurrence of selected bacterial groups in the faeces of piglets fed with Bacillus coagulans as probiotic. J. Basic Microbiol. 39 3–10. [DOI] [PubMed] [Google Scholar]
- Adewumi G. A., Oguntoyinbo F. A., Romi W., Singh T. A., Jeyaram K. (2014). Genome subtyping of autochthonous Bacillus species isolated from Iru, a fermented Parkia biglobosa seed. Food Biotechnol. 28 250–268. 10.1080/08905436.2014.931866 [DOI] [Google Scholar]
- Alkaya B., Laleman I., Keceli S., Ozcelik O., CenkHaytac M., Teughels W. (2016). Clinical effects of probiotics containing Bacillus species on gingivitis: a pilot randomized controlled trial. J. Periodontal. Res. 52 497–504. 10.1111/jre.12415 [DOI] [PubMed] [Google Scholar]
- Alou M. T., Rathored J., Khelaifia S., Michelle C., Brah S., Diallo B. A., et al. (2015). Bacillus rubiinfantis sp. nov. strain mt2T, a new bacterial species isolated from human gut. New Microbes New Infect. 8 51–60. 10.1016/j.nmni.2015.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmeyer S., Kröger S., Vahjen W., Zentek J., Scharek-Tedin L. (2014). Impact of a probiotic Bacillus cereus strain on the jejunal epithelial barrier and on the NKG2D expressing immune cells during the weaning phase of piglets. Vet. Immunol. Immunopathol. 161 57–65. 10.1016/j.vetimm.2014.07.001 [DOI] [PubMed] [Google Scholar]
- Angmo K., Kumari A., Bhalla T. C. (2016). Probiotic characterization of lactic acid bacteria isolated from fermented foods and beverage of Ladakh. LWT Food Sci. Technol. 66 428–435. 10.1016/j.lwt.2015.10.057 [DOI] [Google Scholar]
- Ash C., Farrow J. A. E., Wallbanks S., Collins M. D. (1991). Phylogenetic heterogeneity of the genus Bacillus revealed by comparative analysis of small-subunit-ribosomal RNA sequences. Lett. Appl. Microbiol. 13 202–206. 10.1111/j.1472-765X [DOI] [Google Scholar]
- Bader J., Albin A., Stahl U. (2012). Spore-forming bacteria and their utilisation as probiotics. Benef. Microbes 3 67–75. 10.3920/BM2011.0039 [DOI] [PubMed] [Google Scholar]
- Beaumont M. (2002). Flavouring composition prepared by fermentation with Bacillus spp. Int. J. Food Microbiol. 75 189–196. 10.1016/S0168-1605(01)00706-1 [DOI] [PubMed] [Google Scholar]
- Berthold-Pluta A., Pluta A., Garbowska M. (2015). The effect of selected factors on the survival of Bacillus cereus in the human gastrointestinal tract. Microb. Pathog. 82 7–14. 10.1016/j.micpath.2015.03.015 [DOI] [PubMed] [Google Scholar]
- Bohm M. E., Huptas C., Krey V. M., Scherer S. (2015). Massive horizontal gene transfer, strictly vertical inheritance and ancient duplications differentially shape the evolution of Bacillus cereus enterotoxin operons hbl, cytK and nhe. BMC Evol. Biol. 15:246 10.1186/s12862-015-0529-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butel M. J. (2014). Probiotics, gut microbiota and health. Med. Mal. Infect. 44 1–8. 10.1016/j.medmal.2013.10.002 [DOI] [PubMed] [Google Scholar]
- Cai D., Liu M., Wei X., Li X., Wang Q., Nomura C. T., et al. (2017). Use of Bacillus amyloliquefaciens HZ-12 for high-level production of the blood glucose lowering compound, 1-deoxynojirimycin (DNJ), and nutraceutical enriched soybeans via fermentation. Appl. Biochem. Biotechnol. 181 1108–1122. 10.1007/s12010-016-2272-8 [DOI] [PubMed] [Google Scholar]
- Casula G., Cutting S. M. (2002). Bacillus probiotics: spore germination in the gastrointestinal tract. Appl. Environ. Microbiol. 68 2344–2352. 10.1128/AEM.68.5.2344-2352.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantawannakul P., Oncharoen A., Klanbut K., Chukeatirote E., Lumyong S. (2002). Characterization of proteases of Bacillus subtilis strain 38 isolated from traditionally fermented soybean in northern Thailand. Sci. Asia 28 241–245. 10.2306/scienceasia1513-1874.2002.28.241 [DOI] [Google Scholar]
- Choi J. H., Pichiah P. B. T., Kim M. J., Cha Y. S. (2016). Cheonggukjang, a soybean paste fermented with B. licheniformis 67 prevents weight gain and improves glycemic control in high fat diet induced obese mice. J. Clin. Biochem. Nutr. 59 31–38. 10.3164/jcbn.15-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutting S. M. (2011). Bacillus probiotics. Food Microbiol. 28 214–220. 10.1016/j.fm.2010.03.007 [DOI] [PubMed] [Google Scholar]
- Di Caro S., Tao H., Grillo A., Franceschi F., Elia C., Zocco M. A., et al. (2005). Bacillus clausii effect on gene expression pattern in small bowel mucosa using DNA microarray analysis. Eur. J. Gastroenterol. Hepatol. 17 951–960. 10.1097/00042737-200509000-00011 [DOI] [PubMed] [Google Scholar]
- Doron S., Snydman D. R. (2015). Risk and safety of probiotics. Clin. Infect. Dis. 60 S129–S134. 10.1093/cid/civ085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drago L., Rodighiero V., Celeste T., Rovetto L., De Vecchi E. (2013). Microbiological evaluation of commercial probiotic products available in the United States in 2009. J. Chemother. 22 373–377. 10.1179/joc.2010.22.6.373 [DOI] [PubMed] [Google Scholar]
- Duc L. H., Hong H. A., Barbosa T. M., Henriques A. O., Cutting S. M. (2004). Characterization of Bacillus probiotics available for human use. Appl. Environ. Microbiol. 70 2161–2171. 10.1128/AEM.70.4.2161-2171.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudonné S., Varin T. V., Anhê F. F., Dubé P., Roy D., Pilon G., et al. (2015). Modulatory effects of a cranberry extract co-supplementation with Bacillus subtilis CU1 probiotic on phenolic compounds bioavailability and gut microbiota composition in high-fat diet-fed mice. Pharma Nutr. 3 89–100. 10.1016/j.phanu.2015.04.002 [DOI] [Google Scholar]
- Earl A. M., Eppinger M., Fricke W. F., Rosovitz M. J., Rasko D. A., Daugherty S., et al. (2012). Whole-genome sequences of Bacillus subtilis and close relatives. J. Bacteriol. 194 2378–2379. 10.1128/JB.05675-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellekilde M., Selfjord E., Larsen C. S., Jakesevic M., Rune I., Tranberg B., et al. (2014). Transfer of gut microbiota from lean and obese mice to antibiotic-treated mice. Sci. Rep. 4:5922 10.1038/srep05922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres J. R., Clewell A., Jade K. A., Farber T., Hauswirth J., Schauss A. G. (2009). Safety assessment of a proprietary preparation of a novel probiotic, Bacillus coagulans, as a food ingredient. Food Chem. Toxicol. 47 1231–1238. 10.1016/j.fct.2009.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhry S., Sorrentini I., Ricaa E., De Felice M., Baccigalupi L. (2008). Characterization of spore forming Bacilli isolated from the human gastrointestinal tract. J. Appl. Microbiol. 105 2178–2186. 10.1111/j.1365-2672.2008.03934.x [DOI] [PubMed] [Google Scholar]
- Fan B., Blom J., Klenk H. P., Borriss R. (2017). Bacillus amyloliquefaciens, Bacillus velezensis and Bacillus siamensis form an operational group B. amyloliquefaciens within the B. subtilis species complex. Front. Microbiol. 8:22 10.3389/fmicb.2017.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO/WHO (2001). Health and Nutritional Properties of Probiotics in Food Including Powder Milk With Live Lactic Acid Bacteria. Report of a joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria Rome: Food and Agriculture Organization. [Google Scholar]
- FAO/WHO (2002). Guidelines for the Evaluation of Probiotics in Food. Food and Agriculture Organization of the United Nations and World Health Organization Working Group Report. Rome: Food and Agriculture Organization. [Google Scholar]
- Foligné B., Peys E., Vandenkerckhove J., Van Hemel J., Dewulf J., Breton J., et al. (2012). Spores from two distinct colony types of the strain Bacillus subtilis PB6 substantiate anti-inflammatory probiotic effects in mice. Clin. Nutr. 31 987–994. 10.1016/j.clnu.2012.05.016 [DOI] [PubMed] [Google Scholar]
- Fujiya M., Musch M. W., Nakagawa Y., Hu S., Alverdy J., Kohgo Y., et al. (2007). The Bacillus subtilis quorum-sensing molecule CSF contributes to intestinal homeostasis via OCTN2, a host cell membrane transporter. Cell Host Microbe 1 299–308. 10.1016/j.chom.2007.05.004 [DOI] [PubMed] [Google Scholar]
- Ghani M., Ansari A., Aman A., Zohra R. R., Siddiqui N. N., Qader S. A. U. (2013). Isolation and characterization of different strains of Bacillus licheniformis for the production of commercially significant enzymes. Pak. J. Pharm. Sci. 26 691–697. [PubMed] [Google Scholar]
- Ghelardi E., Celandroni F., Salvetti S., Gueye S. A., Lupetti A., Senesi S. (2015). Survival and persistence of Bacillus clausii in the human gastrointestinal tract following oral administration as spore-based probiotic formulation. J. Appl. Microbiol. 119 552–559. 10.1111/jam.12848 [DOI] [PubMed] [Google Scholar]
- Ghoneim M. A. M., Hassan A. I., Mahmoud M. G., Asker M. S. (2016). Effect of polysaccharide from Bacillus subtilis sp. on cardiovascular diseases and atherogenic indices in diabetic rats. BMC Complement. Altern. Med. 16:112 10.1186/s12906-016-1093-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobi N., Malaikozhundan B., Sekar V., Shanthi S., Vaseeharan B., Jayakumar R., et al. (2016). GFP tagged Vibrio parahaemolyticus Dahv2 infection and the protective effects of the probiotic Bacillus licheniformis Dahb1 on the growth, immune and antioxidant responses in Pangasius hypophthalmus. Fish Shellfish Immunol. 52 230–238. 10.1016/j.fsi.2016.03.006 [DOI] [PubMed] [Google Scholar]
- Green D. H., Wakeley P. R., Page A., Barnes A., Baccigalupi L., Ricca E., et al. (1999). Characterization of two bacillus probiotics. Appl. Environ. Microbiol. 65 4288–4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z., Liu X. M., Zhang Q. X., Shen Z., Tian F. W., Zhang H., et al. (2013). Influence of consumption of probiotics on the plasma lipid profile: a meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 21 844–850. 10.1016/j.numecd.2011.04.008 [DOI] [PubMed] [Google Scholar]
- Haldar L., Gandhi D. N. (2016). Effect of oral administration of Bacillus coagulans B37 and Bacillus pumilus B9 strains on fecal coliforms, Lactobacillus and Bacillus spp. in rat animal model. Vet. World 9 766 10.14202/vetworld.2016.766-772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman T., Troup P., Szabo A., Hungerer C., Jahn D. (1995). The anaerobic life of Bacillus subtilis: cloning of the genes encoding the respiratory nitrate reductase system. FEMS Microbiol. Lett. 131 219–225. 10.1111/j.1574-6968.1995.tb07780.x [DOI] [PubMed] [Google Scholar]
- Hong H. A., Duc le H., Cutting S. M. (2005). The use of bacterial spore formers as probiotics. FEMS Microbiol. Rev. 29 813–835. 10.1016/j.femsre.2004.12.001 [DOI] [PubMed] [Google Scholar]
- Hong H. A., Huang J. M., Khaneja R., Hiep L. V., Urdaci M. C., Cutting S. M. (2008). The safety of Bacillus subtilis and Bacillus indicus as food probiotics. J. Appl. Microbiol. 105 510–520. 10.1111/j.1365-2672.2008.03773.x [DOI] [PubMed] [Google Scholar]
- Hong H. A., Khaneja R., Tam N. M. K., Cazzato A., Tan S., Urdaci M., et al. (2009). Bacillus subtilis isolated from the human gastrointestinal tract. Res. Microbiol. 160 134–143. 10.1016/j.resmic.2008.11.002 [DOI] [PubMed] [Google Scholar]
- Horosheva T. V., Vodyanoy V., Sorokulova I. (2014). Efficacy of Bacillus probiotics in prevention of antibiotic-associated diarrhoea: a randomized, double-blind, placebo-controlled clinical trial. JMM Case Rep. 1 1–6. 10.1099/jmmcr.0.004036 [DOI] [Google Scholar]
- Hosoi T., Kiuchi K. (2003). “Natto – a food made by fermenting cooked soybeans with Bacillus subtilis (natto),” in Handbook of Fermented Functional Foods ed. Farnworth E. R. (Boca Raton, FL: CRC Press; ) 227–245. [Google Scholar]
- Hoyles L., Honda H., Logan N. A., Halket G., La Ragione R. M., McCartney A. L. (2012). Recognition of greater diversity of Bacillus species and related bacteria in human faeces. Res. Microbiol. 163 3–13. 10.1016/j.resmic.2011.10.004 [DOI] [PubMed] [Google Scholar]
- Hyronimus B., Le Marrec C., Sassi A. H., Deschamps A. (2000). Acid and bile tolerance of spore-forming lactic acid bacteria. Int. J. Food Microbiol. 61 193–197. 10.1016/S0168-1605(00)00366-4 [DOI] [PubMed] [Google Scholar]
- Inatsu Y., Nakamura N., Yuriko Y., Fushimi T., Watanasiritum L., Kawamoto S. (2006). Characterization of Bacillus subtilis strains in Thuanao, a traditional fermented soybean food in northern Thailand. Lett. Appl. Microbiol. 43 237–242. 10.1111/j.1472-765X.2006.01966.x [DOI] [PubMed] [Google Scholar]
- Jager R., Shields K. A., Lowery R. P., De Souza E. O., Partl J. M., Hollmer C., et al. (2016). Probiotic Bacillus coagulans GBI-30, 6086 reduces exercise-induced muscle damage and increases recovery. Peer J. 4:e2276 10.7717/peerj.2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong H., Park S. H., Choi S. K. (2016). Draft genome sequences of four plant probiotic Bacillus strains. Genome Announc. 4:e00358–16. 10.1128/genomeA.00358-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph B., Dhas B., Hena V., Raj J. (2013). Bacteriocin from Bacillus subtilis as a novel drug against diabetic foot ulcer bacterial pathogens. Asian Pac. J. Trop. Biomed. 3 942–946. 10.1016/S2221-1691(13)60183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalliomäki M., Salminen S., Arvilommi H., Kero P., Koskinen P., Isolauri E. (2001). Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet 357 1076–1079. 10.1016/S0140-6736(00)04259-8 [DOI] [PubMed] [Google Scholar]
- Karu R., Sumeri I. (2016). Survival of Lactobacillus rhamnosus GG during simulated gastrointestinal conditions depending on food matrix. J. Food Res. 5 57–66. 10.5539/jfr.v5n5p56 [DOI] [Google Scholar]
- Khochamit N., Siripornadulsil S., Sukon P., Siripornadulsil W. (2015). Antibacterial activity and genotypic–phenotypic characteristics of bacteriocin-producing Bacillus subtilis KKU213: potential as a probiotic strain. Microbiol. Res. 170 36–50. 10.1016/j.micres.2014.09.004 [DOI] [PubMed] [Google Scholar]
- Khodadad A., Farahmand F., Najafi M., Shoaran M. (2013). Probiotics for the treatment of pediatric Helicobacter pylori infection: a randomized double blind clinical trial. Iran J. Pediatr. 23 79–84. [PMC free article] [PubMed] [Google Scholar]
- Kniehl E., Becker A., Forster D. H. (2003). Pseudo-outbreak of toxigenic Bacillus cereus isolated from stools of three patients with diarrhoea after oral administration of a probiotic medication. J. Hosp. Infect. 55 33–38. 10.1016/S0195-6701(03)00133-6 [DOI] [PubMed] [Google Scholar]
- Kolsto A. B., Tourasse N. J., Okstad O. A. (2009). What sets Bacillus anthracis apart from other Bacillus species. Annu. Rev. Microbiol. 63 451–476. 10.1146/annurev.micro.091208.073255 [DOI] [PubMed] [Google Scholar]
- Kosak T., Maeda T., Nakada Y., Yukawa M., Tanaka S. (1998). Effect of Bacillus subtilis spore administration on activation of macrophages and natural killer cells in mice. Vet. Microbiol. 60 215–225. 10.1016/S0378-1135(97)00102-8 [DOI] [PubMed] [Google Scholar]
- Kotb E. (2015). Purification and partial characterization of serine fibrinolytic enzyme from Bacillus megaterium KSK-07 isolated from kishk, a traditional Egyptian fermented food. Appl. Biochem. Microbiol. 51 34–43. 10.1134/S000368381501007X [DOI] [Google Scholar]
- Kramer J. M., Gilbert R. J. (1989). “Bacillus cereus and other Bacillus species,” in Foodborne Bacterial Pathogens ed. Doyle M. P. (Boca Raton, FL: CRC Press; ) 21–70. [Google Scholar]
- Krawczyk A. O., de Jong A., Holsappel S., Eijlander R. T., van Heel A., Berendsen E. M., et al. (2016). Genome sequences of 12 spore-forming Bacillus species, comprising Bacillus coagulans, Bacillus licheniformis, Bacillus amyloliquefaciens, Bacillus sporothermodurans, and Bacillus vallismortis, isolated from foods. Genome Announc. 4 103–116. 10.1128/genomeA.00103-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. H., Nam S. H., Seo W. T., Yun H. D., Hong S. Y., Kim M. K., et al. (2012). The production of surfactin during the fermentation of cheonggukjang by potential probiotic Bacillus subtilis CSY191 and the resultant growth suppression of MCF-7 human breast cancer cells. Food Chem. 131 1347–1354. 10.1016/j.foodchem.2011.09.133 [DOI] [Google Scholar]
- Lee Y., Yoshitsugu R., Kikuchi K., Joe G. H., Tsuji M., Nose T., et al. (2016). Combination of soya pulp and Bacillus coagulans lilac-01 improves intestinal bile acid metabolism without impairing the effects of prebiotics in rats fed a cholic acid-supplemented diet. Br. J. Nutr. 116 603–610. 10.1017/S0007114516002270 [DOI] [PubMed] [Google Scholar]
- Lefevre M., Racedo S. M., Ripert G., Housez B., Cazaubiel M., Maudet C. P., et al. (2015). Probiotic strain Bacillus subtilis CU1 stimulates immune system of elderly during common infectious disease period: a randomized, double-blind placebo-controlled study. Immun. Ageing 12 24 10.1186/s12979-015-0051-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Xu Y., Jin L., Li X. (2015). Effects of a probiotic mixture (Bacillus subtilis YB-1 and Bacillus cereus YB-2) on disease resistance and non-specific immunity of sea cucumber, Apostichopus japonicus (Selenka). Aquac. Res. 46 3008–3019. 10.1111/are.12453 [DOI] [Google Scholar]
- London L. E., Kumar A. H., Wall R., Casey P. G., O’Sullivan O., Shanahan F., et al. (2014). Exopolysaccharide-producing probiotic Lactobacilli reduce serum cholesterol and modify enteric microbiota in ApoE-deficient mice. J. Nutr. 144 1956–1962. 10.3945/jn.114.191627 [DOI] [PubMed] [Google Scholar]
- Lopetuso L. R., Scaldaferri F., Franceschi F., Gasbarrini A. (2016). Bacillus clausii and gut homeostasis: state of the art and future perspectives. Expert Rev. Gastroenterol. Hepatol. 10 943–948. 10.1080/17474124 [DOI] [PubMed] [Google Scholar]
- Majeed M., Majeed S., Nagabhushanam K., Natarajan S., Sivakumar A., Ali F. (2016). Evaluation of the stability of Bacillus coagulans MTCC 5856 during processing and storage of functional foods. Int. J. Food Sci. Technol. 51 894–901. 10.1111/ijfs.13044 [DOI] [Google Scholar]
- Mallappa R. H., Rokana N., Duary R. K., Panwar H., Batish V. K., Grover S. (2012). Management of metabolic syndrome through probiotic and prebiotic interventions. Indian J. Endocrinol. Metab. 16 20–27. 10.4103/2230-8210.91178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneerat S., Lehtinen M. J., Childs C. E., Forssten S. D., Alhoniemi E., Tiphaine M., et al. (2014). Consumption of Bifidobacterium lactis Bi-07 by healthy elderly adults enhances phagocytic activity of monocytes and granulocytes. J. Nutr. Sci. 44 1–10. 10.1017/jns.2013.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manhar A. K., Bashir Y., Saikia D., Nath D., Gupta K., Konwar B. K., et al. (2016). Cellulolytic potential of probiotic Bacillus Subtilis AMS6 isolated from traditional fermented soybean (Churpi): An in-vitro study with regards to application as an animal feed additive. Microbiol. Res. 186 62–70. 10.1016/j.micres.2016.03.004 [DOI] [PubMed] [Google Scholar]
- Mohammed Y., Lee B., Kang Z., Du G. (2014). Development of a two-step cultivation strategy for the production of vitamin B12 by Bacillus megaterium. Microb. Cell Fact. 13 102 10.1186/s12934-014-0102-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullany P., Barbosa T. M., Scott K., Roberts A. P. (2004). “Mechanisms of gene transfer and the spread of antibiotic resistance in spore forming organisms in the GI tract,” in Bacterial Spore Formers: Probiotics and Emerging Applications eds Ricca E., Henriques A. O., Cutting S. M. (Norfolk: Horizon Bioscience; ) 113–129. [Google Scholar]
- Muscettola M., Grasso G., Blach-Olszewska Z., Migliaccio P., Borghesi-Nicoletti C., Giarratan M., et al. (1992). Effects of Bacillus subtilis spores on interferon production. Pharmacol. Res. 26 176–177. 10.1016/1043-6618(92)90652-R [DOI] [PubMed] [Google Scholar]
- Muscettola M., Grasso G., Migliaccio P., Gallo V. C. (1991). Plasma interferon-like activity in rabbits after oral administration of Bacillus subtilis spores. J. Chemother. 3 130–132.1875233 [Google Scholar]
- Nagal S., Okimura K., Kaizawa N., Ohki K., Kanatomo S. (1996). Study on surfactin, a cyclic depsipeptide II synthesis of surfactin B2 produced by Bacillus natto KMD 2311. Chem. Phar. Bull. (Tokyo). 44 5–10. 10.1248/cpb.44.5 [DOI] [PubMed] [Google Scholar]
- Nyangale E. P., Farmer S., Cash K., Chernoff D., Gibson G. R. (2015). Bacillus coagulans GBI-30 6086 modulates Faecalibacterium prausnitziiin older men and women. J. Nutr. 145 1446–1452. 10.3945/jn.114.199802 [DOI] [PubMed] [Google Scholar]
- Nyangale E. P., Farmer S., Keller D., Chernoff D., Gibson G. R. (2014). Effect of prebiotics on the fecal microbiota of elderly volunteers after dietary supplementation of Bacillus coagulans GBI-30, 6086. Anaerobe 30 75–81. 10.1016/j.anaerobe.2014.09.002 [DOI] [PubMed] [Google Scholar]
- Ouattara H. G., Reverchon S., Niamke S. L., Nasser W. (2017). Regulation of the synthesis of pulp degrading enzymes in Bacillus isolated from cocoa fermentation. Food Microbiol. 63 255–262. 10.1016/j.fm.2016.12.004 [DOI] [PubMed] [Google Scholar]
- Ozawa K., Yagu-Uchi K., Yamanaka K., Yamashita Y., Ueba K., Miwatani T. (1979). Bacillus natto and Streptococcus faecalis on growth of Candida albicans. Microbiol. Immunol. 23 1147–1156. 10.1111/j.1348-0421.1979.tb00547.x [DOI] [PubMed] [Google Scholar]
- Panwar H., Calderwood D., Grant I. R., Grover S., Green B. D. (2014). Lactobacillus strains isolated from infant faeces possess potent inhibitory activity against intestinal alpha-and beta-glucosidases suggesting anti-diabetic potential. Eur. J. Nutr. 53 1465–1474. 10.1007/s00394-013-0649-9 [DOI] [PubMed] [Google Scholar]
- Panwar H., Calderwood D., Grant I. R., Grover S., Green B. D. (2016). Lactobacilli possess inhibitory activity against dipeptidyl peptidase-4 (DPP-4). Ann. Microbiol. 66 505–509. 10.1007/s13213-015-1129-7 [DOI] [Google Scholar]
- Pinchuk I. V., Bressollier P., Verneuil B., Fenet B., Sorokulova I. B., Megraud F., et al. (2001). In vitro Anti-Helicobacter pylori Activity of the probiotic strain Bacillus subtilis 3 is due to secretion of antibiotics. Antimicrob. Agents Chemother. 45 3156–3161. 10.1128/AAC.45.11.3156-3161.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokesova L., Novakova M., Julak J., Mara M. (1994). Effect of Bacillus firmus and other sporulating aerobic microorganisms on in vitro stimulation of human lymphocytes. A comparative study. Folia Microbiol. 39 501–504. 10.1007/BF02814071 [DOI] [PubMed] [Google Scholar]
- Ramarao N., Lereclus D. (2006). Adhesion and cytotoxicity of Bacillus cereus and Bacillus thuringiensis to epithelial cells are FlhA and PlcR dependent, respectively. Microbes Infect. 8 1483–1491. 10.1016/j.micinf.2006.01.005 [DOI] [PubMed] [Google Scholar]
- Rani R. P., Anandharaj M., Hema S., Deepika R., Ravindran A. D. (2016). Purification of antilisterial aeptide (Subtilosin A) from novel Bacillus tequilensis FR9 and demonstrate their pathogen invasion protection ability using human carcinoma cell line. Front. Microbiol. 7:1910 10.3389/fmicb.2016.01910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranji P., Akbarzadeh A., Rahmati-Yamchi M. (2015). Associations of probiotics with vitamin D and leptin receptors and their effects on colon cancer. Asian Pac. J. Cancer Prev. 16 3621–3627. 10.7314/APJCP.2015 [DOI] [PubMed] [Google Scholar]
- Rao K. P., Chennappa G., Suraj U., Nagaraja H., Raj A. C., Sreenivasa M. Y. (2015). Probiotic potential of Lactobacillus strains isolated from sorghum-based traditional fermented food. Probiotics Antimicrob. Proteins 7 146–156. 10.1007/s12602-015-9186-6 [DOI] [PubMed] [Google Scholar]
- Redman M. G., Ward E. J., Phillips R. S. (2014). The efficacy and safety of probiotics in people with cancer: a systematic review. Ann. Oncol. 25 1919–1929. 10.1093/annonc/mdu106 [DOI] [PubMed] [Google Scholar]
- Rey M. W., Ramaiya P., Nelson B. A., Brody-Karpin S. D., Zaretsky E. J., Tang M., et al. (2004). Complete genome sequence of the industrial bacterium Bacillus licheniformis and comparisons with closely related Bacillus species. Genome Biol. 5:77 10.1186/gb-2004-5-10-r77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripert G., Racedo S. M., Elie A. M., Jacquot C., Bressollier P., Urdaci M. C. (2016). Secreted compounds of the probiotic Bacillus clausii strain O/C inhibit the cytotoxic effects induced by Clostridium difficile and Bacillus cereus toxins. Antimicrob. Agents Chemother. 60 3445–3454. 10.1128/AAC.02815-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan N. J., Deans K., Anderson J. G., Gemmell C. G., Hunter I. S., Chaithong T. (2001). Putative virulence factor expression by clinical and food isolates of Bacillus spp. after growth in reconstituted infant milk formulae. Appl. Environ. Microbiol. 67 3873–3881. 10.1128/AEM.67.9.3873-3881.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah B. N. P., Vasiljevic T., McKechnie S., Donkor O. N. (2014). Effect of probiotics on antioxidant and antimutagenic activities of crude peptide extract from yogurt. Food Chem. 156 264–270. 10.1016/j.foodchem.2014.01.105 [DOI] [PubMed] [Google Scholar]
- Sanchez B., Arias S., Chaignepain S., Denayrolles M., Schmitter J. M., Bressollier P., et al. (2009). Identification of surface proteins involved in the adhesion of a probiotic Bacillus cereus strain to mucin and fibronectin. Microbiology 155 1708–1716. 10.1099/mic.0.025288-0 [DOI] [PubMed] [Google Scholar]
- SCAN (1999). Opinion of of the Scientific Steering Committee on Antimicrobial Resistance. European Commission, Health and Consumer Protection Directorate-General. (SCAN) Scientific Committee on Animal Nutrition. Available at: https://ec.europa.eu/food/sites/food/files/safety/docs/sci-com_ssc_out50_en.pdf [Google Scholar]
- SCAN (2002). Opinion of the Scientific Committee on Animal Nutrition (SCAN) on the use of Bacillus licheniformis NCTC 13123 in Feeding Stuffs for Pigs (product AlCare). European Commission, Health and Consumer Protection Directorate-General. (SCAN) Scientific Committee on Animal Nutrition. Available at: https://ec.europa.eu/food/sites/food/files/safety/docs/animal-feed_additives_rules_scan-old_report_out79.pdf [Google Scholar]
- SCAN (2003). Opinion of the Scientific Committee on Animal Nutrition, on the Criteria for Assessing the Safety of Microorganisms Resistant to Antibiotics of Human Clinical and Veterinary Importance. European Commission, Health and Consumer Protection Directorate-General. (SCAN) Scientific Committee on Animal Nutrition. Available at: https://www.efsa.europa.eu/en/efsajournal/pub/223 [Google Scholar]
- Schierack P., Wieler L. H., Taras D., Herwig V., Tachu B., Hlinak A., et al. (2007). Bacillus cereus var. toyoi enhanced systemic immune response in piglets. Vet. Immunol. Immunopathol. 118 1–11. 10.1016/j.vetimm.2007.03.006 [DOI] [PubMed] [Google Scholar]
- Scott K. P., Antoine J. M., Midtvedt T., van Hemert S. (2015). Manipulating the gut microbiota to maintain health and treat disease. Microb. Ecol. Health Dis. 26 25877 10.3402/mehd.v26.25877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senesi S., Celandroni F., Tavanti A., Ghelardi E. (2001). Molecular characterization and identification of Bacillus clausii strains marketed for use in oral bacteriotherapy. Appl. Environ. Microbiol. 67 834–839. 10.1128/AEM.67.2.834-839.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shida K., Nomoto K. (2013). Probiotics as efficient immuno potentiators: translational role in cancer prevention. Indian J. Med. Res. 138 808–814. [PMC free article] [PubMed] [Google Scholar]
- Shimizu K., Ogura H., Asahara T., Nomoto K., Morotomi M., Tasaki O., et al. (2013). Probiotic/synbiotic therapy for treating critically ill patients from a gut microbiota perspective. Dig. Dis. Sci. 58 23–32. 10.1007/s10620-012-2334-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shobharani P., Padmaja R. J., Halami P. M. (2015). Diversity in the antibacterial potential of probiotic cultures Bacillus licheniformis MCC2514 and Bacillus licheniformis MCC2512. Res. Microbiol. 166 546–554. 10.1016/j.resmic.2015.06.003 [DOI] [PubMed] [Google Scholar]
- Sorokulova I. B., Pinchuk I. V., Denayrolles M., Osipova I. G., Huang J. M., Cutting S., et al. (2008). The safety of two Bacillus probiotic strains for human use. Dig. Dis. Sci. 53 954–963. 10.1007/s10620-007-9959-1 [DOI] [PubMed] [Google Scholar]
- Stein T. (2005). Bacillus subtilis antibiotics: structures, syntheses and specific functions. Mol. Microbiol. 56 845–857. 10.1111/j.1365-2958.2005.04587.x [DOI] [PubMed] [Google Scholar]
- Sudha R. M., Bhonagiri S. (2012). Efficacy of Bacillus coagulans strain Unique IS-2 in the treatment of patients with acute diarrhea. Int. J. Probiotics Prebiotics 7 33–37. [Google Scholar]
- Takano H. (2016). The regulatory mechanism underlying light-inducible production of carotenoids in non phototrophic bacteria. Biosci. Biotechnol. Biochem. 80 1264–1273. 10.1080/09168451.2016.1156478 [DOI] [PubMed] [Google Scholar]
- Tam N. K., Uyen N. Q., Hong H. A., Duc L. H., Hoa T. T., Serra C. R., et al. (2006). The intestinal life cycle of Bacillus subtilis and close relatives. J. Bacteriol. 188 2692–2700. 10.1128/JB.188.7.2692-2700.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamang J. P., Watanabe K., Holzapfel W. H. (2016). Review: diversity of microorganisms in global fermented foods and beverages. Front. Microbiol. 7:377 10.3389/fmicb.2016.00377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Takanaka S., Yoshida K. I. (2014). A second-generation Bacillus cell factory for rare inositol production. Bioengineered 5 331–334. 10.4161/bioe.29897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taras D., Vahjen W., Macha M., Simon O. (2005). Response of performance characteristics and fecal consistency to long-lasting dietary supplementation with the probiotic strain Bacillus cereus var. toyoi to sows and piglets. Arch. Anim. Nutr. 59 405–417. 10.1080/17450390500353168 [DOI] [PubMed] [Google Scholar]
- Terlabie N. N., Sakyi-Dawson E., Amoa-Awua W. K. (2006). The comparative ability of four isolates of Bacillus subtilis to ferment soybeans into dawadawa. Int. J. Food Microbiol. 106 145–152. 10.1016/j.ijfoodmicro.2005.05.021 [DOI] [PubMed] [Google Scholar]
- Tewari V. V., Dubey S. K., Gupta G. (2015). Bacillus clausii for prevention of late-onset sepsis in preterm infants: a randomized controlled trial. J. Trop. Pediatr. 61 377–385. 10.1093/tropej/fmv050 [DOI] [PubMed] [Google Scholar]
- Thakur N., Rokana N., Panwar H. (2016). Probiotics: selection criteria, safety and role in health and disease. J. Innov. Biol. 3 259–270. [Google Scholar]
- Trocino A., Xiccato G., Carraro L., Jimenez G. (2005). Effect of diet supplementation with Toyocerin® (Bacillus cereus var. toyoi) on performance and health of growing rabbits. World Rabbit Sci. 13 17–28. [Google Scholar]
- Urgesi R., Casale C., Pistelli R., Rapaccini G. L., De Vitis I. (2014). A randomized double-blind placebo-controlled clinical trial on efficacy and safety of association of simethicone and Bacillus coagulans (Colinox®) in patients with irritable bowel syndrome. Eur. Rev. Med. Pharmacol. Sci. 18 1344–1353. [PubMed] [Google Scholar]
- Von Mollendorff J. W., Vaz-Velho M., Todorov S. D. (2016). “Boza, a traditional cereal-based fermented beverage: a rich source of probiotics and bacteriocin-producing lactic acid bacteria,” in Functional Properties of Traditional Foods eds Kristbergsson K., Ötles S. (Boston, MA: Springer; ) 157–188. [Google Scholar]
- Wang J., Tang H., Zhang C., Zhao Y., Derrien M., Rocher E., et al. (2015). Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. ISME J. 9 1–15. 10.1038/ismej.2014.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weese J. S., Martin H. (2011). Assessment of commercial probiotic bacterial contents and label accuracy. Can. Vet. J. 52 43. [PMC free article] [PubMed] [Google Scholar]
- Xu D., Cote J. C. (2003). Phylogenetic relationships between Bacillus species and related genera inferred from comparison of 3′ end 16S rDNA and 5′ end 16S–23S ITS nucleotide sequences. Int. J. Syst. Evol. Microbiol. 53 695–704. 10.1099/ijs.0.02346-0 [DOI] [PubMed] [Google Scholar]
- Yang H. J., Kwon D. Y., Kim H. J., Kim M. J., Jung D. Y., Kang H. J., et al. (2014). Fermenting soybeans with Bacillus licheniformis potentiates their capacity to improve cognitive function and glucose homeostaisis in diabetic rats with experimental Alzheimer’s type dementia. Eur. J. Nutr. 54 77–88. 10.1007/s00394-014-0687-y [DOI] [PubMed] [Google Scholar]
- Yang O. O., Kelesidis T., Cordova R., Khanlou H. (2014). Immunomodulation of antiretroviral drug-suppressed chronic HIV-1 infection in an oral probiotic double-blind placebo-controlled trial. AIDS Res. Hum. Retroviruses 30 988–995. 10.1089/aid.2014.0181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. L., Li W. S., Xu D. N., Zheng W. W., Liu Y., Chen J., et al. (2016). Mucosa-reparing and microbiota-balancing therapeutic effect of Bacillus subtilis alleviates dextrate sulfate sodium-induced ulcerative colitis in mice. Exp. Ther. Med. 12 2554–2562. 10.3892/etm.2016.3686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Lv X., Fu J., He C., Hua H., Yan Z. (2016). In vitro inhibitory activity of probiotic products against oral Candida species. J. Appl. Microbiol. 121 254–262. 10.1111/jam.13138 [DOI] [PubMed] [Google Scholar]
- Zheng L. P., Zou T., Ma Y. J., Wang J. W., Zhang Y. Q. (2016). Antioxidant and DNA damage arotecting Activity of exopolysaccharides from the endophytic bacterium Bacillus Cereus SZ1. Molecules 21:174 10.3390/molecules21020174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu K., Hölzel C. S., Cui Y., Mayer R., Wang Y., Dietrich R., et al. (2016). Probiotic Bacillus cereus strains, a potential risk for public health in China. Front. Microbiol. 7:718 10.3389/fmicb.2016.00718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouari R., Abdallah-Kolsi R. B., Hamden K., Feki A. E., Chaabouni K., Makni-Ayadi F., et al. (2015). Assessment of the antidiabetic and antilipidemic properties of Bacillus subtilis SPB1 biosurfactant in alloxan-induced diabetic rats. Pept. Sci. 104 764–774. 10.1002/bip.22705 [DOI] [PubMed] [Google Scholar]


