Abstract
Vibrio cholerae is the cause of cholera, a devastating epidemic and pandemic disease. Despite its importance, the way of its global dissemination is unknown. V. cholerae is abundant in aquatic habitats and is known to be borne by copepods, chironomids and fishes. Our aim was to determine if fish-eating birds act as vectors in the spread of V. cholerae by consuming infected fish. We determined the existence of V. cholerae in the microbiome of 5/7 wild cormorants’ intestine. In three of these V. cholerae-positive wild cormorants, the presence of a gene for cholera toxin (ctxA) was detected. We subsequently tested eight captive, hand-reared cormorants, divided into two equal groups. Prior to the experiment, the feces of the cormorants were V. cholerae-negative. One group was fed exclusively on tilapias, which are naturally infected with V. cholerae, and the other was fed exclusively on goldfish or on koi that were V. cholerae-negative. We detected V. cholerae in the feces of the tilapia-fed, but not in the goldfish/koi-fed, cormorants. Hence, we demonstrate that fish-eating birds can be infected with V. cholerae from their fish prey. The large-scale movements of many fish-eating birds provide a potential mechanism for the global distribution of V. cholerae.
Introduction
Vibrio cholerae is the etiologic agent of cholera, a devastating diarrheal disease which causes epidemics and pandemics. The bacteria are endemic in the aquatic environments1. Copepods and chironomids are both considered natural reservoirs of V. cholerae 1–5. However, it is still not clear how this bacterium spreads all over the world6.
Green and Sanches7 and Frisch et al.8 found that chironomids and copepods can be transferred across waterbodies via waterbirds. Considering these findings we hypothesized that V. cholerae may be dispersed by migratory waterbirds, which consume chironomids or copepods (endozoochory) or carry them externally (epizoochory)6, 9. Support for this hypothesis was also found in the literature10, 11. Furthermore, many fish species feed on copepods and chironomids12, hence may act as a vector of cholera. Indeed, we recently demonstrated that fishes are also reservoirs of V. cholerae 13. This finding was supported by evidence from the literature that cholera cases have been associated with eating, consumption and cleaning of different fish species in different parts of the world14–18.
Because fish are commonly consumed by various waterbird species, they may also create a link between V. cholerae and waterbirds6, 9. Buck19 studied the presence of halophilic Vibrios and Candida albicans from different bird species in the USA and found these pathogens in the feces of one double-crested cormorant (Phalacrocorax carbo). Campylobacter, Escherichia coli and Salmonella were cultured from cloacal and pharyngeal swabs of double-crested cormorant chicks in Canada20.
Great cormorants are known as generalist foragers21 or specialist piscivores22, with a variety of regional23 and seasonal24 diets. They are opportunistic predators that consume a wide range of fish species of diverse size25, 26. Recently, cormorants have increased in number of individuals, thereby causing trouble for commercial and sports fisheries in lakes and rivers all over Eurasia27, 28 and North America (Phalacrocorax auratus)29.
A rise in the numbers of great cormorants arriving from Europe to over-winter in Israel from October to March has been demonstrated. The figures are between 17,000 and 29,000 individuals30. This large number of fish-eating birds causes problems for the fish industry in Israel, as has been described for other countries. As a result, the Israeli authorities allow each fish farm to shoot down up to six cormorants per fish farm per day30.
Here we studied the microbiome composition of the cormorant’s intestine and the possible role of fish infected with V. cholerae in transferring the bacteria to the birds. Our current results reveal that different areas of the cormorant intestine have a qualitatively unique bacterial composition, even though the microbiome of each individual differs from the microbiomes of all the others. Additionally, we have demonstrated that by consuming fish naturally infected with V. cholerae, great cormorants get infected with this bacterial species. This infection may last up to 72 h. Our study opens an exciting new direction for understanding the global spread of V. cholerae.
Results
Analyses of wild great cormorants’ intestine microbiome
Bacterial communities of intestine samples from the seven great cormorants were analyzed by sequencing the V4 variable region of the bacterial 16S ribosomal RNA (rRNA) gene on an Illumina MiSeq platform. Three intestine areas were examined for each bird (total of 20 samples). 1,245,131 quality sequences were obtained. These were classified into 59,299 operational taxonomic units (OTUs) using the cutoff of 97% sequence similarities. Subsampling according to the smallest sample (24,901 sequences) resulted in 498,020 sequences, which were classified into 33,315 OTUs. The dominant OTUs with minimum of 500 sequences in all the samples in total and their taxonomic classification are shown in Fig. 1.
Figure 1.
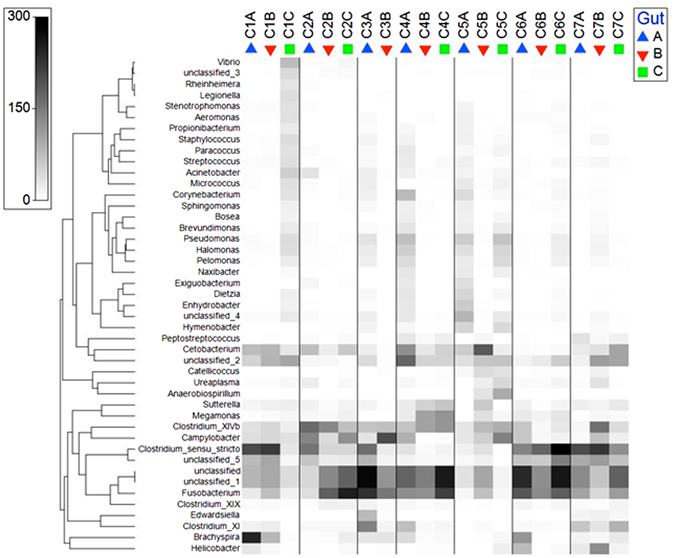
A shade plot presentation of data matrix of all samples showing the dominant OTUs (with minimum of 500 sequences per each OTU in all the samples together) and their taxonomic classification. The different samples are presented in columns. The numbers in the sample name indicate the cormorant individuals; the letters (at the end of the name) A, B, C indicate the three intestine parts: A - esophagus, B - middle, C - cloaca. The grey scale squares are in linear proportion to a square root transformation of the relative abundance of each presented genus or unclassified OTU in a sample. White squares signify the absence of the genera or OTU in a sample. Clustering of the genera (y-axis) was due to similar distribution across the samples. The figure was drawn with the primer 7 software (http://www.primer-e.com).
Rarefaction curves, which describe the OTU numbers as a function of the sampling effort (total sequences), were performed at a phylogenetic distance of 3% sequence similarity for all intestine samples (Fig. S1). Although all samples had at least 40,000 sequences, the rarefaction curve of none of the samples reached an asymptote level, demonstrating that our sampling effort was not sufficient to obtain an accurate estimate of OTU richness.
Overall, 13 phyla were detected. Fusobacteria, Firmicutes and Proteobacteria were the dominant phyla in all samples (Fig. 2a and b). The phylum Fusobacteria was the most abundant in samples that belonged to the esophagus and the cloacal regions along the intestine, the vast majority of reads belonged to the genus Fusobacterium (Fig. 1). Firmicutes was the most abundant phylum in the middle part of the intestine and the majority of reads belonged to the genus Clostridium (Fig. 1). The prevalence of Proteobacteria was 17–19% in all the intestine parts, with Campylobacter as the relatively dominant genus (4.6%) (Fig. 1). Spirochaetes phylum was found in all intestine sections, at relatively lower abundances (esophagus, 9.1%; middle, 1.24%; cloaca, 0.1%). The dominant genus in this phylum was Brachyspira. Actinobacteria was present in the esophagus and the cloaca regions (1.8% and 1%, respectively) with more than one dominant genus (Corynebacterium, Dietzia and Micrococcus) (Fig. 1). Significant differences were observed between the relative abundances of Spirochaetes phylum in the three different parts along the cormorant’s digestive tract (Repeated measures ANOVA: F2,10 = 4.84, p = 0.034). No significant differences were detected among Fusobacteria, Firmicutes, Actinobacteria and Proteobacteria in the different intestine parts in the cormorants’ digestive tracts (p > 0.05) (Figs 1 and 2a).
Figure 2.
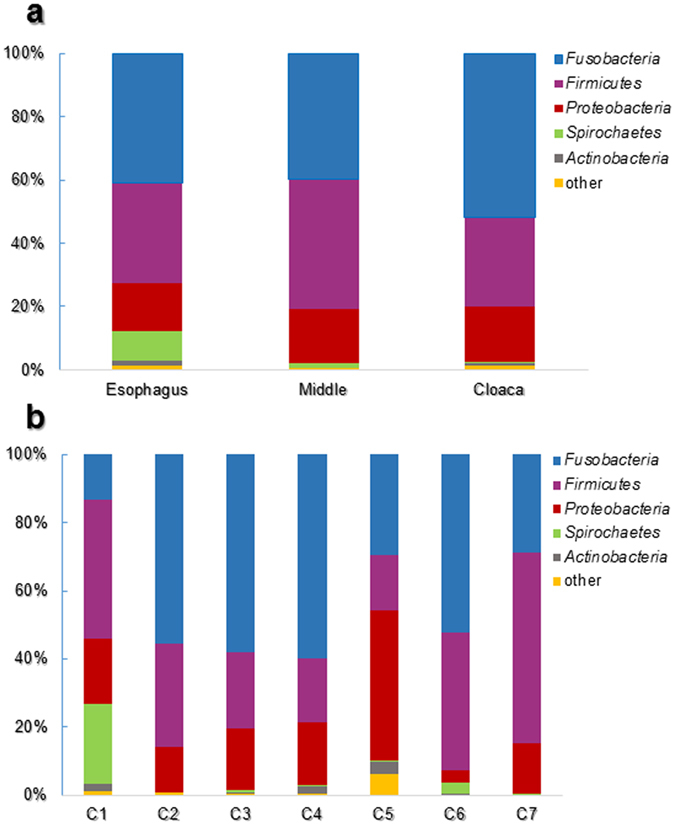
Average OTU abundances at the phyla level. (a) Sum of all the samples from a specific section (esophagus, middle, cloaca) of the cormorant intestine. Fusobacteria, Firmicutes Actinobacteria and Proteobacteria showed no significant differences in the different intestine parts (p > 0.05). However, Spirochaetes phylum evinced significant differences in relative abundance in the different intestine parts (repeated measures ANOVA: F2,10 = 4.84, p = 0.034). (b) Sum of the phyla abundances from the three sampled areas (esophagus, middle, cloaca) for each individual cormorant. Fusobacteria was the most abundant phylum in birds C2, C3, C4 and C6, Firmicutes, the most abundant phylum in birds C1 and C7 and Proteobacteria, the most abundant phylum in bird C5.
Differences among all individuals at the phylum level (Fig. 2b) revealed that Fusobacteria was the most dominant phylum in four out of the seven examined birds. In birds C1 and C7 the dominant phylum was Firmicutes (40.8% and 55.5%, respectively); however, in cormorant C5 the dominant phylum was Proteobacteria (44.1%). 23.6% and 3.2% of the OTUs belonged to phylum Spirochaetes in cormorants C1 and C6, respectively; in all the other birds this phylum was present in less than 1% of the OTUs (Fig. 2b).
According to the Sobs mean (observed number of species in a sample) and Chao1 (species diversity) estimators, OTU richness was the same in the three different intestine sections; the expected bacterial OTU richness was also similar (Table 1). In sum, the different sampled intestine parts were alike in their observed and expected bacterial OTUs. By contrast, when Sobs mean and Chao1 estimators were calculated for the three pooled sections from each bird, OTU richness of cormorant C1 was less than that of the others, and the richness of cormorant C7 was much higher than that of the others (178 ± 54 and 377 ± 15, respectively) (Table S1).
Table 1.
Microbial richness of the three intestine parts in all cormorants (subsampled OTUs at the genera levels).
| Phylogenetic level | Intestine part | A | B | C |
|---|---|---|---|---|
| OTUs | Sobs Mean ± SD | 203 ± 85.0 | 229 ± 111.0 | 267 ± 131.0 |
| Chao1 ± SD | 1138 ± 777.0 | 1221 ± 709.0 | 1387 ± 917.0 | |
| All genera | Sobs Mean ± SD | 202 ± 7.0 | 191 ± 7.0 | 229 ± 8.0 |
| Chao1 ± SD | 217 ± 8.7 | 213 ± 15.6 | 249 ± 10.7 |
The indexes Sobs Mean and Chao1 were calculated on the EstimateS (Version 9.1.0) software. Sobs Mean was calculated as the average number of all taxonomic units in all samples and Chao1 was calculated as the expected taxonomic richness for the complete collection of each intestine part (more details can be found in the Methods section). A - esophagus, B - middle, C - cloaca.
Venn diagram (Fig. 3) presents the shared and unique OTUs in each intestine section where all seven cormorants were pooled. The esophagus section contained the largest portion of unique OTUs, namely 40.7% of them. The middle and the cloaca sections had similar portions (29.6% and 28.1%, respectively). Only 129 OTUs (0.38%) overlapped all three intestine parts, indicating that each intestine section hosted a unique bacterial community. Venn diagram of all the three sections of each bird alone showed a similar profile (data not shown). The Venn diagram does not take into account differences in OTUs’ relative abundances. So when a quantitative approach was taken, using a three-dimensional non-metric multidimensional scaling (nMDS) analysis, the samples of each intestine part in a specific bird clustered together, suggesting that each bird hosts a unique bacterial community (R2 = 0.73, stress value = 0.17) (Fig. 4). Analysis of similarity (ANOSIM) verified the significant differences in the quantitative bacterial communities’ composition in the different cormorant individuals (n = 7, R = 0.505, p < 0.01). Thus, each bird hosts a significantly different microbiome composition and these differences distort and blur the differences in microbiome composition in different “areas” of the gut.
Figure 3.
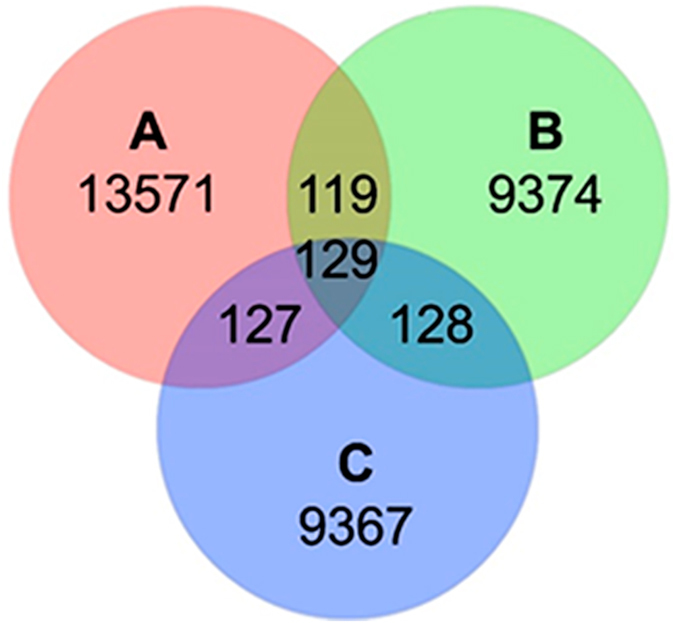
Venn Diagram at a distance of 0.03, illustrating the number of unique and shared subsampled OTUs in the libraries of bacterial communities of the three different cormorant intestine sections (A, B, C). A - esophagus, B - middle, C - cloaca.
Figure 4.
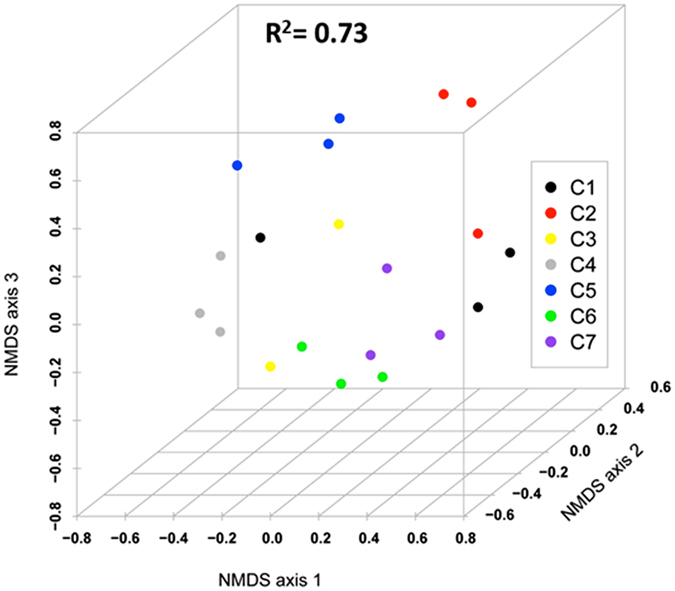
nMDS (nonmetric multidimensional scaling) plot of the entire bacterial community in each intestine sample. OTUs were determined based on 97% read similarities. Samples from the different intestine sections (esophagus, middle, cloaca) of each bird, clustered together. C1–C7 indicate the different cormorant individuals and are marked in different colors (three samples from an individual cormorant) (R2 = 0.73, stress value = 0.17). The different cormorant individuals were found to differ significantly in the intestine microbiome (ANOSIM; n = 7, R = 0.505, p < 0.01).
Culturable and uncultured Vibrios and other potential pathogens
V. cholerae was successfully cultured from only one of the seven wild cormorants’ intestine samples. However, the genus Vibrio was detected in five of the seven birds when an Illumina MiSeq sequencing platform was used. In total, 55 OTUs were identified as Vibrios. To ascertain the OTUs’ phylogenetic position, a phylogenetic tree was generated with representatives of the dominant Vibrio OTUs’ sequences. The tree revealed that all but one of the OTUs clustered together with V. cholerae, V. mimicus and V. metoecus cluster; the exception clustered with V. mediterranei (Fig. 5). Interestingly, 99.8% of the Vibrio OTUs were identified in the cloacal region.
Figure 5.
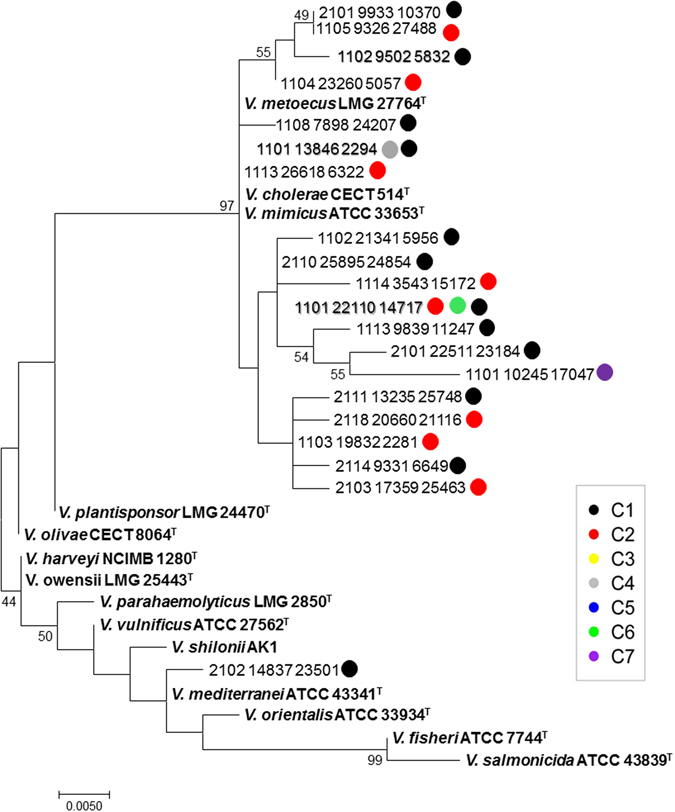
A maximum likelihood tree (generated in MEGA 5.0 software), based on the 16S rRNA gene sequences, showing the nearest neighbors of the most abundant Vibrio OTUs’ sequences. The different cormorant individuals (C1–C7) are marked in different colors and indicate the sequence origin from the different cormorant individuals. Bootstrap values (>50%) resulting from 1,000 replicates are indicated as percentages at branching nodes. Bar, 0.005 substitutions per nucleotide position. No Vibrio OTUs were detected in cormorants 3 and 5. The three most dominant OTUs (1101_22110_14717; 1102_9502_5832 and 1101_13846_2294) were marked in bold. These OTUs comprised of 1,845, 944 and 346 sequences, respectively.
To verify the presence of V. cholerae in the wild cormorant intestine samples, we looked for the presence of two V. cholerae-specific genes (ompW and ctxA) in the intestine DNA extractions. ompW, a specific outer membrane protein gene of V. cholerae was detected in cormorants C1, C2, C4, C6 and C7. These are the same birds in which V. cholerae OTUs were detected (Fig. 5). An interesting result was the detection of ctxA gene (encoding the A subunit of cholera toxin) in wild cormorants C1, C4, and C7 demonstrating that these birds harbored pathogenic strain of V. cholerae.
Among the observed OTUs, genera with potential pathogenicity for humans and/or birds, such as Corynebacterium, Mycobacterium, Campylobacter, Helicobacter, Yersinia, Haemophilus, Clostridium and more, were also detected (Table S2). Overall, genera related to human or bird pathogenic species, were found within the microbial community at a prevalence of 22.1% and 5.8%, respectively in all birds.
Cormorants’ feeding experiments
To demonstrate that fish infected with V. cholerae transfer the bacteria to cormorants, we used cormorants (n = 8) hand-reared in captivity. Prior to the feeding experiments, the cormorants were fed on goldfish (Carassius auratus) or koi (Cyprinos carpio). Culturable V. cholerae was not detected in the intestines of goldfish (n = 10) or koi (n = 10). The same results were obtained (prior to the feeding experiment) for the feces of cormorants that were fed on these fish species. By contrast, the intestine of fresh collected tilapia (Oreochromis niloticus X Oreochromis aureus; n = 10) was found to be V. cholerae-positive.
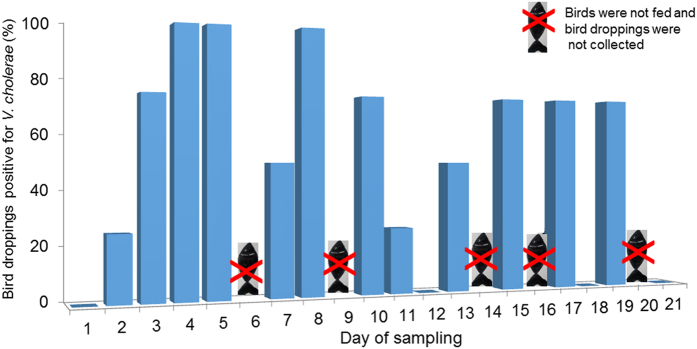
During the experiment, the cormorants were divided into two groups (A, B) of four birds each. In the first experiment, group A was fed exclusively on tilapia and group B was fed exclusively on goldfish or koi. This procedure lasted for three weeks, after which the diet of group A was switched from tilapia to goldfish or koi for at least two weeks more (interval between experiments). Accordingly, in the interval between the experiments all cormorants from both groups A and B were fed on goldfish or koi. In the second experiment, diets were switched between the groups so that group A was fed on goldfish or koi while group B was fed on tilapia. Diet switching between the groups including the intervals between experiments was repeated seven times and included switching the diets between groups A and B. Four experimental repetitions are demonstrated in Figs 6 and S2. The figures show the success in isolating V. cholerae from the birds’ feces that were fed on tilapia. In all cases the feces of the cormorants fed on goldfish and koi were V. cholerae-negative while most of the feces of the cormorants fed on tilapia were V. cholerae-positive. In some experimental repetitions V. cholerae was isolated from birds’ feces within less than two hours of their feeding on tilapia (Fig. S2a–c).
Figure 6.
Hand-reared cormorants feeding experiment. Detailed results for treatment A (birds fed on tilapia fish). On day 1, all the bird droppings were negative for V. cholerae. A fish marked X represents days when the birds were not fed and droppings were not collected (n = 4).
Additionally, during the interval, while the birds’ diet was switched from tilapia to goldfish or koi, their droppings were V. cholerae positive even 72 h after their diet was switched from tilapia to goldfish or koi (experiment repetition no. 6). In experiment repetitions nos. 1 and 7, V. cholerae was detected 48 h after the diet switch, and in experiment repetitions nos. 2 and 4, V. cholerae was detected 24 h after the diet switch (Table 2). In marked contrast, V. cholerae was not isolated from the feces of the birds in the group fed exclusively on goldfish or koi. Hence, these results clearly demonstrate that V. cholerae can survive in cormorants’ digestive tract at least 72 h after ingestion of V. cholerae-infected fish (Table 2).
Table 2.
The persistence of V. cholerae in the cormorants’ feces, days after their diets were switched from tilapia to golden fish or koi.
| Feeding experiment | Time after switching the diet from tilapia to goldfish or koi | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| 1 | + | + | − |
| 2 | + | − | − |
| 3 | − | − | − |
| 4 | + | − | − |
| 5 | − | − | − |
| 6 | + | + | + |
| 7 | + | + | − |
The results demonstrate that V. cholerae can survive in the cormorants’ digestive tract up to 72 h after ingestion of tilapia (fish colonized by V. cholerae).
Virulence genes in V. cholerae isolates
Overall, 48 and 141 V. cholerae strains were isolated from fish and cormorants, respectively. When the presence of virulence genes was screened in the V. cholerae isolates, all isolates from the birds’ feces and the fish intestine were found positive for ompW and toxR genes and negative for ctxA, tcpA and tcpI. In the birds’ feces isolates, 14 different virulence genotypes were observed (Table 3). All isolates were positive for hapA gene. Most strains were positive for hylA gene (98%), 39% were ompU-positive, 26% possessed all examined TTSS genes and only 3.5% demonstrated the presence of zot gene (Table 3). Among the fish isolates, 13 different virulence genotypes were observed. Most isolates were hylA- and hapA-positive (96% and 83%, respectively), about 40% were ompU-positive and possessed all examined TTSS genes, and 23% were zot-positive (Table 3).
Table 3.
Virulence traits of V. cholerae isolates from the cormorants’ droppings (n = 141) and fish intestines (n = 48).
| No. of strains | Genotype prevalence% | Presence or absence of virulent genes* | ||||
|---|---|---|---|---|---|---|
| TTSS* | ompU | hlyA | zot | hapA | ||
| Isolates origin: cormorants’ feces | ||||||
| 1 | 0.7 | + | + | + | + | + |
| 9 | 6.4 | + | + | + | − | + |
| 1 | 0.7 | + | − | + | + | + |
| 24 | 17.0 | + | − | + | − | + |
| 1 | 0.7 | + | − | − | − | + |
| 1 | 0.7 | + | + | − | − | + |
| 7 | 5.0 | +/− | + | + | − | + |
| 1 | 0.7 | +/− | − | + | + | + |
| 23 | 16.3 | +/− | − | + | − | + |
| 1 | 0.7 | +/− | − | − | − | + |
| 36 | 25.5 | − | + | + | − | + |
| 1 | 0.7 | − | − | + | + | + |
| 34 | 24.1 | − | − | + | − | + |
| 1 | 0.7 | − | + | + | + | + |
| Isolates origin: fish intestine | ||||||
| 4 | 8.3 | + | − | + | − | + |
| 3 | 6.3 | +/− | + | + | − | + |
| 1 | 2.1 | +/− | − | + | + | + |
| 10 | 20.8 | +/− | − | + | − | + |
| 2 | 4.2 | +/− | − | + | − | − |
| 1 | 2.1 | +/− | − | − | + | + |
| 5 | 10.4 | − | + | + | + | + |
| 5 | 10.4 | − | + | + | − | + |
| 4 | 8.3 | − | − | + | + | + |
| 6 | 12.5 | − | − | + | − | + |
| 3 | 6.3 | − | + | + | − | − |
| 3 | 6.3 | − | − | + | − | − |
| 1 | 2.1 | − | + | − | − | + |
All the examined isolates were positive for toxR gene and negative for ctxA, tcpA and tcpI genes.
*PCR-based detection of the TTSS cluster (genes; vcsC2, vcsN2, vspD, and vcsV2). (+) positive, (−) negative, (+/−) positive for some but not all the genes in the TTSS cluster.
Discussion
V. cholerae is the cause of the devastating epidemic and pandemic cholera disease. Massive cholera outbreaks are caused by the particular serogroups O1 and O139 which produce the cholera enterotoxin. An example is the case of the Haiti cholera outbreak following the 2010 earthquake31, 32. It is estimated that from 2008 to 2012 ca. 2.9 million people were infected yearly, with a death toll of ca. 95,000 worldwide33. V. cholerae is part of the natural flora and ecology of surface water34, where the bacteria commonly associate with zooplankton, particularly copepods2 and chironomids3–5, which are their potential vectors. It is estimated that both the pathogenic and non-pathogenic serogroups share the same niches34. Despite intensive efforts, the mechanisms that enable the widespread and rapid dispersion of V. cholerae remain an enigma. Some evidence supports our hypothesis6 that fish13 and waterbirds may act as intermediate reservoirs and vectors of V. cholerae strains, including pathogenic V. cholerae O1/O139 serogroups35. Particularly, Hounmanou et al.36 isolated V. cholerae O1 ctxA positive from tilapia. Moreover, V. cholerae was isolated from more than 20 species of waterbirds with pandemic O1 serogroups identified in great blue herons and ring-billed gulls10, 11.
In agreement with this hypothesis, we have demonstrated here that a most common fish-eating bird, the great cormorant, shed V. cholerae within a few hours of consuming fish which are colonized by V. cholerae (Fig. 6). V. cholerae colonization in the birds’ intestine may persist for at least 72 h (Table 2). Support for these birds’ being regarded as vectors of V. cholerae is the fact that the bacterium was isolated from a wild cormorant, and the genus Vibrio was identified in five out of seven wild birds by Illumina sequencing. ompW gene was detected in five wild cormorants, which supports the assumption that these Vibrios OTUs are in fact V. cholerae (Fig. 5). A very interesting result was the direct detection of ctxA in three wild cormorants, demonstrating that the birds harbored pathogenic strains of V. cholerae.
On migrating, great cormorants can cover distances of 500 to 1,000 km/day37, and thus potentially transfer V. cholerae across and between continents. Assuming that different bird species may be infected with different pandemic V. cholerae strains, and that their transmittance occurs under natural conditions, we conclude that birds act as vectors aiding the spread of the disease. Our findings further explain two important aspects: the long-distance transmittance of cholera and the speed at which it spreads.
As far as we know, our study is the first to investigate cormorant intestine microbiota. The rarefaction curves (Fig. S1) did not reach a plateau, suggesting that the actual bacterial diversity is much higher. The Venn diagram (Fig. 3) revealed that only 129 OTUs were found in all three intestinal sections. These results can be explained by the fact that each intestine section has its own unique role in food digestion, and its diverse environmental conditions such as oxygen concentrations, pH, etc., which may lead to the adaptation of a specific bacterial community. The microbiome analyses demonstrated that each individual cormorant possessed its own distinct microbiome (Fig. 4). The uniqueness of each individual can be explained by several factors such as the bird’s age, health status, different food consumption/dietary specialization, different environments, etc. Birds ringing (a numbered tag attachment to the leg or wing) enable birds’ individual identification and provide information regarding their life history including age, migration routes, feeding behavior and more. However, as rings were not detected on the wild cormorants that we sampled, these parameters were not available in the current study. Several studies have demonstrated wide variations in overall gut microbiome in individuals of different populations and from different environments38–40.
Diets can be a major non-genetic factor that governs gut microbiome. The effect of diets on the gut microbiota composition can be explained by two hypotheses; (i) each food item carries a unique bacterial assemblage; (ii) each food item supplies unique chemical compounds that support different bacterial assemblages. Experimental manipulation of avian diets has been shown to alter the gut microbiome within individuals over time, lending support to these hypotheses41–45.
The dominant phyla of the wild cormorants were Fusobacteria, Firmicutes and Proteobacteria (Fig. 2a and b). Actinobacteria and Bacteroidetes were present but much less abundant. Similar results were obtained for the intestinal microbiota of migrating shorebirds and artificial breeding geese46, 47. Wild bar-headed goose (Anser indicus)47 presented a different microbiome, with Firmicutes, Proteobacteria, Actinobacteria and Bacteroidetes as the dominant phyla. Fusobacteria was not detected at all in the gut microbiome of terrestrial avian, and Bacteroidetes and Actinobateria were much more abundant than they were in the cormorants in the current study or in the other waterbirds discussed above48–50.
Species of the Fusobacteria phylum are obligate anaerobic Gram-negative rods51. Three main Fusobacteria OTUs were found in the cormorants: Fusobacterium (39.3%), Cetobacterium (9.6%) and an unclassified OTU (50.9%) (Fig. 1). Fusobacterium and Cetobacterium species were found to inhabit intestinal tracts of humans and animals52, 53. Interestingly, these were identified as the main genera in class Fusobacteria in fecal samples of three shorebird species46.
Some bacterial genera identified in the cormorants’ intestine are potentially human or bird pathogens (Table S2). Cormorants may disseminate these pathogens globally, as was discussed here for V. cholerae and in the literature6, 9, 46, 54. For example: Corynebacterium, Campylobacter, Helicobacter and Clostridium were detected in all the birds’ intestine samples at average frequencies of 0.35%, 4.73%, 0.91% and 22.16%, respectively. Ryu et al.46 also reported the presence of Campylobacter, Helicobacter and Clostridium in fecal samples of red knot, ruddy turnstone and semipalmated sandpiper, collected during a migratory stopover in Delaware Bay.
In the cormorants’ fish-feeding experiments we isolated 48 and 141 V. cholerae strains from fish and cormorants, respectively. These isolates were all non O1/non O139, positive for toxR genes and negative for ctxA, tcpA, and tcpI. The majority ( >90%) were positive for the hapA and hylA genes, and 30% possessed ompU and TTSS genes. zot gene was present in about 20% of the isolates (Table 3). All these genes are associated with the pathogenicity potential of V. cholerae 55–57. For example, the presence of hlyA gene was reported to cause symptoms similar to cholera toxin in hospitalized diarrheal patients56, 58.
In conclusion
Cholera is a devastating disease that spreads globally, but the ways of its dissemination are as yet unknown. Here we showed that cormorants can become infected with V. cholerae by consuming fish infected with this bacterium. Moreover, V. cholerae was isolated from a wild cormorant and was molecularly detected in five. The gene for A subunit of cholera toxin (ctxA) was also detected in three of the wild birds’ intestine samples. The current study is the first evidence that waterbirds can be infected with V. cholerae from their fish prey. The large-scale movements of cormorants as well as many other fish-eating waterbirds provide a potential mechanism for the global distribution of V. cholerae. Further research should be performed in areas endemic to pathogenic strains of V. cholerae and with various bird and fish species.
Methods
Ethics Statement
All methods were performed in accordance with the relevant guidelines and regulations. Great cormorants are subject to controlled culling because of their alleged damage to fisheries30. Israel and all member states of the EU are party to the African-Eurasian Waterbird Agreement (AEWA) under the Convention on Migratory Species (CMS) of the United Nations Environmental Program (UNEP), (www.unep-aewa.org). Hunting permission for controlled waterbirds accords with regulations of wild animals protection 1955 and 1976, [regulation #5 A(2–4), 1976; in Hebrew]. Seven wild cormorants were collected after being shot by fishermen next to their fish ponds. In addition, Cormorants feces were collected from hand-reared birds (n = 8) kept in captivity in a zoological garden at Oranim, Tivon. All procedures were performed under the consent of the cormorants’ owners and under permits of the Israel Nature Parks Authority, University of Haifa Ethics Committee and the Israel Committee for Ethics in Animal Experimentation (permit #033-b4822-1-02/02/2012). All the fish in the current study were obtained at different locations from fishermen selling fresh fish for consumption.
Sampling microbiota in wild great cormorant’s intestine
We examined the intestine of seven great cormorant birds that in Israel are subject to controlled culling because of their alleged damage to fisheries30. Six birds were from Ma’agan Michael (32°33'31.6''N 34°54'37.6''E), and one was from Beit She’an valley (32°29 05.4''N 35°31'45.9''E). They were collected immediately after being shot by fishermen beside their commercial fish pond and brought directly into the lab. Three sections of the intestine were sampled for the presence of V. cholerae and for the microbiome analyses: (A) close to the esophagus (hereinafter esophagus), (B) the middle of the intestine (hereinafter middle), and (C) the cloaca region (hereinafter cloaca). For the microbiome analyses 0.5 gr of the intestine content from each intestine part was transferred to a 2 ml sterile tube that contained 0.5 ml of absolute ethanol. Tubes were kept at −20 °C until DNA extraction. For V. cholerae detection, intestine samples were treated as described below.
DNA extraction from intestine samples
DNA was extracted from the intestinal samples with a DNA isolation kit (DNeasy Blood and Tissue, Qiagene, Germany) according to the manufacturer’s instructions with minor modifications. To obtain DNA without the ethanol residues, the tubes were centrifuged for 30 min at maximum speed, and the ethanol was removed from the tube. Next, 180 µl ELB (Enzymatic lysis buffer- 20 mM Tris HCL pH 8, 2 mM sodium EDTA and 1.2% Triton-X-100) were added to the sample with 20 mg/ml lysozyme (from chicken egg white, SERVE, Germany) and the samples were incubated with shaking for 60 minutes at 37 °C. Extraction then continued according to the manufacturer’s instructions, with storage at −20 °C.
Generation of the 16S rRNA gene library
A set of primers was used to amplify the V4 variable region of the 16S rRNA gene: CS1_515F (ACACTGACGACATGGTTCTACAGTGCCAGCMGCCGCGGTAA) CS2_806R (TACGGTAGCAGAGACTTGGTCTGGACTACHVGGGTWTCTAAT) (primers from: Sigma Aldrich, Israel) as described previously by Caporaso et al.59.
PCR amplification was performed using the EmeraldAmp MAX HS PCR Master Mix (Takara bio Inc, Otsu, Shiga, Japan) in a total reaction of 25 µl. The final concentration of each primer was 0.5 ng/µl. 10–100 ng genomic DNA were added to each PCR reaction. PCR conditions were 95 °C for 5 min, followed by 28 cycles of 30 sec at 95 °C; 45 sec at 55 °C; and 30 sec at 68 °C. The final step was 7 min at 68 °C. Reactions were verified to contain visible amplification using 1.5% agarose gel electrophoresis.
Illumina MiSeq sequencing
Illumina MiSeq sequencing was performed at the DNA Services (DNAS) facility - University of Illinois at Chicago (UIC). The sequencing protocol is described in detail in Aizenberg-Gershtein et al.60 The procedure included a second PCR amplification with a separate primer pair for sample, obtained from the Access Array Barcode Library for Illumina, where each sample received a separate primer set with a unique 10-base barcode (Fluidigm, South San Francisco, CA; Item #100-4876). Pooled, diluted libraries were sequenced with Illumina MiSeq. 600-cycle sequencing kit version 3, and analyzed with Casava 1.8 (pipeline 1.8). Reads were 200 nucleotides in length (paired end, 2 × 200). PhiX DNA was used as a spike-in control. Barcode sequences from Fluidigm were provided to the MiSeq server, and sequences were automatically binned according to 10-base multiplex identifier (MID) sequences. Raw reads were recovered as FASTQ files.
Sequence analysis
Bioinformatics analysis was performed on MOTHUR v.1.33.338. The operational taxonomic unit (OTU)-based approach of the MiSeq Standard Operating Procedure (SOP) was followed61. Any sequences with uncertainties or homopolymers longer than 8 bases were removed from the data set. Sequences were aligned using the SILVA-compatible alignment database available within MOTHUR. Sequences were trimmed to a uniform length of 295 base pairs, and chimeric sequences were removed with Uchime62. Sequences were classified using the MOTHUR-formatted version of the RDP training set (v.9), and clustered into OTUs based on 97% sequence identity; then the entire dataset was randomly subsampled to the minimum number of sequences per sample (lowest number of sequences obtained in a sample): 24,901 sequences per sample.
All the sequence data analyses reported in this paper can be downloaded from the National Center for Biotechnology Information NCBI (https://www.ncbi.nlm.nih.gov/sra) BioProject number PRJNA336254 (items 25–31).
Microbial richness and similarity estimates of wild cormorants
Alpha Diversity
To evaluate the microbial richness of the cormorant’s intestine community, we calculated the estimated “true” species diversity using the chao1 estimator. This index was calculated for the different intestine sections and for each individual bird (when all the sections were pooled), using the subsampled OTUs and genera tables. Chao1 is a nonparametric, abundance-based richness estimator, and was calculated using the observed number of OTUs (sobs) and the fraction between the number of the singletons and doubletons63. This index was calculated using the software EstimateS (Version 9.1.0).
β-diversity
To predict the β-diversity in the different birds and intestine sections (esophagus, middle, cloaca), we processed the data by the MOTHUR program (version 1.33.3) as described previously by Kozich et al.61. The following statistic parameters were calculated: (1) A Venn diagram was created to show the overlapping and unique OTUs found in each intestine part. (2) Ordination plots using the thetaYC distance matrix among samples were plotted to create a three-dimensional non-metric multidimensional scaling (nMDS). Additionally, Analysis of Similarities (ANOSIM) was conducted to test each of the studied wild cormorants (n = 7) for differences in their bacterial communities’ composition. ANOSIM (“R” software with “vegan” package) was applied using OTUs’ relative abundances with Bray-Curtis distance index (permutations = 1000).
Direct detection of V. cholerae from DNA intestine samples
To verify the presence of V. cholerae in the wild cormorant’s intestine we performed a multiplex PCR in accordance with Nandi et al.64.] This PCR identifies the presence of an outer membrane protein gene ompW, which is specific for V. cholerae, and the ctxA gene (A subunit of cholera toxin).
Isolation and Identification of V. cholerae
The presence of culturable V. cholerae was examined in the intestine content samples from the seven wild cormorants (described above), the cormorants’ feces samples (feeding experiments) and from fish intestine samples (fish from the feeding experiments). Thiosulfate-citrate-bile salts-sucrose (TCBS) agar plates (Difco, USA) were used for isolation and cultivation of V. cholerae. Intestine or feces samples were spread directly on the TCBS plates without any enrichment for V. cholerae. TCBS plates were incubated at 37 °C for 24 h and yellow colonies suspected as being V. cholerae were sub-cultured five times on LB agar plates for colonies’ isolation. Isolates’ identity was verified by multiplex PCR assay according to Nandi et al.64. After V. cholerae identification, the isolates were further examined to determine whether they were members of the O1 and O139 serogroups, by slide agglutination with use of two specific antisera: (1) a poly antiserum specific for O1 surface antigen (Difco), and (2) an antiserum specific for O139 surface antigen (Ministry of Health, Israel). Identified isolates were kept in LB with 30% glycerol (Hilabs, Mumbai) at −80 °C.
Cormorants’ feeding experiment
To determine if V. cholerae content in the cormorants’ feces changed with feeding regime, we tested hand-reared birds (n = 8) kept in captivity in a zoological garden at Oranim, Tivon. Prior to the feeding experiments, the cormorants were fed on goldfish (Carassius auratus) or koi (Cyprinos carpio). The fish were kept frozen at −20 °C for at least 30 days before they were used for the feeding experiment. They were thawed before being given to the birds. We screened the intestines of goldfish (n = 10) and koi (n = 10) for culturable V. cholerae according to the protocol described above, and all samples were found negative. Later we screened the feces of all the cormorants and all samples were found V. cholerae-negative. By contrast, the intestines of fresh collected tilapia (Oreochromis niloticus X Oreochromis aureus; n = 10), or tilapia frozen for up to 10 days (at −20 °C), were found positive for V. cholerae.
For the experimental procedure, the cormorants were divided into two separate groups (A and B) and fed on tilapia or goldfish and koi, respectively. The feeding of each group on a given diet lasted two to three weeks, with the cormorants fed four times/week. The presence of V. cholerae in the intestine of the fish (n = 6) during the experimental period was determined weekly. After about three weeks of experiment, the diet of all the cormorants in both groups was switched to goldfish or koi, which were negative for V. cholerae. This interval between experiments lasted up to three weeks. During the interval, the cages and the water were cleaned and cormorant’s feces were checked for the presence of V. cholerae. The next experiment started with diet switching between the groups (A and B: see above) and only after verification that the water and the feces samples were all negative for V. cholerae. The experiments, including the intervals between them, were repeated seven times.
Feces were collected immediately after feeding, as well as occasionally on days when the birds were not fed and during the interval between the experiments. Feces were collected by means of bacteriological sterile needles. Samples were spread directly onto TCBS agar plates which were incubated for 24 h at 37 °C. In parallel, 100 ml water from the pools of both groups was filtered through a 0.2 µm filter (Sartorius, Germany) and filters were placed on TCBS medium for V. cholerae detection. All isolates were treated as described above.
Detection of V. cholerae virulence genes
In addition to ctxA, the presence of other virulence genes was determined in all V. cholerae isolates. The genes were: zonula occludens toxin (zot), El Tor-like hemolysin (hlyA), haemagglutinin/protease (hapA), outer membrane protein (ompU), TCP expression (tcpI and tcpA) and the central regulatory protein (toxR). PCR procedures and primers are described in Halpern et al.5 and Senderovich et al.13. To test the TTSS cluster in this study we used the genes vcsC2, vcsN2, vspD and vcsV2, as described in Chatterjee et al.56 and in Dziejman et al.55.
Electronic supplementary material
Acknowledgements
We would like to thank the two anonymous reviewers for their helpful comments, Avi Bar-Massada for his assistance in generating Figure 4 and Nir Keshales for his technical help in the cormorants feeding experiments. This study was supported by grants from the Israel Science Foundation (ISF, grants no. 1094/12, and 296/16).
Author Contributions
Conceived and designed the experiments: S.L.S., T.L., G.K., I.I., M.H.; Performed the experiments: S.L.S., T.L.; Analyzed the data: S.L.S., G.K., Y.S., I.I., M.H.; Contributed reagents/materials/analysis tools; G.K., I.I., M.H.; Wrote the paper: S.L.S., M.H.; Reviewed and commented: G.K., I.I., M.H.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-08434-8
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Colwell RR, Huq A. Marine ecosystems and cholera. Hydrobiologia. 2001;460:141–145. doi: 10.1023/A:1013111016642. [DOI] [Google Scholar]
- 2.Huq A, et al. Ecological relationships between Vibrio cholerae and planktonic crustacean copepods. Appl. Environ. Microbiol. 1983;45:275–283. doi: 10.1128/aem.45.1.275-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broza M, Halpern M. Chironomids egg masses and Vibrio cholerae. Nature. 2001;412:40. doi: 10.1038/35083691. [DOI] [PubMed] [Google Scholar]
- 4.Halpern M, Broza YB, Mittler S, Arakawa E, Broza M. Chironomid egg masses as a natural reservoir of Vibrio cholerae non-O1 and non-O139 in freshwater habitats. Microb. Ecol. 2004;47:341–349. doi: 10.1007/s00248-003-2007-6. [DOI] [PubMed] [Google Scholar]
- 5.Halpern M, Raats D, Lavion R, Mittler S. Dependent population dynamics between chironomids (nonbiting midges) and Vibrio cholerae. FEMS Microbio. Ecol. 2006;55:98–104. doi: 10.1111/j.1574-6941.2005.00020.x. [DOI] [PubMed] [Google Scholar]
- 6.Halpern M, Senderovich Y, Izhaki I. Waterfowl — the missing link in epidemic and pandemic cholera dissemination? PLoS Pathog. 2008;4:e1000173. doi: 10.1371/journal.ppat.1000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green AJ, Sanches MI. Passive internal dispersal of insect larvae by migratory birds. Biol. Lett. 2006;22:55–57. doi: 10.1098/rsbl.2005.0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frisch F, Green AJ, Figuerola J. High dispersal capacity of a broad spectrum of aquatic invertebrates via waterbirds. Aquat. Sci. 2007;69:568–574. doi: 10.1007/s00027-007-0915-0. [DOI] [Google Scholar]
- 9.Halpern, M. & Izhaki, I. The environmental reservoirs and vector of Vibrio cholerae. In: 389 Holmgren, A. & Borg, G. (Eds). Handbook of disease outbreaks: Prevention, detection and control. 390 Nova, Hauppauge. 309–320 (2010).
- 10.Lee JV, Bashford DJ, Donovan TJ, Furniss AL, West PA. The incidence of Vibrio cholerae in water, animals and birds in Kent, England. J. Appl. Bateriol. 1982;52:281–291. doi: 10.1111/j.1365-2672.1982.tb04852.x. [DOI] [PubMed] [Google Scholar]
- 11.Ogg JE, Ryder RA, Smith HL., Jr. Isolation of Vibrio cholerae from aquatic birds in Colorado and Utah. Appl. Environ. Microbiol. 1989;55:95–99. doi: 10.1128/aem.55.1.95-99.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Njiru N, Okeyo-Owuor JB, Muchiri M, Cowx IG. Shifts in the food of Nile tilapia, Oreochromis niloticus (L.) in Lake Victoria, Kenya. J. African. Ecol. 2004;42:163–170. doi: 10.1111/j.1365-2028.2004.00503.x. [DOI] [Google Scholar]
- 13.Senderovich Y, Izhaki I, Halpern M. Fish as reservoirs and vectors of Vibrio cholerae. PLoS One. 2015;5:e8607. doi: 10.1371/journal.pone.0008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pandit CG, Hora SL. The probable role of the hilsa fish, Hilsa ilisa (Ham) in maintaining cholera endemicity in India. Indian J. Med. Sci. 1951;15:343–356. [Google Scholar]
- 15.McIntyre RC, Tira T, Flood T, Blake PA. Modes of transmission of cholera in a newly infected population on an atoll: Implications for control measures. Lancet. 1979;10:311–314. doi: 10.1016/S0140-6736(79)90719-0. [DOI] [PubMed] [Google Scholar]
- 16.Carvajal GH, Sanchez J, Ayala ME, Hase A. Differences among marine and hospital strains of Vibrio cholerae during Peruvian epidemic. J. Gen. Appl. Microbiol. 1988;l44:27–33. doi: 10.2323/jgam.44.27. [DOI] [PubMed] [Google Scholar]
- 17.Acosta CJ, et al. Cholera outbreak in southern Tanzania: Risk factors and patterns of transmission. Emerg. Infect. Dis. 2001;7:583–587. doi: 10.3201/eid0707.017741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forssman B, et al. Vibrio cholerae O1 El Tor cluster in Sydney linked to imported whitebait. Med. J. Aust. 2007;187:345–347. doi: 10.5694/j.1326-5377.2007.tb01278.x. [DOI] [PubMed] [Google Scholar]
- 19.Buck JD. Occurrence of Candida albicans in fresh gull feces in temperate and subtropical areas. Microbial. Ecology. 1983;9:171–176. doi: 10.1007/BF02015129. [DOI] [PubMed] [Google Scholar]
- 20.Dobbin G, et al. Bacterial flora of free-living double-crested cormorant (Phalacrocorax auritus) chicks on Prince Edward Island, Canada, with reference to enteric bacteria and antibiotic resistance. Comp. Immunol. Microbiol. Infect. Dis. 2005;28:71–82. doi: 10.1016/S0147-9571(04)00059-1. [DOI] [PubMed] [Google Scholar]
- 21.Warke GMA, Day KR, Davidson RD. Cormorant (Phalacrocorax-carbo L.) populations and patterns of abundance at breeding and feeding sites in Northern Ireland, with particular reference to Lough Neagh. Hydrobiologia. 1994;280:91–100. doi: 10.1007/BF00027844. [DOI] [Google Scholar]
- 22.Cramp, S. & Simmons, K. E. L. (Eds). Handbook of the birds of Europe, the Middle East and North Africa. The birds of the Western Palearctic 1. Oxford: Oxford University Press (1977).
- 23.Hald-Mortensen, P. Danske skarvers fødevalg 1992–1994. Danish Forest and Nature Agency, Ministry of Environment and Energy.
- 24.Madsen FJ, Spärck R. On the feeding habits of the southern cormorant in Denmark. Dan. Rev. Game. Biol. 1950;1:45–75. [Google Scholar]
- 25.Russell IC, Cook AC, Kinsman DA, Ives MJ, Lower NJ. Stomach content analysis of Great Cormorants Phalacrocorax carbo at some different fishery types in England and Wales. Vogelwelt. 2003;124:255–259. [Google Scholar]
- 26.Trauttmansdorff J. Analysis of Great Cormorant Phalacrocorax carbo sinensis stomach contents from different areas of Austria and Liechtenstein. Vogelwelt. 2003;124:255–259. [Google Scholar]
- 27.Papazoglou, P., Kreiser, K., Waliczky, Z. & Burfield, I. Birds in the European Union: A status assessment. Birdlife International, Cambridge, UK. 59 http://www.birdlife.org/action/science/species/birds_in_europe/index.html (2004).
- 28.Delany, S. & Scott, D. A. Waterbird Population Estimates, Fourth Edition. Wetlands International, Wageningen, Netherlands. 239 (2006).
- 29.Takahashi T, Kameda K, Kawamura M, Nakajima T. Food habits of great cormorant Phalacrocorax carbo hanedae at Lake Biwa, Japan, with special reference to ayu Plecoglossus altivelis altivelis Tetsumi. Fisheries sci. 2006;72:477–484. doi: 10.1111/j.1444-2906.2006.01175.x. [DOI] [Google Scholar]
- 30.Nemtzov, S. C. Israel-Ukraine cooperation for experimental management of a shared overabundant population of great cormorants (Phalacrocorax carbo). Proceedings of 23rd Vertebrate Pest Conference. University of California, Davis. 108–112 (2008).
- 31.Chin CS, et al. The origin of the Haitian cholera outbreak strain. N. Engl. J. Med. 2011;364:33–42. doi: 10.1056/NEJMoa1012928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katz LS, et al. Evolutionary dynamics of Vibrio cholerae O1 following a singlesource introduction to Haiti. mBio. 2013;4:e00398–13. doi: 10.1128/mBio.00398-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ali M, Nelson AR, Lopez AL, Sackm DA. Updated global burden of cholera in endemic countries. Plos Negl. Trop. Dis. 2015;9:e0003832. doi: 10.1371/journal.pntd.0003832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewin, S. M. Zoological microhabitats of Vibrio cholerae. In: Drasar, B.S. & Forrest, B.D. (Eds). Cholera and the ecology of Vibrio cholerae. London, England: Chapman and Hall. 228–254 (1996).
- 35.Halpern M, Izhaki I. Fish as hosts of Vibrio cholerae. Front. Microbiol. 2017;8:282. doi: 10.3389/fmicb.2017.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hounmanou, Y. M et al. Toxigenic Vibrio cholerae O1 in vegetables and fish raised in wastewater irrigated fields and stabilization ponds during a non-cholera outbreak period in Morogoro, Tanzania: An environmental health study. BMC. res. notes. 466 (2016). [DOI] [PMC free article] [PubMed]
- 37.Yoda K, Tajima T, Sasaki S, Sato K, Niizuma Y. Influence of local wind conditions on the flight speed of the great cormorant Phalacrocorax carbo. Int. J. Zool. 2012;1:1–7. doi: 10.1155/2012/187102. [DOI] [Google Scholar]
- 38.Banks JC, Cary SC, Hogg ID. The phylogeography of Adelie penguin faecal flora. Environ. Microbiol. 2009;11:577–588. doi: 10.1111/j.1462-2920.2008.01816.x. [DOI] [PubMed] [Google Scholar]
- 39.Benskin CM, Rhodes H, Pickup G, Wilson RW, Hartley IR. Diversity and temporal stability of bacterial communities in a model passerine bird, the zebra finch. Mol. Ecol. 2010;19:5531–5544. doi: 10.1111/j.1365-294X.2010.04892.x. [DOI] [PubMed] [Google Scholar]
- 40.Stanley D, Geier MS, Hughes RJ, Denman SE, Moore RJ. Highly variable microbiota development in the chicken gastrointestinal tract. Plos One. 2013;8:e84290. doi: 10.1371/journal.pone.0084290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller MR. Cecal fermentation in mallards in relation to diet. Condor. 1976;8:107–111. doi: 10.2307/1366928. [DOI] [Google Scholar]
- 42.Bedbury HP, Duke GE. Cecal microflora of turkeys fed low or high-fiber diets - enumeration, identification, and determination of cellulolytic activity. Poultry Sci. 1983;62:675–682. doi: 10.3382/ps.0620675. [DOI] [PubMed] [Google Scholar]
- 43.Redig PT. The avian ceca - obligate combustion-chambers or facultative afterburners: The conditioning influence of diet. J. Exp. Zool. 1989;3:66–69. doi: 10.1002/jez.1402520511. [DOI] [PubMed] [Google Scholar]
- 44.Maul J, Gandhi J, Farris J. Community-level physiological profiles of cloacal microbes in songbirds (Order: Passeriformes): Variation due to host species, host diet, and habitat. Microb. Ecol. 2005;50:19–28. doi: 10.1007/s00248-004-0076-9. [DOI] [PubMed] [Google Scholar]
- 45.Hird SM, Carstens BC, Cardiff S, Dittmann DL, Brumfield RT. Sampling locality is more detectable than taxonomy or ecology in the gut microbiota of the brood-parasitic Brown-headed Cowbird (Molothrus ater) Peerj. 2014;2:e321. doi: 10.7717/peerj.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ryu H, et al. Intestinal microbiota and species diversity of Campylobacter and Helicobacter spp. in migrating shorebirds in Delaware Bay. Appl. Environ. Microbiol. 2014;80:1838–1847. doi: 10.1128/AEM.03793-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang W, et al. Comparative analysis of the gastrointestinal microbial communities of bar-headed goose (Anser indicus) in different breeding patterns by high-throughput sequencing. Microbiol. Res. 2016;182:59–67. doi: 10.1016/j.micres.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 48.Xenoulis PG, et al. Molecular characterization of the cloacal microbiota of wild and captive parrots. Vet. Microbiol. 2010;146:320–325. doi: 10.1016/j.vetmic.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 49.Dewar ML, et al. Interspecific variations in the gastrointestinal microbiota in penguins. Microbiol. open. 2013;2:195–204. doi: 10.1002/mbo3.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waite DW, Taylor MW. Characterizing the avian gut microbiota: membership, driving influences, and potential function. Front. Microbiol. 2014;5:223. doi: 10.3389/fmicb.2014.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hofstad, T. The genus Fusobacterium. In Dworkin, M. & Falkow, S. (Eds), The prokaryotes. Springer, Berlin, 1016–1027 (2006).
- 52.Foster G, et al. Cetobacterium ceti gen. nov., sp. nov., a new gram-negative obligate anaerobe from sea mammals. Lett. Appl. Microbiol. 1995;21(3):202–206. doi: 10.1111/j.1472-765X.1995.tb01041.x. [DOI] [PubMed] [Google Scholar]
- 53.Finegold SM, et al. Cetobacterium somerae sp. nov. from human feces and emended description of the genus. Cetobacterium. Syst. Appl. Microbiol. 2003;26:177–181. doi: 10.1078/072320203322346010. [DOI] [PubMed] [Google Scholar]
- 54.Huba’lek Z. Pathogenic microorganisms associated with free-living birds (a review) Acta. Scientiarum Naturalium Brno. 1994;28:1–74. [Google Scholar]
- 55.Dziejman M, et al. Genomic characterization of non-O1, non-O139 Vibrio cholerae reveals genes for a type III secretion system. Proc. Natl. Acad. Sci. USA. 2005;102:3465–3470. doi: 10.1073/pnas.0409918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chatterjee S, et al. Incidence, virulence factors, and clonality among clinical strains of non-O1, non-O139 Vibrio cholerae isolates from hospitalized diarrheal patients in Kolkata, India. J. Clin. Microbiol. 2009;47:1087–1095. doi: 10.1128/JCM.02026-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Debellis L, et al. The Vibrio cholerae cytolysin promotes chloride secretion from intact human intestinal mucosa. PLoS ONE. 2009;4:e5074. doi: 10.1371/journal.pone.0005074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kanoktippornchai B, Chomvarin C, Hahnvajanawong C, Nutrawong T. Role of hlyA positive Vibrio cholerae non-O1/non-O139 on apoptosis and cytotoxicity in a Chinese hamster over cell line. Southeast Asian J. Trop. Med. Public Health. 2014;45:1365–1375. [PubMed] [Google Scholar]
- 59.Caporaso JG, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6:1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aizenberg-Gershtein Y, et al. Pyridine-type alkaloid composition affects bacterial community composition of floral nectar. Sci. Rep. 2015;5:11536. doi: 10.1038/srep11536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013;79(17):5112–20. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hughes JB, Hellmann JJ, Ricketts TH, Bohannan BJM. Counting the uncountable: Statistical approaches to estimating microbial diversity. Appl. Environ. Microbiol. 2001;67:4399–4406. doi: 10.1128/AEM.67.10.4399-4406.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nandi B, et al. Rapid method for species-specific identification of Vibrio cholerae using primers targeted to the gene of outer membrane protein. OmpW. J. Clin. Microbiol. 2008;38:4145–4151. doi: 10.1128/jcm.38.11.4145-4151.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.