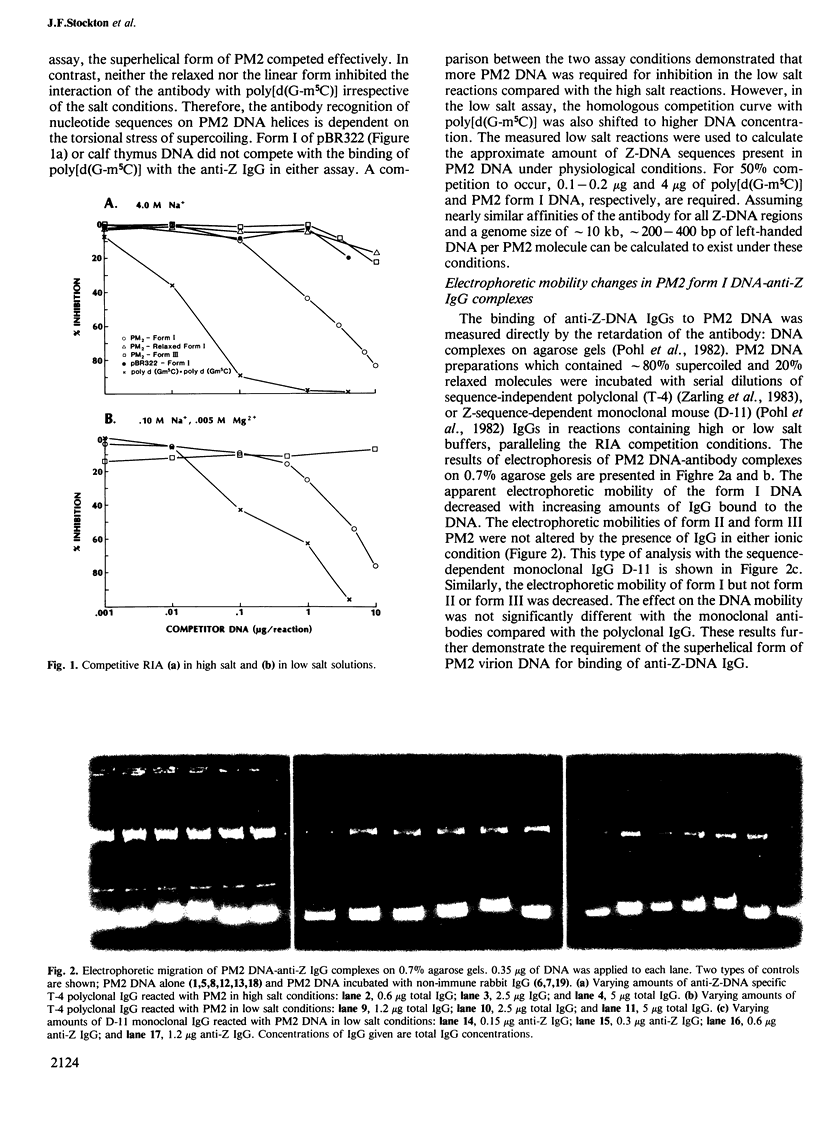
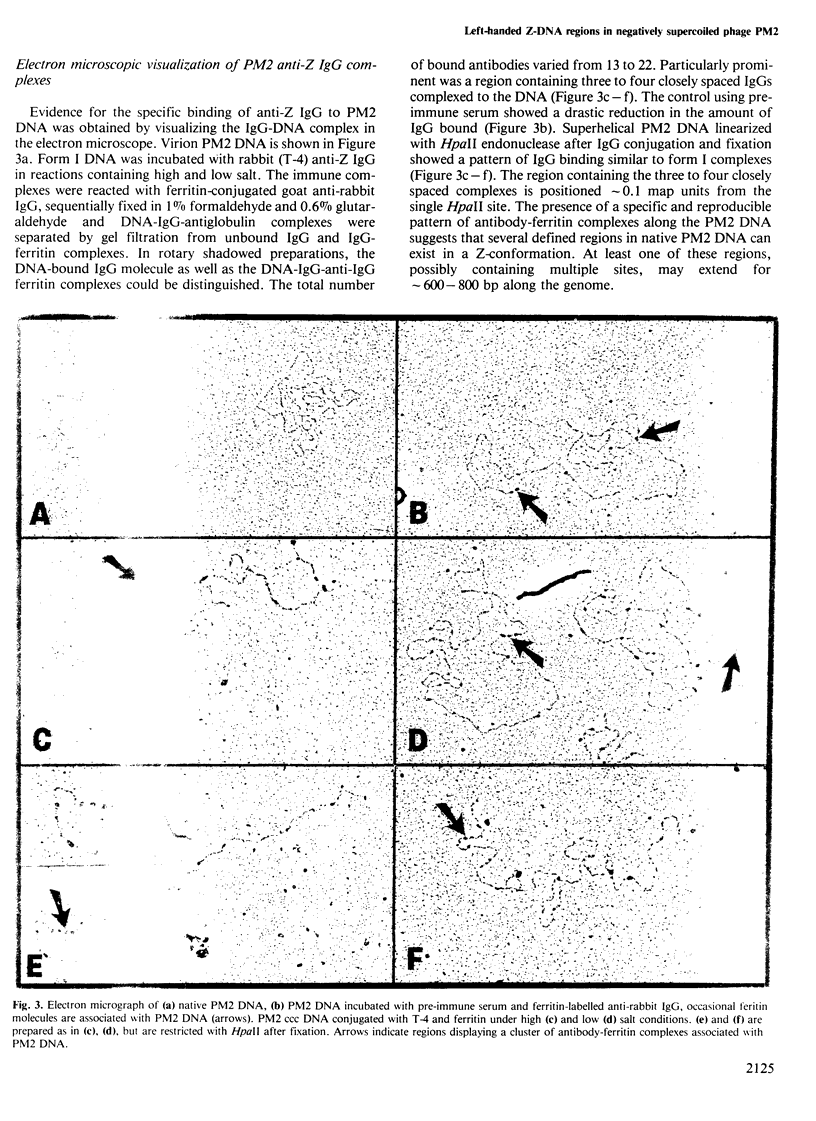
Abstract
Bacteriophage PM2 DNA is a 10-kb covalently closed circular (ccc) molecule with a reported superhelical density of sigma = -0.12. Here we describe the binding of anti-Z-DNA antibodies to PM2 form I DNA under high and low salt conditions. The binding to PM2 DNA has been demonstrated by competitive radioimmunoassay (RIA), retardation of the DNA:antibody complexes in agarose gels and visualization by electron microscopy. The antibody binding is dependent on the degree of negative supercoiling. Thus, PM2 form II and form III did not bind the antibody. The low salt RIA results indicated the presence of 200-400 bp of left-handed DNA per PM2 molecule. This could reduce the effective superhelical density to sigma = -0.04 to -0.08, a range comparable with those found for other ccc DNAs in vivo. Electron microscopy revealed that a maximum of 22 antibody molecules bind to PM2. Single-site restriction with HpaII of the fixed DNA:antibody complex showed a cluster of four to five antibody molecules bound near one end of the linear DNA molecule. The evidence presented indicates that PM2 DNA contains regions of left-handed conformation under physiological conditions (low salt concentration) as well as at high salt concentrations. In addition, electrophoretic analyses of PM2 topoisomers indicate the presence of left-handed regions at superhelical densities less than that of isolated PM2 DNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azorin F., Nordheim A., Rich A. Formation of Z-DNA in negatively supercoiled plasmids is sensitive to small changes in salt concentration within the physiological range. EMBO J. 1983;2(5):649–655. doi: 10.1002/j.1460-2075.1983.tb01479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack C., Bickle T. A., Yuan R. The relation of single-stranded regions in bacteriophage PM2 supercoiled DNA to the early melting sequences. J Mol Biol. 1975 Aug 25;96(4):693–702. doi: 10.1016/0022-2836(75)90146-1. [DOI] [PubMed] [Google Scholar]
- Camerini-Otero R. D., Franklin R. M. Structure and synthesis of a lipid-containing bacteriophage. The molecular weight and other physical properties of bacterophage PM2. Eur J Biochem. 1975 May 6;53(2):343–348. doi: 10.1111/j.1432-1033.1975.tb04074.x. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S. Properties of bacteriophage PM2: a lipid-containing bacterial virus. Virology. 1968 Apr;34(4):738–747. doi: 10.1016/0042-6822(68)90094-9. [DOI] [PubMed] [Google Scholar]
- Griffith J. D., Christiansen G. Electron microscope visualization of chromatin and other DNA-protein complexes. Annu Rev Biophys Bioeng. 1978;7:19–35. doi: 10.1146/annurev.bb.07.060178.000315. [DOI] [PubMed] [Google Scholar]
- Haniford D. B., Pulleyblank D. E. Facile transition of poly[d(TG) x d(CA)] into a left-handed helix in physiological conditions. Nature. 1983 Apr 14;302(5909):632–634. doi: 10.1038/302632a0. [DOI] [PubMed] [Google Scholar]
- Jovin T. M., van de Sande J. H., Zarling D. A., Arndt-Jovin D. J., Eckstein F., Füldner H. H., Greider C., Grieger I., Hamori E., Kalisch B. Generation of left-handed Z-DNA in solution and visualization in polytene chromosomes by immunofluorescence. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):143–154. doi: 10.1101/sqb.1983.047.01.019. [DOI] [PubMed] [Google Scholar]
- Kowalski D. A procedure for the quantitation of relaxed closed circular DNA in the presence of superhelical DNA: an improved fluorometric assay for nicking-closing enzyme. Anal Biochem. 1979 Mar;93(2):346–354. doi: 10.1016/s0003-2697(79)80161-x. [DOI] [PubMed] [Google Scholar]
- Kowalski D., Sanford J. P. Action of mung bean nuclease on supercoiled PM2 DNA. J Biol Chem. 1982 Jul 10;257(13):7820–7825. [PubMed] [Google Scholar]
- Kuhnlein U., Tsang S. S., Edwards J. Cooperative structural transition of PM2 DNA at high ionic strength and its dependence on DNA damages. Nature. 1980 Sep 25;287(5780):363–364. doi: 10.1038/287363a0. [DOI] [PubMed] [Google Scholar]
- Lafer E. M., Möller A., Nordheim A., Stollar B. D., Rich A. Antibodies specific for left-handed Z-DNA. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3546–3550. doi: 10.1073/pnas.78.6.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Morgan A. R. The topological trapping of circular DNAs on agarose: unexpected restrictions on DNA rotation. Can J Biochem. 1978 Jun;56(6):585–591. doi: 10.1139/o78-088. [DOI] [PubMed] [Google Scholar]
- Möller A., Gabriels J. E., Lafer E. M., Nordheim A., Rich A., Stollar B. D. Monoclonal antibodies recognize different parts of Z-DNA. J Biol Chem. 1982 Oct 25;257(20):12081–12085. [PubMed] [Google Scholar]
- Nordheim A., Lafer E. M., Peck L. J., Wang J. C., Stollar B. D., Rich A. Negatively supercoiled plasmids contain left-handed Z-DNA segments as detected by specific antibody binding. Cell. 1982 Dec;31(2 Pt 1):309–318. doi: 10.1016/0092-8674(82)90124-6. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Pardue M. L., Lafer E. M., Möller A., Stollar B. D., Rich A. Antibodies to left-handed Z-DNA bind to interband regions of Drosophila polytene chromosomes. Nature. 1981 Dec 3;294(5840):417–422. doi: 10.1038/294417a0. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Peck L. J., Lafer E. M., Stollar B. D., Wang J. C., Rich A. Supercoiling and left-handed Z-DNA. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):93–100. doi: 10.1101/sqb.1983.047.01.012. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Rich A. The sequence (dC-dA)n X (dG-dT)n forms left-handed Z-DNA in negatively supercoiled plasmids. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1821–1825. doi: 10.1073/pnas.80.7.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordheim A., Tesser P., Azorin F., Kwon Y. H., Möller A., Rich A. Isolation of Drosophila proteins that bind selectively to left-handed Z-DNA. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7729–7733. doi: 10.1073/pnas.79.24.7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck L. J., Nordheim A., Rich A., Wang J. C. Flipping of cloned d(pCpG)n.d(pCpG)n DNA sequences from right- to left-handed helical structure by salt, Co(III), or negative supercoiling. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4560–4564. doi: 10.1073/pnas.79.15.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M., Baehr W., Holbrook J. J. Ethidium bromide as a cooperative effector of a DNA structure. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3805–3809. doi: 10.1073/pnas.69.12.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl F. M. Polymorphism of a synthetic DNA in solution. Nature. 1976 Mar 25;260(5549):365–366. doi: 10.1038/260365a0. [DOI] [PubMed] [Google Scholar]
- Pohl F. M. Salt-induced transition between two double-helical forms of oligo (dC-dG). Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):113–117. doi: 10.1101/sqb.1983.047.01.014. [DOI] [PubMed] [Google Scholar]
- Pohl F. M., Thomae R., DiCapua E. Antibodies to Z-DNA interact with form V DNA. Nature. 1982 Dec 9;300(5892):545–546. doi: 10.1038/300545a0. [DOI] [PubMed] [Google Scholar]
- Pulleyblank D. E., Morgan A. R. The sense of naturally occurring superhelices and the unwinding angle of intercalated ethidium. J Mol Biol. 1975 Jan 5;91(1):1–13. doi: 10.1016/0022-2836(75)90368-x. [DOI] [PubMed] [Google Scholar]
- Rich A. Right-handed and left-handed DNA: conformational information in genetic material. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):1–12. doi: 10.1101/sqb.1983.047.01.003. [DOI] [PubMed] [Google Scholar]
- Schäfer R., Franklin R. M. Structure and synthesis of a lipid-containing bacteriophage. XIX. Reconstitution of bacteriophage PM2 in vitro. J Mol Biol. 1975 Sep 5;97(1):21–34. doi: 10.1016/s0022-2836(75)80019-2. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Brennan R., Chapman K. A., Goodman T. C., Hart P. A., Hillen W., Kellogg D. R., Kilpatrick M. W., Klein R. D., Klysik J. Left-handed DNA helices, supercoiling, and the B-Z junction. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):77–84. doi: 10.1101/sqb.1983.047.01.010. [DOI] [PubMed] [Google Scholar]
- Zimmerman S. B. The three-dimensional structure of DNA. Annu Rev Biochem. 1982;51:395–427. doi: 10.1146/annurev.bi.51.070182.002143. [DOI] [PubMed] [Google Scholar]
- van de Sande J. H., Jovin T. M. Z* DNA, the left-handed helical form of poly[d(G-C)] in MgCl2-ethanol, is biologically active. EMBO J. 1982;1(1):115–120. doi: 10.1002/j.1460-2075.1982.tb01133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]