Abstract
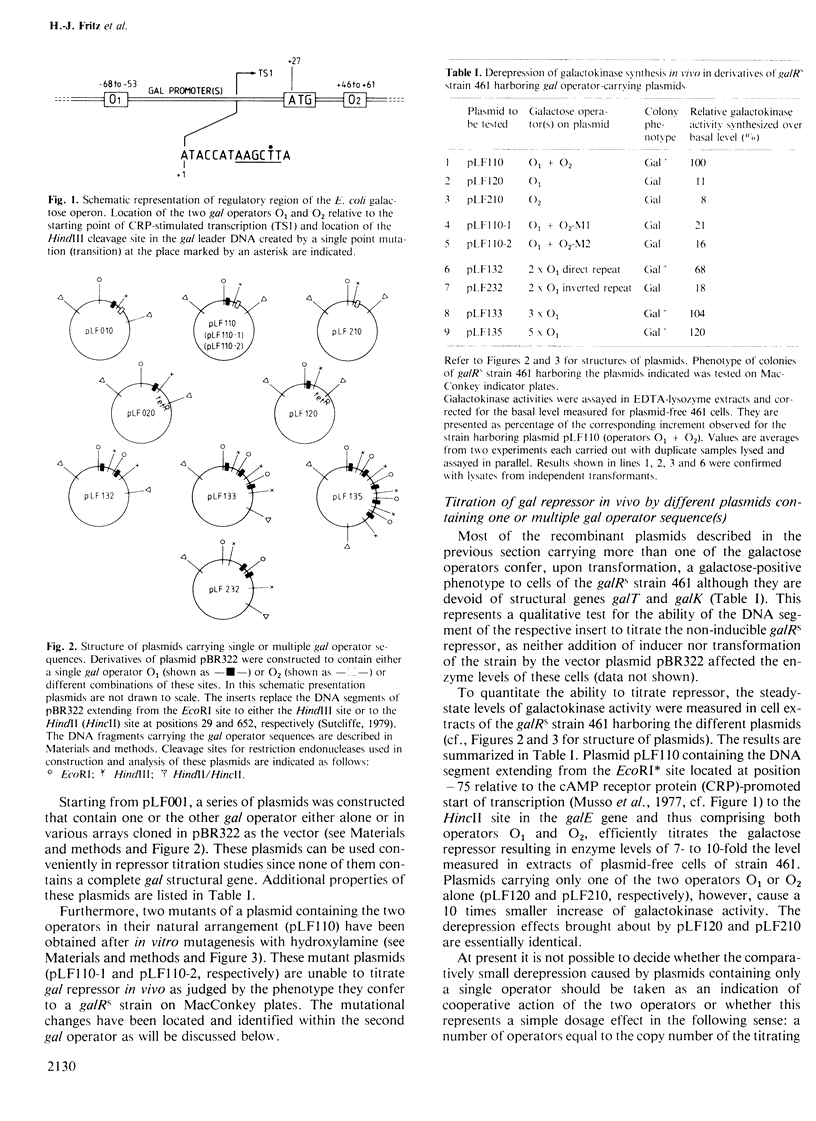
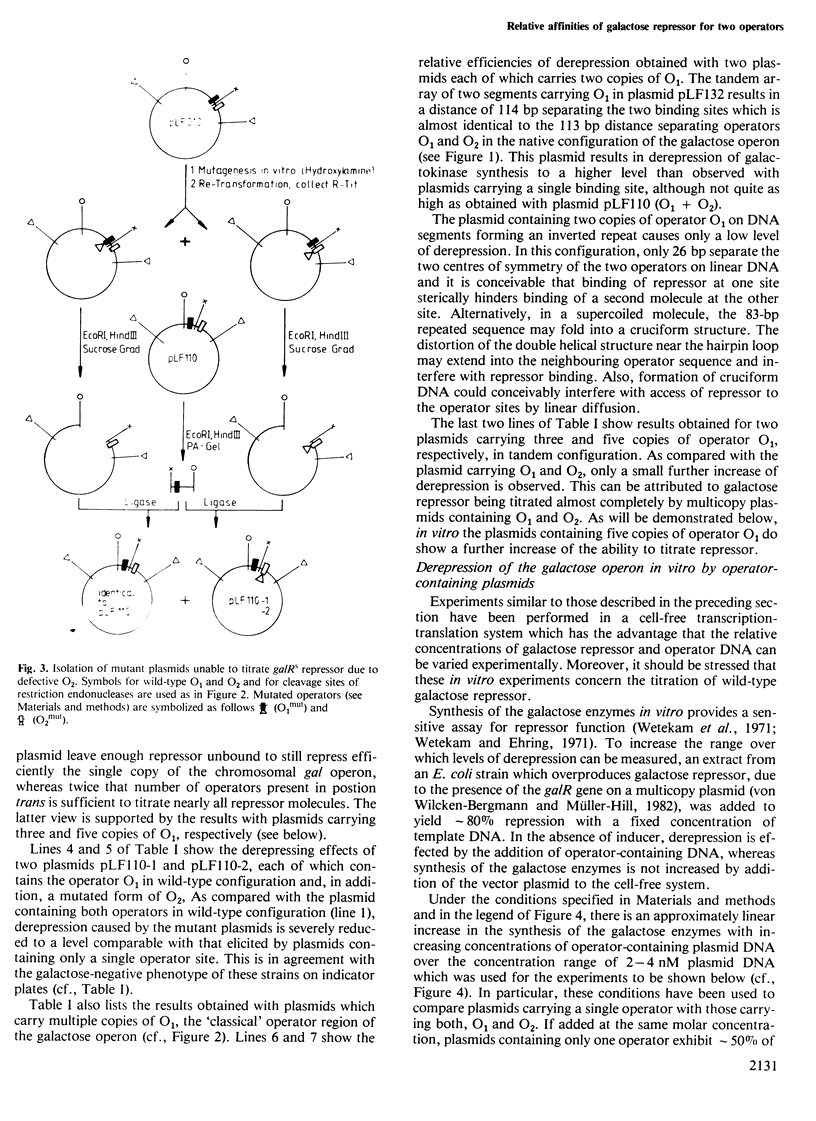
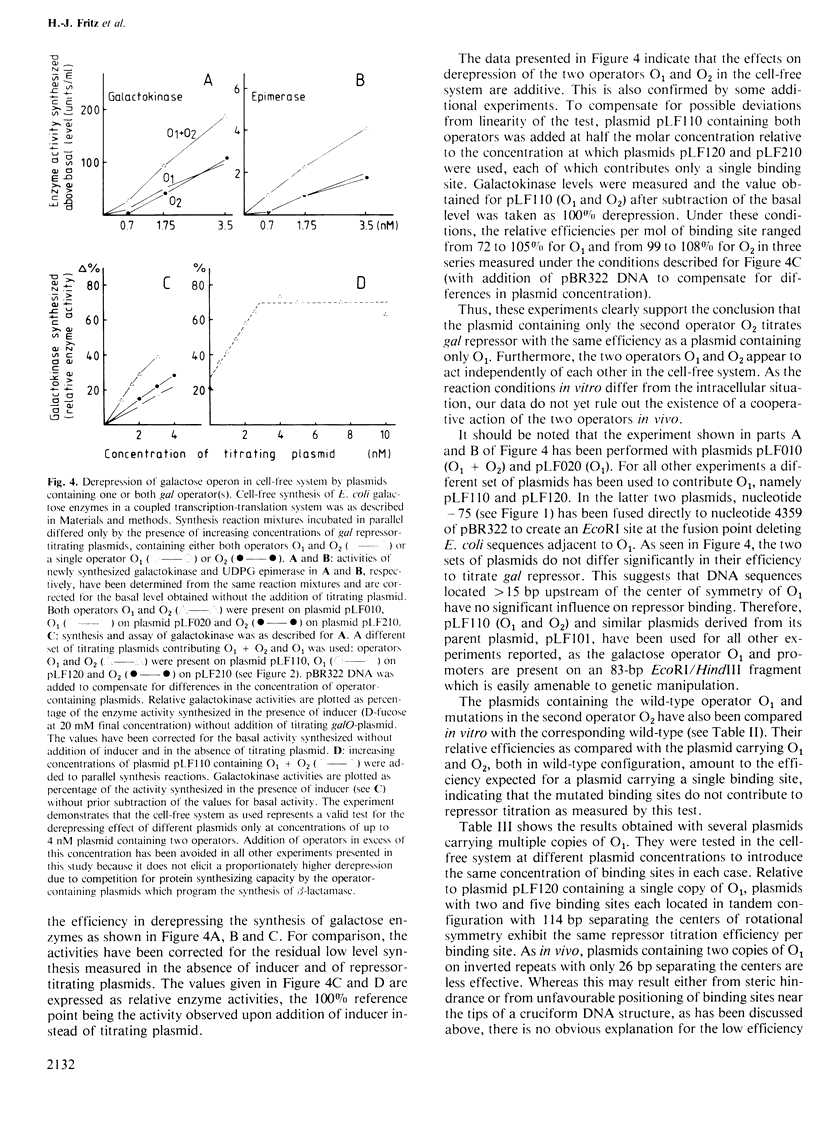
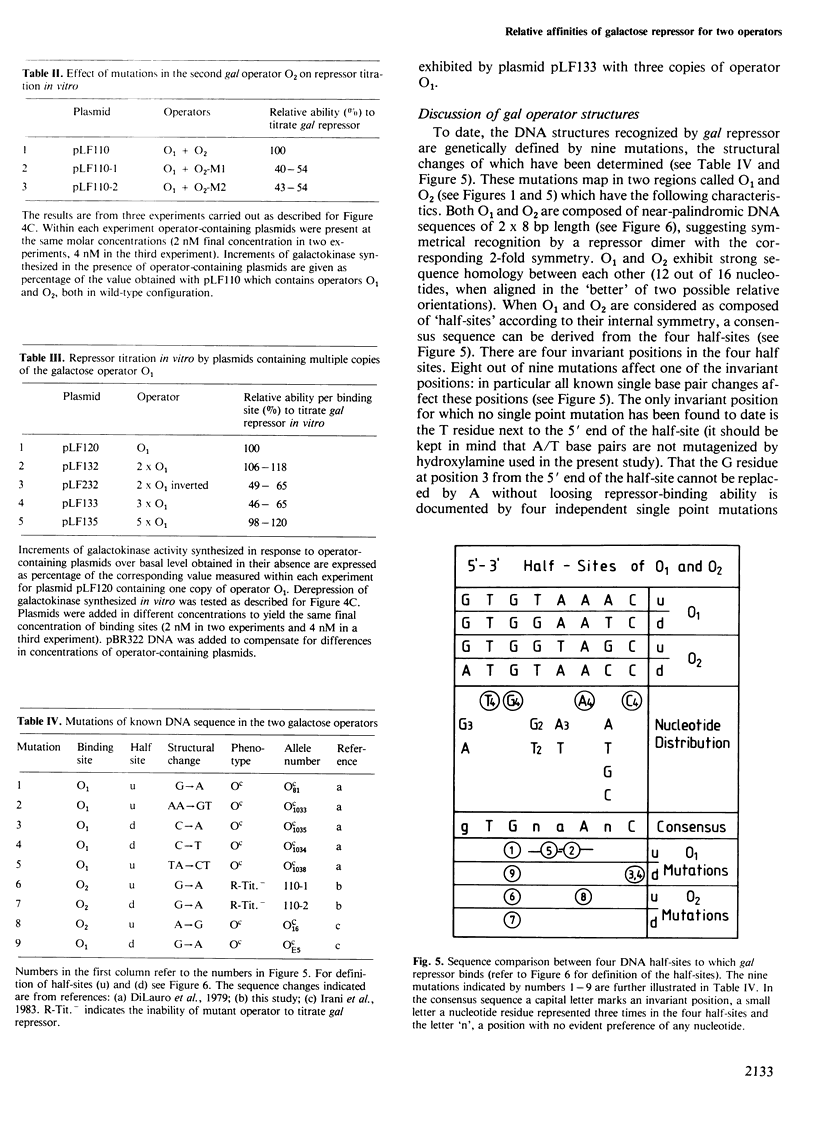
A series of plasmids has been constructed which contain either a single one of the two operators O1 and O2 of the Escherichia coli galactose operon or different combinations thereof. This permits comparison of the two operators with respect to their repressor binding ability. A plasmid containing only the second operator, O2, located within the structural gene galE, was found to titrate repressor in vivo and in vitro with essentially the same efficiency as a plasmid containing only the 'classical' galactose operator, O1, located upstream of the start of transcription. Whereas some cooperativity between the two sites seems possible in vivo, they bind repressor independently under the conditions of the in vitro assay. After hydroxylamine treatment of plasmid DNA in vitro, two different mutations have been isolated each of which inactivates the second operator, O2. Both are GC to AT transitions located at equivalent positions relative to the axis of rotational symmetry within the second gal operator. Subdividing O1 and O2 according to their 2-fold symmetry yields four half-sites with the consensus sequence (5')gTGnaAnC(3'). All known single point mutations of O1 and O2 affect the frame of invariant residues. The two half-sites of the lac operator also coincide with this frame.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Miller W. Modulation of the two promoters of the galactose operon of Escherichia coli. Nature. 1979 Jun 7;279(5713):492–494. doi: 10.1038/279492a0. [DOI] [PubMed] [Google Scholar]
- Besemer J., Herpers M. Suppression of polarity of insertion mutations within the gal operon of E. coli. Mol Gen Genet. 1977 Mar 16;151(3):295–304. doi: 10.1007/BF00268793. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLauro R., Taniguchi T., Musso R., de Crombrugghe B. Unusual location and function of the operator in the Escherichia coli galactose operon. Nature. 1979 Jun 7;279(5713):494–500. doi: 10.1038/279494a0. [DOI] [PubMed] [Google Scholar]
- Echols H. Integrative and excisive recombination by bacteriophage lambda: evidence for an excision-specific recombination protein. J Mol Biol. 1970 Feb 14;47(3):575–583. doi: 10.1016/0022-2836(70)90324-4. [DOI] [PubMed] [Google Scholar]
- Igo-Kemenes T., Hörz W., Zachau H. G. Chromatin. Annu Rev Biochem. 1982;51:89–121. doi: 10.1146/annurev.bi.51.070182.000513. [DOI] [PubMed] [Google Scholar]
- Irani M. H., Orosz L., Adhya S. A control element within a structural gene: the gal operon of Escherichia coli. Cell. 1983 Mar;32(3):783–788. doi: 10.1016/0092-8674(83)90064-8. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Musso R. E., Di Lauro R., Adhya S., de Crombrugghe B. Dual control for transcription of the galactose operon by cyclic AMP and its receptor protein at two interspersed promoters. Cell. 1977 Nov;12(3):847–854. doi: 10.1016/0092-8674(77)90283-5. [DOI] [PubMed] [Google Scholar]
- Müller-Hill B. Lac repressor and lac operator. Prog Biophys Mol Biol. 1975;30(2-3):227–252. doi: 10.1016/0079-6107(76)90011-0. [DOI] [PubMed] [Google Scholar]
- Saedler H., Gullon A., Fiethen L., Starlinger P. Negative control of the galactose operon in E. coli. Mol Gen Genet. 1968;102(1):79–88. doi: 10.1007/BF00341872. [DOI] [PubMed] [Google Scholar]
- Saint-Girons I., Fritz H. J., Shaw C., Tillmann E., Starlinger P. Integration specificity of an artificial kanamycin transposon constructed by the in vitro insertion of an internal Tn5 fragment into IS2. Mol Gen Genet. 1981;183(1):45–50. doi: 10.1007/BF00270136. [DOI] [PubMed] [Google Scholar]
- Sauer R. T., Yocum R. R., Doolittle R. F., Lewis M., Pabo C. O. Homology among DNA-binding proteins suggests use of a conserved super-secondary structure. Nature. 1982 Jul 29;298(5873):447–451. doi: 10.1038/298447a0. [DOI] [PubMed] [Google Scholar]
- Seeman N. C., Rosenberg J. M., Rich A. Sequence-specific recognition of double helical nucleic acids by proteins. Proc Natl Acad Sci U S A. 1976 Mar;73(3):804–808. doi: 10.1073/pnas.73.3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Trinks K., Habermann P., Beyreuther K., Starlinger P., Ehring R. An IS4-encoded protein is synthesized in minicells. Mol Gen Genet. 1981;182(2):183–188. doi: 10.1007/BF00269656. [DOI] [PubMed] [Google Scholar]
- Wetekam W., Ehring R. Coordinate regulation of DNA-dependent cell-free synthesis of uridyltransferase and galactokinase. FEBS Lett. 1971 Nov 1;18(2):271–273. doi: 10.1016/0014-5793(71)80462-3. [DOI] [PubMed] [Google Scholar]
- Wetekam W., Staack K., Ehring R. DNA-dependent in vitro synthesis of enzymes of the galactose operon of Escherichia coli. Mol Gen Genet. 1971;112(1):14–27. doi: 10.1007/BF00266928. [DOI] [PubMed] [Google Scholar]
- Wetekam W., Staack K., Ehring R. Relief of polarity in DNA-dependent cell-free synthesis of enzymes of the galactose operon of Escherichia coli. Mol Gen Genet. 1972;116(3):258–276. doi: 10.1007/BF00269770. [DOI] [PubMed] [Google Scholar]
- Winter R. B., Berg O. G., von Hippel P. H. Diffusion-driven mechanisms of protein translocation on nucleic acids. 3. The Escherichia coli lac repressor--operator interaction: kinetic measurements and conclusions. Biochemistry. 1981 Nov 24;20(24):6961–6977. doi: 10.1021/bi00527a030. [DOI] [PubMed] [Google Scholar]
- von Wilcken-Bergmann B., Koenen M., Griesser H. W., Müller-Hill B. 72 residues of gal repressor fused to beta-galactosidase repress the gal operon of E. coli. EMBO J. 1983;2(8):1271–1274. doi: 10.1002/j.1460-2075.1983.tb01580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wilcken-Bergmann B., Müller-Hill B. Sequence of galR gene indicates a common evolutionary origin of lac and gal repressor in Escherichia coli. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2427–2431. doi: 10.1073/pnas.79.8.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]


