Abstract
Rising CO2 concentration, a driving force of climate change, is impacting global food security by affecting plant physiology. Nevertheless, the effects of elevated CO2 on primary and secondary metabolism in tea plants (Camellia sinensis L.) still remain largely unknown. Here we showed that exposure of tea plants to elevated CO2 (800 µmol mol−1 for 24 d) remarkably improved both photosynthesis and respiration in tea leaves. Furthermore, elevated CO2 increased the concentrations of soluble sugar, starch and total carbon, but decreased the total nitrogen concentration, resulting in an increased carbon to nitrogen ratio in tea leaves. Among the tea quality parameters, tea polyphenol, free amino acid and theanine concentrations increased, while the caffeine concentration decreased after CO2 enrichment. The concentrations of individual catechins were altered differentially resulting in an increased total catechins concentration under elevated CO2 condition. Real-time qPCR analysis revealed that the expression levels of catechins and theanine biosynthetic genes were up-regulated, while that of caffeine synthetic genes were down-regulated in tea leaves when grown under elevated CO2 condition. These results unveiled profound effects of CO2 enrichment on photosynthesis and respiration in tea plants, which eventually modulated the biosynthesis of key secondary metabolites towards production of a quality green tea.
Introduction
Climate change is one of the most important complex factors that greatly impacts global food production. It is predicted that effect of climate change will be intensified over time. For instance, the concentration of atmospheric CO2, an important parameter of climate change, has been increased tremendously in the last century and will be doubled at the end of 21st century (IPCC 2007)1. Studies have revealed that rising atmospheric CO2 concentrations greatly influence plant growth and responses to biotic and abiotic stresses2–4. The general interpretation in favour of rising CO2 is that elevated CO2 stimulates photosynthesis in plants that eventually results in increased yield in terms of quantity. Recent studies have also revealed that plants grown under elevated CO2 maintain a consistently higher leaf dark respiration (mitochondrial respiration), compared with that of ambient CO2 5. Elevated CO2-simulated enhanced respiration can increase crop yield, by providing greater energy to export photoassimilate from source leaves to sink tissues5, 6.
Photosynthesis plays an important role in plant metabolism by synthesizing photoassimilates that are used as substrates for all other biosynthetic pathways7. Respiration utilizes photoassimilate as substrate to generate C-skeleton intermediates, reductants such as NADH and NADPH, and usable energy i.e. ATP as products8. The energy provided by respiration is the source energy for secondary metabolism and the products of respiration serve as the synthetic precursors of secondary metabolites9. Two main biochemical processes such as ribulose-1,5-bis-phosphate (RuBP) carboxylase/oxygenase (RuBisCO) carboxylation and RuBP regeneration strictly control the rate of photosynthesis6. Elevated CO2 not only increases activity of RuBisCO to enhance photosynthetic rate, but also alters partitioning of the photoassimilates for the biosynthesis of secondary metabolites10. Moreover, elevated CO2 increases concentration of non-structural carbohydrate that may stimulate secondary metabolism in plants10, 11. Nonetheless, mitochondrial respiration plays a key role in optimizing adequate photosynthetic rates in plants6. Prior studies showed that increased carbohydrate availability and energy demand under elevated CO2 enhance respiration rate, which helps plant to optimize the allocation of carbon and nutrient for maximizing photosynthesis and plant growth5.
Tea is a fascinating health drink, extensively consumed for its health benefits and astringenic property around the world. Green tea is typically produced from two leaves and a bud of perennial tree tea [Camellia sinensis (L.) O. Kuntze]. The health benefits of green tea and its pleasant taste are due to presence of bioactive compounds predominantly derived from secondary metabolic pathway12–14. The composition of primary metabolite and secondary metabolites determines the ultimate quality of green tea14. Although a number of previous studies have showed that elevated CO2 influences both primary and secondary metabolism in a range of plant species10, 15, 16, one crucial topic that has been ignored is the effect of elevated CO2 on the growth of tea plants and production of secondary metabolites involved in tea quality.
Mostly two groups of chemicals such as tea polyphenols (TP) and amino acids (AA) are considered as main determinants of the taste or pleasant flavor of tea. Catechins are major TP that significantly influence the flavor of green tea, while theanine, an abundant non-protein AA in tea leaves is responsible for its umami taste17. Catechins are well known for its role in preventing cancer, cardiovascular, neurodegenerative and other oxidative stress-related diseases18. Given that catechins are flavan-3-ol type of flavonoid, its synthesis involves participation of the phenylpropanoid and flavonoid pathways. Theanine is used as one of the biosynthetic precursors of catechins19. Health benefits of theanine include reduction of high blood pressure, induction of relaxation and inhibition of the side effects of caffeine17, 19. Caffeine, a secondary metabolite belongs to purine alkaloids, is synthesized in tea plants from purine nucleotides12. The concentration of caffeine in plants is high in young leaves and flowers compared with other plant parts12, 20, 21. Although a moderate amount of caffeine has stimulatory effects on human health, its excessive consumption is often associated with health hazards such as sleep deprivation, tachycardia, abortion and miscarriages22. Therefore, caffeine level in a quality tea is expected to be minimum, so that its consumption would not exceed total dietary threshold. It is evident that biosynthesis of these secondary metabolites that are the key determinants of tea quality occurs through complex as well as inter-connected metabolic pathways that often converge between primary and secondary metabolism. However, the effects of elevated CO2 on the concentrations of tea secondary metabolites and expression of their regulatory genes still remain elusive.
Unlike annual crops, tea plants remain in active production for a long period of time, even for hundred years, which may allow them to experience climate change over the century23. It is believed that long life span of tea plants may lead them to operate massive physiological adaptation instead of genetic modification. Therefore, in the current study, we intend to investigate potential changes in some primary metabolic processes such as photosynthesis and respiration following exposure of tea plants to elevated CO2 for a period of 24 days. In addition, we analyzed the concentrations of various primary metabolites and tea quality-related secondary metabolites coupled with the expression of key genes involved in their biosynthetic pathways. It was hypothesized that elevated CO2 would alter the yield and quality of tea by modulating the primary and secondary metabolism in tea leaves. The results of this study will help us to better understand the preliminary response of tea plants to elevated CO2 at physiological and molecular levels.
Results
Exposure of tea seedlings to elevated CO2 enhances plant growth and biomass accumulation
Many experimental studies have shown that elevated CO2 conditions stimulate plant growth and biomass production in a wide range of plant species2, 11, 15, 16. To clarify this assumption in tea, we exposed tea seedlings to ambient CO2 and elevated CO2 conditions for 24 days. Results showed that elevated CO2 not only increased plant height (by 13.46%), but also promoted dry weights of shoot and root by 24.68 and 67.80%, respectively (Table 1). A positive stimulation in both shoot and root biomass accumulation by elevated CO2 eventually resulted in an increased root to shoot ratio by 27.66% compared with that in ambient CO2.
Table 1.
Effect of elevated CO2 concentration (800 µmol mol−1 for 24 d) on growth and biomass production in tea seedlings.
| Treatments | Plant height (cm) | Leaf DW seedling−1 (g) | Stem DW seedling−1 (g) | Shoot DW seedling−1 (g) | Root DW seedling−1 (g) | Ratio of Root to Shoot |
|---|---|---|---|---|---|---|
| Ambient CO2 | 55.7 ± 3.73 b | 4.8 ± 1.26 b | 7.7 ± 0.98 b | 12.5 ± 2.24 b | 5.9 ± 0.74 b | 0.47 ± 0.031 b |
| Elevated CO2 | 63.2 ± 4.65 a | 7.0 ± 1.33 a | 9.6 ± 1.47 a | 16.7 ± 2.8 a | 9.9 ± 1.32 a | 0.60 ± 0.064 a |
Mean denoted by different letters indicate significant differences between the treatments (P < 0.05). DW, Dry weight.
Elevated CO2 promotes photosynthesis by increasing RuBisCO carboxylation and regeneration capacity
To examine whether increased biomass accumulation under elevated CO2 is associated with photosynthetic performance of tea, we measured net photosynthetic rate at 5 time points over 24 days. Results showed that exposure of plants to elevated CO2 rapidly increased Pn that eventually reached maximum level at 12 day, and then remained more or less stable up to 24 day, indicating an acclimation response of CO2 assimilation capacity to elevated CO2 after 12 day exposure (Fig. 1A). Specifically, elevated CO2 increased Pn by 141.98, 122.25, 136.93 and 87.90% at 6, 12, 18 and 24 day, respectively as compared with that in ambient CO2. We also analyzed the quantum efficiency of PSII photochemistry (ΦPSII) that represents photosynthetic efficiency of tea leaves. Pseudo color images of ΦPSII were shown in Fig. 1B. It is noticeable that the ΦPSII remained stable over the experimental period in tea plants grown under ambient CO2 concentration. However, in tea plants that were grown under elevated CO2, ΦPSII tended to increase, reaching the highest value at 18 d following exposure to elevated CO2. Afterward, ΦPSII declined slightly. In addition, Vcmax increased until 12 day when it reached the highest peak, and then tended to decline over time (Fig. 1C). Similar trend was observed for Jmax under elevated CO2 condition, although notable difference was only found at 12 day under elevated CO2 treatment as compared with that in ambient CO2 (Fig. 1D).
Figure 1.
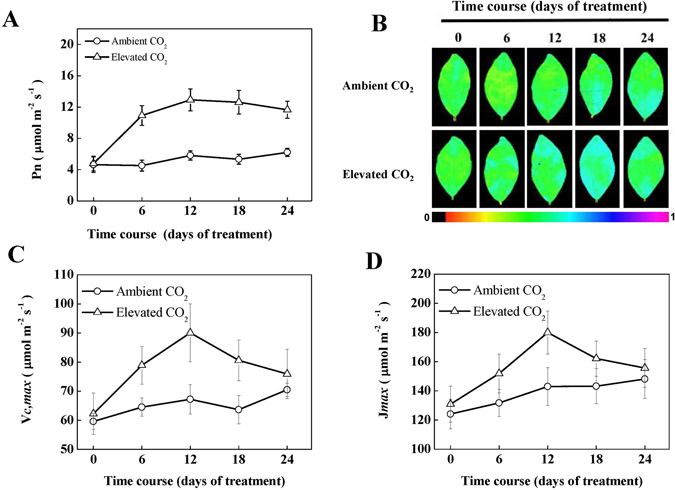
Photosynthetic response of tea plants to elevated CO2. (A) Net photosynthetic rate (Pn), (B) the quantum efficiency of photosystem II (ΦPSII), false colour code depicted in the image ranges from 0 (black) to 1(purple), (C) maximum carboxylation rate of RuBisCO (V cmax), (D) maximum rates of RuBP regeneration (J max). Tea seedlings were exposed to either ambient (380 µmol mol−1) or elevated CO2 concentration (800 µmol mol−1) for 24 days. Measurements were taken at different time-points as mentioned in the respective figures. The results are expressed as the mean values ± SD, n = 6.
Elevated CO2 increases respiration by increasing O2 uptake
An optimum balance between photosynthesis and respiration is required for proper biomass accumulation6, 8. To check whether elevated CO2 also affects respiration, we measured total respiration, SHAM-resistant respiration and CN-resistant respiration. Compared with ambient CO2, elevated CO2 increased total respiration rate by 53.63, 28.88, 52.67 and 32.29% at 6, 12, 18 and 24 day, respectively (Fig. 2A). Similarly, elevated CO2 increased SHAM-resistant respiration by 23.22, 27.70, 46.22 and 28.13% and CN-resistant respiration by 29.61, 32.04, 48.83 and 47.11%, respectively (Fig. 2B). Unlike Pn, maximum total respiration was recorded at 18 d, although respiration rates recorded at 12, 18 and 24 day were not much different, indicating a respiratory acclimation response to elevated CO2. While SHAM-resistant respiration remained stable after 12 day, CN-resistant respiration showed an increasing trend even at 24 day under elevated CO2 (Fig. 2C). Taken together, from the beginning to the end of the experiment, the rates of total respiration, SHAM-resistant and CN-resistant respiration were higher in tea plants grown under elevated CO2 than that under ambient CO2.
Figure 2.
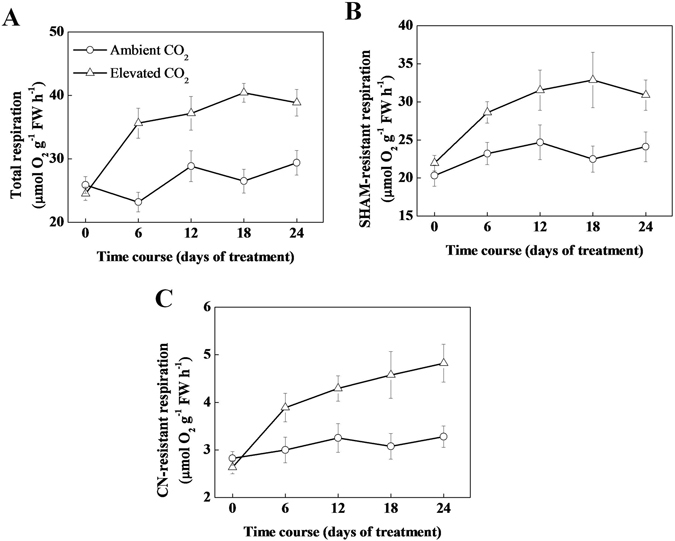
Changes in the O2 uptake rate of tea seedlings grown at ambient (380 µmol mol−1) or elevated CO2 concentration (800 µmol mol−1). (A) Total respiration, (B) salicylhydroxamic acid (SHAM)-resistant respiration, (C) cyanide (CN)-resistant respiration. Tea seedlings were exposed to either ambient (380 µmol mol−1) or elevated CO2 concentration (800 µmol mol−1) for 24 day. Measurements were taken at different time-points as mentioned in the respective figures. The results are expressed as the mean values ± SD, n = 6.
Effect of elevated CO2 on concentration of sugar, starch, carbon and nitrogen
As elevated CO2 stimulated both photosynthesis and respiration in tea leaves, we then looked into carbon and nitrogen metabolism in tea leaves. Elevated CO2 significantly increased the concentration of sugar, sucrose and starch (Fig. 3A–C). While concentration of total carbon increased in tea leaves under elevated CO2, concentration of total nitrogen decreased (Fig. 3D). Such changes in total C and total N eventually resulted in an increased C: N ratio in tea leaves under elevated CO2 conditions.
Figure 3.
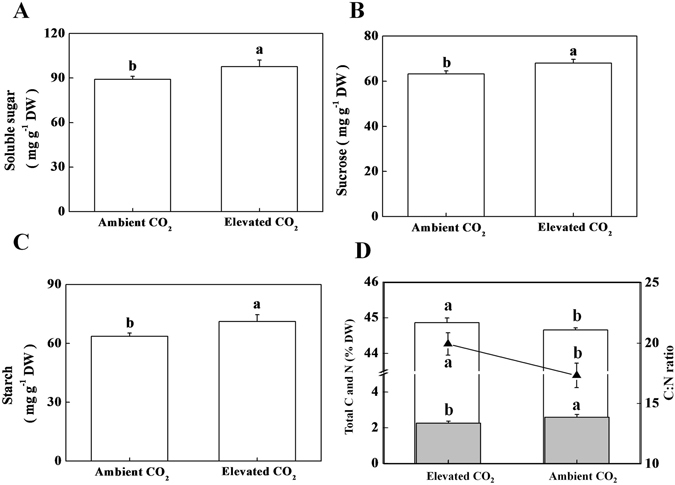
Effect of ambient (380 µmol mol−1) or elevated CO2 concentration (800 µmol mol−1) on carbohydrate, carbon and nitrogen concentration in tea leaves. (A) Soluble sugar concentration, (B) Sucrose concentration, (C) Starch concentration, and (D) Total carbon, total nitrogen and C:N ratio. Histograms with solid fill color (■) and without fill color (□) represent total nitrogen and total carbon, respectively, while line graph (-▲-) represents C: N ratio. Leaf samples were harvested after exposure of tea plants to different concentrations of atmospheric CO2 for 24 days. Data are the means of four replicates (±SD). Mean denoted by different letters indicate significant differences between the treatments (P < 0.05).
Effect of CO2 enrichment on tea quality attributes
Impact of elevated CO2 on tea quality attributes is largely unknown. We determined key bioactive compounds in tea leaves that are responsible for tea quality. Under elevated CO2, total tea polyphenol and amino acid concentration increased by 28.21 and 13.49%, respectively (Fig. 4A and B), while caffeine concentration decreased by 23.64% as compared with that under ambient CO2 (Fig. 4D). We also quantified individual catechins and amino acids concentrations in tea leaves. Results showed that (-)-gallocatechin (GC) and (-)-catechin (C) concentrations were not altered by CO2 enrichment; however, (-)-epigallocatechin (EGC) and (-)-epigallocatechin-3-gallate (EGCG) concentrations were significantly increased following CO2 enrichment, resulting in an overall increase in total catechins content under elevated CO2 (Fig. 4C). Likewise, individual amino acid concentration was differentially modulated by elevated CO2 in tea leaves (Table 2). The concentrations of aspartic acid, theanine, proline, alanine and phenylalanine increased, while that of threonine and serine decreased following exposure of tea plants to elevated CO2. Meanwhile, the concentrations of glutamic acid, glycine, valine, isoleucine, tyrosine, histidine, lysine and arginine were not affected by CO2 enrichment treatment (Table 2).
Figure 4.
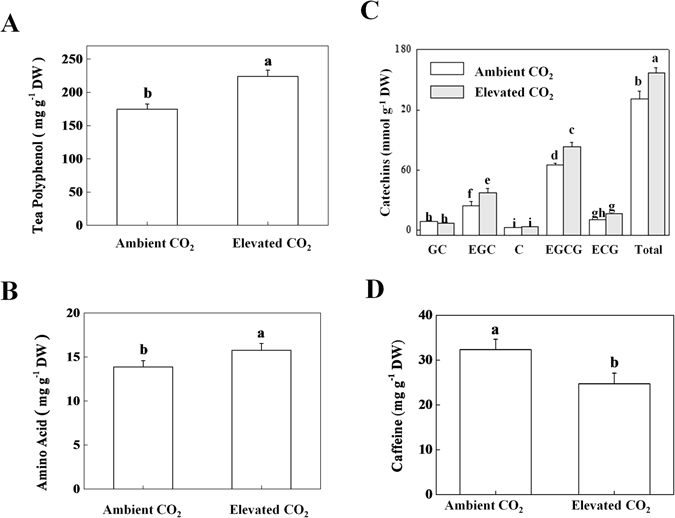
Changes in polyphenol, amino acid, and caffeine concentration in leaves of tea seedlings grown at ambient (380 µmol mol−1) or elevated CO2 concentration (800 µmol mol−1). (A) Total tea polyphenol, (B) total amino acids, (C) Individual catechins, and (D) Caffeine concentrations. Leaf samples were harvested after exposure of tea plants to different concentrations of atmospheric CO2 for 24 days. Data are the means of four replicates (±SD). Mean denoted by different letters indicate significant differences between the treatments (P < 0.05).
Table 2.
Effect of elevated CO2 concentration (800 µmol mol−1 for 24 d) on amino acids concentration in tea leaves.
| Amino acids (mg g−1 DW) | Ambient CO2 | Elevated CO2 |
|---|---|---|
| Aspartic acid (Asp) | 1.124 ± 0.013 b | 1.730 ± 0.029 a |
| Threonine(Thr) | 0.672 ± 0.005 a | 0.448 ± 0.007 b |
| Serine(Ser) | 0.593 ± 0.006 a | 0.467 ± 0.007 b |
| Glutamic acid(Glu) | 1.394 ± 0.021 a | 1.812 ± 0.017 a |
| L-Theanine (Thea) | 14.336 ± 0.571 b | 22.624 ± 0.685 a |
| Proline (Pro) | 0.535 ± 0.011 b | 0.827 ± 0.009 a |
| Glycine(Gly) | 0.224 ± 0.008 a | 0.218 ± 0.005 a |
| Alanine(Ala) | 0.448 ± 0.015 b | 0.677 ± 0.022 a |
| Valine (Val) | 0.856 ± 0.014 a | 0.861 ± 0.009 a |
| Isoleucine (Ile) | 0.433 ± 0.008 a | 0.427 ± 0.014 a |
| Tyrosine (Tyr) | 0.841 ± 0.031 a | 0.863 ± 0.024 a |
| Phenylalanine(Phe) | 0.540 ± 0.011 b | 0.618 ± 0.019 a |
| Histidine (His) | 0.224 ± 0.004 a | 0.228 ± 0.006 a |
| Lysine (Lys) | 0.448 ± 0.010 a | 0.453 ± 0.014 a |
| Arginine (Arg) | 0.672 ± 0.013 a | 0.683 ± 0.009 a |
Mean denoted by different letters indicate significant differences between the treatments (P < 0.05). DW, Dry weight.
Changes in the expressions of catechin, caffeine and theanine synthesis genes under elevated CO2
As we found an increased catechins concentration under elevated CO2, we anticipated that increased concentration of catechins might be attributed to increased biosynthesis of catechins. Therefore, we analyzed expression of key genes in catechins synthesis pathway, such as PHENYLALANINE AMMONIA-LYASE (CsPAL), CINNAMATE 4-HYDROXYLASE (CsC4H), P-COUMARATE:COA LIGASE (Cs4CL), CHALCONE SYNTHASE (CsCHS), CHALCONE ISOMERASE (CsCHI), FLAVANONE 3-HYDROXYLASE (CsF3H), DIHYDROFLAVONOL 4-REDUCTASE (CsDFR), ANTHOCYANIDIN SYNTHASE (CsANS), UDP- GLUCOSE FLAVONOID 3-O-GLUCOSYL TRANSFERASE (CsUFGT), ANTHOCYANIDIN REDUCTASE (CsANR) and LEUACOANTHOCYANIDIN REDUCTASE (CsLAR) by real-time quantitative polymerase chain reaction (qPCR). As shown in Fig. 5, elevated CO2 treatment caused an induction in the gene expression in all steps of the catechins biosynthetic pathway except for CsLAR. For instance, gene expression levels of CsPAL and CsANR, the first and last regulatory genes, respectively, in catechins biosynthetic pathway were upregulated by 5 fold under elevated CO2 as compared with that under ambient CO2. In contrast, transcript of CsLAR was down-regulated by 50% under elevated CO2. Transcript data are more or less in accordance with the endogenous content of individual catechins, implying that elevated CO2 influences catechins biosynthesis at transcription level.
Figure 5.
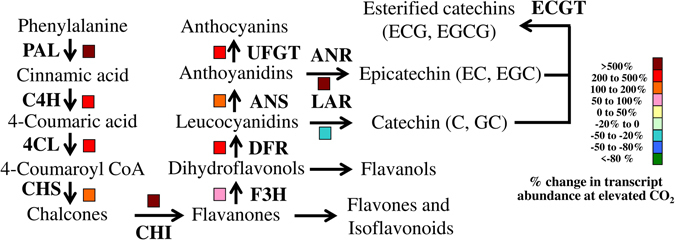
Transcript levels of catechins synthetic pathway-related genes in tea leaves as influenced by ambient (380 µmol mol−1) or elevated CO2 concentration (800 µmol mol−1). Leaf samples were harvested at 24 days following exposure of tea seedlings to different atmospheric CO2 concentrations. Expression levels of genes were analyzed by qPCR using gene-specific primer pairs (Supplementary Table S1). Four biological replicates were used for qPCR analysis.
Theanine is the major tea amino acids accounting for more than 50% of total free amino acid in tea13. To assess whether increased amino acid content under elevated CO2 was attributed to theanine biosynthesis, we analyzed the key genes of theanine synthesis pathway such as GLUTAMINE SYNTHETASE (CsGS), GLUTAMINE: 2-OXOGLUTARATE AMINOTRANSFERASE (CsGOGAT) and THEANINE SYNTHASE (CsTS). Except for CsGOGAT, expression levels of CsGS and CsTS were upregulated under elevated CO2, indicating that CO2 enrichment induced transcription of theanine biosynthetic genes that not only increased content of theanine, but also promoted total free amino acid content in tea leaves (Fig. 6).
Figure 6.
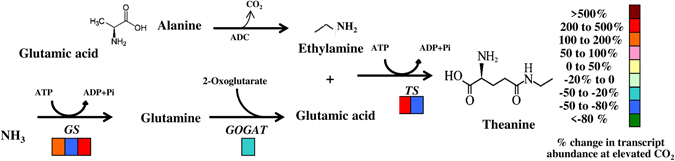
Expression of theanine synthetic pathway-related genes in tea leaves as influenced by ambient (380 µmol mol−1) or elevated CO2 concentration (800 µmol mol−1). Leaf samples were harvested at 24 days following exposure of tea seedlings to different atmospheric CO2 concentrations. Expression levels of genes were analyzed by qPCR using gene-specific primer pairs (Supplementary Table S1). Four biological replicates were used for qPCR analysis.
Finally, we analyzed transcript levels of caffeine synthesis genes such as INOSINE 5’-MONOPHOSPHATE DEHYDROGENASE (TIDH), S-ADENOSYL-L-METHIONINE SYNTHASE (sAMS) and TEA CAFFEINE SYNTHASE 1 (TCS1) following exposure of tea seedling to elevated CO2 for 24 d. Unlike catechins and theanine, genes relating to caffeine synthesis were down-regulated under elevated CO2 (Fig. 7). For instance, transcription of TIDH, the gene involved in encoding TIDH that catalyzes degradation of adenine nucleotides (AMP route) to xanthosine AMP (XAMP route), was decreased by approx. 80% under elevated CO2. Likewise, expression of sAMS gene, which is typically involved in supplying S-adenosl-L-methionine (SAM) from methionine, was also down-regulated by 20–50% under elevated CO2 condition. Consistently, expression of TCS1 that encodes caffeine synthase, the enzyme that catalyzes final two conversions steps of caffeine biosynthesis, was down-regulated by 80% under elevated CO2. Down-regulated expression of caffeine biosynthetic genes under elevated CO2 was in full agreement with the decreased concentration of caffeine in tea leaves.
Figure 7.
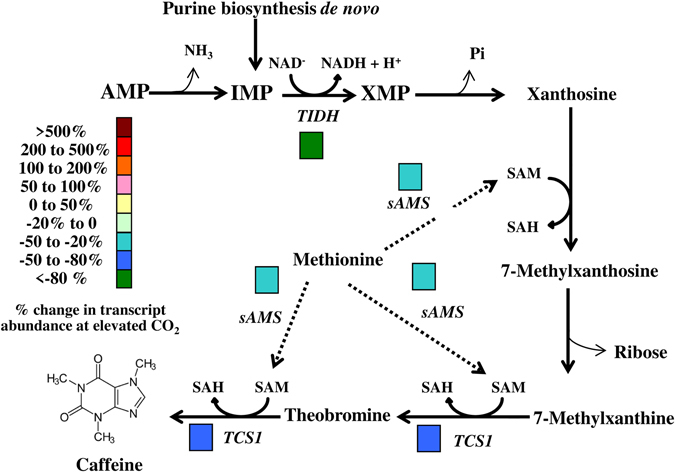
Transcriptional response of caffeine biosynthetic genes to elevated CO2 in tea leaves. Tea seedlings were exposed to either ambient (380 µmol mol−1) or elevated CO2 concentration (800 µmol mol−1) for 24 days. Expression levels of genes were analyzed by qPCR using gene-specific primer pairs (Supplementary Table S1). Four biological replicates were used for qPCR analysis.
Discussion
Rising atmospheric CO2 concentrations have a profound effect on plant growth, development and responses to stresses2, 3, 15, 16. While impact of elevated CO2 has been extensively studied in major food crops, its effect on yield and quality of important beverage crops such as tea remained largely unknown23. In this study, we exposed tea seedlings to elevated level of CO2 for a period of 24 days and monitored primary metabolism-related processes such as photosynthesis and respiration at different time-points. Results showed that CO2 enrichment improved both photosynthesis and respiration in tea plants, albeit a photosynthetic acclimation response was noticed after 6 day exposure. On one hand, elevated CO2 increased photosynthesis and respiration towards increased biomass accumulation, while one the other hand, enhancement in photosynthesis and respiration perhaps altered resource allocation towards secondary metabolism, leading to an increased biosynthesis of tea total polyphenols (TP), amino acids (AA), catechins and theanine, but a decreased content of caffeine. qPCR analysis of the catechins, theanine and caffeine biosynthetic genes further confirmed the stimulatory effects of elevated CO2 at transcriptional level. Our results suggest that rising CO2, a driving force of climate change not only improves primary metabolism, but also promotes secondary metabolism towards production of a quality green tea.
In Arabidopsis, elevated CO2 causes a metabolic perturbation that compels plants to increase its functions or activity by consuming or storing photoassimilates16. In the current study, elevated CO2 might also increase production and consumption of photoassimilates in tea plants by enhancing net photosynthesis and respiration rate, respectively (Figs 1–3). It is to be noted that an enhancement in photosynthesis under elevated CO2 could provide increased levels of substrates for glycolysis and a significant increase in TCA cycle intermediates might contribute to increased C-partitioning to respiration or for other relevant anabolic pathways16. However, a photosynthetic acclimation response was noticed following 12 day CO2 enrichment (Fig. 1). Earlier studies showed that exposure of plants to long-term CO2 enrichment may induce photosynthetic acclimation24, which is in agreement with our current observation. The acclimation response of Pn, was more or less accompanied with values of ΦPSII, Vcmax and Jmax. As Pn is dependent on RuBisCO carboxylation and RuBP regeneration rate6, 15, a close association between Pn, Vcmax and Jmax suggests that elevated CO2 perhaps stimulates RuBisCO carboxylation and RuBP regeneration rate to positively affect CO2 assimilation rate. Importantly, elevated CO2 increased plant growth in tea plants (Table 1). An increased plant growth due to elevated CO2 may stimulate growth respiration proportionally6. In addition, an enhancement in photosynthesis by elevated CO2 may increase carbohydrate availability and energy demand which necessitate plant to increase its respiration rate5. Therefore, the enhanced respiration rate under elevated CO2 was attributed to increased photosynthetic rate in tea plants (Fig. 1).
In the current study, CO2 enrichment remarkably increased contents of polyphenols including catechins (Fig. 4). The biosynthesis of catechins through phenylpropanoid and flavonoid pathways is dependent on the primary metabolism that supplies initial compounds required to run phenylpropanoid pathway14, 25. We found that elevated CO2 increased primary metabolites such as sugar, sucrose and starch in tea leaves (Fig. 3A–C). Moreover, carbon to nitrogen ratio was increased in tea leaves under elevated CO2 (Fig. 3D). As per carbon-nutrient balance theory, CO2 enrichment increases the carbon to nitrogen ratio and thus a greater amount of carbohydrates can be allocated to secondary metabolism in plants26. In addition, many experimental studies have shown that elevated CO2 conditions increase carbon-rich structural compounds and secondary metabolites in a range of plant species1, 10, 15, 16. It is worth mentioning that catechins are C-rich secondary metabolites. As C capture through photosynthesis was remarkably induced under elevated CO2, it is highly likely that increased C supply towards secondary metabolic pathway can be a potential reason for increased production of C-based secondary metabolites such as catechins under elevated CO2 condition.
To get a better insight into elevated CO2-modulated catechins biosynthesis, we analyzed the transcript levels of key genes of catechins biosynthetic pathway. The first committed step in the biosynthesis of catechins, is deamination of L-phenylalanine to trans cinnamic acid, catalyzed by the enzyme PAL. PAL is encoded by CsPAL in tea25. In the current study, consistent with catechins content, gene expression level of CsPAL was upregulted by 5-fold under elevated CO2 condition (Fig. 5). In tobacco, elevated CO2 (1000 ppm) significantly increased activity of PAL at both lower- and higher N-supply10. However, the effect of elevated CO2 on PAL activity was more pronounced at the lower N-supply. In case of tea, N-deficiency leads to increased accumulation of catechins especially epicatechins, which was associated with upregulated expression of CsPAL and other key genes (CsCHS, CsCHI, CsDFR, CsANS and CsANR) in catechin biosynthetic pathway27. In Arabidopsis, effect of short-term elevated CO2 on expression of genes involved in nitrogen metabolism may resemble the perturbation caused by N-deficiency16. In the current study, total nitrogen concentration in tea leaves was decreased under elevated CO2 (Fig. 3D). Therefore, it is quite plausible that elevated CO2-induced enhanced photosynthesis and/or perturbed N-metabolism might lead to increased production of catechins in tea plants.
Notably, except for CsLAR, other key regulatory genes in catechins biosynthetic pathway such as CsC4H, Cs4CL, CsCHS, CsCHI, CsF3H, CsDFR, CsANS, CsUFGT and CsANR all were upregulated under elevated CO2 (Fig. 5). At the final step of catechins biosynthesis, CsLAR catalyzes conversion of leucocyanidins into catechins (C, GC), while CsANR catalyzes conversion of anthoyanidins into epicatechins (EC, EGC)25. In line with suppression of CsLAR expression, the concentrations of GC and C were slightly decreased or remained unaltered, respectively under elevated CO2 in tea leaves (Fig. 4C). By contrast, upregulation of CsANR under elevated CO2 resulted in increased EGC concentration. Subsequently, gallylation of epicatechins caused an increased accumulation of EGCG and ECG under elevated CO2 conditions in tea leaves. As epicatechins constitute about 90% of total catechins in tea leaves, an enhancement in epicatechins content ultimately increased total catechins content under elevated CO2 28. In albino tea plants, the expression levels of CsPAL, CsF3H and CsFLS are correlated with the endogenous concentration of catechins, where PAL is considered as a core regulator that controls biosynthesis of catechins29. In our study, elevated CO2 which is an important environmental cue, might directly or indirectly influence the transcription of all key genes of catechins biosynthetic pathway including CsPAL and thus resulted in increased levels of epicatechins and total catechins in tea leaves (Fig. 4C).
Furthermore, total amino acid and theanine concentrations increased in tea leaves when grown under elevated CO2 condition (Fig. 4, Table 2). The concentration of theanine is closely associated with the expression of its key biosynthetic genes namely TS1 and TS2 that encode theanine synthetase30. In addition, other two enzymes such as glutamine synthetase (GS) and glutamine: 2-oxoglutarate aminotransferase (GOGAT) catalyze the initial steps of NH3 assimilation into glutamic acid, are also considered as key determinant of theanine biosynthesis. In the current study, elevated CO2 sharply induced gene expression levels of TS and GS in tea leaves, which eventually resulted in increased theanine concentration as compared with that in ambient CO2-grown tea plants. Environmental stresses such as salt treatment could influence theanine biosynthesis. Increased theanine content under salt treatment was found to be associated with increased expression of theanine synthetase protein in tea leaves31. qPCR data of theanine biosynthetic genes are well in accord with the content of theanine (Fig. 6, Table 2). For multi-faceted health benefits of theanine, high concentration of theanine in tea leaves is considered as a sign of good quality. Our results suggest that CO2 enrichment can be considered as a potential approach to enhance theanine concentration in tea.
By way of contrast, the caffeine content was dramatically decreased following exposure of tea plants to elevated CO2. Caffeine is N-rich secondary metabolite, and its biosynthesis depends on the flow of N-based compounds toward secondary metabolic pathway12. Previous studies showed that elevated CO2 sharply decreased the levels of N-rich secondary metabolites such as nicotine at limited N-supply in tobacco10. This effect was presumably related to changes in primary nitrogen metabolism, as elevated CO2 typically decreased nitrate, ammonium, amino acids and protein under low and intermediate N-supply. Although, we noticed a sharp decrease in concentration of caffeine and total nitrogen at elevated CO2 grown tea plants, concentration of total amino acids increased in tea leaves (Figs 3D and 4A and D). The possibility of direct or indirect suppression of caffeine synthesis due to altered N metabolism under elevated CO2 cannot be ignored. Previous reports also showed that shading substantially increased caffeine content in tea leaves, implying that environmental cue has remarkable effect on the biosynthesis of caffeine11. From qPCR analysis, it becomes evident that elevated CO2 sharply down-regulated key genes involved in the biosynthesis of caffeine. Suppression of sAMS could suppress methylation steps of caffeine biosynthesis. Because SAM functions as methyl donor in the three methylation steps (Xanthosine to 7-methylxanthosine, 7-Methylxanthine to theobromine and finally theobromine to caffeine) in the caffeine biosynthetic pathway (Fig. 7), whereas SAM is converted to S-adenosyl-L-homocysteine (SAH)12. Similarly, down-regulation of TIDH and TCS1, the first and the last regulatory genes in caffeine biosynthesis under elevated CO2 further confirmed the potential reasons of decreased caffeine concentration under elevated CO2 condition in tea leaves.
To sum up, elevated CO2 induced photosynthesis and subsequently contents of carbohydrates such as starch, sucrose and sugar (Figs 1 and 3). At the same time, respiration was also induced by elevated CO2 (Fig. 2). Since carbohydrate is utilized in the process of respiration to produce energy, pyruvate and some other intermediates8, which are used in some anabolic pathways such as biosynthesis of amino acid, it is highly possible that an increase in respiration eventually stimulates amino acid biosynthesis. Here, the contents of EGC, EGCG and theanine were induced by elevated CO2, while content of caffeine was decreased (Fig. 4). It is interpreted in the carbon-nutrient balance hypothesis that under elevated CO2, excess carbon products that are not required for primary metabolic functions, will be allocated for biosynthesis of secondary metabolites, which eventually result in increased carbon-based secondary metabolites and subsequently decreased N-based secondary metabolites in plants32. These also explain a potential reason of elevated CO2-induced increased catechins and decreased caffeine concentrations in our current study. Results of qPCR analysis of catechins, theanine and caffeine biosynthetic genes were in good agreement with the biochemical data (Figs 5–7). As low caffeine and high theanine contents are desired for a better quality tea, it is quite possible that rising CO2 may improve green tea quality in the face of climate change. It will be interesting to further explore the molecular mechanisms that cause such biochemical changes in tea leaves under elevated CO2 condition.
Materials and Methods
Plant material and growth conditions
Seedlings of Longjing 43, a well-known green tea (Camellia sisnensis L.) cultivar, were grown in pots. Two years old tea seedlings were exposed to atmospheric CO2 at either 380 μmol mol−1 or 800 μmol mol−1, corresponding to the “ambient CO2” and “elevated CO2” treatments, respectively, in controlled-environment growth chambers (Conviron, Winnipeg, Canada). The growth conditions were as follows: the photosynthetic photo flux density (PPFD)- 600 μmol m−2 s−1, photoperiod- 14/10 h (day/night), day/night air temperature- 26/22 °C and relative humidity- 80%. CO2 enrichment treatment lasted for 24 day, while data for photosynthesis- and respiration-related parameters were recorded at 0, 6, 12, 18 and 24 day. There were 80 seedlings under each treatment, which were placed in four randomized blocks, representing four replicates. Thus, each replicate consisted of 20 pots. Pot placement within specified CO2 condition was randomized every 2 day. Meanwhile, seedlings were fertilized with Hoagland’s nutrient solution every 2 day. For harvesting samples, young leaves were collected from each block and pooled together separately.
Estimation of photosynthesis, RuBisCO carboxylation capacity and ΦPSII
Net CO2 assimilation rate (Pn) was measured on 3rd fully expanded leaves using an open-flow infrared gas analyzer adapted with light and temperature control systems (Li-COR 6400, Li-COR, Lincoln, NE, USA). Following method of von Caemmerer and Farquhar33, rate of CO2 assimilation/intercellular CO2 concentration (A/Ci) curves were measured in which the leaf temperature and PPFD were maintained at 25 °C and 1800 µmol m−2 s−1, respectively. The maximum carboxylation rate of RuBisCO (V cmax) and maximum rates of RuBP regeneration (J max) were estimated by fitting a maximum-likelihood regression below and above the inflexion of the A/Ci response according to the method described by Ethier and Livingston34.
The photochemical efficiency of photosystem II (ΦPSII) was determined by an imaging pulse amplitude modulated (PAM) fluorimeter (IMAG-MAXI; Heinz Walz, Effeltrich, Germany) and calculated according to Genty et al.35.
Measurement of leaf respiration by O2 uptake
To determine leaf respiration, the O2 uptake by leaf segments was measured using a Clark-type liquid-phase oxygen electrode (Oxygraph-lab, Hansatech, UK)36. In brief, the plants were dark adapted for 30 min to avoid any light-enhanced photosynthesis; afterward, 0.1 g leaf samples were cut into pieces for measuring respiration at 25 °C in 2 mL of air-saturated 20 mM potassium phosphate buffer (pH 6.8). When oxygen uptake reached a constant rate, potassium cyanide (1 mM) or salicylhydroxamic acid (SHAM, 20 mM) was added for the estimation of cyanide (CN)- or SHAM-resistant respiration, respectively. Likewise, when a constant rate of O2 uptake was attained in the buffer without any reagents, the sucrose-induced leaf respiration was analyzed by adding 110 mM sucrose37.
Determination of tea polyphenols and total amino acids quantification
The harvested leaf samples were immediately placed into an oven run at 105 °C for 15 min and then transferred to 80 °C until they were completely dried. The powdered dry samples were used for determination of tea polyphenols and amino acids. Total tea polyphenols was extracted and determined spectrophotometrically according to the method described by the International Organization for Standardization (ISO) 14502-138. Gallic acid was used as standard. Briefly, the diluted sample extract (1.0 mL) was transferred to tubes in duplicate, where each tube contained 5.0 mL of a 1/10 dilution of Folin-Ciocalteu’s reagent in water. Afterward, 4.0 mL sodium carbonate solution (7.5% w/v) was added into each tube. The tubes were kept at room temperature for 60 min before absorbance at 765 nm was measured against water.
Amino acids from tea leaf sample (0.5 g) were extracted in 80% ethanol at 80 °C. Following evaporation, dried samples were dissolved in 0.02 N HCl. Amino acid content was determined using a Hitachi L-8900 amino acid analyzer (Hitachi, Japan). In brief, amino acids, separated by cation-exchange chromatography, were subjected to postcolumn reaction with ninhydrin reagent and detected spectrophotometrically as described previously elsewhere39.
Quantification of catechins, caffeine and individual amino acids
The concentrations of caffeine and catechins in the extract was determined with a HPLC system (Waters 590, Waters Corp., Milford, MA, USA) equipped with a Hypersil ODS2 C18 column (5 ml, 4.6 mm × 250 mm, 35 °C) at 280 nm as previously described39. Solvents A (2% acetic acid) and B (acetonitrile) were run in linear gradients with A decreasing from 93% to 55% within 20 min and maintained for 5 min thereafter at a rate of 1.4 mL min−1. The concentrations of caffeine and catechins were quantified by their peak areas against those of standards prepared from authentic compounds.
An automatic amino acid analyzer (Hitachi L-8900, Japan) was used to measure individual amino acids including theanine (Thea), phenylalanine (Phe), aspartic acid (Asp), arginine (Arg), threonine (Thr), serine (Ser), valine (Val), alanine (Ala), proline (Pro) and γ-aminobutyric acid (GABA). Amino acids were measured by adding 5 mL of tea extract with 5 mL of sulfosalicylic acid and centrifuging the mixture at 13000 rpm for 5 min to facilitate the reaction. The mixture was filtered through a 0.20 μm nylon filter membrane and run using the amino acid analyzer40, 41.
Determination of sugar, starch, total C and total N concentration
Soluble sugar and starch concentrations were determined by anthrone colorimetry in a spectrophotometer (SHIMADZU UV-2550, Kyoto, Japan) as described by Buysse and Merckx42. Total C and total N were measured by Vario MAX CN analyzer (Elementar Co. Ltd., Germany).
RNA isolation and real-time qPCR assay
Total RNA from tea leaves was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instruction. Genomic DNA in RNA samples was removed using a purifying column. Reverse transcription was done using Superscript II (Invitrogen) following the manufacturer’s protocol. The primers used for transcript analysis have been listed in Supplementary Table S1. qPCR analysis was carried out using the StepOnePlus Real-Time PCR system (Applied Biosystems, Foster City, CA, USA) with Power SYBR Green PCR Master Mix (Applied Biosystems). The PCR conditions consisted of denaturation at 95 °C for 3 min, followed by 40 cycles of denaturation at 95 °C for 30 s, annealing at 58 °C for 30 s and extension at 72 °C for 30 s. Transcript abundance was normalized to actin, and relative gene expression was calculated following formulae of Livak and Schmittgen43. Four biological replicates were used for qPCR analysis.
Statistical analysis
At least four independent replicates were conducted for each determination. The data were subjected to analysis of variance using SAS 8.0 software package (SAS Institute, Cary, NC), and the means were compared using Tukey’s test at the P < 0.05 level.
Electronic supplementary material
Acknowledgements
This work was supported by the Innovation Project of the Chinese Academy of Agricultural Sciences (CAAS-ASTIP-2015-TRICAAS) and the National Natural Science Foundation of China (project Nos 41171218, 31600561, 31550110201).
Author Contributions
X.L. and W.H. conceived and designed the research; X.L., G.A., Z.L., L.Z., J.W., C.S., P.Y. and L.Z. performed the experiments and analyzed the data; W.H. provided crucial reagents and supervised the study; G.A. and L.X. wrote the manuscript. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Xin Li and Lan Zhang contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-08465-1
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.IPCC. Climate change 2007: the physical science basis. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Solomon, S., Qin, D., Manning, M.,Chen, Z., Marquis, M., Averyt, K. B., Tignor, M., Miller, H. L. (Eds). Cambridge University Press, Cambridge, UK (2007).
- 2.Li X, et al. Carbon dioxide enrichment alleviates heat stress by improving cellular redox homeostasis through an ABA-independent process in tomato plants. Plant Biol. 2015;17:81–89. doi: 10.1111/plb.12211. [DOI] [PubMed] [Google Scholar]
- 3.Z., S. et al. Antagonism between phytohormone signalling underlies the variation in disease susceptibility of tomato plants under elevated CO2. J. Exp. Bot. 66, 1951–1963 (2015). [DOI] [PMC free article] [PubMed]
- 4.Li, X. et al. Decreased biosynthesis of jasmonic acid via lipoxygenase pathway compromised caffeine-induced resistance to Colletotrichum gloeosporioides under elevated CO2 in tea seedlings. Phytopathology (2016). [DOI] [PubMed]
- 5.Li X, et al. Stimulated leaf dark respiration in tomato in an elevated carbon dioxide atmosphere. Sci. Rep. 2013;3:3433. doi: 10.1038/srep03433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amthor JS. Terrestrial higher-plant response to increasing atmospheric CO2 in relation to the global carbon cycle. Glob. Change Biol. 1995;1:243–274. doi: 10.1111/j.1365-2486.1995.tb00025.x. [DOI] [Google Scholar]
- 7.Lewis CE, Noctor G, Causton D, Foyer CH. Regulation of assimilate partitioning in leaves. Aust. J. Plant Physiol. 2000;27:507–519. [Google Scholar]
- 8.Amthor, J.S. Plant respiratory responses to elevated carbon dioxide partial pressure. Adv. Carbon Dioxide Eff. Res. 35–77 (1997).
- 9.Brooks A, Farquhar GD. Effect of temperature on the CO2/O2 specificity of ribulose-1,5-bisphosphate carboxylase/oxygenase and the rate of respiration in the light. Estimates from gas-exchange measurements on spinach. Planta. 1985;165:397–406. doi: 10.1007/BF00392238. [DOI] [PubMed] [Google Scholar]
- 10.Matros A, et al. Growth at elevated CO2 concentrations leads to modified profiles of secondary metabolites in tobacco cv. SamsunNN and to increased resistance against infection with potato virus Y. Plant Cell Environ. 2006;29:126–137. doi: 10.1111/j.1365-3040.2005.01406.x. [DOI] [PubMed] [Google Scholar]
- 11.Sun Y, Cao H, Yin J, Kang LE, Ge F. Elevated CO2 changes the interactions between nematode and tomato genotypes differing in the JA pathway. Plant Cell Environ. 2010;33:729–739. doi: 10.1111/j.1365-3040.2010.02118.x. [DOI] [PubMed] [Google Scholar]
- 12.Ashihara H, Sano H, Crozier A. Caffeine and related purine alkaloids: biosynthesis, catabolism, function and genetic engineering. Phytochemistry. 2008;69:841–856. doi: 10.1016/j.phytochem.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 13.Mu W, Zhang T, Jiang B. An overview of biological production of L-theanine. Biotechnol. Advan. 2015;33:335–342. doi: 10.1016/j.biotechadv.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Tounekti T, Joubert E, Hernández I, Munné-Bosch S. Improving the polyphenol content of tea. Crit. Rev. Plant Sci. 2013;32:192–215. doi: 10.1080/07352689.2012.747384. [DOI] [Google Scholar]
- 15.Ghasemzadeh A, Jaafar HZ. Effect of CO2 enrichment on synthesis of some primary and secondary metabolites in ginger (Zingiber officinale Roscoe) Int. J. Mol. Sci. 2011;12:1101–1114. doi: 10.3390/ijms12021101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li P, et al. Arabidopsis transcript and metabolite profiles: ecotype-specific responses to open-air elevated [CO2] Plant Cell Environ. 2008;31:1673–1687. doi: 10.1111/j.1365-3040.2008.01874.x. [DOI] [PubMed] [Google Scholar]
- 17.Kaneko S, Kumazawa K, Masuda H, Henze A, Hofmann T. Molecular and sensory studies on the umami taste of Japanese green tea. J. Agric. Food Chem. 2006;54:2688–2694. doi: 10.1021/jf0525232. [DOI] [PubMed] [Google Scholar]
- 18.Bordoni A, et al. Green tea protection of hypoxia/reoxygenation injury in cultured cardiac cells. J. Nutr. Biochem. 2002;13:103–111. doi: 10.1016/S0955-2863(01)00203-0. [DOI] [PubMed] [Google Scholar]
- 19.Too JC, Kinyanjui T, Wanyoko JK, Wachira FN. Effect of sunlight exposure and different withering durations on theanine levels in tea (Camellia sinensis) Food. Nutr. Sci. 2015;06:1014–1021. doi: 10.4236/fns.2015.611105. [DOI] [Google Scholar]
- 20.Filho O, Mazzafera P. Caffeine does not protect coffee against the leaf miner Perileucoptera coffeella. J. Chem. Ecol. 2000;26:1447–1464. doi: 10.1023/A:1005587725704. [DOI] [Google Scholar]
- 21.Li Z-X, et al. Developmental changes in carbon and nitrogen metabolism affect tea quality in different leaf position. Plant Physiol. Biochem. 2016;106:327–335. doi: 10.1016/j.plaphy.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 22.Glade MJ. Caffeine-Not just a stimulant. Nutrition. 2010;26:932–938. doi: 10.1016/j.nut.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Larson C. Reading the tea leaves for effects of climate change. Science. 2015;348:953–954. doi: 10.1126/science.348.6238.953. [DOI] [PubMed] [Google Scholar]
- 24.Goufo P, et al. Rice (Oryza sativa L.) phenolic compounds under elevated carbon dioxide (CO2) concentration. Environ. Exp. Bot. 2014;99:28–37. doi: 10.1016/j.envexpbot.2013.10.021. [DOI] [Google Scholar]
- 25.Rani A, Singh K, Ahuja PS, Kumar S. Molecular regulation of catechins biosynthesis in tea [Camellia sinensis (L.) O. Kuntze] Gene. 2012;495:205–210. doi: 10.1016/j.gene.2011.12.029. [DOI] [PubMed] [Google Scholar]
- 26.Ibrahim MH, Jaafar HZ. Enhancement of leaf gas exchange and primary metabolites under carbon dioxide enrichment up-regulates the production of secondary metabolites in Labisia pumila seedlings. Molecules. 2011;16:3761–3777. doi: 10.3390/molecules16053761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan K, Fan D, Ding Z, Su Y, Wang X. Cs-miR156 is involved in the nitrogen form regulation of catechins accumulation in tea plant (Camellia sinensis L.) Plant Physiol. Biochem. 2015;97:350–360. doi: 10.1016/j.plaphy.2015.10.026. [DOI] [PubMed] [Google Scholar]
- 28.Hong G, et al. Biosynthesis of catechin components is differentially regulated in dark-treated tea (Camellia sinensis L.) Plant Physiol. Biochem. 2014;78:49–52. doi: 10.1016/j.plaphy.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 29.Xiong L, et al. Dynamic changes in catechin levels and catechin biosynthesis-related gene expression in albino tea plants (Camellia sinensis L.) Plant Physiol. Biochem. 2013;71:132–143. doi: 10.1016/j.plaphy.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 30.Deng W-W, Ogita S, Ashihara H. Biosynthesis of theanine (γ-ethylamino-l-glutamic acid) in seedlings of Camellia sinensis. Phytochem. Lett. 2008;1:115–119. doi: 10.1016/j.phytol.2008.06.002. [DOI] [Google Scholar]
- 31.Deng WW, Wang S, Chen Q, Zhang ZZ, Hu XY. Effect of salt treatment on theanine biosynthesis in Camellia sinensis seedlings. Plant Physiol. Biochem. 2012;56:35–40. doi: 10.1016/j.plaphy.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Bryant JP, Chapin FS, Klein DR. Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos. 1983;40:357–68. doi: 10.2307/3544308. [DOI] [Google Scholar]
- 33.von Caemmerer S, Farquhar GD. Some Relationships between the biochemistry of photosynthesis and the gas-exchange of leaves. Planta. 1981;153:376–387. doi: 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
- 34.Ethier GJ, Livingston NJ. On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar-von Caemmerer-Berry leaf photosynthesis model. Plant Cell Environ. 2004;27:137–153. doi: 10.1111/j.1365-3040.2004.01140.x. [DOI] [Google Scholar]
- 35.Genty B, Briantais JM, Baker NR. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta-Gen. Subj. 1989;990:87–92. doi: 10.1016/S0304-4165(89)80016-9. [DOI] [Google Scholar]
- 36.Millenaar FF, Gonzalez‐Meler MA, Siedow JN, Wagner AM. & Lambers, H. Role of sugars and organic acids in regulating the concentration and activity of the alternative oxidase in Poa annua roots. J. Exp. Bot. 2002;53:1081–1088. doi: 10.1093/jexbot/53.371.1081. [DOI] [PubMed] [Google Scholar]
- 37.Noguchi K, Nakajima N, Terashima I. Acclimation of leaf respiratory properties in Alocasia odora following reciprocal transfers of plants between high- and low-light environments. Plant Cell Environ. 2001;24:831–839. doi: 10.1046/j.1365-3040.2001.00728.x. [DOI] [Google Scholar]
- 38.ISO 14502-1. Determination of substances characteristic of green and black tea. Part 1: Content of total polyphenols in tea. Colorimetric method using Folin-Ciocalteu reagent. Switzerland (2005).
- 39.Chen XH, et al. Photosynthesis, yield, and chemical composition of Tieguanyin tea plants (Camellia sinensis (L.) O. Kuntze) in response to irrigation treatments. Agric. Water Manage. 2010;97:419–425. doi: 10.1016/j.agwat.2009.10.015. [DOI] [Google Scholar]
- 40.Su YL, Leung LK, Huang Y, Chen ZY. Stability of tea theaflavins and catechins. Food Chem. 2003;83:189–195. doi: 10.1016/S0308-8146(03)00062-1. [DOI] [Google Scholar]
- 41.Wang HF, Tsai YS, Lin ML, Ou ASM. Comparison of bioactive components in GABA tea and green tea produced in Taiwan. Food Chem. 2006;96:648–653. doi: 10.1016/j.foodchem.2005.02.046. [DOI] [Google Scholar]
- 42.Buysse J, Merckx R. An improved colorimetric method to quantify sugar content of plant tissue. J. Exp. Bot. 1993;44:1627–1629. doi: 10.1093/jxb/44.10.1627. [DOI] [Google Scholar]
- 43.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


