Abstract
Background and Purpose
The non‐psychoactive phytocannabinoid cannabidiol (CBD) can affect the pharmacological effects of Δ9‐tetrahydrocannabinol (THC). We tested the possible synergy between CBD and THC in decreasing mechanical sensitivity in a mouse model of paclitaxel‐induced neuropathic pain. We also tested the effects of CBD on oxaliplatin‐ and vincristine‐induced mechanical sensitivity.
Experimental Approach
Paclitaxel‐treated mice (8.0 mg·kg−1 i.p., days 1, 3, 5 and 7) were pretreated with CBD (0.625–20.0 mg·kg−1 i.p.), THC (0.625–20.0 mg·kg−1 i.p.) or CBD + THC (0.04 + 0.04–20.0 + 20.0 mg·kg−1 i.p.), and mechanical sensitivity was assessed on days 9, 14 and 21. Oxaliplatin‐treated (6.0 mg·kg−1 i.p., day 1) or vincristine‐treated mice (0.1 mg·kg−1 i.p. days 1–7) were pretreated with CBD (1.25–10.0 mg·kg−1 i.p.), THC (10.0 mg·kg−1 i.p.) or THC + CBD (0.16 mg·kg−1 THC + 0.16 mg·kg−1 CBD i.p.).
Key Results
Both CBD and THC alone attenuated mechanical allodynia in mice treated with paclitaxel. Very low ineffective doses of CBD and THC were synergistic when given in combination. CBD also attenuated oxaliplatin‐ but not vincristine‐induced mechanical sensitivity, while THC significantly attenuated vincristine‐ but not oxaliplatin‐induced mechanical sensitivity. The low dose combination significantly attenuated oxaliplatin‐ but not vincristine‐induced mechanical sensitivity.
Conclusions and Implications
CBD may be potent and effective at preventing the development of chemotherapy‐induced peripheral neuropathy, and its clinical use may be enhanced by co‐administration of low doses of THC. These treatment strategies would increase the therapeutic window of cannabis‐based pharmacotherapies.
Abbreviations
- CB1
cannabinoid 1 receptor subtype
- CB2
cannabinoid 2 receptor subtype
- CBD
cannabidiol
- CIPN
chemotherapy‐induced peripheral neuropathy
- Eadd
predicted additive effect level
- Eobs
actual effect level observed
- THC
δ9‐tetrahydrocannabinol
Tables of Links
| TARGETS | |
|---|---|
| GPCRs a | Voltage–gated ion channels b |
| CB1 receptor | TRPV1 channels |
| CB2 receptor | |
| 5‐HT1A receptor |
| LIGANDS | |
|---|---|
| Cannabidiol | SR144528 |
| Cisplatin | Δ9‐Tetrahydrocannabinol |
| Paclitaxel | Vincristine |
| SR141716 | WIN55,212 |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,bAlexander et al., 2015a,b).
Introduction
Chemotherapy‐induced peripheral neuropathy (CIPN) is a serious dose‐limiting adverse effect associated with several commonly used chemotherapeutic agents, including taxanes, platinum agents and vinca alkaloids. The exact mechanism of CIPN has not been fully elucidated and can differ across classes of chemotherapeutic agents. In general, these agents probably initiate toxicity by affecting cellular microtubules, disrupting mitochondrial function and/or impairing DNA synthesis. These assaults on peripheral nerves can lead to peripheral and central nitroxidative stress and inflammation, sensitization and spontaneous activity of peripheral nerve fibres and hyperexcitability in the dorsal column of the spinal cord (Hu and McLachlan, 2002), leading to ascending pain pathway sensitization (Peters et al., 2007). In most cases, CIPN is only partly reversible with cessation of treatment and, in the worst cases, damage can be permanent. To date, no one drug or drug class is considered to be safe and effective for treatment of CIPN (Lynch et al., 2004). It is therefore necessary to identify novel therapies to prevent or treat CIPN.
Cannabinoids suppress neuropathic pain induced by traumatic nerve injury, toxic insults and metabolic changes (Guindon and Hohmann, 2008). We have recently reported that the non‐psychoactive phytocannabinoid cannabidiol (CBD) prevents the development of paclitaxel‐induced mechanical sensitivity in mice (Ward et al., 2011; Ward et al., 2014). The mixed CB1/ CB2 receptor agonist WIN55,212 also attenuates paclitaxel‐ and vincristine‐induced neuropathic pain through both CB1 and CB2 receptor mechanisms (Pascual et al., 2005; Rahn et al., 2007), while selective CB2 receptor agonism has also been shown to attenuate cisplatin‐ and paclitaxel‐induced neuropathic pain (Deng et al., 2012). While several of CBD's overt effects are similar to cannabinoids acting at CB1 and/or CB2 receptors, such as Δ9‐tetrahydrocannabinol (THC), CBD has very low affinity for these receptors. Instead, CBD interacts with several other receptors and intracellular messengers that have been identified as targets for its pharmacological effects. For example, we demonstrated that agonism at the 5‐HT1A receptor may underlie CBD's protective effect against development of paclitaxel‐induced mechanical sensitivity, as its protective effect was lost in the presence of the 5‐HT1A receptor antagonist WAY100635, but not the CB1 receptor antagonist SR141716 or the CB2 receptor antagonist SR144528 (Ward et al., 2014). Others have identified a role for the activation of TRPV1 channels (Comelli et al., 2008) and suppression of ROS and other inflammatory mediators (Costa et al., 2007) in the attenuation of CIPN by CBD, in rodent models.
Therefore, while CBD and cannabinoid receptor agonists both attenuate chemotherapy‐induced neuropathic pain in rodent models, these agents are likely to work through different sites of action. This suggests that when co‐administered, these compounds may interact in a manner that deviates from additive, supporting the commonly held ‘entourage effect’ concept that components of the marijuana plant interact synergistically in producing there pharmacological effects. Such potential interactions between the phytocannabinoids CBD and THC have been investigated since the 1970s in a range of animal models and with mixed results. By and large, these studies have focused on the ‘tetrad’ ofcannabinoid effects (catalepsy, locomotor activity, hypothermia and antinociception). These are classical CB1 receptor‐mediated behavioural pharmacological effects, reliably measured in rodents (Smith et al., 1994) that are typically not observed after administration of CBD. Therefore, these experiments are characterizing whether ineffective doses of CBD have no effect, increase or decrease, the effects of THC on these behaviours. Moderate to high doses of CBD, which are ineffective on their own, had no effect (Jones and Pertwee, 1972; Ham and De Jong, 1975), increased (Varvel et al., 2006; Hayakawa et al., 2008) or decreased (Welburn et al., 1976; Formukong et al., 1988) tetrad behaviours with little consistency between studies. Most pertinent to the present experiments, moderate to high (10–50 mg·kg−1) doses of CBD, lacking antinociceptive effects on their own, did increase the antinociceptive potency of THC, while lower doses (e.g. 3 mg·kg−1) did not (Karniol and Carlini, 1973; Varvel et al., 2006; Hayakawa et al., 2008). However, to our knowledge, no published studies have determined whether effective anti‐neuropathic doses of CBD and THC alone interact in an additive, synergistic or subadditive manner when combined. The drug Sativex (Nabixomols), a defined extract of the Cannabis plant that contains THC, CBD and a range of non‐cannabinoid chemicals native to the plant, was developed by GWPharma based on the tenet that the combination of THC, CBD and other chemicals in cannabis‐based medicinal extracts may produce a therapeutic effect greater than the sum of its parts. This is an exciting notion that needs to be empirically tested.
In the present set of experiments, we examined if the mixed CB1/CB2 receptor agonist THC was as potent and efficacious as CBD in preventing paclitaxel‐induced mechanical sensitivity alone and if the combination of CBD and THC produced synergistic effects. We also tested the efficacy of selected doses of CBD, THC and in combination, to prevent mechanical sensitivity following oxaliplatin or vincristine administration.
Methods
Animals
All animal care and experimental procedures complied with the guidelines of the Temple University Institutional Animal Care and Use Committee and were as humane as possible. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath & Lilley, 2015) and US NIH guidelines for reporting experiments involving animals. The total number of animals used was 344.
Male C57Bl6 mice weighing 18–20 g (Taconic Farms, Cranbury NJ, USA) were acclimatized to the temperature‐ and humidity‐controlled vivarium and housed in groups of four for at least 5 days before initiation of the study. Mice were housed under a reverse 12 h light/dark cycle (lights off 9:00 h), with access to food and water ad libitum. Following experimentation, all mice were killed by CO2 asphyxiation.
Mechanical allodynia
Paclitaxel study
Baseline sensitivity to mechanical stimuli was assessed prior to drug treatment using von Frey monofilaments (0.07–2.0 g) and the up‐down method, as described previously (Ward et al., 2011; Ward et al., 2014). Chemotherapeutic agents, cannabinoid compounds and/or vehicle were then administered on experimental days 1, 3, 5 and 7. Paclitaxel was given at a dose of 8.0 mg·kg−1 per injection. Fifteen minutes prior to paclitaxel injection, mice were first pretreated with vehicle or a range of CBD (0.625–20 mg·kg−1), THC (0.625–20 mg·kg−1) or CBD + THC combination (0.04 + 0.04–20.0 + 20.0 mg·kg−1) doses. Mechanical sensitivity was reassessed on days 9, 14 and 21. Three paclitaxel + vehicle groups were tested throughout the duration of the study: one alongside the CBD dose–response groups, one alongside the THC dose–response groups and one alongside the combination groups, to ensure that the effect of paclitaxel remained consistent throughout the course of the study.
Oxaliplatin study
Baseline sensitivity to mechanical stimuli was assessed prior to drug treatment in mice as described above. A dose of 6 mg·kg−1 oxaliplatin was administered once. CBD (1.25–10.0 mg·kg−1), THC (10.0 mg·kg−1) or THC + CBD (0.16 mg·kg−1 THC + 0.16 mg·kg−1 CBD) was administered 15 min prior to the single oxaliplatin injection. Mechanical sensitivity was reassessed on days 2, 4, 7 and 10.
Vincristine study
Baseline sensitivity to mechanical stimuli was assessed prior to drug treatment in mice as described above. A dose of 0.1 mg·kg−1·day−1 vincristine was administered once daily for 7 days. CBD (1.25–10.0 mg·kg−1), THC (10.0 mg·kg−1) or THC + CBD (0.16 mg·kg−1 THC + 0.16 mg·kg−1 CBD) was administered 15 min prior to each vincristine injection. Mechanical sensitivity was reassessed on days 5, 10, 15 and 22.
Mice were randomized to their treatment groups, and all behavioural experimenters were blinded to the treatment conditions of the mice during behavioural testing. One experimenter placed the mice in the allodynia testing cubbies for habituation, while the other experimenter conducted allodynia testing procedure outside the room. Mice are used because they are a common species used for this model, and we have used them in our CIPN research for the past decade. Sample sizes were n = 6–12 per group for all studies (n = 12 for combination experiment due to higher variability in the combination data), based on our and others’ past results. Dosing regimens for each chemotherapeutic agent differ based on the optimal dosing regimens to produce reliable and robust mechanical sensitivity based on published data from rodent models.
Data and statistical analysis
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). For statistical analyses and graphical presentation of data, a percent baseline sensitivity value for each mouse is calculated and the mean percent baseline sensitivity (±SEM) was determined for each treatment group. Baseline sensitivity for each mouse was calculated as their sensitivity threshold value on test day divided by their sensitivity threshold value at baseline multiplied by 100. Therefore, 100% baseline sensitivity represents no development of mechanical sensitivity following treatment, with lower % values equating to the development of increasing sensitivity to mechanical stimulation. One‐way repeated measures ANOVAs and Dunnett's post hoc comparisons (GraphPad Prism 6) were used to determine doses significantly different from vehicle treatment. P < 0.05 was used as the limit of statistical significance.
Additionally, in order to determine dose‐effect levels of the ascending limb of the CBD and THC dose‐effect curves, as well as the CBD + THC dose combinations, for dose‐equivalence analyses (see below), paclitaxel data from day 14 for each animal were also transformed into percent maximum possible effect (%MPE ±SEM). To determine %MPE for each mouse, the relevant paclitaxel group's percent baseline value is subtracted from the mouse's percent baseline value, as the paclitaxel effect will represent 0% MPE. In addition, a scaling multiplier is calculated by subtracting the paclitaxel group's percent baseline value from 100% and multiplying dividing this number into 100. The final calculation for each mouse is as follows: (percent baseline value − paclitaxel percent baseline value) × (100/100‐paclitaxel baseline value). For example, on day 14, the first‐run paclitaxel percent baseline average was 39.8%. The percent baseline sensitivity of a mouse in the 2.5 mg·kg−1 CBD group on day 14 was 71.4%. The above formula was used to determine the %MPE for that mouse (52.4%) when the paclitaxel value was set to 0%. Derived values exceeding 100% were capped at 100%, and derived values in the negative were assigned 0%. Linear regression analysis (GraphPad Prism 6) was then used to determine the ED50 values for the ascending limb of the CBD, THC and CBD + THC dose–response curves.
Dose‐equivalence analysis was used to predict the combined effects of CBD and THC, administered in a 1:1 ratio, based on their effects alone on the ascending limbs of their dose response curves. Briefly, in dose‐equivalence analysis, for each CBD dose, an effect‐equivalent dose of THC is identified. This matched THC dose is added to the actual THC dose in each combination to be tested so that the sum is the predicted effective dose for that combination. The test for significance for the difference between the predicted additive effect level (Eadd) and the actual effect level observed (Eobs) is based on Student's t distribution (see Tallarida, 2000, Tallarida, 2012) for details).
Materials
Paclitaxel solution (Teva Parenteral Medicines, dissolved in 1:1 mixture of alcohol and Cremophor), oxaliplatin (Teva Parenteral Medicines) and vincristine (Hospira Worldwide) were purchased from the Temple University Pharmacy (Philadelphia, PA, USA). Paclitaxel was then diluted with sterile saline to 0.8 mg·mL−1. Oxaliplatin was dissolved in water to a concentration of 0.6 mg·mL−1. Vincristine was dissolved in saline to a concentration of 0.01 mg·mL−1. Laboratory‐synthesized CBD was provided by INSYS Therapeutics, Inc. (Austin, TX) and THC was provided by the National Institute on Drug Abuse drug supply programme (Bethesda, MD, USA). CBD and THC were dissolved in a mixture of ethyl alcohol, Cremophor and saline; 1:1:18, v/v. All agents were injected i.p. in a volume of 10 μL·g−1 of body weight. For combination testing, CBD and THC were dissolved together into a single solution and administered by a single injection.
Results
Reproducibility of paclitaxel‐induced mechanical sensitivity
As we have reported previously, administration of paclitaxel (8.0 mg·kg−1 per injection, administered on experimental days 1, 3, 5 and 7) produced robust and reliable mechanical sensitivity in C57Bl/6 mice (Figure 1). This effect lasted for at least 21 days post‐initiation of administration with peak allodynic effects occurring between days 9 and 14. Because of the long duration of the study, multiple paclitaxel + vehicle groups were run to determine that paclitaxel administration was producing a consistent level of mechanical sensitivity across all phases of CBD and THC alone and combination testing.
Figure 1.
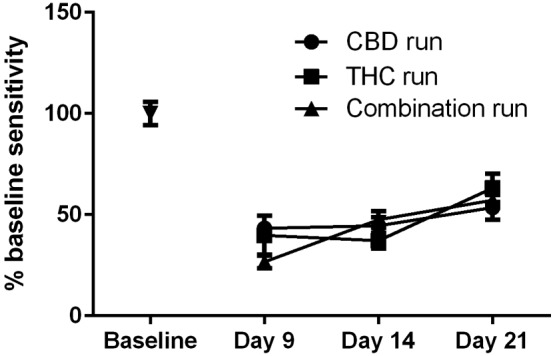
Effect of paclitaxel on mechanical sensitivity in three separate groups of mice on different days. Paclitaxel (8.0 mg·kg−1 per injection) is administered on experimental days 1, 3, 5 and 7 following baseline measurement of mechanical sensitivity. Three separate groups of paclitaxel‐treated mice were run in order to provide appropriate non‐cannabinoid treated control data along the length of the study. n = 8 per group.
Dose‐response effects of CBD or THC on paclitaxel‐induced mechanical sensitivity
Treatment with either CBD (Figure 2A) or THC (Figure 2B) produced a biphasic, dose‐related attenuation of paclitaxel‐induced mechanical sensitivity. For CBD treated groups, analysis of the results from day 9 with one‐way ANOVA indicated a significant effect of CBD treatment [F (8, 62) = 4.566]. Dunnett's multiple comparisons test indicated significant effects of the 1.0 and 20 mg·kg−1 doses of CBD. For the results from day 14, one‐way ANOVA indicated a significant effect of CBD treatment [F (8, 62) = 2.693] Dunnett's multiple comparisons test indicated significant effects of the 1.0 and 20 mg·kg−1 doses of CBD. Analysis of the results from day 21 indicated no significant effect of CBD treatment [one‐way ANOVA; F (8, 62) < 1.0].
Figure 2.
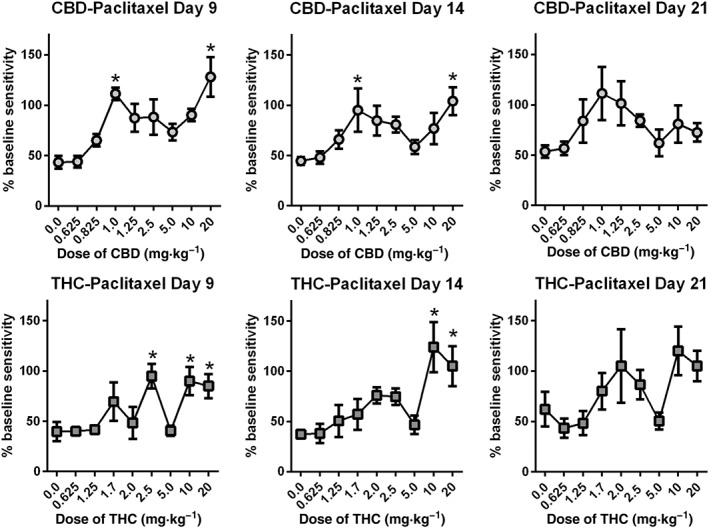
Effect of CBD or THC on paclitaxel‐induced mechanical sensitivity 9, 14 and 21 days after the start of treatment. Paclitaxel alone (8.0 mg·kg−1 per injection) or following pretreatment with CBD (A) or THC (B) (0.625–20 mg·kg−1 i.p.) was administered on experimental days 1, 3, 5 and 7, following baseline measurement of mechanical sensitivity. Mechanical sensitivity was tested 9, 14 and 21 days following initiation of treatment. n = 8 per group, with the exception of the 10 mg·kg−1 CBD group which was n = 7 due to one mouse found dead in home cage. *P < 0.05, significantly different from ??? ; Dunnett's multiple comparisons test.
For the THC treated groups, analysis of the results from day 9 indicated a significant effect of THC treatment [one‐way ANOVA ; F (7, 56) = 3.819]. Dunnett's multiple comparisons test indicated significant effect of the 2.5 and 10 mg·kg−1 doses of THC. Results from day 14 indicated a significant effect of THC treatment [one‐way ANOVA F (7, 56) = 2.810] and Dunnett's multiple comparisons test indicated significant effect of the 10 mg·kg−1 dose of THC. For Day 21, one‐way ANOVA indicated a nearly significant effect of THC treatment [F (7, 56) = 2.104, P = 0.058].
Dose‐response effects of CBD + THC combination on paclitaxel‐induced mechanical sensitivity
Treatment with CBD + THC in a 1:1 ratio based on dose produced a biphasic, dose‐related attenuation of paclitaxel‐induced mechanical sensitivity (Figure 3). This effect was more potent than either agent given alone. Analysis of the results from CBD + THC treatment on day 14, indicated a significant effect of CBD + THC treatment [one‐way ANOVA; F (10, 113) = 2.576,] and Dunnett's multiple comparisons test indicated a statistically significant effect of the combined 0.31 mg·kg−1 (0.16 mg·kg−1 CBD + 0.16 mg·kg−1 THC) dose compared with paclitaxel treatment alone. We focused on the day 14 combination effect because this is the time point of peak allodynia, but the combination treatments at days 9 and 21 showed a similar leftward shift of the dose‐response curve (data not shown).
Figure 3.
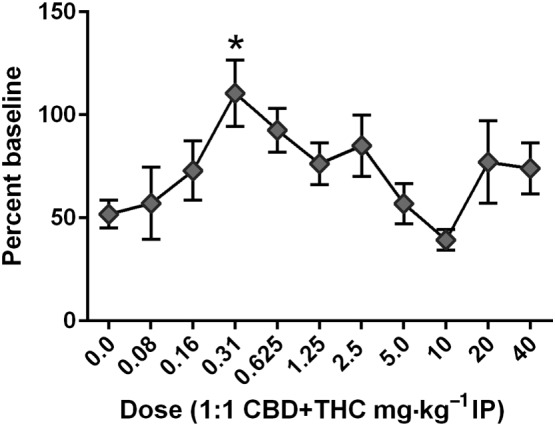
Effect of CBD + THC on paclitaxel‐induced mechanical sensitivity 14 days after the start of treatment. Paclitaxel alone (8.0 mg·kg−1 per injection) or following pretreatment with CBD + THC (combined doses 0.08–40.0 mg·kg−1 per injection i.p.) is administered on experimental days 1, 3, 5 and 7 following baseline measurement of mechanical sensitivity. n = 12 per group, with the xexception of the 10 mg·kg−1 dose, where two mice were removed from the study due to skin lesions from a dominant cage mate. *P < 0.05, significantly different from paclitaxel alone; one‐way ANOVA with Dunnett's multiple comparisons test.
Comparison of single, compared with combined, phytocannabinoid treatment on paclitaxel‐induced mechanical sensitivity
To determine whether the 1:1 CBD + THC combination produced protective effects in the CIPN model that deviated from simple additivity, predicted effect levels for these dose combinations were first determined from the dose effects of each agent alone. To do this, raw data for each animal from the initial ascending limbs of the CBD and THC dose‐response curves (0.625–2.5 mg·kg−1) were transformed into %MPE values, as described in the Methods. Linear regression analysis of the ascending limb indicated an ED50 for CBD of 1.014 mg·kg−1 and for THC of 1.8 mg·kg−1 (Figure 4A, B). To determine the predicted additive effect of doses of CBD and THC given in a 1:1 ratio, effect levels of each dose/drug in the combination were determined using the following dose effect equations derived for each for each drug: for CBD and for THC. These effect levels were added together to determine the Eadd for each dose combination tested (Figure 4C). The Eadds were then compared graphically and statistically to the actual effect levels determined by the experiment (Eobs, Figure 4C). Student's t‐test indicated a significant difference between the predicted additive and observed effect levels.
Figure 4.
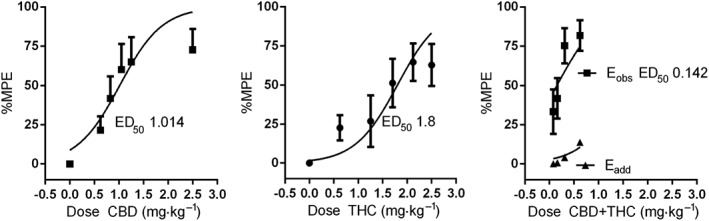
Linear regression analysis of ascending limbs of the observed single and combined agent dose–response curves and predictive additive curve. Data were transformed to %MPE (see text for details) in order to determine relative effective dose levels. Eobs is the observed dose effect levels of CBD + THC combinations along the ascending limb of the combination dose response curve. Eadd is the predicted additive dose effect levels of those same doses based on the ascending limb of the single agent dose response curves.
Effect of CBD on oxaliplatin‐induced mechanical sensitivity
The effect of CBD (1.25–10.0 mg·kg−1 i.p.) on oxaliplatin‐induced mechanical sensitivity was tested in 40 male C57Bl/6 mice. Two‐way ANOVA indicated a significant effect of time [F (3, 140) = 7.505] and treatment [F (4140) = 4.260,]. There was no significant interaction between time and treatment [F (12, 140) = 1.239 (Figure 5A) and Dunnett's post test indicated no specific significant differences for any dose of CBD, compared with oxaliplatin alone. The effect of 10.0 mg·kg−1 THC was also tested in a separate group of animals, and two‐way ANOVA indicated a significant effect of time [F (3, 48) = 5.780] and a non‐significant attenuated mechanical sensitivity [F (1, 48) = 2.912] (Figure 5B). The effect of the low dose combination 0.16 mg·kg−1 THC + 0.16 mg·kg−1 CBD produced a significant effect of treatment [F (1, 48) = 7.180] and of time [F (3, 48) = 7.155] (Figure 5C).
Figure 5.
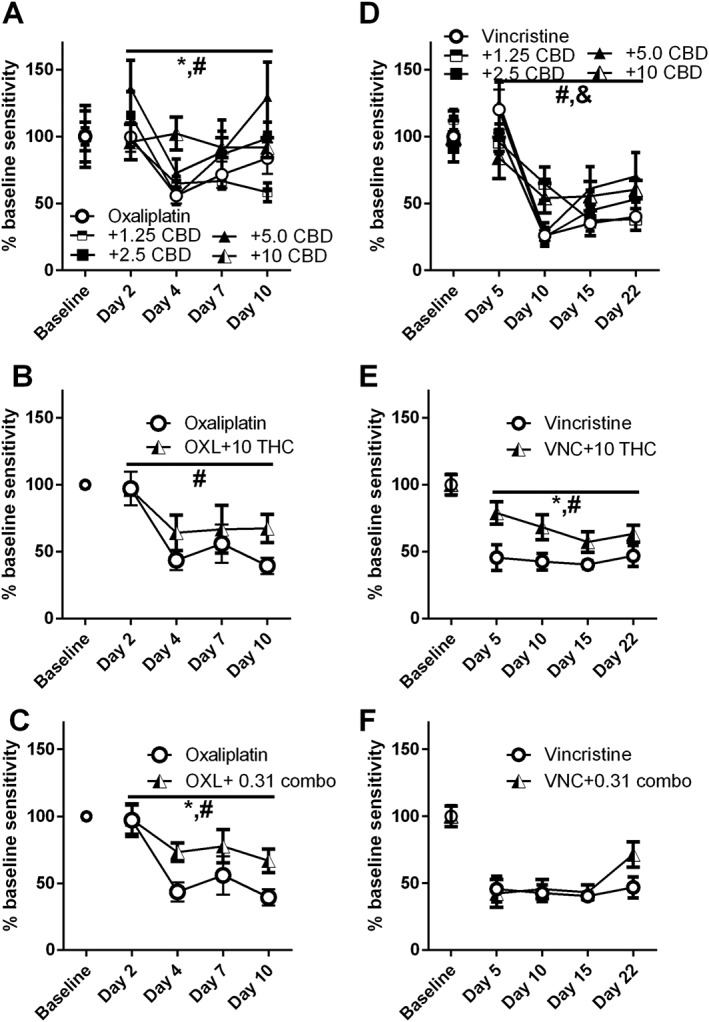
Effect of CBD, THC and their combination on oxaliplatin‐ or vincristine‐induced mechanical sensitivity. Oxaliplatin (6.0 mg·kg−1 i.p.) alone or following pretreatment with CBD (doses 1.25–10.0 mg·kg−1 per injection i.p.), THC (10.0 mg·kg−1 i.p.) or THC + CBD (0.16 mg·kg−1 THC + 0.16 mg·kg−1 CBD i.p.) was administered on experimental day 1 following baseline measurement of mechanical sensitivity. Vincristine (0.1 mg·kg−1 i.p.) alone or following pretreatment with CBD (doses 1.25–10.0 mg·kg−1 per injection i.p.), THC (10.0 mg·kg−1 i.p.) or THC + CBD (0.16 mg·kg−1 THC + 0.16 mg·kg−1 CBD i.p.) was administered on experimental days 1–7 following baseline measurement of mechanical sensitivity. n = 8 per group, with the exception of the oxaliplatin group in B and C, which is n = 6 due to two mice being killed because of urinary blockage. # P < 0.05, significant main effect of time; * P < 0.05, significant main effect of treatment; & P < 0.05, significant interaction.
Effect of CBD on vincristine‐induced mechanical sensitivity
The effect of CBD (1.25–10.0 mg·kg−1 ip) on vincristine‐induced mechanical sensitivity was tested in 40 additional male C57Bl/6 mice. Two‐way ANOVA indicated a significant effect of time [F (3, 140) = 32.00] but not of treatment [F (4, 140) = 1.163] (Figure 5D). The effect of 10.0 mg·kg−1 THC was also tested in a separate group of animals, and two‐way ANOVA indicated a significant effect of treatment [F (1, 56) = 18.82] (Figure 5E). The low dose combination (0.16 mg·kg−1 THC + 0.16 mg·kg−1 CBD) did not attenuate vincristine‐induced mechanical sensitivity [F (1,56) = 1.550] (Figure 5F).
Discussion
Previously, we have already demonstrated that pretreatment with the phytocannabinoid CBD prevented the development of behavioural symptoms of CIPN in a mouse mode (Ward et al., 2011, 2014). The main objectives of the present study were to demonstrate whether the primary phytocannabinoid THC shows similar potency and efficacy in the mouse CIPN model and to determine if CBD and THC interact in a manner deviating from additivity in this model. This is important because, while the entourage effects of cannabinoids working together as more than a sum of their parts have been anecdotally discussed at length, few rigorous studies have been executed to demonstrate such a phenomenon. These data in the present study were subjected to equivalence analyses to quantitatively determine whether the two dominant phytocannabinoids can work synergistically to prevent the development of neuropathic pain in a mouse model of CIPN.
Initially, it was critical to determine the relative potency of each compound alone to produce comparable anti‐allodynic effects. As shown in Figure 2, both phytocannabinoids show very similar triphasic dose–response curves with two apparent peaks in efficacy, one within a dose range of 1.0–2.5 mg·kg−1 and the other within the 10–20 mg·kg−1 range. Biphasic effects of cannabinoids have been reported (Grisham and Ferraro, 1972; Kwiatkowska et al., 2004; Margulies and Hammer, 1991), and one study has reported triphasic effects of THC on locomotor activity across a wide range of doses (Sanudo‐Pena et al., 2000). In female mice dosed daily with CBD for 14 days, paclitaxel‐induced mechanical sensitivity was attenuated at the 10 mg·kg−1 dose but not the 5.0 mg·kg−1 dose (Ward et al., 2011). In a follow‐up experiment, female mice were treated with CBD (2.5 or 5.0 mg·kg−1) only prior to each paclitaxel injection (days 1, 3, 5 and 7), and both CBD doses attenuated the development of paclitaxel‐induced mechanical sensitivity, but through a 5‐HT1A but not a CB1 or CB2 receptor‐mediated mechanism (Ward et al., 2014). Our present results with synthetic CBD (manufactured by INSYS Rx) showed that doses as low as 1.0 mg·kg−1 i.p. fully blocked the development of mechanical sensitivity, following paclitaxel administration in male mice. Taken together, potency differences we have obtained with CBD in the CIPN model may be dependent on sex as has been determined with THC (Tseng et al., 2004) or on source of compound. In the present study, efficacy was again observed at 10 and 20 mg·kg−1 i.p., showing a triphasic dose–response curve. Such effects of these higher doses have also been reported in other pain (Costa et al., 2004; Ward et al., 2011) and CNS injury models (Kwiatkoski et al., 2012). The reason(s) underlying these triphasic effects need to be further examined. One possibility, made more plausible by the fact that CBD has such a range of putative therapeutic sites of action, is that the two ascending limbs of the CBD dose–response curve represent the compound working by two distinct mechanisms. For example, we have previously demonstrated that the anti‐neuropathic effects of CBD (5.0 mg·kg−1 every other day for 4 days) may be mediated by agonist activity at the 5‐HT1A receptor, while Comelli et al. (2008) reported that these effects of CBD (10.0 mg·kg−1 daily for 14 days) may be mediated by the TRPV1 cation channel.
In the present model, THC produced triphasic effects, comparable to those observed for CBD. The 2.5 mg·kg−1 dose of THC fully blocked the development of mechanical sensitivity following paclitaxel administration, as did higher doses of 10 and 20 mg·kg−1. It is unlikely that motor impairment was responsible for these high dose effects of THC, as the final cannabinoid doses were given on day 7 and anti‐allodynic responses were measured up to day 21. We are unaware of a comprehensive preclinical study demonstrating dose–response effects of the partial CB1/CB2 receptor agonist THC in a rodent model of neuropathic pain, which is surprising given that targeting CB1 and CB2 receptors to produce antinociception and anti‐inflammation, respectively, has been investigated extensively in animal models. Previous studies demonstrated that full agonists at CB1 (Vera et al., 2013) and CB2 receptors (Deng et al., 2015) can attenuate chemotherapy‐induced neuropathic pain, but the primary mechanism of action of the partial mixed CB1/ CB2 receptor agonist THC in this model remains to be determined. Taken together, these experiments reveal that CBD and THC are roughly equipotent and equi‐effective at preventing the development of mechanical sensitivity induced by paclitaxel administration.
For the next phase of the study, a dose‐equivalence analysis experimental design (where combinations are paired by dose), as opposed to a dose‐addition analysis design (where combinations are paired by effect level), was implemented to model the cannabis extract Sativex, which contains nearly equal amounts of THC (2.7 mg) and CBD (2.5 mg) per spray delivery. When equal dose pairs of THC and CBD were administered to mice receiving paclitaxel, the cannabinoid combination treatment was more potent than either CBD or THC treatment alone. A combined dose of 0.32 mg·kg−1 (0.16 mg·kg−1 THC: 0.16 mg·kg−1 CBD) was fully effective at preventing paclitaxel‐induced mechanical sensitivity 14 days after initiation of drug administration. Linear regression analyses revealed an ED50 of 1.014 mg·kg−1 for CBD and for THC, an ED50 of 1.8 mg·kg−1 and, for the combination THC + CBD, a much decreased ED50 of 0.142 mg·kg−1. Interestingly, the combined dose‐effect curve also maintained its triphasic dose response function. These results reveal a significant interaction between THC and CBD and demonstrate for the first time quantitatively that these phytocannabinoids act in a synergistic manner to exert shared physiological effects, in this case to prevent the development of neuropathic pain in a rodent model. As mentioned in the Introduction, several previous studies have investigated the effect of predominantly ineffective doses of CBD on established behavioural pharmacological effects of THC. In mice alone, results have pointed to a lack of interaction, a potentiation of THC effects or an attenuation of THC effects across studies, even on the same behavioural endpoint [e.g. catalepsy (Jones and Pertwee, 1972; Karniol and Carlini, 1973; Ham and De Jong, 1975; Formukong et al., 1988; Hayakawa et al., 2008)]. Specifically regarding pain, interaction studies have been carried out again in rodent models, showing that CBD was ineffective, with findings that CBD can increase the antinociceptive potency of THC (Varvel et al., 2006) but it decreased the attenuation of formic acid‐induced writhing by THC (Welburn et al., 1976). Because previous reports have focused on relatively high doses of CBD in animal models, where CBD is typically ineffective, it is difficult to compare the present degree of observed synergy with these earlier studies. Overall, our present synergistic results are critical because they demonstrate and reinforce several key points. Firstly, the effects of the interactions between phytocannabinoids, be they additive, synergistic or sub‐additive, are highly dependent on the physiological endpoint. Secondly, ultra‐low doses of these phytocannabinoids may be effective in clinical populations for the treatment of neuropathic pain, allowing for a more favourable therapeutic index with reduced adverse effects. Finally, THC and CBD are likely to be working through separate, facilitative mechanisms of action which, if elucidated, could lead to the development of a wider class of safe and effective anti‐neuropathic pharmacotherapies. It is generally recognized now that the initial receptor sites of action of these two compounds are distinct. As mentioned, the effects of THC in this model could be mediated by CB1, CB2 or both receptor subtypes. Regarding CBD, a role for 5‐HT1A receptors (Ward et al., 2014), glycine channels (Xiong et al., 2014) or TRPV1 channels (Costa et al., 2004),among others, have been shown to be involved in the anti‐neuropathic actions of CBD. The present synergistic results suggest that co‐activation by CND and THC of these distinct sites leads either to increased potency of these compounds to stimulate their intracellular signalling pathways or to the stimulation of alternative pathways that are not activated by either compound alone. Moreover, these interactions could occur by CBD and THC activating separate receptors on the same cell types, or by co‐modulating distinct cell types. The cell types primarily responsible for these anti‐neuropathic and anti‐inflammatory effects include not only neurons, but also microglia, astrocytes and/or other cell types associated with the immune system. These possibilities provide for a range of experimental approaches and directions in order to elucidate possible mechanisms at the cellular, receptor and signalling levels. Lastly, changes in pharmacokinetics of each compound may also play a role in the increased potency of the combination, as some have found that CBD can decrease THC metabolism and increase plasma and brain THC levels (Jones and Pertwee, 1972; Bornheim et al., 1995).
To further our understanding of the anti‐allodynic efficacy of CBD, THC and their combination, we examined whether CBD treatment would lead to protection from mechanical sensitivity resulting from two additional chemotherapeutic agents, oxaliplatin and vincristine. This suggestion of selectivity is also important to consider given the different mechanisms of action of oxaliplatin, vincristine and paclitaxel in their chemotherapeutic and chemotoxic effects. Differential effects of cannabinoids on these various induced neuropathies might speak to the generalization of a treatment class across chemotoxic neuropathies and to more distinct mechanisms of action of CBD, THC and their combination, as they relate to specific chemotoxic neuropathies. Our results to date suggest that CBD is effective at preventing oxaliplatin‐induced mechanical sensitivity, but the dose–response relationship is shifted to the right, again suggesting a different mechanism of action of CBD (and perhaps oxaliplatin) in this model. One reported difference between these chemotherapeutic agents in the preclinical models is the involvement of non‐neuronal cell types in CIPN. For example, there are reports that paclitaxel administration is associated with microglial activation in the dorsal horn of the spinal cord (Pevida et al. 2013) and infiltration of T‐cells into the dorsal root ganglia, whereas oxaliplatin activated spinal astrocytes but not microglia (Robinson et al., 2014).THC at 10 mg·kg−1 produced a non‐significant attenuation of oxaliplatin‐induced mechanical sensitivity, and perhaps more importantly, we again observed a very potent, significant effect of the low dose combination 0.16 mg·kg−1 THC + 0.16 mg·kg−1 CBD. Interestingly, CBD was ineffective at preventing vincristine‐induced mechanical sensitivity. It should be noted that in the vincristine/CBD experiment, vincristine induced a much more profound percent baseline effect on mechanical allodynia than that observed with paclitaxel and oxaliplatin, so the lack of CBD efficacy after vincristine may be due to an insurmountable level of neuropathy in these mice. THC at 10 mg·kg−1 was also effective at preventing vincristine‐induced mechanical sensitivity, while in this case, the low dose combination (0.16 mg·kg−1 THC + 0.16 mg·kg−1 CBD) did not attenuate it. This may again be due to different underlying mechanisms for sensory sensitivity induced by the different chemotherapeutic agents, with some mechanistic targets being less sensitive to CBD or combined treatment than others.
In summary, these experiments demonstrate quantitatively for the first time that CBD and THC work synergistically to prevent the development of neuropathic pain using a mouse model of chemotherapy‐induced peripheral neuropathy. We and others identified more than one non‐cannabinoid receptor‐mediated mechanisms underlying the efficacy of CBD in this model, while related data suggest that THC is likely to act through CB1 and CB2 receptors in this assay. Further work is warranted to determine the mechanism of synergy between THC and CBD, from the cellular to receptor to intracellular signalling level, to enhance our ability to develop novel safe and effective treatment strategies for CIPN and other neuropathic pain disorders.
Author contributions
K.K., A.M. and A.S.‐M.performed behavioural testing and was involved in statistical analysis and critical review of the manuscript; R.F.T. and R.J.T. assisted in the conception and design of the work and in critical review of the manuscript; S.J.W. was responsible for the conception and design of the work. She also was responsible for the majority of the statistical analyses and the drafting of the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Acknowledgements
This work was supported by INSYS Therapeutics and R03DA034761 (S.J.W.) and dedicated to the memory of Dr Ronald J. Tallarida and his invaluable contributions to pharmacology and the investigation of drug interactions.
King, K. M. , Myers, A. M. , Soroka‐Monzo, A. J. , Tuma, R. F. , Tallarida, R. J. , Walker, E. A. , and Ward, S. J. (2017) Single and combined effects of Δ9‐tetrahydrocannabinol and cannabidiol in a mouse model of chemotherapy‐induced neuropathic pain. British Journal of Pharmacology, 174: 2832–2841. doi: 10.1111/bph.13887.
References
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Catterall WA, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Voltage‐gated ion channels. Br J Pharmacol 172: 5904–5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornheim LM, Kim KY, Li J, Perotti BY, Benet LZ (1995). Effect of cannabidiol pretreatment on the kinetics of tetrahydrocannabinol metabolites in mouse brain. Drug Metab Dispos 23: 825–831. [PubMed] [Google Scholar]
- Comelli F, Giagnoni G, Bettoni I, Colleoni M, Costa B (2008). Antihyperalgesic effect of a Cannabis sativa extract in a rat model of neuropathic pain: mechanisms involved. Phytother Res 22: 1017–1024. [DOI] [PubMed] [Google Scholar]
- Costa B, Giagnoni G, Franke C, Trovato AE, Colleoni M (2004). Vanilloid TRPV1 receptor mediates the antihyperalgesic effect of the nonpsychoactive cannabinoid, cannabidiol, in a rat model of acute inflammation. Br J Pharmacol 143: 247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa B, Trovato AE, Comelli F, Giagnoni G, Colleoni M (2007). The non‐psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur J Pharmacol 556: 75–83. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Guindon J, Cornett BL, Makriyannis A, Mackie K, Hohmann AG (2015). Chronic cannabinoid receptor 2 activation reverses paclitaxel neuropathy without tolerance or cannabinoid receptor 1‐dependent withdrawal. Biol Psychiatry 77: 475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Guindon J, Vemuri VK, Thakur GA, White FA, Makriyannis A et al. (2012). The maintenance of cisplatin‐ and paclitaxel‐induced mechanical and cold allodynia is suppressed by cannabinoid CB(2) receptor activation and independent of CXCR4 signaling in models of chemotherapy‐induced peripheral neuropathy. Mol Pain 8: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formukong EA, Evans AT, Evans FJ (1988). Inhibition of the cataleptic effect of tetrahydrocannabinol by other constituents of Cannabis sativa L. J Pharm Pharmacol 40: 132–134. [DOI] [PubMed] [Google Scholar]
- Grisham MG, Ferraro DP (1972). Biphasic effects of 9 ‐tetrahydrocannabinol on variable interval schedule performance in rats. Psychopharmacologia 27: 163–169. [DOI] [PubMed] [Google Scholar]
- Guindon J, Hohmann AG (2008). Cannabinoid CB2 receptors: a therapeutic target for the treatment of inflammatory and neuropathic pain. Br J Pharmacol 153: 319–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham MT, De Jong Y (1975). Absence of interaction between delta9‐tetrahydrocannabinol (delta‐THC) and cannabidiol (CBD) in aggression, muscle control and body temperature experiments in mice. Psychopharmacologia 41: 169–174. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Mishima K, Hazekawa M, Sano K, Irie K, Orito K et al. (2008). Cannabidiol potentiates pharmacological effects of delta(9)‐tetrahydrocannabinol via CB(1) receptor‐dependent mechanism. Brain Res 1188: 157–164. [DOI] [PubMed] [Google Scholar]
- Hu P, McLachlan EM (2002). Macrophage and lymphocyte invasion of dorsal root ganglia after peripheral nerve lesions in the rat. Neuroscience 112: 23–38. [DOI] [PubMed] [Google Scholar]
- Jones G, Pertwee RG (1972). A metabolic interaction in vivo between cannabidiol and 1‐tetrahydrocannabinol. Br J Pharmacol 45: 375–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karniol IG, Carlini EA (1973). Pharmacological interaction between cannabidiol and delta 9‐tetrahydrocannabinol. Psychopharmacologia 33: 53–70. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkoski M, Guimaraes FS, Del‐Bel E (2012). Cannabidiol‐treated rats exhibited higher motor score after cryogenic spinal cord injury. Neurotox Res 21: 271–280. [DOI] [PubMed] [Google Scholar]
- Kwiatkowska M, Parker LA, Burton P, Mechoulam R (2004). A comparative analysis of the potential of cannabinoids and ondansetron to suppress cisplatin‐induced emesis in the Suncus murinus (house musk shrew). Psychopharmacology (Berl) 174: 254–259. [DOI] [PubMed] [Google Scholar]
- Lynch JJ 3rd, Wade CL, Zhong CM, Mikusa JP, Honore P (2004). Attenuation of mechanical allodynia by clinically utilized drugs in a rat chemotherapy‐induced neuropathic pain model. Pain 110: 56–63. [DOI] [PubMed] [Google Scholar]
- Margulies JE, Hammer RP Jr (1991). Delta 9‐tetrahydrocannabinol alters cerebral metabolism in a biphasic, dose‐dependent manner in rat brain. Eur J Pharmacol 202: 373–378. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual D, Goicoechea C, Suardiaz M, Martin MI (2005). A cannabinoid agonist, WIN 55,212‐2, reduces neuropathic nociception induced by paclitaxel in rats. Pain 118: 23–34. [DOI] [PubMed] [Google Scholar]
- Peters CM, Jimenez‐Andrade JM, Jonas BM, Sevcik MA, Koewler NJ, Ghilardi JR et al. (2007). Intravenous paclitaxel administration in the rat induces a peripheral sensory neuropathy characterized by macrophage infiltration and injury to sensory neurons and their supporting cells. Exp Neurol 203: 42–54. [DOI] [PubMed] [Google Scholar]
- Pevida M, Lastra A, Hidalgo A, Baamonde A, Menendez L (2013). Spinal CCL2 and microglial activation are involved in paclitaxel‐evoked cold hyperalgesia. Brain Res Bull 95: 21–27. [DOI] [PubMed] [Google Scholar]
- Rahn EJ, Makriyannis A, Hohmann AG (2007). Activation of cannabinoid CB1 and CB2 receptors suppresses neuropathic nociception evoked by the chemotherapeutic agent vincristine in rats. Br J Pharmacol 152: 765–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CR, Zhang H, Dougherty PM (2014). Astrocytes, but not microglia, are activated in oxaliplatin and bortezomib‐induced peripheral neuropathy in the rat. Neuroscience 274: 308–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanudo‐Pena MC, Romero J, Seale GE, Fernandez‐Ruiz JJ, Walker JM (2000). Activational role of cannabinoids on movement. Eur J Pharmacol 391: 269–274. [DOI] [PubMed] [Google Scholar]
- Smith PB, Compton DR, Welch SP, Razdan RK, Mechoulam R, Martin BR (1994). The pharmacological activity of anandamide, a putative endogenous cannabinoid, in mice. J Pharmacol Exp Ther 270: 219–227. [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44 (Database Issue): D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida RJ (2000). Drug synergism and dose‐effect data analysis. Chapman and Hall/CRC, Roca Raton. [Google Scholar]
- Tallarida RJ (2012). Revisiting the isobole and related quantitative methods for assessing drug synergism. J Pharmacol Exp Ther 342: 2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng AH, Harding JW, Craft RM (2004). Pharmacokinetic factors in sex differences in delta 9‐tetrahydrocannabinol‐induced behavioral effects in rats. Behav Brain Res 154: 77–83. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Wiley JL, Yang R, Bridgen DT, Long K, Lichtman AH et al. (2006). Interactions between THC and cannabidiol in mouse models of cannabinoid activity. Psychopharmacology (Berl) 186: 226–234. [DOI] [PubMed] [Google Scholar]
- Vera G, Cabezos PA, Martin MI, Abalo R (2013). Characterization of cannabinoid‐induced relief of neuropathic pain in a rat model of cisplatin‐induced neuropathy. Pharmacol Biochem Behav 105: 205–212. [DOI] [PubMed] [Google Scholar]
- Ward SJ, McAllister SD, Kawamura R, Murase R, Neelakantan H, Walker EA (2014). Cannabidiol inhibits paclitaxel‐induced neuropathic pain through 5‐HT(1A) receptors without diminishing nervous system function or chemotherapy efficacy. Br J Pharmacol 171: 636–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SJ, Ramirez MD, Neelakantan H, Walker EA (2011). Cannabidiol prevents the development of cold and mechanical allodynia in paclitaxel‐treated female C57Bl6 mice. Anesth Analg 113: 947–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welburn PJ, Starmer GA, Chesher GB, Jackson DM (1976). Effect of cannabinoids on the abdominal constriction response in mice: within cannabinoid interactions. Psychopharmacologia 46: 83–85. [DOI] [PubMed] [Google Scholar]
- Xiong W, Chen SR, He L, Cheng K, Zhao YL, Chen H et al. (2014). Presynaptic glycine receptors as a potential therapeutic target for hyperekplexia disease. Nat Neurosci 17: 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]


