Abstract
The biology of H2S is a still developing area of research and several biological functions have been recently attributed to this gaseous molecule in many physiological systems, including the cardiovascular, urogenital, respiratory, digestive and central nervous system (CNS). H2S exerts anti‐inflammatory effects and can be considered an endogenous mediator with potential effects on gastrointestinal motility. During the last few years, we have investigated the role of H2S as a regulator of gastrointestinal motility using both animal and human tissues. The aim of the present work is to review published data regarding the potential role of H2S as a signalling molecule regulating physiopathological processes in gastrointestinal motor function. H2S is endogenously produced by defined enzymic pathways in different cell types of the intestinal wall including neurons and smooth muscle. Inhibition of H2S biosynthesis increases motility and H2S donors cause smooth muscle relaxation and inhibition of propulsive motor patterns. Impaired H2S production has been described in animal models with gastrointestinal motor dysfunction. The mechanism(s) of action underlying these effects may include several ion channels, although no specific receptor has been identified. At this time, even though there is much experimental evidence for H2S as a modulator of gastrointestinal motility, we still do not have conclusive experimental evidence to definitively propose H2S as an inhibitory neurotransmitter in the gastrointestinal tract, causing nerve‐mediated relaxation.
Abbreviations
- 3‐MPST
3‐mercaptopyruvate sulfurtransferase
- AOAA
amino‐oxyacetic acid
- CBS
cystathionine β‐synthase
- CSE
cystathionine γ‐lyase
- Ethe1
sulphur dioxygenase [ethylmalonic encephalopathy 1]
- GI
gastrointestinal
- HA
hydroxylamine
- ICC
interstitial cells of Cajal
- KATP channels
ATP‐sensitive potassium channels
- KO
knockout
- l‐NNA
Nω‐nitro‐l‐arginine
- MP
myenteric plexus
- nNOS
neuronal NOS
- NSAIDs
non‐steroidal anti‐inflammatory drugs
- ODQ
1H‐[1,2,4]oxadiazolo[4,3‐a]quinoxalin‐1‐one
- PAG
l‐propargylglycine
- PLP
pyridoxal phosphate
- RMP
resting membrane potential
- sGC
soluble guanylyl cyclase
- SKCa
small‐conductance calcium‐activated potassium channels
- SMCs
smooth muscle cells
- SMP
submuscular plexus
- SQR
sulphide quinone reductase
- TTX
tetrodotoxin
Introduction
Hydrogen sulphide (H2S) is a toxic gas that may lead to inhibition of the mitochondrial cytochrome c oxidase (Reiffenstein et al., 1992). However, it is also an endogenous gasomediator with potential physiological roles in a wide range of systems, including the cardiovascular, urogenital, respiratory, digestive systems and the CNS (Abe and Kimura, 1996; Patacchini et al., 2004; Trevisani et al., 2005; Yang et al., 2008; d'Emmanuele et al., 2009; Wallace et al., 2010; Gur et al., 2015). In the vascular system, H2S acts as an inhibitory endothelium‐derived factor with similar functions to NO, causing smooth muscle relaxation and hypotension (Skovgaard et al., 2011). Regarding the gastrointestinal (GI) tract, H2S has been proposed as an anti‐inflammatory mediator (Fiorucci and Distrutti, 2011; Vandiver and Snyder, 2012; Takeuchi et al., 2015; Wallace et al., 2015) and as an endogenously synthesized molecule through‐specific enzymic pathways with potential effects on GI motility (Jimenez, 2010). H2S produced by luminal bacteria has the potential to modify GI function and participates in motility disorders when intestinal microbiota is altered. The epithelium plays an important role as a barrier between the internal and external milieu. Nowadays, many authors consider H2S to be an inhibitory neurotransmitter in the GI tract with functions similar to those of NO. However, this needs a discussion based on experimental data. During the last years, our research group has been investigating the role of H2S as a regulator of GI motility using both animal and human tissues. The aim of the present review is to analyse published data regarding the potential role of H2S as a signalling molecule regulating physiopathological processes in GI motility.
Synthesis of H2S in the GI tract
In mammalian cells, two pyridoxal phosphate (PLP)‐dependent enzymes are responsible for the synthesis of H2S from the amino acid l‐cysteine: cystathionine β‐synthase (CBS) and cystathionine γ‐lyase (CSE) (Cavallini et al., 1962; Braunstein et al., 1971; Stipanuk and Beck, 1982; Yang et al., 2008). A third route of H2S synthesis involving L‐cysteine is the one performed by the enzyme 2‐oxoglutarate aminotransferase in cooperation with 3‐mercaptopyruvate sulfurtransferase (3‐MPST) (Stipanuk and Beck, 1982; Shibuya et al., 2009a, b) (Table 1). Recently, a new pathway for H2S biosynthesis has been reported using d‐cysteine as a substrate (Shibuya et al., 2013). Although the mechanisms regulating H2S release remain unclear, it has been proposed that H2S might be synthesized on demand or, alternatively, released from sulphur stores in response to physiological signals. Selective activation of CSE by calcium–calmodulin has been suggested (Yang et al., 2008) although opposite results have also been published (Mikami et al., 2013). Release of H2S in response to reducing conditions has been reported as well (Ishigami et al., 2009; Kimura, 2010). In the latter, H2S might be stored in the cytoplasm as bound sulphane sulphur, a divalent sulphur bound with other sulphur atoms present in intracellular proteins (Ishigami et al., 2009; Kimura, 2010).
Table 1.
Enzymes responsible for H2S production in mammalian cells
| Nomenclature (EC number) | Common Abbreviation | Endogenous substrates | Cofactors | Inhibitors |
|---|---|---|---|---|
| Cystathionine β‐synthase (4.2.1.22) | CBS |
L‐cysteine l‐homocysteine |
Pyridoxal phosphate | AOAA |
| Cystathionine γ‐lyase (4.4.1.1) | CSE | L‐cysteine | Pyridoxal phosphate | Aminoethoxyvinylglycine |
| AOAA | ||||
| β‐cyano‐l‐alanine | ||||
| Propargylglycine | ||||
| L‐cysteine : 2 oxoglutarate aminotransferase (4.4.1.13) | CAT | L‐cysteine | Pyridoxal Phosphate | Compound 3a |
| 3‐mercaptopyruvate sulfurtransferase (2.8.1.2) | 3‐MPST | 3‐mercaptopyruvic acid | Zn2+ | – |
CAT and 3‐MPST function in combination to generate H2S.
Adapted from (Alexander et al., 2015a).
CAT, Cysteine:2‐oxoglutarate aminotransferase.
Toutle et al. 2013.
Both CBS and CSE are localized along the entire GI tract in mammals (Table 2). CSE is expressed in neurons of both the submucosal (SMP) and myenteric plexuses (MPs) as well as in certain subclasses of interstitial cells of Cajal (ICC) (Linden et al., 2008; Schicho et al., 2006). Both enzymes are expressed in the epithelium and muscle wall in the rat colon (Hennig and Diener, 2009; Gil et al., 2011). CBS immunoreactivity is detected in enteric neurons from guinea pigs and humans (Schicho et al., 2006; Quan et al., 2015). Similar results have been reported in the murine colon with expression of these two enzymes in a wide variety of cellular types (Linden et al., 2008; Hennig and Diener, 2009; Martin et al., 2010; Liu et al., 2013). 3‐MPST and CSE are expressed in smooth muscle cells (SMCs) isolated from the rabbit stomach, suggesting that both enzymes might participate in H2S synthesis in smooth muscle (Nalli et al., 2015). According to these data, several types of intestinal cells possess the enzymic machinery to produce H2S (Table 2).
Table 2.
Distribution of CSE, CBS and 3‐MPST in the GI tract
| Species | Organ | Enzyme | Cell type/layer | Method | References |
|---|---|---|---|---|---|
| Mouse | Stomach | CBS/CSE | SMC | IHC/WB | Han et al., 2011 Huang et al., 2013 |
| Small intestine | CBS/CSE | SMC (tunica muscularis) | WB | Guo et al., 2012 | |
| Colon | CBS | Lamina propia | RT‐PCR/IHC | Linden et al., 2008 | |
| CSE | MP (lamina propia and SMC diffuse) | ||||
| Guinea Pig | Ileum and Colon | CBS | MP,SMC | IHC | Schicho et al., 2006 |
| CSE | MP, SMP, ICC | ||||
| Human | Colon | CBS/CSE | SMP | IHC | Schicho et al., 2006 |
| CBS/CSE | Epithelium | WB | Martin et al., 2010 | ||
| Rat | Stomach | CBS/CSE | Epithelium (mucosa) | RT‐PCR | Fiorucci et al., 2005 |
| Jejunum | CBS/CSE | MP | IHC | Kasparek et al., 2012 | |
| Colon | CBS/CSE | Epithelium, SMC | IHC | Hennig and Diener, 2009 | |
| CBS | Muscularis mucosa, SMP, lamina propia | IHC/WB | Martin et al., 2010 | ||
| CSE | General diffuse | ||||
| CBS | Epithelium, SMC (diffuse) | IHC | Gil et al., 2011 | ||
| CSE | MP, SMP, SMC (mucosa and submucosa diffuse) | ||||
| CBS | MP, epithelium (SMC diffuse) | IHC | Liu et al., 2013 | ||
| CSE | MP, SMC (mucosa and submucosa diffuse) | ||||
| Rabbit | Stomach | CSE/3‐MPST | SMC | RT‐PCR/WB | Nalli et al., 2015 |
Enzymes: CSE, 3‐MPST. Techniques: IHC, immunohistochemistry; RT‐PCR; WB, Western blot. Cells/layers: SMC, MP, SMP, ICC.
Experimental approaches to investigate the functional role of H2S in GI function
An important limitation in the characterization of the role played by H2S in GI motility is the lack of a clearly identified receptor to be targeted as a possible pharmacological approach. For NO, in contrast, although it has many signalling pathways, soluble guanylyl cyclase (sGC) has been identified as its primary target leading to smooth muscle hyperpolarization and relaxation in the GI tract (Lies et al., 2013, 2014; Mane et al., 2014b). However, several experimental approaches have been used to investigate the putative role of H2S in GI physiology.
H2S synthesis inhibition
The first approach is to characterize the role of endogenous H2S by blocking its production. This can be achieved through the use of H2S synthesis inhibitors (Table 1). l‐propargylglycine (PAG), an inhibitor of CSE; amino‐oxyacetic acid (AOAA), an inhibitor of both CBS and CSE; and hydroxylamine (HA), a CBS inhibitor (Wang, 2002; Szabo, 2007; Linden et al., 2010), are the most commonly used inhibitors of H2S biosynthesis. AOAA and HA are non‐selective PLP‐dependent enzyme inhibitors, whereas PAG is an irreversible inhibitor of CSE (John and Charteris, 1978; Sun et al., 2009; Linden et al., 2010). These compounds have been widely used in experiments with tissue homogenates and at a cellular level (Stipanuk and Beck, 1982; Hosoki et al., 1997; Szabo, 2007; Linden et al., 2010). This experimental approach has the limitation of the selectivity of the pharmacological tools available and the presence of multiple pathways of H2S synthesis (Whiteman et al., 2011; Asimakopoulou et al., 2013).
Genetically modified animals
Another experimental approach that blocks H2S production is the use of genetically modified animals that lack a specific synthesis pathway. This is an interesting approach since CSE knockout (KO) mice have been used to demonstrate that endogenous H2S maintains a smooth muscle relaxation and hypotension in the vascular system (Yang et al., 2008), although hypertension was not observed in CSE KO mice used in a similar study (Ishii et al., 2010). This might be due to the fact that different H2S synthesis pathways are present in the GI tract and the three gasomediators [NO, carbon monoxide (CO) and H2S] might have overlapping and interacting functions.
H2S donors
A third approach is the use of compounds that increase the concentration of H2S. This can be achieved by using H2S donors such as sodium hydrosulphide (NaHS) or H2S slow‐releasing organic compounds and H2S precursors such as L‐cysteine or by blocking the degradation pathway of H2S. NaHS is widely used to study the biological effects of H2S (Hosoki et al., 1997; Wang, 2002; Szabo, 2007; Linden et al., 2010). In the case of NaHS and L‐cysteine, one of the crucial points of discussion is the concentration of the compound. The limit between physiological, pharmacological and even toxic concentrations is unknown. Moreover, the effects obtained with NaHS incubation are not always equivalent to those obtained with promotion of endogenous H2S synthesis (Figure 1). L‐cysteine might be binding to other receptors in the plasma membrane of different cell types.
Figure 1.
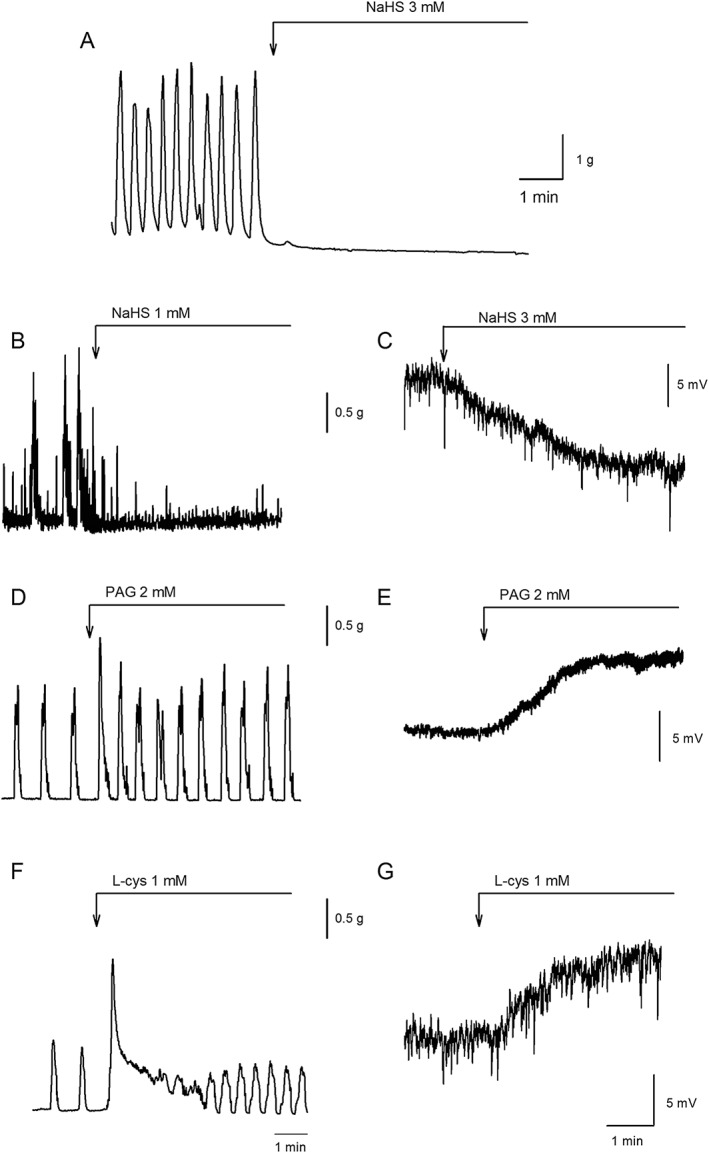
Muscle bath recordings showing the effect of NaHS on spontaneous contractions in the human colon [(A) NaHS at 3 mM; Martinez‐Cutillas et al., 2015] and rat colon [(B) NaHS at 1 mM; Gil et al., 2013]. (C) Intracellular microelectrode recording showing the effect of NaHS (1 mM) on the RMP of the rat mid‐colon (obtained from Gil et al., 2013). Mechanical (left) and intracellular recording (right) showing the increase of spontaneous motility and the depolarization of the RMP elicited by PAG (2 mM) (D, E) and L‐cysteine (l‐cys; 1 mM) (F, G) in the rat colon (unpublished results).
H2S and smooth muscle contractility
Experiments performed with colonic samples in which the mucosa and submucosa were removed demonstrated that H2S can be enzymically produced from L‐cysteine in the mouse and rat colon (Linden et al., 2008; Gil et al., 2011). PAG and AOAA significantly reduced H2S production (Linden et al., 2008; Gil et al., 2011). Therefore, these experiments demonstrate that under these experimental conditions, H2S is endogenously produced by defined enzymic pathways in the colonic wall.
In vitro, intestinal preparations have the ability to ‘spontaneously’ release inhibitory neurotransmitters from enteric motor neurons (Gil et al., 2010). Therefore, incubation with the neuronal blocker tetrodotoxin (TTX) causes smooth muscle depolarization and enhances the frequency and amplitude of spontaneous contractions due to the inhibition of the neuronal inhibitory tone. Similar results are observed with the inhibitor of neuronal NOS (nNOS), Nω‐nitro‐l‐arginine (L‐NNA) or the sGC blocker ODQ, showing that NO is the responsible for the inhibitory neuronal tone (Gil et al., 2010). If H2S also contributes to smooth muscle inhibition, smooth muscle depolarization and increase of tone and/or an increase in spontaneous contractions should be observed after inhibition of H2S synthesis. Interestingly, PAG causes smooth muscle depolarization and increases the frequency of spontaneous contractions in rat colonic circular muscle (Figure 1), whereas AOAA caused a mild increase in muscle contraction without major changes in the membrane potential (Gil et al., 2013). A second study reported that both PAG and AOAA increased spontaneous contractions in both circularly and longitudinally oriented rat colonic preparations (Liu et al., 2013). This suggests that H2S, synthesized by CSE and possibly also by CBS, is tonically inhibiting colonic motility. Interestingly, the depolarization and motility increase observed with PAG are still observed after neuronal blockade with TTX (Gil et al., 2011), suggesting that H2S synthesis is not dependent on sodium‐mediated action potentials in neurons and, therefore, a potential non‐ neuronal source of H2S might be present in the GI tract.
In human colonic samples, the presence of an inhibitory neuronal tone is still under discussion (Jimenez et al., 2014). Several issues such as regional differences or differences in sample handling (i.e. time interval from extraction to experimentation) can be important to detect a functional inhibitory neuronal tone in vitro. Incubation with PAG and AOAA causes a smooth muscle depolarization and a transient increase in tone and amplitude of spontaneous contractions (Martinez‐Cutillas et al., 2015), suggesting that, as previously shown in rats, H2S contributes to an endogenous neuronal tone in human colonic samples. However, these compounds are non‐selective inhibitors of CSE and CBS and show effects on several other enzymes and receptors (John and Charteris, 1978; Teague et al., 2002; Szabo, 2007; Whiteman et al., 2011). Therefore, the interpretation of the results obtained with these pharmacological tools must always be carried out with the support of other experimental findings (Szabo, 2007; Jimenez, 2010; Whiteman et al., 2011).
It is important to note that HA is not only a H2S‐producing enzyme inhibitor but also has been described as an NO donor (Iversen et al., 1994; Correia et al., 2000). For instance, HA causes smooth muscle hyperpolarization leading to an inhibition of spontaneous contractility in both the rat and human colons (Gil et al., 2011; Martinez‐Cutillas et al., 2015). This response is sensitive to the sGC inhibitor ODQ. Accordingly, we strongly recommend not using HA as an inhibitor of CBS in biological processes involving the GI tract where the role of NO is extremely relevant.
Participation of H2S in the transwall gradient of smooth muscle membrane potential
The resting membrane potential (RMP) of the colonic muscle is graded through the colonic muscle wall; that is, SMCs located near the SMP are more hyperpolarized than the cells near the MP (Sha et al., 2010). Several factors such as ICC‐SMP themselves that are electrically coupled to smooth muscle or inhibitory mediators released by SMP neurons may contribute to setting this gradient in the RMP of the circular muscle. An excellent work using haem oxygenase‐2‐KO (Sha et al., 2010) and CSE KO mice (Sha et al., 2014) demonstrated that the transwall gradient is probably due to CO and potentiated by H2S. Both NO and CO are possibly released by SMP neurons. CO and H2S produced by the mucosa itself might also contribute to inhibit motility in the circular layer (Martin‐Cano et al., 2014). The consequence of this organization is that SMCs near the SMP are considerably more hyperpolarized than the cells near the MP and have the ability to oscillate at a frequency paced by ICC‐SMP (Mane et al., 2014b).
Effect of NaHS on GI function
In the GI tract, NaHS exerts pro‐secretory effects both through neuronal mechanisms involving afferent neurons and by direct stimulation of the intestinal epithelium (Schicho et al., 2006; Hennig and Diener, 2009; Krueger et al., 2010; Pouokam et al., 2011). Both anti‐nociceptive and pro‐nociceptive effects have been observed in response to NaHS when administrated intraperitoneally and intracolonically respectively (Distrutti et al., 2006; Matsunami et al., 2009; Schemann and Grundy, 2009). Anti‐inflammatory properties have also been described for H2S as administration of NaHS accelerates healing of gastric ulcers and significantly contributes to the resolution of colitis (Wallace et al., 2007, 2009, 2012).
Regarding its role in modulating smooth muscle activity, contractile but more often inhibitory responses have been reported. For example, in the guinea pig and mouse stomach, NaHS causes a dual effect, producing contraction at low concentrations and relaxation at high concentrations (Zhao et al., 2009; Han et al., 2011). Spontaneous circular smooth muscle contractions recorded in vitro in rat and human colonic preparations are concentration‐dependently inhibited by NaHS (Gallego et al., 2008) (Figure 1). NaHS concentration‐dependently relaxed circular muscle strips of mouse fundus and distal colon, contracted by PGF2α (Dhaese and Lefebvre, 2009; Dhaese et al., 2010). NaHS also exerted relaxant effects on guinea pig, rabbit and rat ileum and jejunum preparations (Hosoki et al., 1997; Teague et al., 2002; Nagao et al., 2011, 2012; Kasparek et al., 2012). However, the concentrations of NaHS used to induce relaxation in the GI tract are high and the physiological relevance of this action is still unknown (Figures 1 and 2).
Figure 2.
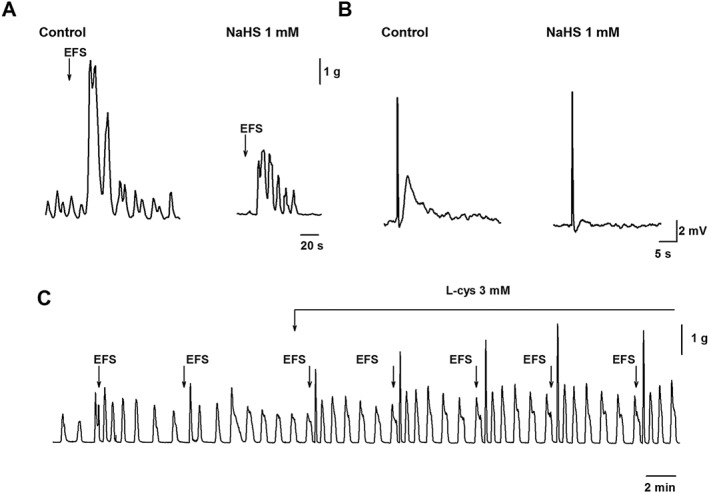
(A) Effect of NaHS (1 mM) on cholinergic contractions in the human colon (Martinez‐Cutillas et al., 2015) and on the excitatory junction potential (B) in the rat colon (Gil et al., 2013). (C) Increase of the amplitude of cholinergic contractions elicited by L‐cysteine (l‐cys; 3 mM) in the rat colon (unpublished results). EFS, electrical field stimulation.
Inorganic sulphide salts such as NaHS induce a short‐lasting increase in H2S concentration that can reach non‐physiological concentrations, and furthermore, they can be easily oxidized. For these disadvantages to be solved, organic slow‐releasing H2S agents such as GYY4137 have been developed. However, the effect of these compounds on GI motility has not yet been tested.
Effect of NaHS on intestinal motor patterns
NaHS inhibits peristaltic activity in the mouse small intestine and colon (Gallego et al., 2008). In rats, video recordings of spontaneous active colonic segments reveal two types of movements: (i) low‐frequency high‐amplitude aboral propulsive motor movements and (ii) high‐frequency non‐propulsive low‐amplitude contractions (ripples) (Huizinga et al., 2011). Propulsive movements cause outflow of intraluminal contents, and consequently, their most likely function is to propel pellets in an aboral direction. In contrast, ripples probably participate in segmentation motor patterns responsible for mixing movements (Huizinga et al., 2011). Both motility patterns are probably related to the presence of two pacemaker systems in the colon (Pluja et al., 2001; Alberti et al., 2005; Mane et al., 2014b). NaHS produces a decrease of propulsive contractions without major changes on ripples (Gil et al., 2013). It is important to notice that both rhythmic activities are differently affected by smooth muscle hyperpolarization, which is a potential effect of H2S. A second potential effect of NaHS is inhibition of neurally mediated excitatory responses involving post‐junctional mechanisms. A third potential effect of NaHS is a direct effect on pacemaker activity.
Smooth muscle hyperpolarization
Activation of ATP‐sensitive potassium (Kir 6.1 and 6.2; KATP) channels by H2S has been proposed in a wide variety of studies with vascular SMCs (Zhao et al., 2001; Cheng et al., 2004; Dombkowski et al., 2004; d'Emmanuele et al., 2009). Participation of these channels in the relaxant effect of NaHS has also been reported in the colon (Zhao et al., 2001; Distrutti et al., 2006; Gallego et al., 2008; Nagao et al., 2012; Liu et al., 2013). Both KATP and small‐conductance calcium‐activated potassium (KCa2.2/KCa2.3; SKCa) channels might participate in smooth muscle hyperpolarization in both human and rat colonic tissues (Gallego et al., 2008; Gil et al., 2013). sGC may indirectly participate in the mediation of NaHS responses in the colon by releasing NO from nitrosothiols, as observed in the brain (Ondrias et al., 2008). Also, H2S inhibits phosphodiesterase activity and, therefore, accumulation of cGMP takes place in post‐junctional cells (Bucci et al., 2010). All these mechanisms might account for the crosstalk between NO and H2S pathways. A complex interaction between H2S and NO also occurs in the vascular system (Dunn et al., 2016) where the crosstalk between the two gaseous compounds has been more extensively studied (Figure 1).
Inhibition of excitatory neurally mediated responses
In addition to muscle hyperpolarization, H2S might also produce its inhibitory effects by inhibiting excitatory neuromuscular transmission. In the rat colon, NaHS is able to inhibit atropine‐sensitive excitatory junction potentials and contractions elicited by electrical field stimulation (Gil et al., 2013) (Figure 2). Similar results have been observed in the human colon where NaHS reduced both cholinergic (Figure 2) and tachykinergic neural responses. In contrast, purinergic inhibitory junction potentials were not affected (Martinez‐Cutillas et al., 2015). NaHS also reduced carbachol‐ and neurokinin A‐ evoked responses, suggesting that NaHS effects are at a post‐junctional level (Martinez‐Cutillas et al., 2015). Smooth muscle contractions are calcium‐calmodulin dependent and due to the activation of myosin light‐chain kinase and inhibition of the myosin‐light chain phosphatase (MLCP). Rho kinase and protein kinase C (PKC) inhibit MLCP, causing a sustained contraction. Exogenous (NaHS) and endogenous (L‐cysteine) H2S reduced carbachol‐induced contractions in isolated rabbit gastric SMCs. This reduction is due to the activation of MLCP and inhibition of Rho kinase and PKC activities leading to the dephosphorylation of the myosin light chain and inhibition of contraction (Nalli et al., 2015). These results suggest that H2S might be inhibiting contractions by targeting specific post‐junctional pathways (Figure 2).
Effect on pacemaker activity
Electrophysiological experiments and intracellular calcium analysis demonstrated that high concentrations of NaHS (0.5–1 mM) are needed to inhibit pacemaker currents in cultured ICC isolated from the mouse small intestine (Parajuli et al., 2010). However, with low concentrations of NO donors, low concentrations of NaHS potentiate the inhibitory effect exerted by NO on the pacemaker system, suggesting a possible interaction between mediators (Yoon et al., 2011). Despite the effects observed in isolated ICC, NaHS at a concentration of 1 mM does not modify the amplitude, duration or frequency of slow‐wave activity originated in ICC‐SMP in whole thickness preparations from rat colon (Gil et al., 2013). In fact, neither hyperpolarization nor dihydropyridines modify electrical slow‐wave activity in intact tissue (Mane et al., 2014b). In contrast, propulsive contractions are reduced by NaHS (Gallego et al., 2008; Gil et al., 2013). This inhibition of low‐frequency contractions has been attributed to the hyperpolarization of the smooth muscle (Gil et al., 2013) and/or a direct effect on l‐type calcium channels (Cav1.2) (Quan et al., 2015) needed for the generation of the pacemaker, which is nifedipine‐sensitive.
Mechanism of action
NaHS exerts its biological effects through a wide variety of mechanisms of action that include activation of cAMP‐dependent pathways (Kimura, 2000); activation of the MLCP (Dhaese and Lefebvre, 2009; Nagao et al., 2012); opening of KATP channels (Gallego et al., 2008; Zhao et al., 2009; Nagao et al., 2012), SKCa channels (Gallego et al., 2008), Nav1.5 voltage‐dependent sodium channels (Strege et al., 2011), Cav3.2‐T‐type channels (Matsunami et al., 2009), TRPV1 and TRPA1 cation channels (Schicho et al., 2006; Macpherson et al., 2007; Krueger et al., 2010); and inhibition of phosphodiesterase activity (Bucci et al., 2010). Recently, it has been demonstrated using patch clamp experiments that NaHS inhibits l‐type calcium channels in rat colonic SMCs. This effect might also be responsible for its inhibitory effect on spontaneous contractions. However, inhibition of large‐conductance calcium‐activated potassium channels (KCa1.1) has also been reported (Quan et al., 2015). Why is the effect of H 2 S so diverse? It has been hypothesized that sulfhydration of different proteins modulating a wide variety of cellular functions might explain the ‘promiscuity’ of H2S (Mustafa et al., 2009a, b; Paul and Snyder, 2015).
Comparison of responses to L‐cysteine and to NaHS
L‐cysteine is the precursor of H2S synthesis, and as previously mentioned, it is often used to stimulate endogenous H2S production. Ideally, the response obtained with endogenous H2S production should be similar to the response obtained with exogenous NaHS (Nalli et al., 2015). Although this might be the case in some studies, in others, different or even opposite results have been reported (Figures 1 and 2). Although NaHS inhibited contractile activity in the rat small intestine, L‐cysteine did not (Nagao et al., 2011; Kasparek et al., 2012). Furthermore, in the rat colon, recent experiments performed in our laboratory show that, whereas NaHS inhibits motility, L‐cysteine increases spontaneous contractions (Figure 1). Moreover, L‐cysteine increased atropine sensitive nerve‐mediated contractions (Figure 2), whereas NaHS decreased them (Figure 2). One possible explanation is that, because of the high concentrations of L‐cysteine (i.e. 1 to 10 mM) needed to measure H2S production (Gil et al., 2013) and to observe an inhibitory effect (Yamane et al., 2014), this amino‐acid could target many receptors and channels (Kendig et al., 2014).
H2S degradation
Enzymes involved in the degradation of H2S are crucial in the termination of H2S signalling. H2S can be metabolized to thiosulphate by the serial action of three mitochondrial enzymes: sulphide quinone reductase (SQR), sulphur dioxygenase [ethylmalonic encephalopathy 1 (Ethe1)] and sulphur transferase (Hildebrandt and Grieshaber, 2008; Tiranti et al., 2009). This functional unit of enzymes has been described in the mitochondria of colonic epithelial cells and is probably responsible for the degradation of luminal H2S (Mimoun et al., 2012). Interestingly, SQR has been identified in the muscle layer and MP of the mouse colon. In addition, pharmacological blockade of SQR induces an increase of the tissue levels of H2S (Linden et al., 2012). However, Ethe1 and sulphur transferase have not been detected in colonic muscle cells. Therefore, it is possible that there are other downstream enzymes for the degradation of H2S in this tissue (Linden et al., 2012) although the level of H2S degradation in the musculature is negligible, when compared with that in the mucosa (Flannigan et al., 2013).
Bacteria as a potential source of H2S in the GI tract
In the large intestine, luminal bacteria also represent a potential source of H2S (Blachier et al., 2010). However, despite the fact that high concentrations of H2S are present in the colon (mM range), the vast majority of this H2S is bound to luminal contents (Jorgensen and Mortensen, 2001; Levitt et al., 2002). Thus, low levels (~60 μM in the human colon, measured with spectrophotometry) of free H2S are available in the colonic lumen (Jorgensen and Mortensen, 2001; Mimoun et al., 2012). Furthermore, luminal H2S is quickly oxidized to thiosulphate by colonic epithelial cells (Furne et al., 2001; Ramasamy et al., 2006; Goubern et al., 2007; Mimoun et al., 2012). Therefore, under physiological conditions, the concentration of H2S that reaches the submucosa and the muscle layers could be much lower. Accordingly, NaHS infused into the lumen is not able to cause motor changes in the colonic mechanical activity in rats (Gil et al., 2013). Therefore, it can be hypothesized that this source of H2S will not be able to modify colonic functions when the integrity of the barrier is preserved. Further studies are needed to evaluate if under pathological conditions that imply barrier disruption or impairment of epithelium metabolism, the H2S produced in the lumen can reach the effector cell and consequently modify motility. Interestingly, instability in microbiota has been recently reported in patients with flatulence. In these patients, Bacteroides fragilis or Bilophila wadsworthia correlated with number of gas evacuations or volume of gas evacuated respectively. Bilophila wadsworthia has strong catalase activity and produces H2S from sulphur‐containing amino acids. Excessive gas including H2S production can participate in physiopathological abdominal symptoms including distention and pain (Pozuelo et al., 2015). Figure 3 is a schematic overview of the potential role of H2S on GI function.
Figure 3.
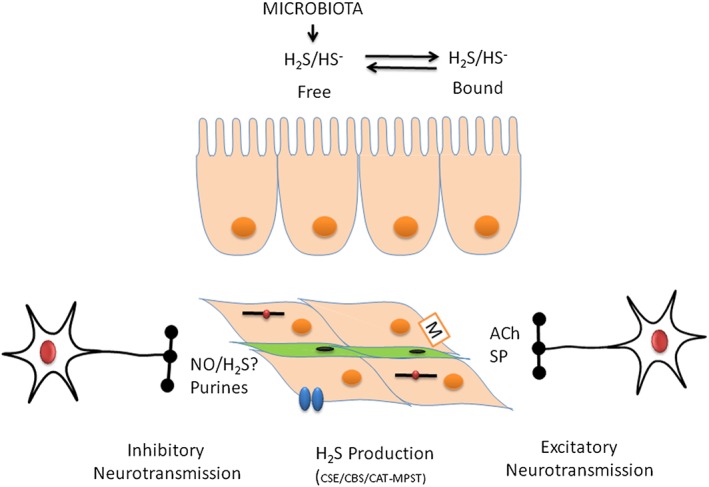
H2S is produced by luminal bacteria. Enterocytes participate in H2S detoxification. H2S can be produced by different cell types including neurons, SMCs or interstitial cells. H2S causes smooth muscle relaxation possibly acting on different mechanisms including the contractile apparatus, channels (Kir6, KCa, Cav) and receptors. Smooth muscle hyperpolarization and inhibition of nerve‐mediated contractions are potential mechanisms to inhibit propulsion. Neurally mediated relaxation is mediated by NO and purines. More experiments are needed to determine if H2S is an inhibitory neurotransmitter in the GI tract. CAT, L‐cysteine: 2‐oxoglutarate aminotransferase; SP, substance P.
H2S in motility dysfunction
Few data are available on a possible role for endogenous H2S in GI motility dysfunction. Both central and peripheral mechanisms may contribute to the physiopathological processes underlying esophageal motility, gastric emptying or colonic hypermotility.
Achalasia is an oesophageal motor disorder characterized by aperistalsis of the oesophageal body and impaired relaxation of the lower oesophageal sphincter. Accordingly, mechanisms that participate in pre‐ or post‐junctional nerve‐mediated relaxation could be impaired in achalasia. Lack of functional nNOS has been described in the lower oesophageal sphincter (Mearin et al., 1993; Shteyer et al., 2015) and a mutation in sGC disrupts NO signalling, causing achalasia (Wallace et al., 2016). Regarding the H2S pathway, reduced expression of both CBS and CSE has been reported in patients with achalasia (Zhang et al., 2015). However, it is unknown if the loss of H2S producing enzymes is the consequence of the loss of myenteric neurons (De Giorgio et al., 1999).
H2S enhances gastric emptying in rats through a peripheral mechanism that involves pyloric relaxation (Medeiros et al., 2012). Neurons expressing CBS have been detected in the dorsal motor nucleus of the vagus, and central administration of NaHS inhibits gastric motility and enhances gastric secretion (Sun et al., 2015). Recently, decreased H2S production has been associated with gastroparesis in an experimental model of diabetic rats (Mard et al., 2016). This is consistent with a dual effect of NaHS on gastric contractility producing contraction at low concentrations and relaxation at high concentrations (Zhao et al., 2009; Han et al., 2011; Mard et al., 2016).
Colonic hypermotility has been associated with decreased H2S synthesis in an experimental model of stress in rats. Under these experimental conditions, both lower H2S production and CBS/CSE down‐regulation were observed. This lower production was also accompanied by lower NaHS smooth muscle sensitivity associated with up‐regulation of KATP channels (Liu et al., 2013). CBS and CSE were also down‐regulated in a model of partial ileal obstruction with ICC loss, although these changes have been associated with inflammation with TNFα as the central mediator (Guo et al., 2012). Increase of H2S during inflammation has shown to decrease the proliferation of smooth muscle during ulcerative colitis in rats (Wallace et al., 2009), which will definitely also affect GI motility. More studies should be conducted to ascertain if there is a role for H2S in abnormal GI motility and whether this gaseous mediator is a key factor in diseases affecting the GI tract or not.
H2S as a potential therapeutic agent
The aim of the present review is not to discuss the role of H2S as a potential therapeutic molecule. However, there is solid experimental evidence to suggest that H2S is as a potential anti‐inflammatory mediator, in combination with non‐steroidal anti‐inflammatory drugs (NSAIDs). NSAIDs that release H2S have enhanced activity and/or improved safety profiles. Gaseous mediators improve blood flow, reduce oxidative stress, prevent GI mucosa injury, enhance anti‐inflammatory effects of NSAIDS and promote resolution of inflammation, angiogenesis and epithelialization (see Sulaieva and Wallace, 2015).
Final remarks: can we consider H2S an inhibitory neurotransmitter in the GI tract?
In spite of many reports of H2S as an inhibitory gasotransmitter in the enteric nervous system, with functions similar to those of NO, we strongly believe that we do not have enough experimental evidence to support this conclusion. For H2S to be considered an inhibitory neurotransmitter, it should be demonstrated that stimulation of inhibitory motor neurons releases H2S and that the release is blocked by Na+ channel blockers such as TTX. Pre‐junctional calcium channel blockers such as ω‐Conotoxin GVIA that block nerve‐mediated relaxation should also block H2S release. Moreover, it is well known that NOS inhibitors such as L‐NNA decrease nerve‐mediated relaxation, and to our knowledge, this has never been reported for H2S synthesis inhibitors. Another important limitation to demonstrate the putative role of H2S as an inhibitory gasotransmitter is the lack of a specific post‐junctional receptor. A classical experimental approach with in vitro preparations is tissue incubation with ODQ (sGC inhibitor) that blocks nitrergic inhibitory responses, and animals with cell‐specific deletion of sGC have impaired nitrergic neurotransmission (Lies et al., 2014). This experimental approach identifies the receptor and possible post‐junctional pathways (ICC vs. SMCs) that contribute to nitrergic nerve‐mediated relaxation (Lies et al., 2015). None of these experiments can be carried out if post‐junctional receptors are not identified. We have recently performed a variety of experiments by using different voltage and frequencies of stimulation and by measuring electrophysiological post‐junctional responses. In these, L‐NNA and MRS2500 totally blocked inhibitory responses in a wide variety of experimental conditions, and therefore, inhibitory neurotransmission in the GI tract can be said to involve NO and a purine acting on P2Y1 receptors (Mane et al., 2014a,b; Mane et al., 2016). In the context of H2S, we do not have sufficient clear experimental evidence to demonstrate that H2S is an inhibitory gasotransmitter in the GI tract leading to nerve‐mediated smooth muscle relaxation (Figure 3). Further experiments with more selective pharmacological tools are needed to identify the exact physiological role of H2S in motor function and dysfunction.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015a,b,c).
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
This work has been funded by the Ministerio de Ciencia e Innovación (Spain) (BFU2009‐11118) and by the Agència de Gestió d'Ajuts Universitaris de Catalunya (grant: 2011 CTP 00032).
Jimenez, M. , Gil, V. , Martinez‐Cutillas, M. , Mañé, N. , and Gallego, D. (2017) Hydrogen sulphide as a signalling molecule regulating physiopathological processes in gastrointestinal motility. British Journal of Pharmacology, 174: 2805–2817. doi: 10.1111/bph.13918.
References
- Abe K, Kimura H (1996). The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci 16: 1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti E, Mikkelsen HB, Larsen JO, Jimenez M (2005). Motility patterns and distribution of interstitial cells of Cajal and nitrergic neurons in the proximal, mid‐ and distal‐colon of the rat. Neurogastroenterol Motil 17: 133–147. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Catterall WA, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Voltage‐gated ion channels. Br J Pharmacol 172: 5904–5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015c). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asimakopoulou A, Panopoulos P, Chasapis CT, Coletta C, Zhou Z, Cirino G et al. (2013). Selectivity of commonly used pharmacological inhibitors for cystathionine beta synthase (CBS) and cystathionine gamma lyase (CSE). Br J Pharmacol 169: 922–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blachier F, Davila AM, Mimoun S, Benetti PH, Atanasiu C, Andriamihaja M et al. (2010). Luminal sulfide and large intestine mucosa: friend or foe? Amino Acids 39: 335–347. [DOI] [PubMed] [Google Scholar]
- Braunstein AE, Goryachenkova EV, Tolosa EA, Willhardt IH, Yefremova LL (1971). Specificity and some other properties of liver serine sulphhydrase: evidence for its identity with cystathionine‐synthase. Biochim Biophys Acta 242: 247–260. [DOI] [PubMed] [Google Scholar]
- Bucci M, Papapetropoulos A, Vellecco V, Zhou Z, Pyriochou A, Roussos C et al. (2010). Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler Thromb Vasc Biol 30: 1998–2004. [DOI] [PubMed] [Google Scholar]
- Cavallini D, Mondovi B, De MC, Scioscia‐Santoro A (1962). The mechanism of desulphhydration of cysteine. Enzymologia 24: 253–266. [PubMed] [Google Scholar]
- Cheng Y, Ndisang JF, Tang G, Cao K, Wang R (2004). Hydrogen sulfide‐induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol 287: H2316–H2323. [DOI] [PubMed] [Google Scholar]
- Correia NA, Oliveira RB, Ballejo G (2000). Pharmacological profile of nitrergic nerve‐, nitric oxide‐, nitrosoglutathione‐ and hydroxylamine‐induced relaxations of the rat duodenum. Life Sci 68: 709–717. [DOI] [PubMed] [Google Scholar]
- De Giorgio R, Di Simone MP, Stanghellini V, Barbara G, Tonini M, Salvioli B et al. (1999). Esophageal and gastric nitric oxide synthesizing innervation in primary achalasia. Am J Gastroenterol 94: 2357–1262. [DOI] [PubMed] [Google Scholar]
- Dhaese I, Lefebvre RA (2009). Myosin light chain phosphatase activation is involved in the hydrogen sulfide‐induced relaxation in mouse gastric fundus. Eur J Pharmacol 606: 180–186. [DOI] [PubMed] [Google Scholar]
- Dhaese I, Van CI, Lefebvre RA (2010). Mechanisms of action of hydrogen sulfide in relaxation of mouse distal colonic smooth muscle. Eur J Pharmacol 628: 179–186. [DOI] [PubMed] [Google Scholar]
- Distrutti E, Sediari L, Mencarelli A, Renga B, Orlandi S, Antonelli E et al. (2006). Evidence that hydrogen sulfide exerts antinociceptive effects in the gastrointestinal tract by activating KATP channels. J Pharmacol Exp Ther 316: 325–335. [DOI] [PubMed] [Google Scholar]
- Dombkowski RA, Russell MJ, Olson KR (2004). Hydrogen sulfide as an endogenous regulator of vascular smooth muscle tone in trout. Am J Physiol Regul Integr Comp Physiol 286: R678–R685. [DOI] [PubMed] [Google Scholar]
- Dunn WR, Alexander SP, Ralevic V, Roberts RE (2016). Effects of hydrogen sulphide on smooth muscle. Pharmacol Ther 158: 101–113. [DOI] [PubMed] [Google Scholar]
- d'Emmanuele V, Sorrentino R, Maffia P, Mirone V, Imbimbo C, Fusco F et al. (2009). Hydrogen sulfide as a mediator of human corpus cavernosum smooth‐muscle relaxation. Proc Natl Acad Sci U S A 106: 4513–4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorucci S, Antonelli E, Distrutti E, Rizzo G, Mencarelli A, Orlandi S et al. (2005). Inhibition of hydrogen sulfide generation contributes to gastric injury caused by anti‐inflammatory nonsteroidal drugs. Gastroenterology 129: 1210–1224. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Distrutti E (2011). COXIBs, CINODs and H(2)S‐releasing NSAIDs: current perspectives in the development of safer non steroidal anti‐inflammatory drugs. Curr Med Chem 18: 3494–3505. [DOI] [PubMed] [Google Scholar]
- Flannigan KL, Ferraz JG, Wang R, Wallace JL (2013). Enhanced synthesis and diminished degradation of hydrogen sulfide in experimental colitis: a site‐specific, pro‐resolution mechanism. PLoS One 8: e71962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furne J, Springfield J, Koenig T, DeMaster E, Levitt MD (2001). Oxidation of hydrogen sulfide and methanethiol to thiosulfate by rat tissues: a specialized function of the colonic mucosa. Biochem Pharmacol 62: 255–259. [DOI] [PubMed] [Google Scholar]
- Gallego D, Clave P, Donovan J, Rahmati R, Grundy D, Jimenez M et al. (2008). The gaseous mediator, hydrogen sulphide, inhibits in vitro motor patterns in the human, rat and mouse colon and jejunum. Neurogastroenterol Motil 20: 1306–1316. [DOI] [PubMed] [Google Scholar]
- Gil V, Gallego D, Grasa L, Martin MT, Jimenez M (2010). Purinergic and nitrergic neuromuscular transmission mediates spontaneous neuronal activity in the rat colon. Am J Physiol Gastrointest Liver Physiol 299: G158–G169. [DOI] [PubMed] [Google Scholar]
- Gil V, Gallego D, Jimenez M (2011). Effects of inhibitors of hydrogen sulphide synthesis on rat colonic motility. Br J Pharmacol 164: 485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil V, Parsons S, Gallego D, Huizinga J, Jimenez M (2013). Effects of hydrogen sulphide on motility patterns in the rat colon. Br J Pharmacol 169: 34–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goubern M, Andriamihaja M, Nubel T, Blachier F, Bouillaud F (2007). Sulfide, the first inorganic substrate for human cells. FASEB J 21: 1699–1706. [DOI] [PubMed] [Google Scholar]
- Guo H, Gai JW, Wang Y, Jin HF, Du JB, Jin J (2012). Characterization of hydrogen sulfide and its synthases, cystathionine beta‐synthase and cystathionine gamma‐lyase, in human prostatic tissue and cells. Urology 79: 483–485. [DOI] [PubMed] [Google Scholar]
- Gur S, Kadowitz PJ, Sikka SC, Peak TC, Hellstrom WJ (2015). Overview of potential molecular targets for hydrogen sulfide: a new strategy for treating erectile dysfunction. Nitric Oxide 50: 65–78. [DOI] [PubMed] [Google Scholar]
- Han YF, Huang X, Guo X, Wu YS, Liu DH, Lu HL et al. (2011). Evidence that endogenous hydrogen sulfide exerts an excitatory effect on gastric motility in mice. Eur J Pharmacol 673: 85–95. [DOI] [PubMed] [Google Scholar]
- Hennig B, Diener M (2009). Actions of hydrogen sulphide on ion transport across rat distal colon. Br J Pharmacol 158: 1263–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt TM, Grieshaber MK (2008). Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J 275: 3352–3361. [DOI] [PubMed] [Google Scholar]
- Hosoki R, Matsuki N, Kimura H (1997). The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun 237: 527–531. [DOI] [PubMed] [Google Scholar]
- Huang X, Meng XM, Liu DH, Wu YS, Guo X, Lu HL et al. (2013). Different regulatory effects of hydrogen sulfide and nitric oxide on gastric motility in mice. Eur J Pharmacol 720: 276–285. [DOI] [PubMed] [Google Scholar]
- Huizinga JD, Martz S, Gil V, Wang XY, Jimenez M, Parsons S (2011). Two independent networks of interstitial cells of cajal work cooperatively with the enteric nervous system to create colonic motor patterns. Front Neurosci 5: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishigami M, Hiraki K, Umemura K, Ogasawara Y, Ishii K, Kimura H (2009). A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid Redox Signal 11: 205–214. [DOI] [PubMed] [Google Scholar]
- Ishii I, Akahoshi N, Yamada H, Nakano S, Izumi T, Suematsu M (2010). Cystathionine gamma‐Lyase‐deficient mice require dietary cysteine to protect against acute lethal myopathy and oxidative injury. J Biol Chem 285: 26358–26368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen HH, Gustafsson LE, Leone AM, Wiklund NP (1994). Smooth muscle relaxing effects of NO, nitrosothiols and a nerve‐induced relaxing factor released in guinea‐pig colon. Br J Pharmacol 113: 1088–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez M (2010). Hydrogen sulfide as a signaling molecule in the enteric nervous system. Neurogastroenterol Motil 22: 1149–1153. [DOI] [PubMed] [Google Scholar]
- Jimenez M, Clave P, Accarino A, Gallego D (2014). Purinergic neuromuscular transmission in the gastrointestinal tract; functional basis for future clinical and pharmacological studies. Br J Pharmacol 171: 4360–4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John RA, Charteris A (1978). The reaction of amino‐oxyacetate with pyridoxal phosphate‐dependent enzymes. Biochem J 171: 771–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen J, Mortensen PB (2001). Hydrogen sulfide and colonic epithelial metabolism: implications for ulcerative colitis. Dig Dis Sci 46: 1722–1732. [DOI] [PubMed] [Google Scholar]
- Kasparek MS, Linden DR, Farrugia G, Sarr MG (2012). Hydrogen sulfide modulates contractile function in rat jejunum. J Surg Res 175: 234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendig DM, Hurst NR, Bradley ZL, Mahavadi S, Kuemmerle JF, Lyall V et al. (2014). Activation of the umami taste receptor (T1R1/T1R3) initiates the peristaltic reflex and pellet propulsion in the distal colon. Am J Physiol Gastrointest Liver Physiol 307: G1100–G1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H (2000). Hydrogen sulfide induces cAMP and modulates the NMDA receptor. Biochem Biophys Res Commun 267: 129–133. [DOI] [PubMed] [Google Scholar]
- Kimura H (2010). Hydrogen sulfide: from brain to gut. Antioxid Redox Signal 12: 1111–1123. [DOI] [PubMed] [Google Scholar]
- Krueger D, Foerster M, Mueller K, Zeller F, Slotta‐Huspenina J, Donovan J et al. (2010). Signaling mechanisms involved in the intestinal pro‐secretory actions of hydrogen sulfide. Neurogastroenterol Motil 22: 1224–1220. [DOI] [PubMed] [Google Scholar]
- Levitt MD, Springfield J, Furne J, Koenig T, Suarez FL (2002). Physiology of sulfide in the rat colon: use of bismuth to assess colonic sulfide production. J Appl Physiol (1985) 92: 1655–1660. [DOI] [PubMed] [Google Scholar]
- Lies B, Beck K, Keppler J, Saur D, Groneberg D, Friebe A (2015). Nitrergic signalling via interstitial cells of Cajal regulates motor activity in murine colon. J Physiol 593: 4589–4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lies B, Groneberg D, Friebe A (2014). Toward a better understanding of gastrointestinal nitrergic neuromuscular transmission. Neurogastroenterol Motil 26: 901–912. [DOI] [PubMed] [Google Scholar]
- Lies B, Groneberg D, Gambaryan S, Friebe A (2013). Lack of effect of ODQ does not exclude cGMP signalling via NO‐sensitive guanylyl cyclase. Br J Pharmacol 170: 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DR, Furne J, Stoltz GJ, Abdel‐Rehim MS, Levitt MD, Szurszewski JH (2012). Sulphide quinone reductase contributes to hydrogen sulphide metabolism in murine peripheral tissues but not in the CNS. Br J Pharmacol 165: 2178–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DR, Levitt MD, Farrugia G, Szurszewski JH (2010). Endogenous production of H2S in the gastrointestinal tract: still in search of a physiologic function. Antioxid Redox Signal 12: 1135–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DR, Sha L, Mazzone A, Stoltz GJ, Bernard CE, Furne JK et al. (2008). Production of the gaseous signal molecule hydrogen sulfide in mouse tissues. J Neurochem 106: 1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Luo H, Liang C, Xia H, Xu W, Chen J et al. (2013). Actions of hydrogen sulfide and ATP‐sensitive potassium channels on colonic hypermotility in a rat model of chronic stress. PLoS One 8: e55853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF et al. (2007). Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 445: 541–545. [DOI] [PubMed] [Google Scholar]
- Mane N, Gil V, Martinez‐Cutillas M, Clave P, Gallego D, Jimenez M (2014a). Differential functional role of purinergic and nitrergic inhibitory cotransmitters in human colonic relaxation. Acta Physiol (Oxf) 212: 293–305. [DOI] [PubMed] [Google Scholar]
- Mane N, Gil V, Martinez‐Cutillas M, Martin MT, Gallego D, Jimenez M (2014b). Dynamics of inhibitory co‐transmission, membrane potential and pacemaker activity determine neuromyogenic function in the rat colon. Pflugers Arch 466: 2305–2321. [DOI] [PubMed] [Google Scholar]
- Mane N, Viais R, Martinez‐Cutillas M, Gallego D, Correia‐de‐Sa P, Jimenez M (2016). Inverse gradient of nitrergic and purinergic inhibitory cotransmission in the mouse colon. Acta Physiol (Oxf) 216: 120–131. [DOI] [PubMed] [Google Scholar]
- Mard SA, Ahmadi I, Ahangarpour A, Gharib‐Naseri MK, Badavi M (2016). Delayed gastric emptying in diabetic rats caused by decreased expression of cystathionine gamma lyase and H2 S synthesis: in vitro and in vivo studies. Neurogastroenterol Motil 28: 1677–1689. [DOI] [PubMed] [Google Scholar]
- Martin GR, McKnight GW, Dicay MS, Coffin CS, Ferraz JG, Wallace JL (2010). Hydrogen sulphide synthesis in the rat and mouse gastrointestinal tract. Dig Liver Dis 42: 103–109. [DOI] [PubMed] [Google Scholar]
- Martin‐Cano FE, Camello PJ, Pozo MJ (2014). Characterization of the motor inhibitory role of colonic mucosa under chemical stimulation in mice. Am J Physiol Gastrointest Liver Physiol 306: G614–G621. [DOI] [PubMed] [Google Scholar]
- Martinez‐Cutillas M, Gil V, Mane N, Clave P, Gallego D, Martin MT et al. (2015). Potential role of the gaseous mediator hydrogen sulphide (H2S) in inhibition of human colonic contractility. Pharmacol Res 93: 52–63. [DOI] [PubMed] [Google Scholar]
- Matsunami M, Tarui T, Mitani K, Nagasawa K, Fukushima O, Okubo K et al. (2009). Luminal hydrogen sulfide plays a pronociceptive role in mouse colon. Gut 58: 751–761. [DOI] [PubMed] [Google Scholar]
- Mearin F, Mourelle M, Guarner F, Salas A, Riveros‐Moreno V, Moncada S et al. (1993). Patients with achalasia lack nitric oxide synthase in the gastro‐oesophageal junction. Eur J Clin Invest 23: 724–728. [DOI] [PubMed] [Google Scholar]
- Medeiros JV, Bezerra VH, Lucetti LT, Lima‐Junior RC, Barbosa AL, Tavares BM et al. (2012). Role of KATP channels and TRPV1 receptors in hydrogen sulfide‐enhanced gastric emptying of liquid in awake mice. Eur J Pharmacol 693: 57–63. [DOI] [PubMed] [Google Scholar]
- Mikami Y, Shibuya N, Ogasawara Y, Kimura H (2013). Hydrogen sulfide is produced by cystathionine γ‐lyase at the steady‐state low intracellular Ca(2+) concentrations. Biochem Biophys Res Commun 431: 131–135. [DOI] [PubMed] [Google Scholar]
- Mimoun S, Andriamihaja M, Chaumontet C, Atanasiu C, Benamouzig R, Blouin JM et al. (2012). Detoxification of H(2)S by differentiated colonic epithelial cells: implication of the sulfide oxidizing unit and of the cell respiratory capacity. Antioxid Redox Signal 17: 1–10. [DOI] [PubMed] [Google Scholar]
- Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK et al. (2009a). H2S signals through protein S‐sulfhydration. Sci Signal 2: ra72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa AK, Gadalla MM, Snyder SH (2009b). Signaling by gasotransmitters. Sci Signal 2: re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao M, Duenes JA, Sarr MG (2012). Role of hydrogen sulfide as a gasotransmitter in modulating contractile activity of circular muscle of rat jejunum. J Gastrointest Surg 16: 334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao M, Linden DR, Duenes JA, Sarr MG (2011). Mechanisms of action of the gasotransmitter hydrogen sulfide in modulating contractile activity of longitudinal muscle of rat ileum. J Gastrointest Surg 15: 12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalli AD, Rajagopal S, Mahavadi S, Grider JR, Murthy KS (2015). Inhibition of RhoA‐dependent pathway and contraction by endogenous hydrogen sulfide in rabbit gastric smooth muscle cells. Am J Physiol Cell Physiol 308: C485–C495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondrias K, Stasko A, Cacanyiova S, Sulova Z, Krizanova O, Kristek F et al. (2008). H(2)S and HS(−) donor NaHS releases nitric oxide from nitrosothiols, metal nitrosyl complex, brain homogenate and murine L1210 leukaemia cells. Pflugers Arch 457: 271–279. [DOI] [PubMed] [Google Scholar]
- Parajuli SP, Choi S, Lee J, Kim YD, Park CG, Kim MY et al. (2010). The inhibitory effects of hydrogen sulfide on pacemaker activity of interstitial cells of cajal from mouse small intestine. Korean J Physiol Pharmacol 14: 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patacchini R, Santicioli P, Giuliani S, Maggi CA (2004). Hydrogen sulfide (H2S) stimulates capsaicin‐sensitive primary afferent neurons in the rat urinary bladder. Br J Pharmacol 142: 31–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul BD, Snyder SH (2015). H2S: a novel gasotransmitter that signals by sulfhydration. Trends Biochem Sci 40: 687–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluja L, Alberti E, Fernandez E, Mikkelsen HB, Thuneberg L, Jimenez M (2001). Evidence supporting presence of two pacemakers in rat colon. Am J Physiol Gastrointest Liver Physiol 281: G255–G266. [DOI] [PubMed] [Google Scholar]
- Pouokam E, Steidle J, Diener M (2011). Regulation of colonic ion transport by gasotransmitters. Biol Pharm Bull 34: 789–793. [DOI] [PubMed] [Google Scholar]
- Pozuelo M, Panda S, Santiago A, Mendez S, Accarino A, Santos J et al. (2015). Reduction of butyrate‐ and methane‐producing microorganisms in patients with irritable bowel syndrome. Sci Rep 5: 12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan X, Luo H, Liu Y, Xia H, Chen W, Tang Q (2015). Hydrogen sulfide regulates the colonic motility by inhibiting both L‐type calcium channels and BKCa channels in smooth muscle cells of rat colon. PLoS One 10: e0121331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy S, Singh S, Taniere P, Langman MJ, Eggo MC (2006). Sulfide‐detoxifying enzymes in the human colon are decreased in cancer and upregulated in differentiation. Am J Physiol Gastrointest Liver Physiol 291: G288–G296. [DOI] [PubMed] [Google Scholar]
- Reiffenstein RJ, Hulbert WC, Roth SH (1992). Toxicology of hydrogen sulfide. Annu Rev Pharmacol Toxicol 32: 109–134. [DOI] [PubMed] [Google Scholar]
- Schemann M, Grundy D (2009). Role of hydrogen sulfide in visceral nociception. Gut 58: 744–747. [DOI] [PubMed] [Google Scholar]
- Schicho R, Krueger D, Zeller F, Von Weyhern CW, Frieling T, Kimura H et al. (2006). Hydrogen sulfide is a novel prosecretory neuromodulator in the guinea‐pig and human colon. Gastroenterology 131: 1542–1552. [DOI] [PubMed] [Google Scholar]
- Sha L, Farrugia G, Linden DR, Szurszewski JH (2010). The transwall gradient across the mouse colonic circular muscle layer is carbon monoxide dependent. FASEB J 24: 3840–3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha L, Linden DR, Farrugia G, Szurszewski JH (2014). Effect of endogenous hydrogen sulfide on the transwall gradient of the mouse colon circular smooth muscle. J Physiol 592 (Pt 5): 1077–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya N, Koike S, Tanaka M, Ishigami‐Yuasa M, Kimura Y, Ogasawara Y et al. (2013). A novel pathway for the production of hydrogen sulfide from d‐cysteine in mammalian cells. Nat Commun 4: 1366. [DOI] [PubMed] [Google Scholar]
- Shibuya N, Mikami Y, Kimura Y, Nagahara N, Kimura H (2009a). Vascular endothelium expresses 3‐mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J Biochem 146: 623–626. [DOI] [PubMed] [Google Scholar]
- Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, Ishii K et al. (2009b). 3‐Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal 11: 703–714. [DOI] [PubMed] [Google Scholar]
- Shteyer E, Edvardson S, Wynia‐Smith SL, Pierri CL, Zangen T, Hashavya S et al. (2015). Truncating mutation in the nitric oxide synthase 1 gene is associated with infantile achalasia. Gastroenterology 148: 533–536. [DOI] [PubMed] [Google Scholar]
- Skovgaard N, Gouliaev A, Aalling M, Simonsen U (2011). The role of endogenous H2S in cardiovascular physiology. Curr Pharm Biotechnol 12: 1385–1393. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipanuk MH, Beck PW (1982). Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem J 206: 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strege PR, Bernard CE, Kraichely RE, Mazzone A, Sha L, Beyder A et al. (2011). Hydrogen sulfide is a partially redox‐independent activator of the human jejunum Na+ channel, Nav1.5. Am J Physiol Gastrointest Liver Physiol 300: G1105–G1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulaieva O, Wallace JL (2015). Gaseous mediator‐based anti‐inflammatory drugs. Curr Opin Pharmacol 25: 1–6. [DOI] [PubMed] [Google Scholar]
- Sun HZ, Yu KH, Ai HB (2015). Role of hydrogen sulfide within the dorsal motor nucleus of the vagus in the control of gastric function in rats. Neurogastroenterol Motil 27: 618–626. [DOI] [PubMed] [Google Scholar]
- Sun Q, Collins R, Huang S, Holmberg‐Schiavone L, Anand GS, Tan CH et al. (2009). Structural basis for the inhibition mechanism of human cystathionine gamma‐lyase, an enzyme responsible for the production of H(2)S. J Biol Chem 284: 3076–3085. [DOI] [PubMed] [Google Scholar]
- Szabo C (2007). Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov 6: 917–935. [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Ise F, Takahashi K, Aihara E, Hayashi S (2015). H2S‐induced HCO3‐ secretion in the rat stomach–involvement of nitric oxide, prostaglandins, and capsaicin‐sensitive sensory neurons. Nitric. Oxide. 46: 157–164. [DOI] [PubMed] [Google Scholar]
- Teague B, Asiedu S, Moore PK (2002). The smooth muscle relaxant effect of hydrogen sulphide in vitro: evidence for a physiological role to control intestinal contractility. Br J Pharmacol 137: 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiranti V, Viscomi C, Hildebrandt T, Di MI, Mineri R, Tiveron C et al. (2009). Loss of ETHE1, a mitochondrial dioxygenase, causes fatal sulfide toxicity in ethylmalonic encephalopathy. Nat Med 15: 200–205. [DOI] [PubMed] [Google Scholar]
- Trevisani M, Patacchini R, Nicoletti P, Gatti R, Gazzieri D, Lissi N et al. (2005). Hydrogen sulfide causes vanilloid receptor 1‐mediated neurogenic inflammation in the airways. Br J Pharmacol 145: 1123–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandiver M, Snyder SH (2012). Hydrogen sulfide: a gasotransmitter of clinical relevance. J Mol Med (Berl) 90: 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JL, Caliendo G, Santagada V, Cirino G (2010). Markedly reduced toxicity of a hydrogen sulphide‐releasing derivative of naproxen (ATB‐346). Br J Pharmacol 159: 1236–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JL, Dicay M, McKnight W, Martin GR (2007). Hydrogen sulfide enhances ulcer healing in rats. FASEB J 21: 4070–4076. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Ferraz JG, Muscara MN (2012). Hydrogen sulfide: an endogenous mediator of resolution of inflammation and injury. Antioxid Redox Signal 17: 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JL, Ianaro A, Flannigan KL, Cirino G (2015). Gaseous mediators in resolution of inflammation. Semin Immunol 27: 227–233. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Vong L, McKnight W, Dicay M, Martin GR (2009). Endogenous and exogenous hydrogen sulfide promotes resolution of colitis in rats. Gastroenterology 137: 569–578 578. [DOI] [PubMed] [Google Scholar]
- Wallace S, Guo DC, Regalado E, Mellor‐Crummey L, Banshad M, Nickerson DA et al. (2016). Disrupted Nitric Oxide signaling due to GUCY1A3 mutations increases the risk for moyamoya disease, achalasia and hypertension. Clin Genet 90: 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R (2002). Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J 16: 1792–1798. [DOI] [PubMed] [Google Scholar]
- Whiteman M, Le TS, Chopra M, Fox B, Whatmore J (2011). Emerging role of hydrogen sulfide in health and disease: critical appraisal of biomarkers and pharmacological tools. Clin Sci (Lond) 121: 459–488. [DOI] [PubMed] [Google Scholar]
- Yamane S, Kanno T, Nakamura H, Fujino H, Murayama T (2014). Hydrogen sulfide‐mediated regulation of contractility in the mouse ileum with electrical stimulation: roles of L‐cysteine, cystathionine beta‐synthase, and K+ channels. Eur J Pharmacol 740: 112–120. [DOI] [PubMed] [Google Scholar]
- Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K et al. (2008). H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma‐lyase. Science 322: 587–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon PJ, Parajuli SP, Zuo DC, Shahi PK, Oh HJ, Shin HR et al. (2011). Interplay of hydrogen sulfide and nitric oxide on the pacemaker activity of interstitial cells of cajal from mouse small intestine. Chonnam Med J 47: 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhao W, Zheng Z, Wang T, Zhao C, Zhou G et al. (2015). Reduction of hydrogen sulfide synthesis enzymes in the esophagus of patients with achalasia: effect of hydrogen sulfide in achalasia. Neurogastroenterol Motil 27: 1274–1281. [DOI] [PubMed] [Google Scholar]
- Zhao P, Huang X, Wang ZY, Qiu ZX, Han YF, Lu HL et al. (2009). Dual effect of exogenous hydrogen sulfide on the spontaneous contraction of gastric smooth muscle in guinea‐pig. Eur J Pharmacol 616: 223–228. [DOI] [PubMed] [Google Scholar]
- Zhao W, Zhang J, Lu Y, Wang R (2001). The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J 20: 6008–6016. [DOI] [PMC free article] [PubMed] [Google Scholar]


