Abstract
Angiotensin-converting enzyme inhibitor (ACE-I)–induced angioedema can be life-threatening without emergent intervention. The putative mediator is believed to be bradykinin, similar to hereditary angioedema, so these patients respond poorly to corticosteroids and antihistamines. This study was designed to determine characteristics and clinical outcomes of patients presenting to an emergency department (ED) with ACE-I angioedema. This was a retrospective chart review of 100 patients presenting to the ED from 2007 to 2008 with an ICD-9 code of 995.1 (angioedema) or 995.2 (drug-induced angioedema). Two hundred fifty-two patients with these ICD-9 codes were identified and placed in random order, and the first 100 meeting inclusion criteria were included. Statistical analysis was primarily descriptive. All 100 patients had an ICD-9 code of 995.1 (angioedema). Patients presented in every month, with spring months (April–June) having the most presentations (32%). The median age was 59 years, 75% were African American, and 66% were admitted to the hospital. Two patients (2%) required endotracheal intubation. Lisinopril was the most commonly prescribed ACE-I (84%). The most common symptom was moderate lip and tongue swelling (89%) followed by mild difficulty breathing (12%). Tongue swelling was significantly associated with admission. Time from symptom onset to ED presentation was not associated with need for admission. Concomitant medications did not differ between admitted and discharged patients. ACE-I angioedema is associated with significant morbidity and health care use because many patients require hospitalization, suggesting an unmet need for novel therapies targeted to treat this condition.
Keywords: Angioedema, angiotensin-converting enzyme inhibitor, discharge, emergency department, hospitalization, lip swelling, lisinopril, seasonality, timing, tongue swelling
Angioedema is a self-limiting, localized swelling of the skin and/or mucosal membranes resulting from extravasation of fluid into the interstitium. It can be acute or chronic and is usually transient but often recurrent. It usually involves surfaces that have loose connective tissue such as the face, lips, mouth, throat, larynx, and uvula.1 Occasionally, bowel mucosa and visceral organs may be involved.1 Although most cases of angioedema are self-limiting, reactions can be severe and even life-threatening, especially if it involves the swelling of the tongue, glottis, and/or larynx.2,3
Angioedema induced by angiotensin-converting enzyme inhibitors (ACE-I) is a potentially life-threatening event. With the increased use of these agents for a number of chronic medical conditions, the incidence of ACE-I–induced angioedema is expected to increase.4 In 2001, 35–40 million prescriptions were written for ACE-I worldwide.5 The mechanism for ACE-induced angioedema is believed to involve the accumulation of bradykinin similar to hereditary angioedema (HAE).6,7 It is estimated that angioedema occurs in ∼0.1–2.2% of patients treated with ACE-I.8–11 A higher incidence of ACE-I–induced angioedema has been observed in women and African Americans and in patients with heart failure for both ACE-I and angiotensin II receptor blockers (ARBs).12–14
Because of extensive use of ACE-I in common chronic diseases including hypertension, diabetes, and heart failure, ACE-I has become the most common cause of angioedema in patients presenting to the emergency department (ED). There are several descriptive retrospective studies of patients visiting the ED with ACE-I–induced angioedema.15–19 Among these previous retrospective studies, only one study investigated the presentation, management, and predictors for hospitalization.15 The purpose of this study was to further define the characteristics of patients presenting to the ED with ACE-I–induced angioedema associated with hospitalization. A description of characteristics associated with hospital admission is important, because the wide usage and effectiveness of ACE-Is for a spectrum of clinical disorders and their low cost makes them a very important therapeutic class that will likely be used by millions of people for many years to come. Therefore, increasing our understanding of the presentation and natural course of ACE-I angioedema in different institutions throughout the United States is warranted.
METHODS
Study Design
This was a retrospective medical record review of patients who presented to the ED of a busy, level 1 tertiary care referral center in the Midwest from 2007 to 2008 with an ICD-9 code of either 995.1 (angioedema) or 995.2 (drug-induced angioedema). Trained chart reviewers, composed of a physician and nurse, screened all charts to determine whether the angioedema was ACE-I induced and met inclusion criteria for a complete ED chart review. The main criteria for inclusion were presentation to the ED with facial, tongue, lip, or laryngeal angioedema, currently taking an agent that included an ACE-I alone or in combination with another blood pressure medication (i.e., hydrochlorothiazide [HCTZ]), and having a discharge diagnosis or hospital admission diagnosis consistent with ACE-I–induced angioedema. We identified 287 visits from 252 unique patients with ICD codes 995.1 or 995.2. Of these patients 156 failed and 131 passed the initial screen for ACE-I angioedema. These visits were placed in random order and abstractors were instructed to review the complete ED chart of eligible cases until 100 cases were completed. Discordant charts (i.e., where one reviewer indicated the case should be included and the other did not) were adjudicated by a third physician investigator. Information extracted from each chart included gender sex, age, race, ACE-I usage, time to onset of symptom, presenting signs and symptom, concomitant medication use, ED treatment, outcome of treatment, and patient disposition. The two reviewers abstracted data from each chart independently. Data were entered into a detailed case report form that included prespecified data definitions. Approval for this study was obtained from our Institutional Review Board.
Statistical Analysis
Values for all measurements are expressed as medians and ranges or as frequencies and percents. Presenting signs and symptoms and subsequent treatments were compared between discharged and admitted patients by computing differences and 95% CIs for differences. No patients were excluded from this analysis. Data were analyzed using Statistical Package for the Social Sciences, (SPSS Version 18.0; SPSS, Inc., Chicago, IL).
RESULTS
For the 100 ED patients with ACE-I–induced angioedema, the median age was 59 years (25–90 years). About one-half (53%) were women, and most (75%) were African American. Vital signs on presentation to the ED showed a mean respiratory rate of 18 breaths/min (SD, 3) heart rate of 86 beats/min (SD, 15), a systolic blood pressure of 149 mmHg (SD, 26), diastolic blood pressure of 87 mmHg (SD, 16), and oxygen saturation of 98% (SD 2%). The most common symptom exhibited by patients was moderate tongue swelling (89%), followed by mild difficulty breathing (12%). In the ED most patients were treated with corticosteroids (87%), H1-antagonists (89%) and H2-antagonists (74%). Only one patient was treated with epinephrine (1%). Inhaled β2-agonists were used in 4% of patients. The majority of patients required inpatient hospital admission (66%). Two of these patients (2%) required ventilatory support in the ED. Patients presented throughout the year, although seasonal variation was observed with the greatest number of patients presenting during the Spring months (April–June; 32%; Fig. 1).
Figure 1.
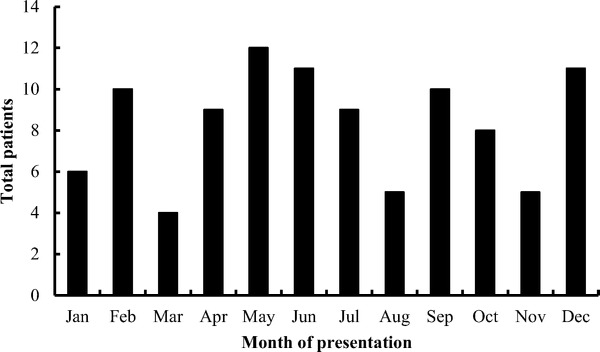
Monthly frequency of angiotensin-converting enzyme inhibitor (ACE-I)-induced angioedema presenting to the emergency department (ED) over 1 year.
Lisinopril was the most commonly prescribed ACE-I in patients with angioedema (86%), followed by enalapril (5%), benazepril/amlodipine (2%), benazepril (2%), lisinopril/HCTZ (2%), ramipril (2%), and moexipril/HCTZ (1%). Among the 37 patients for whom duration of ACE-I use was available, approximately one-half had been taking the ACE-I <3 months and one-half were on it for >1 year (Table 1).
Table 1.
Demographics by disposition
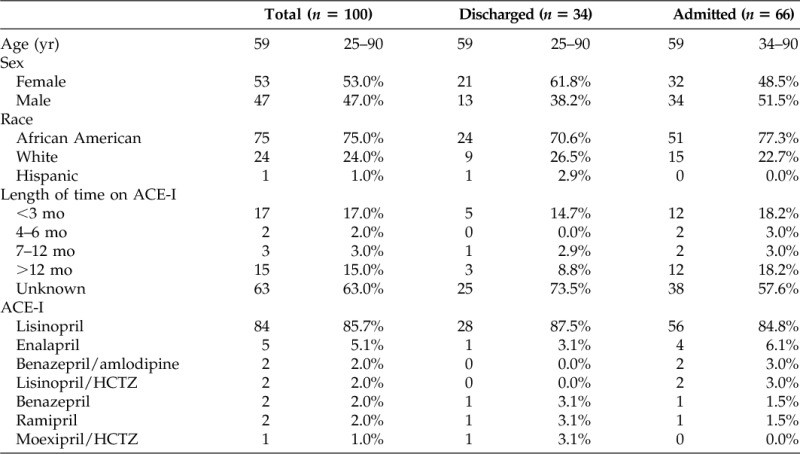
Data are presented in median and range of frequency and percent.
ACE-I = angiotensin-converting enzyme inhibitor; HCTZ = hydrochlorothiazide.
There were few statistically significant differences in clinical features of patients with ACE-I angioedema admitted to the hospital or discharged home. Tongue swelling was more common in admitted patients, compared with discharged patients (47% versus 21%; difference in proportions 26%; 95% CI, 7–42%; p < 0.01). In contrast, oral/lip swelling was somewhat more common in discharged patients, although this did not reach statistical significance (77% versus 62%; difference in proportions 14%; 95% CI, −5–31%). Symptom onset time was poorly documented and could be determined for only 32 patients. In these patients, the time from symptom onset to ED presentation was not associated with the need for hospitalization (median, 237 minutes in admitted patients versus 237 minutes in discharged patients; 95% CI, −407.77–405.77; Table 2). Concomitant medications that patients were taking did not differ between admitted and discharged patients (data not shown). Although angioedema can also be induced by ARBs the focus of this study was on angioedema induced by ACE-I. Only three patients in this analysis were documented to be taking an ACE-I concomitantly with an ARB. However, a recent meta-analysis comparing angioedema and cough induced by ACE-I and ARBs versus placebo found that these intolerances for ARBs were no greater than placebo.20
Table 2.
Baseline symptoms and ED treatment by disposition
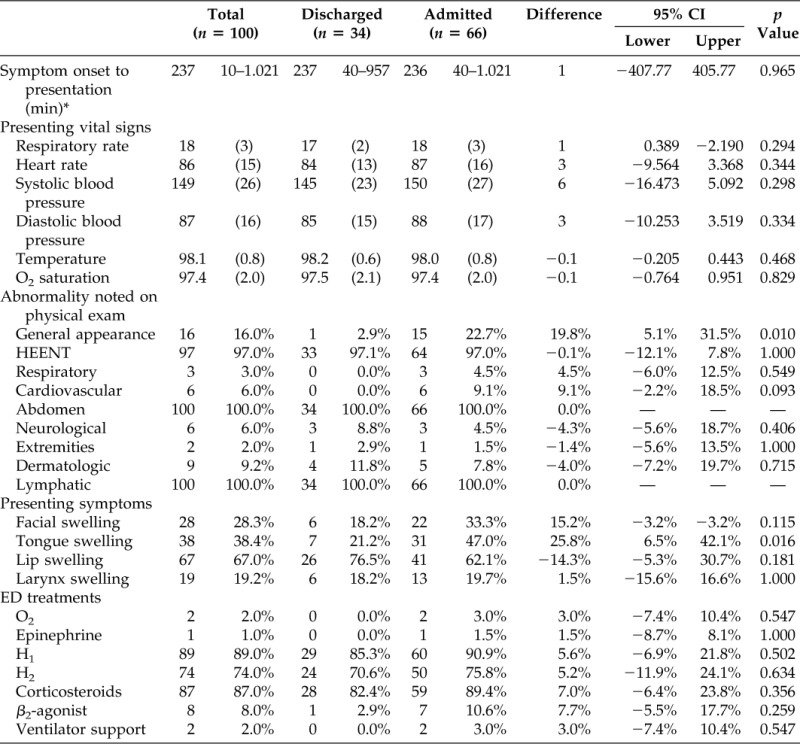
*n = 32.
ED = emergency department; HEENT = head, eyes, ears, nose and throat.
DISCUSSION
The major findings of our study were as follows: (1) patients with ACE-I–induced angioedema presented every month during the year but a seasonal increase during the spring months (April–June) was observed, (2) lisinopril was the most common ACE-I associated with angioedema, (3) the most common symptom associated with hospitalization was tongue/laryngeal swelling, and (4) the time from symptom onset to ED presentation was not associated with need for admission.
The observed seasonal variation in presentations by patients with ACE-I angioedema has been reported by other investigators who have postulated a possible role for atopy in triggering these attacks by further stressing the complement system.13,21 Unfortunately, in our study, information regarding the patient's atopic status was not available. However, further study of involvement of seasonal allergy in the pathogenesis of ACE-I–induced angioedema is warranted.
Lisinopril has previously been shown to be the most frequently prescribed ACE-I.15,22,23 Our data mirror this, with 84% of patients taking lisinopril at presentation to the ED. This observation likely reflects the low cost and high efficacy of this agent and is not caused by its unique structural characteristics.
Most cases of ACE-I angioedema are thought to occur within hours to a week after starting the medication. Studies have reported that 50–60% of patients experienced angioedema in the 1st week after initiating the use of an ACE-I.2,16 However, in rare instances ACE-I angioedema can occur as long as 5 years after first starting this medication.21 The results of our study are consistent with these previous reports, suggesting that ACE-I angioedema can occur more than a year after the patient starts taking the medication, although the lack of documented information about duration of medication use in this study limits our ability to estimate the proportion of persons for whom there is a significant delay to the first angioedema attack.
ACE-I–induced angioedema is thought to occur secondary to accumulation of bradykinin levels.24,25 In the kinin pathway, high–molecular-weight kininogen comes into contact with a negatively charged surface. Activated Hageman factor then cleaves kininogen to form kallikrein, which degrades high–molecular-weight kininogen to form bradykinin, which activates bradykinin 2 receptors to induce vessel dilation and increase vascular permeability.22 ACE normally regulates the production of bradykinin by inhibiting kallikrein. Inhibition of ACE in addition to the impaired ability to degrade bradykinin has been postulated as the reason why susceptible individuals taking these medications swell. X-propeptidase, the enzyme important for degrading bradykinin, has been reported to be deficient in patients with ACE-I–induced angioedema.26 In addition, the enzyme dipeptidyl peptidase IV, which is responsible for degradation of substance P, has been found to be significantly decreased in patients with ACE-I–induced angioedema and has been speculated to predispose individuals taking an ACE inhibitor to develop angioedema.27–29 Reduced activity of this enzyme leads to an accumulation of substance P resulting in increased vascular permeability and edema.27
Our study cohort included a disproportionate number of African Americans and women. If this cohort were truly reflective of underlying disease epidemiology, this would be consistent with previous incidence reports.5,15,22,28 African Americans have an increased risk of ACE-I–induced angioedema, independent of ACE-I dose, although the reason for their increased risk has not been completely elucidated. Differences in the kallikrein-kinin system have been reported, which might contribute to their increased risk of ACE-I–induced angioedema.30–32 It has also been postulated that African Americans may have lower levels of bradykinin than white populations, as evidenced by their decreased urinary kallikrein levels but greater physiological sensitivity to bradykinin.29–31 We note that our study was not designed to assess incidence of ACE-I–induced angioedema and so it would not be appropriate to use our data in support of incident statements.
Lip and tongue swelling and shortness of breath were the most common symptoms manifested by patients in our study. Other studies have also reported that ACE-I–induced angioedema has a predilection for the head and neck.2,5 Banerji reported that shortness of breath was one of the most commonly documented presenting symptoms of ACE-I–induced angioedema, which is consistent with our findings.15 Although the duration of lip, tongue, and pharyngeal swelling was not available in this study, previous reports indicate that it typically resolves within 24–48 hours.33
Two-thirds (66%) of the patients in our cohort were admitted to hospital, likely because of tongue and laryngeal swelling. This admission rate is slightly higher than previously reported for a tertiary care center study.15 Tongue and laryngeal swelling reflects the severity of angioedema. Patients with these symptoms tend to be observed more cautiously and receive more aggressive treatment. Therefore, these patients are usually admitted to the hospital. We also noted that time from symptom onset to presentation was not associated with subsequent hospital admission in either study. Although two patients in the current cohort required intubation, there were no reported deaths secondary to asphyxia.
ACE-I–induced angioedema has been reported to account for one-third of angioedema-related admissions from the ED.15,16,18,34 Consistent with our findings, many of these patients require inpatient hospitalization for management of upper airway angioedema and airway compromise.35 Effective treatment of ACE-I–induced angioedema is essential for reducing morbidity and potential mortality as well as to reduce health care use. First and foremost, the ACE-I should be discontinued. Patients with ACE-I angioedema should be monitored for recurrent angioedema for several weeks because they appear to be at increased risk for angioedema episodes irrespective of the non–ACE-I replacement therapy initiated.36 Conventional therapies such as H1- and H2-antagonists, oral corticosteroids, and epinephrine have not been effective in preventing the progression of these attacks in the majority of patients, supporting a key role for bradykinin as the putative mediator.37 There are a handful of cases reporting successful treatment of ACE-induced angioedema with fresh frozen plasma, which was used because there were no other options.38–41 Plasma-derived C1-inhibitor has also been reported to be effective for ACE-I angioedema.42,43 Newer therapies that either target kallikrein (ecallantide) or block the bradykinin 2 receptor (icatibant) are now approved in the United States for the treatment of HAE, a rare orphan disorder, also mediated by bradykinin.44,45 Recent cases have been reported that the bradykinin receptor 2 antagonist (icatibant) was effective for the treatment of ACE-I angioedema.37,46–48 Randomized clinical trials are currently underway to determine the effectiveness of these medications in ACE-I–induced angioedema (ClinTrials.gov ID-NCT01036659).
This report adds to the existing and still emerging literature, because it confirms as well as contradicts data reported by other investigators. With the advent of new therapies being investigated to treat ACE-I angioedema, it is important to create further awareness of this problem among the medical community so research and development continues to progress. Using an alternative antihypertensive agent is certainly an option. However, ACE inhibitors are excellent therapeutic agents that are well tolerated for the vast majority of patients and have many long-term benefits.
Limitations
Our findings should be interpreted with respect to the limitations inherent in our design. First, because this was a retrospective study, information that would have been useful to help further explain some of our observations such as atopic status of the patients and duration of their attacks was not available. Second, we are not able to discern whether some of these patients could have had undiagnosed HAE or acquired angioedema because C4 or C1 esterase inhibitor levels were not measured; the definition of ACE-I–induced angioedema was based simply on the concurrence of angioedema and taking an ACE-I. Third, the true incidence of laryngeal involvement would not be found unless all of the patients underwent direct examination of the laryngeal structures, and so our data are subject to workup bias. Perhaps our increased rate of admission was caused by patient-reported symptoms associated with airway involvement, even if the airway structures were not directly visualized. Fourth, this study was small, with only 100 patients. Our analytic approach, therefore, has focused on describing the patient cohort and management of the cohort. When comparisons have been made, we have provided estimates of effect size rather than statistical tests in part to avoid emphasizing differences that might be the result of a type I error due to multiple testing. Finally, recording bias is always a potential problem in retrospective studies. For example, the poor reporting in time from symptom onset was not inconsistent between discharged and admitted patients and there is potential for nonrandom missing data. We attempted to overcome these biases by using prespecified methods for handling missing data, rigorous chart review methodology, cross-checking of charts, and adjudication by a third investigator.
CONCLUSIONS
In summary, our results suggest ACE-I–induced angioedema is associated with significant morbidity and health care use because many patients require hospitalization. This suggests an unmet need for novel therapies targeted to treat this condition.
Footnotes
Presented at 2011 Annual Meeting of the American College of Allergy, Asthma, and Immunology, Boston, Massachusetts, November 5, 2011
JA Bernstein is a PI, consultant, and speaker for ViroPharma, Dyax, Shire, and CSL-Behring; a PI for Pharming; on the Editorial Board of the Journal of Angioedema and the Medical Advisory Board of the HAEA organization. J Moellman has done consulting work for CSL-Behring and is a Co-PI with JA Bernstein for Dyax investigating the safety and efficacy of ecallantide in ace inhibitor–induced angioedema. S Collins and CJ Lindsell are Co-PIs with JA Bernstein for Dyax investigating the safety and efficacy of ecallantide in ace inhibitor induced angioedema. JA Bernstein, S Collins, CJ Lindsell, and J Moellman have also received an unrestricted educational grant from Dyax, Shire, Pharming, and CSL-Behring to develop consensus guidelines for the Evaluation and Management of Angioedema in the ED. The remaining authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Bernstein JA. Update on angioedema: Evaluation, diagnosis, and treatment. Allergy Asthma Proc 32:408–412, 2011. [DOI] [PubMed] [Google Scholar]
- 2. Vleeming W, van Amsterdam JG, Stricker BH, de Wildt DJ. ACE inhibitor-induced angioedema. Incidence, prevention, and management. Drug Saf 18:171–188, 1998. [DOI] [PubMed] [Google Scholar]
- 3. Vasekar M, Craig TJ. ACE inhibitor-induced angioedema. Curr Allergy Asthma Rep 12:72–78. [DOI] [PubMed] [Google Scholar]
- 4. Holm JP, Ovesen T. Increasing rate of angiotensin-converting enzyme inhibitor-related upper airway angio-oedema. Dan Med J 59:A4449, 2012. [PubMed] [Google Scholar]
- 5. Sondhi D, Lippmann M, Murali G. Airway compromise due to angiotensin-converting enzyme inhibitor-induced angioedema: Clinical experience at a large community teaching hospital. Chest 126:400–404, 2004. [DOI] [PubMed] [Google Scholar]
- 6. Nussberger J, Cugno M, Amstutz C, et al. Plasma bradykinin in angio-oedema. Lancet 351:1693–1697, 1998. [DOI] [PubMed] [Google Scholar]
- 7. Caballero T, Baeza ML, Cabanas R, et al. Consensus statement on the diagnosis, management, and treatment of angioedema mediated by bradykinin. Part I. Classification, epidemiology, pathophysiology, genetics, clinical symptoms, and diagnosis. J Investig Allergol Clin Immunol 21:333–347, 2011. [PubMed] [Google Scholar]
- 8. Warner KK, Visconti JA, Tschampel MM. Angiotensin II receptor blockers in patients with ACE inhibitor-induced angioedema. Ann Pharmacother 34:526–528, 2000. [DOI] [PubMed] [Google Scholar]
- 9. Kostis JB, Packer M, Black HR, et al. Omapatrilat and enalapril in patients with hypertension: The Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens 17:103–111, 2004. [DOI] [PubMed] [Google Scholar]
- 10. Makani H, Messerli FH, Romero J, et al. Meta-analysis of randomized trials of angioedema as an adverse event of renin-angiotensin system inhibitors. Am J Cardiol 110:383–391. [DOI] [PubMed] [Google Scholar]
- 11. Toh S, Reichman ME, Houstoun M, et al. Comparative risk for angioedema associated with the use of drugs that target the renin-angiotensin-aldosterone system. Arch Intern Med 15:1–8, 2012. [DOI] [PubMed] [Google Scholar]
- 12. Brown NJ, Ray WA, Snowden M, Griffin MR. Black Americans have an increased rate of angiotensin converting enzyme inhibitor-associated angioedema. Clin Pharmacol Ther 60:8–13, 1996. [DOI] [PubMed] [Google Scholar]
- 13. Israili ZH, Hall WD. Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy. A review of the literature and pathophysiology. Ann Intern Med 117:234–242, 1992. [DOI] [PubMed] [Google Scholar]
- 14. Makani H, Messerli FH, Romero J, et al. Meta-analysis of randomized trials of angioedema as an adverse event of renin-angiotensin system inhibitors. Am J Cardiol 110:383–391, 2012. [DOI] [PubMed] [Google Scholar]
- 15. Banerji A, Clark S, Blanda M, et al. Multicenter study of patients with angiotensin-converting enzyme inhibitor-induced angioedema who present to the emergency department. Ann Allergy Asthma Immunol 100:327–332, 2008. [DOI] [PubMed] [Google Scholar]
- 16. Agah R, Bandi V, Guntupalli KK. Angioedema: The role of ACE inhibitors and factors associated with poor clinical outcome. Intensive Care Med 23:793–796, 1997. [DOI] [PubMed] [Google Scholar]
- 17. Megerian CA, Arnold JE, Berger M. Angioedema: 5 years' experience, with a review of the disorder's presentation and treatment. Laryngoscope 102:256–260, 1992. [DOI] [PubMed] [Google Scholar]
- 18. Pigman EC, Scott JL. Angioedema in the emergency department: The impact of angiotensin-converting enzyme inhibitors. Am J Emerg Med 11:350–354, 1993. [DOI] [PubMed] [Google Scholar]
- 19. Bluestein HM, Hoover TA, Banerji AS, et al. Angiotensin-converting enzyme inhibitor-induced angioedema in a community hospital emergency department. Ann Allergy Asthma Immunol 103:502–507, 2009. [DOI] [PubMed] [Google Scholar]
- 20. Caldeira D, David C, Sampaio C. Tolerability of angiotensin-receptor blockers in patients with intolerance to angiotensin-converting enzyme inhibitors: A systematic review and meta-analysis. Am J Cardiovasc Drugs 12:263–277, 2012. [DOI] [PubMed] [Google Scholar]
- 21. Byrd JB, Woodard-Grice A, Stone E, et al. Association of angiotensin-converting enzyme inhibitor-associated angioedema with transplant and immunosuppressant use. Allergy 65:1381–1387, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Malde B, Regalado J, Greenberger PA. Investigation of angioedema associated with the use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Ann Allergy Asthma Immunol 98:57–63, 2007. [DOI] [PubMed] [Google Scholar]
- 23. Piller LB, Ford CE, Davis BR, et al. Incidence and predictors of angioedema in elderly hypertensive patients at high risk for cardiovascular disease: A report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). J Clin Hypertens (Greenwich) 8:649–656, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Molinaro G, Cugno M, Perez M, et al. Angiotensin-converting enzyme inhibitor-associated angioedema is characterized by a slower degradation of des-arginine(9)-bradykinin. J Pharmacol Exp Ther 303:232–237, 2002. [DOI] [PubMed] [Google Scholar]
- 25. Anderson MW, deShazo RD. Studies of the mechanism of angiotensin-converting enzyme (ACE) inhibitor-associated angioedema: The effect of an ACE inhibitor on cutaneous responses to bradykinin, codeine, and histamine. J Allergy Clin Immunol 85:856–858, 1990. [DOI] [PubMed] [Google Scholar]
- 26. Adam A, Cugno M, Molinaro G, et al. Aminopeptidase P in individuals with a history of angio-oedema on ACE inhibitors. Lancet 359:2088–2089, 2002. [DOI] [PubMed] [Google Scholar]
- 27. Lefebvre J, Murphey LJ, Hartert TV, et al. Dipeptidyl peptidase IV activity in patients with ACE-inhibitor-associated angioedema. Hypertension 39:460–464, 2002. [DOI] [PubMed] [Google Scholar]
- 28. Byrd JB, Adam A, Brown NJ. Angiotensin-converting enzyme inhibitor-associated angioedema. Immunol Allergy Clin North Am 26:725–737, 2006. [DOI] [PubMed] [Google Scholar]
- 29. Byrd JB, Shreevatsa A, Putlur P, et al. Dipeptidyl peptidase IV deficiency increases susceptibility to angiotensin-converting enzyme inhibitor-induced peritracheal edema. J Allergy Clin Immunol 120:403–408, 2007. [DOI] [PubMed] [Google Scholar]
- 30. Gainer JV, Nadeau JH, Ryder D, Brown NJ. Increased sensitivity to bradykinin among African Americans. J Allergy Clin Immunol 98:283–287, 1996. [DOI] [PubMed] [Google Scholar]
- 31. Gainer JV, Stein CM, Neal T, et al. Interactive effect of ethnicity and ACE insertion/deletion polymorphism on vascular reactivity. Hypertension 37:46–51, 2001. [DOI] [PubMed] [Google Scholar]
- 32. Rosenbaum DA, Pretorius M, Gainer JV, et al. Ethnicity affects vasodilation, but not endothelial tissue plasminogen activator release, in response to bradykinin. Arterioscler Thromb Vasc Biol 22:1023–1028, 2002. [DOI] [PubMed] [Google Scholar]
- 33. Chiu AG, Newkirk KA, Davidson BJ, et al. Angiotensin-converting enzyme inhibitor-induced angioedema: A multicenter review and an algorithm for airway management. Ann Otol Rhinol Laryngol 110:834–840, 2001. [DOI] [PubMed] [Google Scholar]
- 34. Gabb GM, Ryan P, Wing LM, Hutchinson KA. Epidemiological study of angioedema and ACE inhibitors. Aust N Z J Med 26:777–782, 1996. [DOI] [PubMed] [Google Scholar]
- 35. Al-Khudari S, Loochtan MJ, Peterson E, Yaremchuk KL. Management of angiotensin-converting enzyme inhibitor-induced angioedema. Laryngoscope 121:2327–2334, 2011. [DOI] [PubMed] [Google Scholar]
- 36. Beltrami L, Zanichelli A, Zingale L, et al. Long-term follow-up of 111 patients with angiotensin-converting enzyme inhibitor-related angioedema. J Hypertens 29:2273–2277, 2011. [DOI] [PubMed] [Google Scholar]
- 37. Fast S, Henningsen E, Bygum A. Icatibant is a new treatment option in life-threatening angioedema triggered by angiotensin-converting enzyme inhibitor. Ugeskrift Laeger 173:2574–2575, 2011. [PubMed] [Google Scholar]
- 38. Karim MY, Masood A. Fresh-frozen plasma as a treatment for life-threatening ACE-inhibitor angioedema. J Allergy Clin Immunol 109:370–371, 2002. [DOI] [PubMed] [Google Scholar]
- 39. Warrier MR, Copilevitz CA, Dykewicz MS, Slavin RG. Fresh frozen plasma in the treatment of resistant angiotensin-converting enzyme inhibitor angioedema. Ann Allergy Asthma Immunol 92:573–575, 2004. [DOI] [PubMed] [Google Scholar]
- 40. Hassen GW, Kalantari H, Parraga M, et al. Fresh frozen plasma for progressive and refractory angiotensin-converting enzyme inhibitor-induced angioedema. J Emerg Med 2012. DOI: 10.1016/j.jemermed.2012.07.055 (Epub ahead of print.) [DOI] [PubMed] [Google Scholar]
- 41. Stewart M, McGlone R. Fresh frozen plasma in the treatment of ACE inhibitor-induced angioedema. BMJ Case Rep 2012. DOI: 10.1136/bcr-2012–006849 (Epub ahead of print.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nielsen EW, Gramstad S. Angioedema from angiotensin-converting enzyme (ACE) inhibitor treated with complement 1 (C1) inhibitor concentrate. Acta Anaesthesiol Scand 50:120–122, 2006. [DOI] [PubMed] [Google Scholar]
- 43. Gelee B, Michel P, Haas R, et al. Angiotensin-converting enzyme inhibitor-related angioedema: emergency treatment with complement C1 inhibitor concentrate. Rev Med Intern 29:516–519, 2008. [DOI] [PubMed] [Google Scholar]
- 44. Riedl MA. Update on the acute treatment of hereditary angioedema. Allergy Asthma Proc 32:11–16, 2011. [DOI] [PubMed] [Google Scholar]
- 45. Zuraw BL. HAE therapies: Past present and future. Allergy Asthma Clin Immunol 6:23, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gallitelli M, Alzetta M. Icatibant: A novel approach to the treatment of angioedema related to the use of angiotensin-converting enzyme inhibitors. Am J Emerg Med 30:1664.e1–1664.e2, 2012. [DOI] [PubMed] [Google Scholar]
- 47. Javaud N, Fain O, Bernot B, et al. Bradykinin-mediated angioedema secondary to angiotensin converting enzyme: Initiate treatment from the prehospital phase. Ann Fr Anesth Reanim 30:848–850, 2011. [DOI] [PubMed] [Google Scholar]
- 48. Illing EJ, Kelly S, Hobson JC, Charters S. Icatibant and ACE inhibitor angioedema. BMJ Case Rep 2012. DOI: 10.1136/bcr-2012–006646 (Epub ahead of print.) [DOI] [PMC free article] [PubMed] [Google Scholar]


