Abstract
Serum 25-hydroxyvitamin D (25[OH]D) concentrations are positively associated with pneumococcal antibody titers (PATs) in subjects with atopy or asthma. Little is known about the association of serum 25(OH)D concentrations and the waning of PATs over time in subjects with or without atopy. This study was designed to determine whether serum 25(OH)D concentrations are associated with waning of PATs and if such relationship is modified by atopic conditions. The study was designed as a prospective cohort study, which followed 20 asthmatic patients and 19 individuals without asthma for an average of 12 months. We measured PATs and serum 25(OH)D concentrations at baseline and at a subsequent follow-up visit. Asthma was ascertained by predetermined criteria. The association between serum 25(OH)D concentrations and PATs was determined by Pearson's correlation coefficient and a least square model. Of the 39 children and adults, 21(53%) were male subjects, all were white, and 6 (15%) were children. There was an overall negative correlation between serum 25(OH)D concentrations and the decrease of PATs during follow-up (r = −0.47; p = 0.004), suggesting that higher 25(OH)D concentrations were associated with a reduction in waning of PATs over time. Controlling for follow-up duration and pneumococcal colonization, these trends were significant among asthmatic patients but not in individuals without asthma. Similar trends were observed for individuals with or without other atopic conditions. Serum 25(OH)D concentrations are inversely associated with the waning of PATs over time, especially individuals with asthma and other atopy conditions. These study findings deserve further investigation.
Keywords: 25(OH)D, Allergic rhinitis, asthma, atopic dermatitis, atopy, pneumococcal antibody titer, vitamin D
The prevalence of vitamin D deficiency (≤20 ng/mL) in U.S. adults (40–100%) and children (32–52%) including asthmatic patients is common depending on gender, ages, ethnicity, diet, and lifestyle as reported in recent studies.1–3 Vitamin D has been reported to have multiple health benefits independent of its effect on calcium and phosphorus transport. The 2011 Institute of Medicine Report on dietary requirement for calcium and vitamin D suggested a key role of calcium and vitamin D in skeletal health but an inconclusive causal relationship between vitamin D or calcium and nonskeletal outcomes (e.g., inflammatory disease or cardiovascular disease).4
Asthma is the most common chronic disease in children and a major cause of morbidity in adults in the United States and worldwide.5,6 25-Hydroxyvitamin D (25[OH]D) has been reported to be related to asthma. It has been reported to be associated with risk of asthma and asthma exacerbation,3,7–10 treatment responses,11,12 and immune mechanism,13 although the study results have been inconsistent.11,14–16 The role of vitamin D in the development and exacerbation of asthma and its potential treatment has been extensively investigated.3,7–9,11,14,17,18 Although a significant role for vitamin D in innate immunity has been established,19–21 the adverse effect of vitamin D deficiency on risk of pneumonia has been reported to be independent of cathelicidin and β-defensin 2.22 There is scant information on the role of vitamin D in adaptive immune function such as humoral immune response to pneumococcal polysaccharide antigens.23
Studying the role of vitamin D in adaptive immune function might address important clinical and public health issues, given the increased risks of serious pneumococcal disease in asthmatic patients and the current recommendation of a single dose of 23-valent pneumococcal polysaccharide vaccine to adult asthmatic patients,24–26 the reported suboptimal humoral immune responses to pneumococcal polysaccharide antigens,27,28 and the potential impact of vitamin D on pneumococcal antibody titers (PATs).29 Regarding the latter, we recently reported that although vitamin D concentrations did not directly correlate with PATs, the impact of vitamin D on PATs was significantly modified by asthma status and other atopic conditions.29 However, because this study was cross-sectional, it was unable to address our study question, whether vitamin D affects a waning of PATs over time and whether such waning is still influenced by atopic conditions. To address this question, we conducted a prospective cohort study by following 39 children and adults with and without asthma.
METHODS
Study Design
This was a prospective cohort study that followed the original cohort of children and adults for about 1 year and the original study results have been recently reported.29 We examined the 23 serotype-specific pneumococcal antibody levels at baseline and subsequent follow-up visits and serum 25(OH)D concentrations at baseline. Subsequently, we determined the correlation between changes of PATs and serum 25(OH)D concentrations at baseline and performed stratified analysis by asthma status and other atopic conditions. Asthma status was ascertained by applying predetermined criteria for asthma and other atopic conditions were based on a physician diagnosis documented in medical records.30 The study was approved by the Institutional Review Board at the Mayo Clinic, and informed consent was obtained.
Study Subjects
The details of the original cohort were recently reported.29 Briefly, we used the same enrollment and exclusion criteria as were used in previous studies.31–33 The original study enrolled a convenience sample of 21 asthmatic patients and 23 individuals without asthma who had received medical care at the Mayo Clinic in Rochester, MN. Exclusion criteria included (1) the presence of moderate or severe disability, cerebral palsy, syndromes and nasopharyngeal disorders affecting swallowing, ear/nose/throat disorders affecting the anatomy of the nose and pharynx, documented or suspected immune deficiency, and immunosuppressive therapy (these conditions were based on a previous study)32; (2) those without research authorization for use of medical records; (3) receipt of blood products or immunoglobulin within 3 months; (4) documented pneumococcal diseases (e.g., acute otitis media, acute sinusitis, and community acquired pneumonia) with antibiotic treatment within 1 month before enrollment; (5) non-Olmsted County, MN, residents; (6) pregnancy; and (7) clinical conditions making asthma ascertainment difficult (e.g., cystic fibrosis, α-1-anti-trypsin deficiency, severe kyphoscoliosis, and pulmonary fibrosis). Of these 44 subjects, a total of 5 subjects were excluded due to emigration from the community (n = 2), refusal to participate (n = 2), and ineligibility (n = 1) leaving 39 subjects (20 asthmatic patients and 19 individuals without asthma). These 39 subjects were followed for a median duration of 12 months (interquartile range [IQR], 11–19 months).
Measurement of Serotype-Specific Antipneumococcal Polysaccharide IgG levels and Serum 25(OH)D Concentrations
Details of the measurement of pneumococcal antibodies and 25(OH)D were previously reported.27,29 Briefly, measurement of 23 serotype-specific antipneumococcal IgG levels was determined by the Mayo Clinic Clinical Immunology Laboratory using ELISA.34 A serotype-specific antipneumococcal polysaccharide antibody (IgG) concentration of ≥1.3 μg/mL was considered positive.29,35 For analysis, we coded the individual serotype-specific pneumococcal antibody concentrations as a binary variable (0 versus 1) and summed the number of positive serotype-specific antibody levels (possible range, 0–23). Because data for PATs and 25(OH)D concentrations did not follow a Gaussian distribution, the change of PATs and 25(OH)D concentrations were natural-log transformed. Serum 25(OH)D concentrations (ng/mL) were determined at Mayo Clinic clinical laboratory using mass spectrometry at baseline.36
Ascertainment of Asthma Status and Other Atopic Conditions
The ascertainment criteria for asthma and other atopic conditions were previously reported.27,29 These criteria are summarized in Table 1 and these criteria have been extensively used in previous research.27,31,37–39 Atopic dermatitis and allergic rhinitis were determined by a physician diagnosis documented in the medical record at the time of enrollment.
Table 1.
Definition of asthma
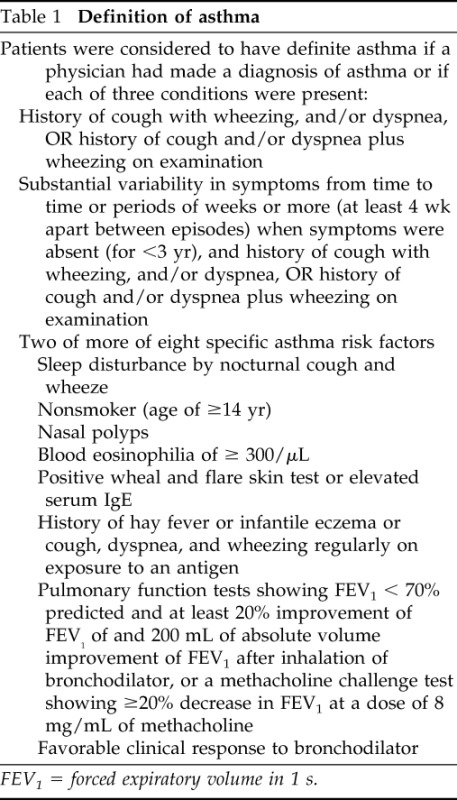
FEV1 = forced expiratory volume in 1 s.
Determination of Atopic Sensitization Status
We determined allergic sensitization for subjects by measuring allergen-specific IgE levels for the common allergens in our community including house-dust mite, elm, oak, cat, ragweed, Alternaria, and grass pollens. This test was performed at the Mayo Clinic clinical laboratory, using the Phadia immunoCAP system (Phadia, Uppsala, Sweden). Allergen-specific IgE levels of ≥0.35 kU/L were considered positive.
Statistical Analysis
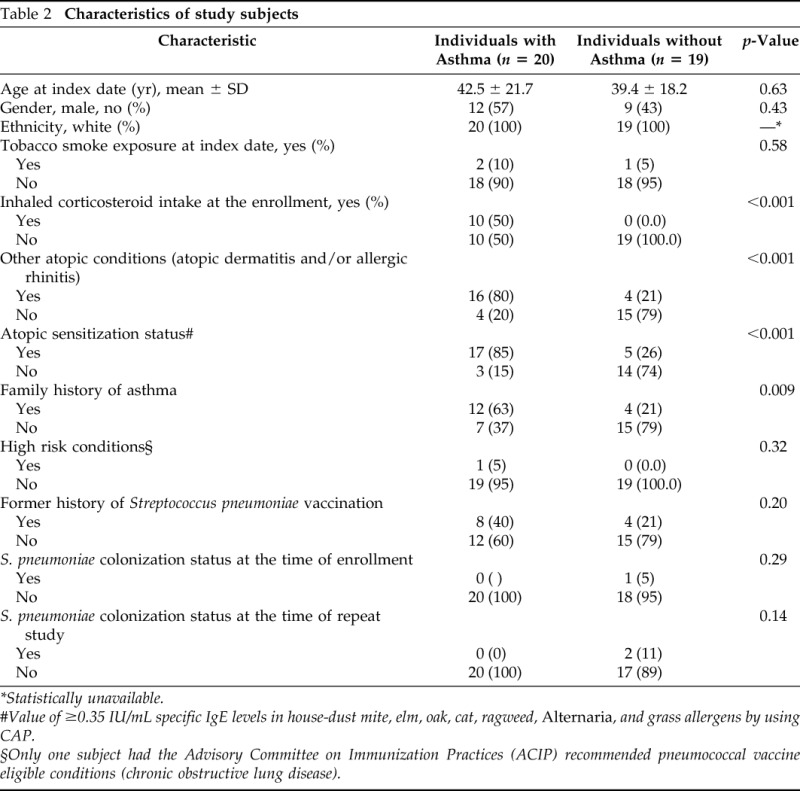
We compared subjects with and without asthma using descriptive statistics as summarized in Table 2. We compared differences in sociodemographic and clinical characteristics between subjects included in and excluded from the current study. We used Student's t-test for continuous variables and chi-square or Fisher exact test for categorical tests. Because change (ΔT0-T1) of pneumococcal antibody levels at baseline (T0) and follow-up visit (T1) and serum 25(OH)D concentrations (ng/mL) did not follow Gaussian distribution, both were natural-log transformed. To determine the influence of serum 25(OH)D concentrations at baseline on PATs, nonparametric ANOVA (Kruskal-Wallis test) was used to make an overall comparison in PATs among subjects with different 25(OH)D concentrations stratified by tertile (three groups). The p values were corrected for multiple comparisons by using Bonferroni correction. In addition, we determined the correlation between serum 25(OH)D concentrations at baseline and change of pneumococcal antibody levels during the follow-up period by determining Pearson's correlation coefficients. We stratified this correlation by asthma status and other atopic conditions. Because of different follow-up durations and the potential influence of pneumococcal colonization on PATs, data were fitted to a least square model to estimate adjusted parameters (β-coefficient) controlling for the follow-up duration and pneumococcal colonization status during the follow-up period. All statistical significance was tested at a two-sided α-error of 0.05. All analyses were performed using Stata Version 11 (Stata Corp., College Station, TX).
Table 2.
Characteristics of study subjects
*Statistically unavailable.
#Value of ≥0.35 IU/mL specific IgE levels in house-dust mite, elm, oak, cat, ragweed, Alternaria, and grass allergens by using CAP.
§Only one subject had the Advisory Committee on Immunization Practices (ACIP) recommended pneumococcal vaccine eligible conditions (chronic obstructive lung disease).
RESULTS
Study Subjects
There were no significant differences between the original cohort and the study subjects included in this study with regard to age, gender, ethnicity, asthma status, 25(OH)D concentrations, and PATs except atopic condition other than asthma (higher proportion of atopic conditions other than asthma among the present study cohort; p = 0.030). The characteristics of the study subjects included in the present study are described in Table 2. Of the 39 subjects, 23 (52.3%) were male subjects, 36 (81.8%) were white, and 20 (51%) were asthmatic patients. Six subjects (15%), were <18 years of age with a median age of 10.9 years (IQR, 8.1–12.6 years), and 33 (85%) were adults with a median age of 45 years (IQR, 33.2–58.6). Of the 39 subjects, 20 subjects had atopic conditions other than asthma: 3 (8%) had atopic dermatitis, 13(33%) had allergic rhinitis, 4 (10%) had both conditions, and 22 subjects had atopic sensitizations (56.4%). There was only one subject that had positive pneumococcal colonization at enrollment, and two subjects developed pneumococcal colonization during the study follow-up period.
Association between Serum 25(OH)D Concentrations and Changes of PATs
Characteristics associated with changes (waning) in PATs over the median duration of 12 months (IQR, 11–19 months) are summarized in Table 3. The log-transformed mean decrease in serotype-specific pneumococcal antibody levels between baseline and follow-up was 2.56 ± 0.13 (SD), and the log-transformed mean of 25(OH)D concentrations at baseline was 3.46 ± 0.40 (SD; 31.8 ± 1.49 ng/mL). The relationship between serum 25(OH)D concentrations at baseline (tertile) and changes in pneumococcal antibody levels over time are summarized in Table 4. Overall, there was a significant difference in PATs among subjects with different serum 25(OH)D concentrations (p = 0.031). There were trends that a higher concentration of 25(OH)D at baseline was associated with reduction in changes (ΔT0-T1) of PATs during the follow-up period. Compared with the median PATs (2.62) among subjects with the lowest concentration of serum 25(OH)D at baseline (tertile 1), subjects with the highest concentration of serum 25(OH)D at baseline (tertile 3) had the lowest decrease of PATs during the follow-up period (2.50; p = 0.006).
Table 3.
Characteristics of subjects and the log-transformed mean changes (ΔT0–1) of serotype-specific PATs between baseline and follow-up visit
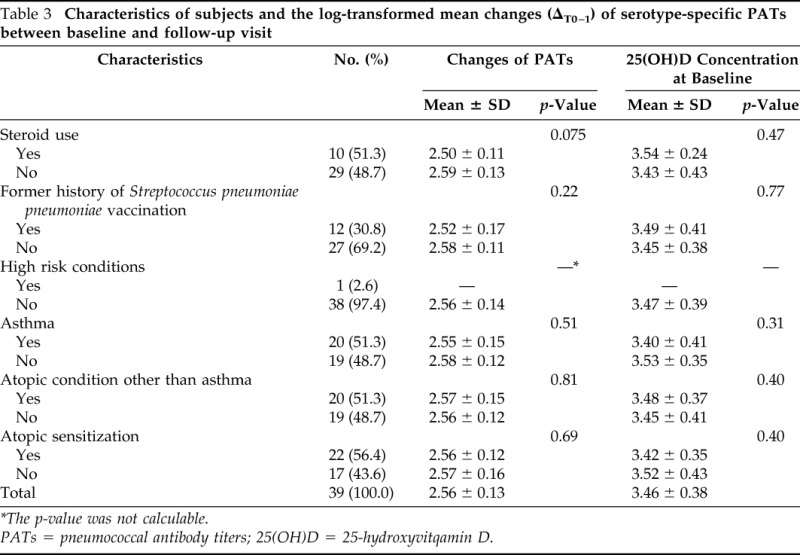
*The p-value was not calculable.
PATs = pneumococcal antibody titers; 25(OH)D = 25-hydroxyvitqamin D.
Table 4.
Natural log-transformed change (waning) of pneumococcal antibody levels over ∼1-yr period among all subjects
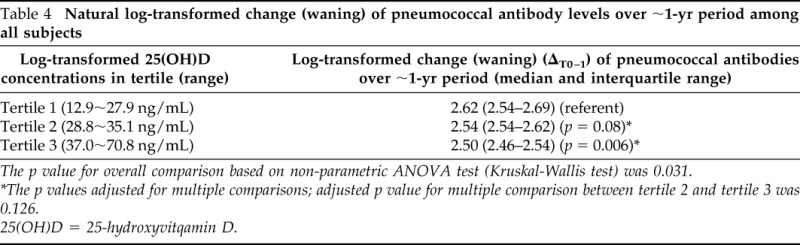
The p value for overall comparison based on non-parametric ANOVA test (Kruskal-Wallis test) was 0.031.
*The p values adjusted for multiple comparisons; adjusted p value for multiple comparison between tertile 2 and tertile 3 was 0.126.
25(OH)D = 25-hydroxyvitqamin D.
Correlation between Change in PATs and Serum (25OH)D Concentration Stratified by Atopic Conditions
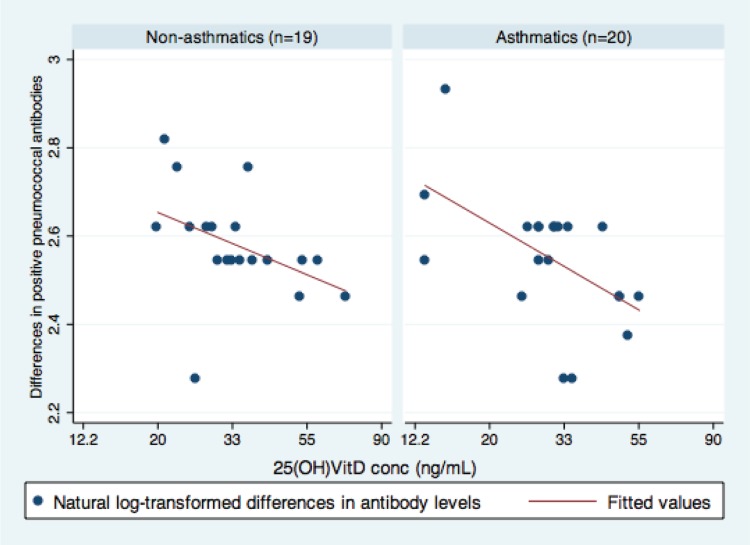
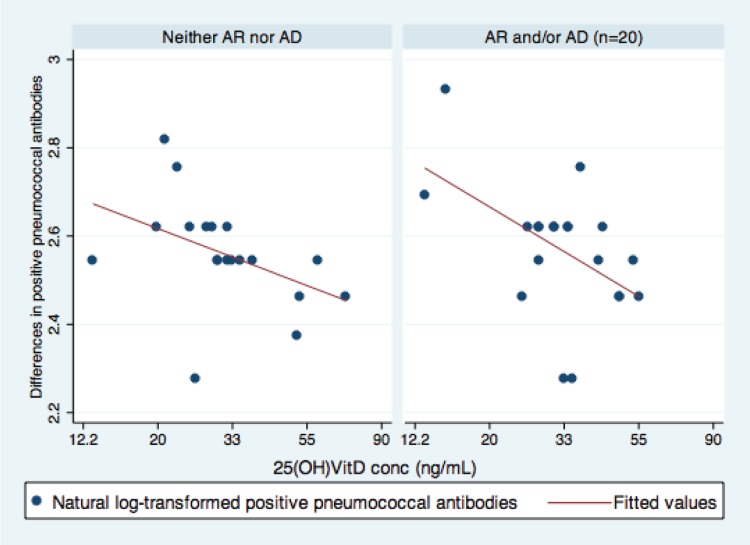
There was a significant correlation between serum 25(OH)D concentrations at baseline and the changes in PATs during the follow-up period (r = −0.47; p = 0.003). The stratified analyses by asthma and other atopic conditions are displayed in Figs. 1 and 2. This correlation was stronger in asthmatic patients (r = −0.55; p = 0.013) than individuals without asthma (r = −0.41; p = 0.08; Fig. 1). However, the interaction term between asthma and 25(OH)D was not significant (p = 0.599). Similar trends were observed in other atopic conditions (r = −0.51 and p = 0.023 for atopic individuals, and r = −0.44 and p = 0.06 for nonatopic individuals; Fig. 2). We did not find such a trend for atopic sensitization (r = −0.29 and p = 0.18 for those with atopic sensitizations versus r = −0.64 and p = 0.006 for those without sensitizations).
Figure 1.
Association between serum 25-hydroxyvitamin D (25[OH]D) concentrations and the differences in pneumococcal antibodies titers (PATs) during follow-up. Overall (γ = −0.47; p = 0.004), this illustrates asthmatic patients (adj. β, −0.19; 95% CI, −0.34 ∼ −0.04; p = 0.017) and individuals without asthma (adj. β, −0.13, 95% CI, −0.31 ∼ −0.04; p = 0.13). The x-axis: log-transformed 25(OH)D concentrations (range, 2.57–4.26). The y-axis: log-transformed number of serotype positive pneumococcal antibody levels (range, 2.28–2.93). Data were fitted to a least square model.
Figure 2.
Association between serum 25-hydroxyvitamin D (25[OH]D) concentrations and the differences in pneumococcal antibodies titers (PATs) during follow-up in subjects with and without other atopic conditions based on a physician diagnosis documented in a medical record. This illustrates other atopic conditions (adj. β, −0.19; 95% CI, −0.36 to −0.02; p = 0.034) and without atopic conditions (adj. β, −0.13; 95% CI, −0.28 to 0.03; p = 0.107). The x-axis: log-transformed 25(OH)D concentrations (range, 2.57–4.26). The y-axis: log-transformed number of serotype positive pneumococcal antibody levels (range, 2.28–2.93). Data were fitted to a least square model. The correlation between serum 25(OH)D concentrations and the number of serotype-positive pneumococcal antibody levels was modified by clinically defined AD and/or AR. AD, atopic dermatitis; AR, allergic rhinitis.
To adjust for the duration and pneumococcal colonization, data were fitted to least square models. Serum 25(OH)D concentrations at baseline mitigated waning of PATs during the follow-up period among asthmatic patients (adj. β, −0.19; 95% CI, −0.34 to −0.04; p = 0.017) but not among individuals without asthma (adj. β, −0.13; 95% CI, −0.31 to −0.04; p = 0.13). We found similar trends for subjects with other atopic conditions (adj. β, −0.19; 95% CI, −0.36 to −0.02; p = 0.034) but not among those without such conditions (adj. β, −0.13; 95% CI, −0.28 to −0.03; p = 0.107).
DISCUSSION
We previously showed a significant relationship between serum vitamin D concentrations and pneumococcal polysaccharide antibody titers, which was modified by the presence of atopic conditions29; in asthmatics and atopics, 25(OH)D concentration was positively correlated with PATs, whereas it was inversely correlated in individuals without asthma and other atopic conditions. However, we did not observe such effect modification by asthma status on the relationship between 25(OH)D and changes in PATs in this present study.
In this present study, we examined the impact of 25(OH)D concentrations on change (waning) of PATs over time and the potential effect modification by asthma or atopy status as observed before. Subjects with a higher concentration of 25(OH)D at baseline (37.0 ∼ 70.8 ng/mL) had a smaller reduction (ΔT0-T1) in PATs over a 1-year period (median change [IQR], 2.50 [2.46–2.54]) compared with those with a lower concentration of 25(OH)D (2.62 [2.54–2.69]; overall value of p = 0.031) as summarized in Table 4. In addition, we also observed an inverse correlation between 25(OH)D concentrations at baseline and change in PATs over a 1-year period (Figs. 1 and 2). Overall, there was an inverse correlation between 25(OH)D concentrations at baseline and changes (reduction) of PATs over about a 1-year period (r = −0.47; p = 0.004). This correlation, however, was significantly modified by asthma status (r = −0.55 and p = 0.013 for asthmatic patients and r = −0.41 and p = .079 for individuals without asthma here). However, the interaction term was statistically not significant (p = 0.599), suggesting no significant interaction or effect modification. After adjustment for follow-up duration and exposure to pneumococcal colonization, this association remained significant, however, only among asthmatic patients (adj. β, −0.19; 95% CI, −0.34 to −0.04; p = 0.017), but not in individuals without asthma (adj. β, −0.13; 95% CI, −0.31 to −0.04; p = 0.13). Similar trends were observed for other atopic conditions (adj. β, −0.19, 95% CI, −0.36 to −0.02, and p = 0.034 versus adj. β, −0.13, 95% CI, −0.28 to −0.03, and p = 0.107) based on a physician diagnosis documented in the medical record. Potential founders such as comorbid conditions or atopic sensitization did not significantly influence these results (see Table 3). As far as we aware, this is the first prospective study to assess the association between 25(OH)D concentrations and waning of PATs. A few earlier cross-sectional studies showed a positive influence of vitamin D on hepatitis B40 and inactivated influenza vaccine responses41 and total IgG levels42 among patients with chronic health conditions. For example, Zitt et al. reported that vitamin D deficiency was relatively common in patients with chronic kidney disease and patients with vitamin D deficiency had poorer seroconversion rate and lower antibody titers to hepatitis B vaccination compared with those without vitamin deficiency.40 A recent study showed sun exposure was inversely correlated with risk of IPD. Therefore, the literature supports our study findings and suggests the potential immunomodulatory role of vitamin D in adaptive immune functions. However, whether vitamin D supplementation reduces risk of pneumococcal disease through increasing pneumococcal antibody response and decreasing waning of pneumococcal antibodies needs to be determined in future study.
The mechanisms for how 25(OH)D affects waning of PATs need to be investigated given the role of other micronutrients in immune functions.43 Although our previous study showed a significant effect modification (interaction) by asthma status on the relationship between 25(OH)D and pneumococcal antibody levels by asthma status, we did not observe such effect modification by asthma status in the present study. This could be caused by a small sample size and a relatively small effect size stemming from a short follow-up duration.
The main strength of our study was its prospective study design, showing results consistent with our earlier cross-sectional study except a significant effect modification by asthma or atopy status. Nevertheless, our study has limitations. Our study was based on a small sample size, although significant associations were found. The findings need to be replicated in a prospective study with a larger sample size. Study subjects were all white limiting its generalizability of findings. Our unstandardized baseline measurement of 25(OH)D concentration may have introduced unwanted variation in our analyses because vitamin D levels are known to be influenced by season, latitude, and dietary intake.2,44,45
In conclusion, serum 25(OH)D concentrations may be associated with reduction of the waning of PATs over time, especially those with asthma or other atopic conditions. Vitamin D may be an important immune modulator that affects humoral immunity depending on the underlying immune milieu. Our study findings call for replication and further elucidation of underlying mechanisms.
ACKNOWLEDGMENTS
The authors are grateful for secretarial support from Elizabeth Krusemark and research support from the staff of the Pediatric Asthma Epidemiology Research Unit. They are grateful to the Rochester Epidemiology Project, supported by the National Institutes of Aging (R01-AG034676), for allowing us access to the medical record of the research participants involved in this study.
Footnotes
Funded by a grant from the T. Denny Sanford Collaborative Research Fund, a partnership between Sanford Health and Mayo Clinic and the Bridge Award from Mayo Foundation
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res 31:48–54, 2011. [DOI] [PubMed] [Google Scholar]
- 2. Holick MF. Vitamin D deficiency. N Engl J Med 357:266–281, 2007. [DOI] [PubMed] [Google Scholar]
- 3. Brehm JM, Schuemann B, Fuhlbrigge AL, et al. Serum vitamin D levels and severe asthma exacerbations in the Childhood Asthma Management Program study. J Allergy Clin Immunol 126:52–58, e5, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J Clin Endocrinol Metab 96:53–58, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005–2009. Natl Health Stat Rep 32:1–14, 2011. [PubMed] [Google Scholar]
- 6. Asher MI, Montefort S, Björkstén B, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 368:733–743, 2006. [DOI] [PubMed] [Google Scholar]
- 7. Erkkola M, Kaila M, Nwaru BI, et al. Maternal vitamin D intake during pregnancy is inversely associated with asthma and allergic rhinitis in 5-year-old children. Clin and Exp Allergy 39:875–882, 2009. [DOI] [PubMed] [Google Scholar]
- 8. Freishtat RJ, Iqbal SF, Pillai DK, et al. High prevalence of vitamin D deficiency among inner-city African American youth with asthma in Washington, DC. J Pediatr 156:948–952, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brehm JM, Celedon JC, Soto-Quiros ME, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am Journal Respir Crit Care Med 179:765–771, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Keet CA, McCormack MC, Peng RD, Matsui EC. Age- and atopy-dependent effects of vitamin D on wheeze and asthma. J Allergy Clin Immunol 128:414–416.e5, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Devereux G, Wilson A, Avenell A, et al. A case-control study of vitamin D status and asthma in adults. Allergy 65:666–667, 2010. [DOI] [PubMed] [Google Scholar]
- 12. Wu AC, Tantisira K, Li L, et al. Effect of vitamin D and inhaled corticosteroid treatment on lung function in children. Am J Respir Crit Care Med 186:508–513, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frieri M, Valluri A. Vitamin D deficiency as a risk factor for allergic disorders and immune mechanisms. Allergy Asthma Proc 32:438–444, 2011. [DOI] [PubMed] [Google Scholar]
- 14. Hollams EM, Hart PH, Holt BJ, et al. Vitamin D and atopy and asthma phenotypes in children: A longitudinal cohort study. Eur Respir J 38:1320–1327, 2011. [DOI] [PubMed] [Google Scholar]
- 15. Camargo CA, Ingham T, Wickens K, al Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics 127:e180–e187, 2011. [DOI] [PubMed] [Google Scholar]
- 16. Cremers E, Thijs C, Penders J, et al. Maternal and child's vitamin D supplement use and vitamin D level in relation to childhood lung function: the KOALA Birth Cohort Study. Thorax 66:474–480, 2011. [DOI] [PubMed] [Google Scholar]
- 17. Majak P, Olszowiec-Chlebna M, Smejda K, Stelmach I. Vitamin D supplementation in children may prevent asthma exacerbation triggered by acute respiratory infection. J Allergy Clin Immunol 127:1294–1296, 2011. [DOI] [PubMed] [Google Scholar]
- 18. Frieri M. The role of vitamin D in asthmatic children. Curr Allergy asthma Rep 11:1–3, 2011. [DOI] [PubMed] [Google Scholar]
- 19. Hata TR, Kotol P, Jackson M, et al. Administration of oral vitamin D induces cathelicidin production in atopic individuals. J Allergy Clin Immunol 122:829–831, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bhan I, Camargo CA, Jr, Wenger J, et al. Circulating levels of 25-hydroxyvitamin D and human cathelicidin in healthy adults. J Allergy Clin Immunol 127:1302–1304 e1, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hiemstra PS. The role of epithelial beta-defensins and cathelicidins in host defense of the lung. Exp Lung Res 33:537–542, 2007. [DOI] [PubMed] [Google Scholar]
- 22. Leow L, Simpson T, Cursons R, et al. Vitamin D, innate immunity and outcomes in community acquired pneumonia. Respirology 16:611–616, 2011. [DOI] [PubMed] [Google Scholar]
- 23. Chambers ES, Hawrylowicz CM. The impact of vitamin D on regulatory T cells. Curr Allergy Asthma Rep 11:29–36, 2011. [DOI] [PubMed] [Google Scholar]
- 24. Talbot TR, Hartert TV, Mitchel E, et al. Asthma as a risk factor for invasive pneumococcal disease. N Engl J Med 352:2082–2090, 2005. [DOI] [PubMed] [Google Scholar]
- 25. Centers for Disease Control and Prevention (CDC), Advisory Committee on Immunization Practices. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR Morb Mortal Wkly Rep 59:1102–1106, 2010. [PubMed] [Google Scholar]
- 26. Lee HJ, Kang JH, Henrichsen J, et al. Immunogenicity and safety of a 23-valent pneumococcal polysaccharide vaccine in healthy children and in children at increased risk of pneumococcal infection. Vaccine 13:1533–1538, 1995. [DOI] [PubMed] [Google Scholar]
- 27. Jung JA, Kita H, Dhillon R, et al. Influence of asthma status on serotype-specific pneumococcal antibody levels. Postgrad Med 122:116–124, 2010. [DOI] [PubMed] [Google Scholar]
- 28. van den Biggelaar AH, Pomat WS, Phuanukoonnon S, et al. Effect of early carriage of Streptococcus pneumoniae on the development of pneumococcal protein-specific cellular immune responses in infancy. Pediatr Infect Dis J 31:243–248, 2012. [DOI] [PubMed] [Google Scholar]
- 29. Lee J, Zhao H, Fenta Y, et al. Serum 25-hydroxyvitamin D is associated with enhanced pneumococcal antibody levels in individuals with asthma. Allergy Asthma Proc 32:445–452, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Molis WE, Bagniewski S, Weaver AL, et al. Timeliness of diagnosis of asthma in children and its predictors. Allergy 63:1529–1535, 2008. [DOI] [PubMed] [Google Scholar]
- 31. Juhn YJ, Kita H, Yawn BP, et al. Increased risk of serious pneumococcal disease in patients with asthma. J Allergy Clin Immunol 122:719–723, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goldblatt D, Hussain M, Andrews N, et al. Antibody responses to nasopharyngeal carriage of Streptococcus pneumoniae in adults: A longitudinal household study. J Infect Dis 192:387–393, 2005. [DOI] [PubMed] [Google Scholar]
- 33. Hanania NA, Sockrider M, Castro M, et al. Immune response to influenza vaccination in children and adults with asthma: Effect of corticosteroid therapy. J Allergy Clin Immunol 113:717–724, 2004. [DOI] [PubMed] [Google Scholar]
- 34. Jacob GL, Homburger HA. Simultaneous QUANTITATIVE MEASUREMENT of IgG antibodies to Streptococcus pneumoniae serotypes by microsphere phtomometry. J Allergy Clin Immunol 113:pS288, 2004. [Google Scholar]
- 35. Sorensen RU, Leiva LE, Javier FC, III, et al. Influence of age on the response to Streptococcus pneumoniae vaccine in patients with recurrent infections and normal immunoglobulin concentrations. J Allergy Clin Immunol 102:215–221, 1998. [DOI] [PubMed] [Google Scholar]
- 36. Roth HJ, Schmidt-Gayk H, Weber H, Niederau C. Accuracy and clinical implications of seven 25-hydroxyvitamin D methods compared with liquid chromatography-tandem mass spectrometry as a reference. Ann Clin Biochem 45:153–159, 2008. [DOI] [PubMed] [Google Scholar]
- 37. Silverstein MD, Reed CE, O'Connell EJ, et al. Long-term survival of a cohort of community residents with asthma. N Engl J Med 331:1537–1541, 1994. [DOI] [PubMed] [Google Scholar]
- 38. Hunt LW, Jr, Silverstein MD, Reed CE, et al. Accuracy of the death certificate in a population-based study of asthmatic patients. JAMA 269:1947–1952, 1993. [PubMed] [Google Scholar]
- 39. Juhn YJ, Weaver A, Katusic S, Yunginger J. Mode of delivery at birth and development of asthma: A population-based cohort study. J Allergy Clin Immunol 116:510–516, 2005. [DOI] [PubMed] [Google Scholar]
- 40. Zitt E, Sprenger-Mähr H, Knoll F, et al. Vitamin D deficiency is associated with poor response to active hepatitis B immunisation in patients with chronic kidney disease. Vaccine 30:931–935, 2012. [DOI] [PubMed] [Google Scholar]
- 41. Chadha MK, Fakih M, Muindi J, et al. Effect of 25-hydroxyvitamin D status on serological response to influenza vaccine in prostate cancer patients. Prostate 71:368–372, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pincikova T, Nilsson K, Moen IE, et al. Inverse relation between vitamin D and serum total immunoglobulin G in the Scandinavian Cystic Fibrosis Nutritional Study. Eur J Clin Nutr 65:102–109, 2011. [DOI] [PubMed] [Google Scholar]
- 43. Taylor CE, Camargo CA., Jr Impact of micronutrients on respiratory infections. Nutr Rev 69:259–269, 2011. [DOI] [PubMed] [Google Scholar]
- 44. Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med 169:626–632, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ginde AA, Mansbach JM, Camargo CA., Jr Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med 169:384–390, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]