Abstract
In many developing countries, jaundice is the common symptom of hepatic diseases which are a major cause of mortality. The use of natural product-based therapies is very popular for such hepatic disorders. A great number of medicinal plants have been utilized for this purpose and some facilitated the discovery of active compounds which helped the development of new synthetic drugs against jaundice. However, more epidemiological studies and clinical trials are required for the practical implementation of the plant pharmacotherapy of jaundice. The focus of this second part of our review is on several of the most prominent plants used against jaundice identified in the analysis performed in the first part of the review viz. Andrographis paniculata (Burm.f.) Nees, Silybum marianum (L.) Gaertn., Terminalia chebula Retz., Glycyrrhiza glabra L. and some species of genus Phyllanthus. Furthermore, we discuss their physiological effects, biologically active ingredients, and the potential mechanisms of action. Some of the most important active ingredients were silybin (also recommended by German commission), phyllanthin and andrographolide, whose action leads to bilirubin reduction and normalization of the levels of relevant serum enzymes indicative for the pathophysiological status of the liver.
Keywords: jaundice, bilirubin, oxidative stress, traditional use, phytoconstituents, serum enzymes, alkaline phosphatase
The Medicinal Plants of Prime Importance for the Treatment of Jaundice
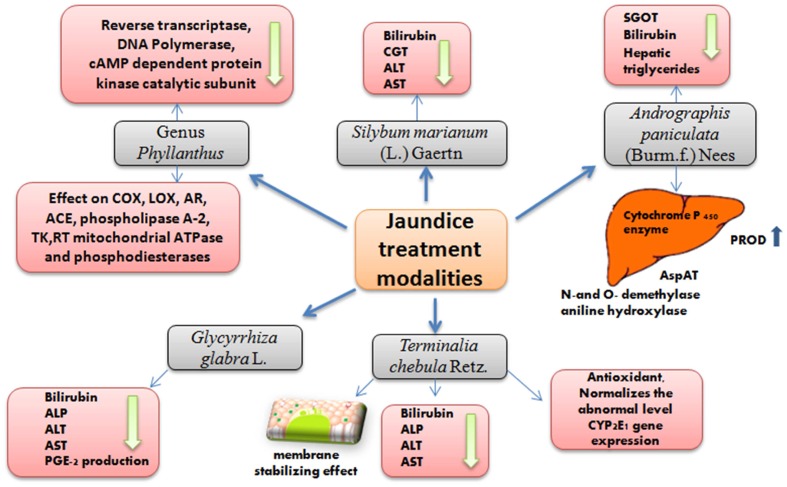
In the first part of the review, we presented an overview of the history, symptoms and causes of jaundice and the significance and diversity of medicinal plants used in its treatment. We also presented an exhaustive list of 207 plant species from 20 countries used for the treatment of jaundice. These plants were mainly indicated by different ethnopharmacological or ethnobotanical surveys. Based on their most popular use, several of the 207 plants classified in five different families are described in the current review based on their therapeutic profiles with special reference to jaundice and hepato-protective mechanism (Figure 1).
FIGURE 1.
Overview of observed effects associated with the use of the reviewed plants for jaundice treatment.
Andrographis paniculata (Burm.f.) Nees
Andrographis paniculata (Burm.f.) Nees is a medicinal plant which belongs to the family Acanthaceae. The major biologically active chemical constituents of the plant are diterpene lactones, either free, or glycosidated. These include andrographolide, deoxyandrographolide, neoandrographolide, andrographiside, andrographanoside, etc. (Figure 2) (Xu, 1986; Thai Pharmacopoeia Committee, 1995; WHO, 1999). It is used as a cure for a broad spectrum of diseases and utilized traditionally for centuries in folk medicine, mainly in Asia (Jarukamjorn et al., 2006). Aerial parts of A. paniculata are included in traditional remedies, and are being used for a broad range of disorders, more specifically as hepatic stimulant and hepatoprotective agent along with other liver disorders and jaundice (Kapil et al., 1993; Trivedi and Rawal, 2000). The medicinal effect of the aerial parts of the plant is represented by their in vitro and in vivo anti-hepatotoxic activities (Gupta et al., 1990; Chander et al., 1995). These studies demonstrated marked effect of the A. paniculata and its diterpenes andrographolide and neoandrographolide on the alkaline phosphatase (ALP), serum lipoprotein-X, GPT, GOT, bilirubin and also the effect of A. paniculata on the enterotoxin in animal models. These studies also showed promising protective effects of the diterpenes andrographolide and neoandrographolide in hepatic damage. However, further clinical studies with sufficient number of subjects are required to further prove the efficacy of these compounds in humans.
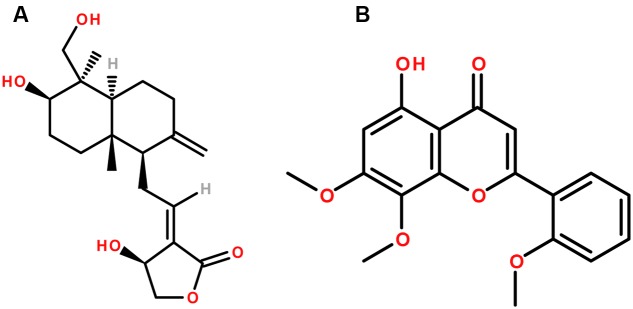
FIGURE 2.
The chemical structures of phytoconstituents of Andrographis paniculata (A) Andrographolide and (B) Andrographin.
Various studies have been carried out to explore the therapeutic effect of this plant, particularly its antiviral (Calabrese et al., 2000), anti-inflammatory (Shen et al., 2002), immune stimulatory (Puri et al., 1993; Iruretagoyena et al., 2004), and anti-cancer effects (Kumar et al., 2004). A. paniculata extract, and in particular its active compound andrographolide and its related analogs were shown to exhibit effects on various hepatic enzymes like N-and O-demethylase, aniline hydroxylase (Choudhury and Poddar, 1984), AspAT, alanine aminotransferase (ALT) (Trivedi and Rawal, 2000), DT-diaphorase (DTD) and glutathione S-transferase (GST) (Singh et al., 2001). The aqueous extract of A. paniculata significantly increased the pentoxyresorufin O-dealkylase (PROD) activity, suggesting that A. paniculata constituents might affect hepatic cytochrome P-450 enzyme (Jarukamjorn et al., 2006).
CCl4 induced hepatotoxicity in mice was reduced by methanol extract of A. paniculata and the histopathological liver changes were reversed (Handa and Sharma, 1990), as well as the elevated concentrations of different enzymes like SGOT; bilirubin and hepatic triglycerides were reduced by andrographolide (Handa and Sharma, 1990; Sharma et al., 1991). Andrographolide also exerted a prominent protective effect against hepatotoxicity caused by various substances viz. D-Gal N (Saraswat et al., 1995), ethanol (Pramyothin et al., 1994), paracetamol (Visen et al., 1993), and CCl4 (Kapil et al., 1993). Comparison showed that andrographolide exhibits higher efficiency than the standard hepatoprotective agent silymarin (Kapil et al., 1993; Visen et al., 1993). However, it is still not clear if the extract, or the pure substance has higher efficiency based on their inhibition on CCl4 and paracetamol toxicity (Choudhury and Poddar, 1984; Visen et al., 1993). This can be due to presence of several complex phytochemicals in the extract, which can lead to synergistic, or antagonistic effects during bioactivation and detoxification (Singh et al., 2001).
A report on modulation potential of phases I and II enzymes and antioxidant enzymes by A. paniculata revealed a remarkable effect on hepatic metabolic enzymes. The activities of DTD and GST were increased in mouse liver and other organs by an 80% hydroalcoholic extract of the plant and thus strengthening the xenobiotic metabolism toward detoxification, which plays an important role in chemoprevention and cytoprotection (Singh et al., 2001). The effect of the plant was also correlated with numerous GST, DTD inducing phytoconstituents, having an important role against chemical-induced carcinogenesis (Singh et al., 2001). The intracellular GSH concentration, which is of major importance for detoxification of xenobiotics is also modulated by Andrographis usage (Ketterer, 1988; Meister, 1994).
In a clinical study by Chturvedi et al. (1983) outstanding results by using A. paniculata for the treatment of infectious hepatitis were achieved. Improvements in appetite, jaundice, fever and epigastric discomfort were observed in all patients within 4 weeks. A significant reduction in serum bilirubin level (up to 10-fold) was recorded as well. Apart from this, ALP, SGPT and SGOT, were also significantly improved, suggesting that A. paniculata might be considered as the cheapest and the most beneficial cure for infectious hepatitis (Chturvedi et al., 1983). Although these results appear promising, it should be considered that this study conducted more than three decades ago had low number of studied subjects. The lack of statistical power and the insufficient data by the former experiments require the conduction of larger clinical trials to elucidate the effects of the active compounds. It should be considered that next to the major constituents andrographolide and andrographin there may be also other compounds modulating the bioactivities of the plant. Therefore, further studies on the synergistic and antagonistic effects of the plant compounds combined with knowledge about their deeper molecular mechanisms are required to unravel the mode of action of A. paniculata.
In brief, A. paniculata seems effective in various liver diseases such as viral and toxic hepatitis and intra and extra hepatic cholestasis, that would reduce jaundice (Deng et al., 1982; Handa and Sharma, 1990; Visen et al., 1993). However, there are also reports about its compounds cytotoxicity (Nanduri et al., 2004) and toxicity for the male reproductive system (Akbarsha and Murugaian, 2000). Therefore, further clinical studies are required to establish the safety and clinical efficacy of the long term use.
Genus Phyllanthus
The genus Phyllanthus belongs to the Euphorbiaceae family and is widely distributed throughout the tropical and subtropical zones being described for the first time in 1773 by Linnaeus. The genus comprises of about 550–1200 species (Unander et al., 1990, 1991, 1992, 1995; Mabberley, 2008; Cruz-Vega et al., 2009). A number of species of this genus have been used since ancient times to treat a broad spectrum of diseases such as the hepatitis B virus (HBV) infection, bone disorders (Piva et al., 2009), diabetes (Kusirisin et al., 2009), intestinal infections and disturbance of the kidney and urinary bladder (Morton, 1981; Oliver-Bever, 1983; Unander et al., 1990, 1991, 1992, 1995; Calixto et al., 1998; Cruz-Vega et al., 2009). Evidences for utilization of this genus are present in the aboriginal texts of Ayurveda (also known as “Ancient Science of Life”), which is one of the ancient traditional medicine systems of the world, native to India for more than 2000 years. Apart from India, these species are also used in other locations viz. Central and South America, Philippines, Guam, Nigeria, Cuba, China, and Africa (Thyagarajan et al., 1988).
A variety of bioactive molecules have been isolated and characterized from a large number of species of this genus (Figure 3). Phytochemical studies revealed the presence of lignans, alkaloids, tannins, lactones, steroids, and flavonoids (Pettit et al., 1982a,b; Foo and Wong, 1992; Bachmann et al., 1993; Foo, 1995; Filho et al., 1996; Calixto et al., 1998). Therapeutic activities have been reported from phytosterols like β-sitosterol, stigmasterols, and campesterol, present in Phyllanthus species having antinociceptive action among other effects. Furthermore, rutin in Phyllanthus emblica, P. niruri, and P. amarus is associated with analgesic and anti-inflammatory activity (Alcaraz and Jiménez, 1988; Pathak et al., 1991; Santos et al., 1994); ellagic acid found in P. emblica and P. niruri acts as an aldose reductase inhibitor (Shimizu et al., 1989; Unander et al., 1991); geraniin has antiallergic, analgesic and ACE-inhibitor activity; and quercetin has mitochondrial ATPase, phosphorylase and tyrosine kinase inhibition, analgesic activity, cyclooxygenase (COX) inhibition; phospholipase A-2 inhibition and mutagenic effect on bacteria (Suolinna et al., 1974; Beretz et al., 1978; Shisheva and Shechter, 1992; Duarte et al., 1993; Lindahl and Tagesson, 1993; Morales and Lozoya, 1994; Filho et al., 1996; Qian-Cutrone et al., 1996; Calixto et al., 1998; Ullah et al., 2013). Niruriside present in P. niruri has HIV transcriptase inhibitory activity (Hussain et al., 1995; Qian-Cutrone et al., 1996). Positive effect in the treatment of genitourinary infections, HBV infection, diabetes and airborne disease management are attributed as well to P. niruri L. (Perry and Metzger, 1980; Oliver-Bever, 1983; Calixto et al., 1998). In vitro studies showed that the reverse transcriptase from human immuno deficiency virus type-I (HIV-RT) was inhibited by the aqueous extract of P. niruri (Ogata et al., 1992; Naik and Juvekar, 2003).
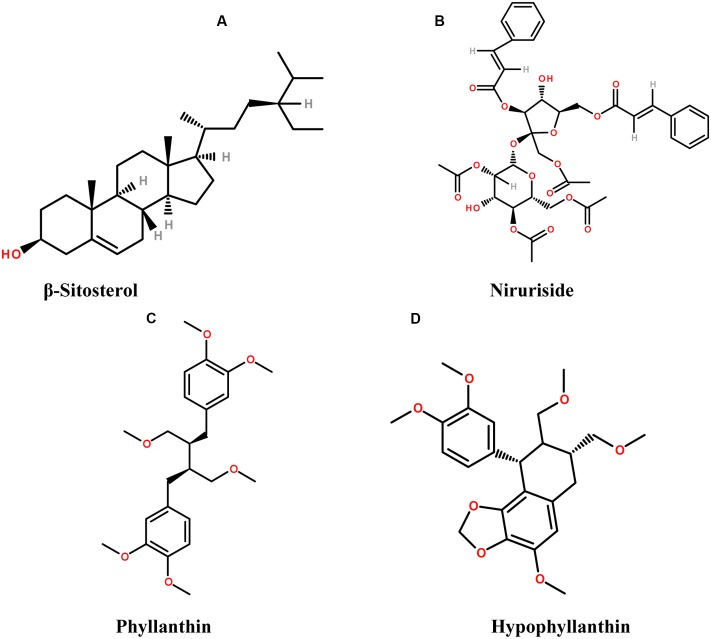
FIGURE 3.
The chemical structures of phytoconstituents present in species from Phyllanthus genus (A) β-sitosterol, (B) Niruriside, (C) Phyllanthin, and (D) Hypophyllanthin.
Phyllanthus niruri, is an erect, small herb, indigenous to Amazon rainforest, South India, China and South Africa (Girach et al., 1994). P. niruri (wh. pl.) is used in different disease conditions like diabetes, jaundice, tumors, kidney stones, influenza etc. Moreover, the use of this plant for prevention of hepatotoxicity, viral, bacterial infection and inhibition of HBV is also reported (Chopra et al., 1956; Bagalkotkar et al., 2006). P. niruri is present worldwide in different traditional systems of medicine. It is known by several names in different medicinal systems such as “Tamalaki” or “Bhumyan amalaki” in Ayurveda, which means “resembling Indian gooseberry” (Indian gooseberry is P. emblica which is known as ‘Amalaki’ in Sanskrit), P. niruri also known as “Dukong” in Malaysia, “Chanca Piedra” in Spanish, which means ‘breaker of stone’ due to its lithotriptic activity that has been used as an effective remedy to eliminate different types of stones like, kidney and gall stones (Bagalkotkar et al., 2006). It has also exhibited non-concentration dependent inhibition on CaOX crystals formation (Campos and Schor, 1999). The plant is also known as “Quebra Pedra” in Brazil, where it is considered as an excellent remedy for renal disorders, bladder associated infections and hydropsy (Dias et al., 1995; Wang et al., 1995; Wang, 2000). It is also known as “Pitiriasi,” or “Budhatri” and is used as a household remedy for anemia, jaundice, tuberculosis, extreme thirst, respiratory disorders etc. in India (Dhar et al., 1968). The work by Otawa in 1891 led to the isolation of the compound phyllanthin (Row et al., 1966). Phyllanthin and hypophyllanthin are lignans and the hepatoprotective and anti-genotoxic activity of P. niruri is attributed to their presence (Row et al., 1966). However, the hepatoprotective activity is not exclusively due to the presence of hypophyllanthin and phyllanthin as these constituents are present only in P. amarus (Khatoon et al., 2006) but other Phyllanthus species such as P. polyphyllus, P. acidus, and P. fraternus also exhibit the hepatoprotective activity and the presence of hydroxyl rich compositions and antioxidant activity may be correlated with the hepatoprotective effect (Mao et al., 2016).
The first medically oriented research on Phyllanthus plants was conducted by Indian and Brazilian teams due to the traditional use of the plants by indigenous people in these areas (Unander and Blumberg, 1991; Bagalkotkar et al., 2006). The effect of P. niruri on jaundice among children was reported by a group of Indian scientists (Dixit et al., 1982; Bagalkotkar et al., 2006). Damage of hepatic mouse tissues was found to be counteracted by the protein fraction of P. niruri. Some of the potent antioxidants from this plant such as rutin, quercetin are also beneficial in colonic inflammation treatment (Gálvez et al., 1995, 1997; Cruz et al., 1998; Sanchez de Medina et al., 2002); gallocatechin, isolated by tissue culture of P. niruri (Ishimaru et al., 1992) and catechins in general have suppressive effect on the growth of hepatic and colon epithelial cancer cell lines (Uesato et al., 2001). Moreover, the flavone glycoside nirurin [5,6,7,4-tetrahydroxy-8-(3-methylbat-2-enyl)] and some volatile constituents were also reported as present in the plant (Bagalkotkar et al., 2006). P. niruri gained particular attention globally due to its anti HBV activity during late 1980s (Venkateswaran et al., 1987).
The extract of P. amarus Schumach. & Thonn. demonstrated steady inhibition on the HBV surface antigen in vitro. Most of the plant extracts of this genus are capable of acting as reverse transcriptase inhibitors and DNA polymerase inhibitors and, thus, preventing the replication of HBV (Thyagarajan et al., 1988; Unander and Blumberg, 1991; Ogata et al., 1992; Unander et al., 1995; Lee et al., 1996; Calixto et al., 1998; Padmalatha et al., 2009). The chemical constituents responsible for HBV suppression are still not well known, although ellagic acid may have a role for this effect. Further reports showed that hydrolysable fraction from P. amarus acts via inhibition of cAMP dependent protein kinase catalytic subunit (Polya et al., 1995). Clinical studies revealed that P. amarus extract had very little, or no adverse effect on the patients (Thyagarajan et al., 1988; Wang et al., 2005).
The toxic and therapeutic effects of other plants of this genus like P. urinaria L. and P. niruri were examined in 123 patients and significant improvements were observed in those with chronic hepatitis (Wang et al., 2005). In a study performed by Yeh et al. (1993), a reversible inhibition of cellular proliferation and suppression of HBV surface antigen was observed in human hepatoma cell lines (HepA2). The biological effect of P. amarus extract and its medical use in jaundice treatment was explained by reducing the mRNA level of the HBV surface antigen (Wang et al., 2005). The HBV suppression by plant extracts of Phyllanthus origin is a well-known fact. However, the discovery of active molecules and their mechanism of action is a matter of future studies. This will allow improving liver function and resolving jaundice by their more efficient analogs, or by combination with other compounds. In addition to the spectrum of favorable activities of this genus, the clastogenic effect of nickel chloride on mouse bone marrow cells is antagonized by P. emblica and P. niruri extracts (Agarwal et al., 1992); and potential hypoglycemic, diuretic and hypotensive effects of P. amarus were also observed in a human clinical study (Srividya and Periwal, 1995). The hepatoprotective activity of different Phyllanthus species was also evaluated in animal models (Syamasundar et al., 1985; Dhir et al., 1990; Gulati et al., 1995; Prakash et al., 1995). Liver injury induced by CCl4 was counteracted by P. niruri and P. urinaria, but not by P. simplex, and normalization of the elevated serum levels of transaminases (SGOT and GPT) in rat liver was observed (Syamasundar et al., 1985; Prakash et al., 1995); quercetin, which is one of the main component of P. emblica, exhibited hepatoprotective effect after paracetamol and country-made liquor intake in mice, and P. niruri also led to similar result (Umarani et al., 1985; Gulati et al., 1995; Unander et al., 1995). It is assumed that most of the phytoconstituents present in this genus, as described earlier, interact with key regulatory enzymes viz. COX, LOX, AR, ACE, phospholipase A-2, tyrosine kinase (TK), reverse transcriptase (RT) mitochondrial ATPase and phosphodiesterases (Calixto et al., 1998). The clinical study of Thyagarajan et al. (1988) found weaker responses to HBV treatment by P. amarus extracts in males vs. females, however, the number of individuals was insufficient for statistical significance of this difference.
In conclusion, it is evident that the plants of genus Phyllanthus have a great potential as therapeutics against jaundice. However, there is a need of proper clinical trials aimed toward the establishment of safety and efficacy of the genus Phyllanthus species in a standardized way.
Silybum marianum (L.) Gaertn. (milk thistle)
Silybum marianum (L.) Gaertn. (Carduus marianus L.), belonging to the family Asteraceae, or Compositae, has been used for more than 2000 years, in particular as a remedy for hepatobiliary disorders since 16th century (Flora et al., 1998; Schuppan et al., 1999). The plant is commonly known as the milk thistle, Our lady’s thistle and St. Mary’s thistle (Schuppan et al., 1999; Wellington and Jarvis, 2001). One of the most important hepatoprotective agent, silymarin is obtained from milk thistle fruits and seeds. The plant extract has a variety of phytoconstituents including silybin, or sometimes incorrectly called silibinin, which are flavonolignans, as well as isosilibin, silychristin, and silidianin (Figure 4). The plant is widely spread in the United States and it became officially used in clinical practice after 1969 (Morazzoni and Bombardelli, 1995; Flora et al., 1998; Šimanek et al., 2000).
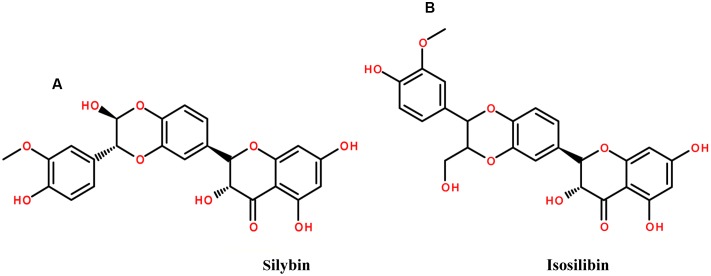
FIGURE 4.
The chemical structures of phytoconstituents of Milk thistle (A) Silybin and (B) isosilibin.
A study by Flora et al. (1996) revealed that 31% of patients were taking OTC milk thistle as an alternative agent for liver diseases. Dioscorides, the famous Greek herbalist, wrote about the use of tea from milk thistle seed against snake poison/bite (Greive, 1981). The use of milk thistle against hyperbilirubinemia was described by Pliny The Elder (AD 23–79) (Foster, 1991). Later, in 1596, Gerarde considered milk thistle as the best remedy against black bile or melancholy (Hobbs, 1987). Furthermore, in 1787 the seeds and the roots of the plant were noted as an excellent remedy to treat liver and spleen obstruction and to cure jaundice along with expelling stones (Greive, 1981).
General features of flavonoids are their membrane stabilizing effect and free radical capture, which defines their biological activity. Silybin, being a flavolignan is considered as the most efficient phytochemical responsible for different therapeutic activities of the plant viz. hepatoprotective, antiangiogenic, chemoprotective etc. (Gazak et al., 2007). Fruits and seeds are considered to be the richest in active constituents along with leaves (Harnisch and Stolze, 1983; Hobbs, 1987; Flora et al., 1998). Silybin is the major component of silymarin, constituting about 50–70% of the silymarin extract (Loguercio and Festi, 2011). The effect of silymarin against liver diseases of different etiology such as hepatobiliary diseases was reported during 1960s (Wagner et al., 1974; Luper, 1998; Pradhan and Girish, 2006). Silymarin has been extensively studied for its different pharmacokinetic and pharmacodynamic properties, and mechanism of liver protective action (Ramellini and Meldolesi, 1974; Miadonna et al., 1987; Feher et al., 1989; Barzaghi et al., 1990; Wellington and Jarvis, 2001).
Moreover, the effect of silymarin on liver cirrhosis was investigated in several randomized double blind clinical studies (Salmi and Sarna, 1982; Feher et al., 1989; Ferenci et al., 1989; Bunout et al., 1992; Parés et al., 1998). Some studies revealed a significant increase in the patient survival rate after the treatment with silymarin (Ferenci et al., 1989; Parés et al., 1998). It was also reported that slightly elevated levels of serum bilirubin are normalized by silymarin treatment and CGT, ALT, and AST are decreased significantly (Feher et al., 1989). However, another study showed that 420 mg/day dose of silymarin did not inflict significant effect on the serum level of total bilirubin, ALP and, AST, in patients with primary biliary cirrhosis (Angulo et al., 2000). The opposite result was obtained at the same dose, i.e., 420 mg/day, in a randomized, multicentric, double blind study with larger number of patients (n = 59). Significant reduction of serum AST and bilirubin was recorded as comparable to placebo in patients with acute viral hepatitis A or B (Magliulo et al., 1978). Serum ALT level was decreased significantly in patients with chronic hepatitis by studies including 180 patients with chronic persistent hepatitis (Tănăsescu et al., 1987).
The daily dose of 12–15 g of crude herb, or 200–400 mg of silymarin (counted as silybin) is recommended by German commission E (Meyer et al., 1999). Tablets, or capsules with a dose of 70 or 140 mg, or milk thistle fruit infusion are also used (Fleming, 1998; Meyer et al., 1999; Wellington and Jarvis, 2001). The drug should be avoided for children below age of 12 years due to lack of proper clinical tests for its effect. Several other parameters like enhancement of SOD activity, as well as SOD expression in erythrocytes and lymphocytes along with the increase of glutathione and glutathione peroxidase levels are also associated with silymarin intake (Wellington and Jarvis, 2001). Consequently, silymarin is becoming a more popular OTC herbal preparation in Europe due to its liver regeneration properties. A large number of patients with different liver disease are taking this drug with other prescribed medications in United States as well (Leng-Peschlow, 1994; Morazzoni and Bombardelli, 1995; Schuppan et al., 1999; Šimanek et al., 2000; Wellington and Jarvis, 2001).
Terminalia chebula Retz.
Terminalia chebula Retz. originates from India (Choi et al., 2015) and belongs to the family Combretaceae commonly known as “chebulic myrobalan” and “Haritaki” (Tasduq et al., 2006; Yeasmin et al., 2016). T. chebula contains significant amount of phenolic and flavonoid compounds. Some of the main constituents are 2,4-chebulyl-β-D-glucopyranose, ellagic acid, gallic acid and chebulic ellagitannins (Figure 5) (Juang et al., 2004; Panunto et al., 2010; Li et al., 2014).
FIGURE 5.
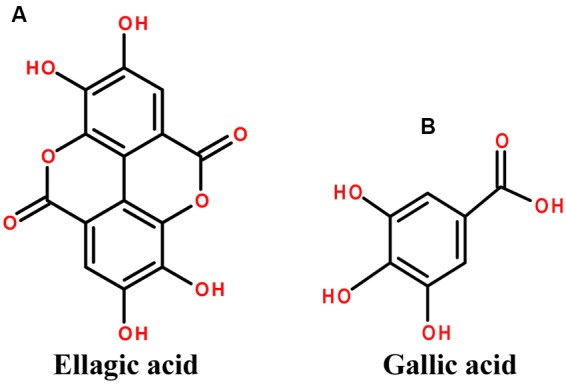
The chemical structures of phytoconstituents of Terminalia chebula (A) Ellagic acid and (B) Gallic acid.
The plant is well recognized for its various medicinal uses, and it is one of the main ingredient of the important Ayurvedic formulation “Triphala” (three myrobalan fruits) (Anand et al., 1994). Ayurvedic Pharmacopoeia of India prescribes this formulation to cure kidney and liver dysfunctions (Chatterjee and Pakrashi, 1991; Mhaskar et al., 2000). The use of dried ripe fruit of T. chebula is reported in Ayurveda as antitussive, cardiotonic, homeostatic, diuretic and laxative (Lee H.-S. et al., 2005; Sarkar et al., 2012). There are several formulations with T. chebula as the main ingredient, for example the official formulation in Ayurvedic Pharmacopoeia of India named “Triphala Ghrita”, which is a mixture of T. chebula, T. bellirica, Phyllanthus emblica and 19 other compounds found to have beneficial effect on jaundice (Kamala). The plant is also the main ingredient of the polyherbal hepatoprotective drug HP-1 (Tasaduq et al., 2003).
Together with the suppressive effect on hepatic fibrosis, the fruit extract of T. chebula inhibits the lipid peroxidase and affects the iron chelation associated protein oxidation. Reduction in ALT, AST, ALP and total bilirubin level indicates protection of liver damage (Sharma and Rathore, 2010; Sarkar et al., 2012; Yeasmin et al., 2016). The antioxidative role of the 70 % methanolic extract is due to its chelating properties for iron ion and thus decreasing the toxicity in iron overload states (Harrison, 1977; Sarkar et al., 2012). Aqueous fruit extract of T. chebula was tested against severe acute liver injury by t-BHP (tert-butyl hydroperoxide) in mice (Choi et al., 2015). The t-BHP causes rigorous necrosis, damage of hepatic tissues and significant elevations of serum enzymes like LDH, AST, and ALT. T. chebula fruit extract normalizes the liver enzyme levels and exhibits antioxidant effects overall providing liver protection (Owoyele et al., 2001; Fu et al., 2010; Choi et al., 2015; Yeasmin et al., 2016).
T. chebula extract also normalizes the abnormal level of CYP2E1 gene expression, which is mainly activated during drug metabolism process (Rush et al., 1985; Choi et al., 2015). Altogether with the hepatoprotective effect in different models (Tasduq et al., 2006; Sarkar et al., 2012), the immune modulatory action mediated by chebulagic acid in T. chebula is also reported (Lee S. et al., 2005). Additionally, due to its antioxidant activity and bilirubin level lowering effect T. chebula extract ensures hepatoprotection against paracetamol-induced damage. The reduction in serum bilirubin level is the most important evidence supporting the traditional use of the plant against jaundice.
Hepatotoxicity associated with prolonged use of rifampicin (RIF), isoniazid (INH) and pyrazinamide (PZA), which are used in a combination for the chemoprophylaxis and treatment of tuberculosis (Wong et al., 2000), is common. The side effects of anti-tuberculosis drugs on liver were reduced after the use of T. chebula fruit extract containing 0.250% chebuloside on an oral administration for 12 weeks in rats (Tasduq et al., 2006). Membranes (cytoplasmic and microsomal) were found as the main target of action for T. chebula extract. Hepatoprotective effect of T. chebula is also based on effects on the Na+, K+ -ATPase and CYP2E1, similarly to silymarin (Mourelle et al., 1989; Tasduq et al., 2006).
Glycyrrhiza glabra L.
Genus Glycyrrhiza belongs to the family Fabaceae, and consists of around 30 species. The plants of this genus are perennial herbs and are native to Mediterranean region and Asia, from Iran to Southern Russia. They are also cultivated throughout Europe and Asia (Blumenthal et al., 2000; Asl and Hosseinzadeh, 2008). The most common plant of this genus is Glycyrrhiza glabra L. also known as licorice. It has been utilized for relief of catarrh of the respiratory organs since ancient Egyptian times and described in the Ebers papyrus (1552 B.C.) and Codex Hammurabi (2100 B.C.). It also appears in “De Materia Medica” of Dioscorides (40–90 A.D.) in Rome and “De Causis Plantarum” and “De Historia Plantarum” of Theophrastus (371–286 B.C.) in Greece. According to Dioscorides manuscript entitled “Glukoriza” (sweet root), the expressed sap of its root is used for the liver, stomach, and kidney related ailments (Shibata, 2000). Flavonoid content from the root was found to exert antiulcerogenic and spasmolytic activity (Kitagawa et al., 1994; Fukai et al., 1996; Evans, 2009). Several isoflavonoid derivatives such as shinpterocarpin, glabrone, glabrene, glabridin, lico-isoflavones A and B etc. are also present in licorice (Williamson, 2003; Asl and Hosseinzadeh, 2008). The ancient medicinal use of licorice has been documented in the Chinese medical book “Shang-Han-Za-Bing-Lun” as well. Among 113 prescriptions of Shang-Han-Lun, 80% contain licorice as a significant constituent (Shibata, 2000). The cultivation of this plant in England has been traced back to the sixteenth century (Evans, 2009). The plant is utilized for its medicinal activity since 500 BC and described as ‘the grandfather of the herbs’ (Ody, 2000). It is known under different names like licorice, gancao, yasthi-madhu, kanzoh and sweet root (Blumenthal et al., 2000; Nomura et al., 2002; Asl and Hosseinzadeh, 2008). G. glabra L., G. bucharica Regel, G. foetida Desf., G. aspera Pall., G. echinata L., and G. inflata Batalin are some of the species of this genus among which three have varieties: G. glabra viz. G. glabra var typica (Spanish and Italian licorice), G. glabra var glandulifera (Russian licorice) and G. glabra var violacea (Turkish licorice) (Nomura et al., 2002). Substantial research has been carried out for the main medicinal effects of licorice since 1990 largely by the Japanese scientists, since the drug is widely used in the traditional medicine of Japan, brought earlier from China (Wang et al., 1996; Arase et al., 1997; Shibata, 2000; van Rossum et al., 2001; Hidaka et al., 2007; Evans, 2009; Eerdunbayaer et al., 2014; Ohno et al., 2014).
The sweet taste of licorice is due to the triterpenoid saponin known as glycyrrhizin (glycyrrhizic acid) (Figure 6). Further, the glycyrrhizinic acid is diglucopyranosiduronic acid of the glycyrrhetinic acid, which has a triterpenoid structure (Kokate et al., 2003; Evans, 2009). Flavonoids are the cause of the yellow color of licorice, and they were recognized in 1978 for their inhibitory effect on gastric acid secretion (Dastagir and Rizvi, 2016). Flavonoids include liquiritin, isoliqueritin (a chalcone), liquiritigenin, neoliuirtin, rhamnoliuirtin and others (Williamson, 2003; Asl and Hosseinzadeh, 2008).
FIGURE 6.
Chemical structure of glycyrrhizin (glycyrrhizic acid): the main active constituent of glycyrrhiza.
The leaves, roots and rhizomes of licorice have been used traditionally for different purposes including wound healing (Dafni et al., 1984), cough (Fujita et al., 1995) cystitis (Yarnell, 1997), tuberculosis (Arseculeratne et al., 1985), gastric ulcer (Varshney et al., 1983) and diabetes (Rajurkar and Pardeshi, 1997). Licorice shows different pharmacological activities such as hepatoprotective (Nakamura et al., 1985; Lin and Tome, 1988; Nose et al., 1994; Lin et al., 1999; Al-Qarawi et al., 2001; Jeong et al., 2002; Lee et al., 2009; Huo et al., 2011), anti-inflammatory (Finney and Somers, 1958; Ohuchi and Tsurufuji, 1982; Yu et al., 2015; Yang et al., 2016), antiviral, antimicrobial (Shebl et al., 2012; Sabouri Ghannad et al., 2014; Wang et al., 2015) and immunomodulatory effect (Kimura et al., 1992; Tandon et al., 2002; Raphael and Kuttan, 2003). Glycyrrhizin is widely used for treatment of different types of hepatitis (Yamamoto et al., 1958; Suzuki et al., 1977). However, there are reports of aldosteronism effect when used in enormous doses (Ulmann et al., 1975; Epstein et al., 1977; Ishikawa and Saito, 1980). Pharmacokinetic studies of glycyrrhizin were also carried out in patients with hepatitis and liver cirrhosis and a monophasic decline in plasma concentration of glycyrrhizin was found with 6.0 h elimination half-life (t1/2) and 7.9 ml h-1 kg-1 total body clearance (CLtot) (Tanaka et al., 1993; Yamamura et al., 1995). Another pharmacokinetic study in rats with D-Gal N induced hepatic disease didn’t show significant change in bioavailability of glycyrrhizin (Wang et al., 1996).
Use of licorice extract for peptic ulcer was reported by Revers in 1946. Studies have been conducted extensively to investigate the effect of glycyrrhizin alone, or in combination with other drugs against chronic hepatitis (Takahara et al., 1994; Arase et al., 1997; Lau et al., 2000; Tandon et al., 2001), and subacute hepatitis (caused mainly by HBV and HEV). Duration of illness and other fatal complications of subacute liver failure due to HEV are also reduced by intravenous glycyrrhizin therapy along with marked decline in elevated bilirubin concentration (Tandon et al., 2002). One of the popular preparations containing glycyrrhizin, L-cysteine and glycine that have been used in Japan for more than six decades is with the trade name of “Stronger Neo-Minophagen C” (SNMC). Initially the preparation was used as an antiallergic and antidote to toxic substances. Later on, the medication was used intravenously for chronic hepatitis, plummeting serum AST (GOT) and ALT (GPT) levels in patients. A double-blind clinical trial of SNMC for treatment of chronic hepatitis was carried out by Suzuki and coworkers and observed significant decline in plasma transaminase activity and improvement of the liver cells in histological samples from the SNMC treated group (Suzuki et al., 1977). Intravenously administered glycyrrhizin is rapidly eliminated from sera, and is transformed to glycyrrhetinic monoglucuronide by means of hepatic β-glucuronidase. The intravenous administration of SNMC also led to significant decline of elevated AST and ALT levels in hepatitis patients. Significant suppression of the release of AST from hepatocytes was also found in isolated rat hepatocytes by glycyrrhizin and glycyrrhetinic acid incubated with anti-liver cell membrane antibody (Shiki et al., 1992; Shibata, 2000). Yoshikawa et al. (1997) using an antigen-specific murine CD4+ T hybridoma cell line, showed that glycyrrhizin restrains immune-mediated cytotoxicity against hepatocytes, and thus explaining the reduction of AST and ALT elevated plasma levels. These were the results of apoptosis of hepatocytes resulting from liver injury (Hiramatsu et al., 1994; Mita et al., 1994). Anti-Fas antibody-induced elevation of ALT in mice was counteracted by glycyrrhizin and it was hypothesized that ALT decrease in the chronic hepatitis C virus (HCV) patients treated with SNMC might be due to the inhibition of Fas-mediated hepatic injury. The anti-inflammatory effect of SNMC is believed to be mediated by its membrane protecting activity and it is associated with a lower transaminase level in chronic hepatitis patients (Watari, 1973; Utsumi, 1984). Glycyrrhizic acid inhibits cisplatin efflux from the cells and reverses cisplatin resistance in HCC cell (Wakamatsu et al., 2007). The glycyrrhizic acid effect is associated with reduced immunosuppression, cell cycle arrest, induction of autophagy and apoptosis of the HCC cells (Satomi et al., 2005). The mechanism of action of glycyrrhizic acid is via its binding to glycyrrhizic acid membrane receptors on the hepatocytes and glycyrrhetinic acid-modified novel drug delivery system for HCC was consequently developed based on the promising activity pattern of this molecule (Cai et al., 2016). Several clinical trials were also performed on SNMC to see the effect of glycyrrhizin in hepatitis and hepatocarcinogenesis (Suzuki, 1983; Hino et al., 1994; Arase et al., 1997; van Rossum et al., 1999; Ikeda et al., 2006; Veldt et al., 2006). These trials revealed the effectiveness of SNMC in the prevention of liver carcinogenesis and the effect of glycyrrhizin in decreasing the HCC, improvement of plasma transaminase activity and effect on chronic HCV infected patients with non response toward interferon. Further, glycyrrhizin therapy exhibited normalization of ALAT levels and showed less incidence of HCC.
Licorice also possesses preventive role on the development of hepatocellular carcinoma (HCC) in HCV-associated chronic hepatitis patients (Arase et al., 1997; Miyakawa and Iino, 2001). Patients with long-term abnormal serum levels of α-fetoprotein (AFP) after transfusion have high probability of HCC and histological aggravation (Ikeda et al., 1993). They could benefit using licorice to maintain normal liver function.
Based on their biological activity and efficiency in the cure of some hepatic diseases, including HCC, there is increasing interest toward the use of herbs and natural products, in the treatment and prevention of these disorders. The reviewed plants are widely used in hepatic disorders treatment and their biologically active components have been extensively studied. However, more investigations are required in order to clarify their specificity and improve their efficiency by revealing their molecular targets and mechanism of action. Further clinical studies should confirm their ability to cure jaundice and standardize their medical inference.
Conclusion
In this review we discussed in details some of the most important plants used for the treatment of jaundice. The remarkable potential of medicinal plants used in the context of gastrointestinal disorders is already well known and their utilization is quite common in ethnomedicine, however, the experimental verifications are limited. Some of the salient features are the beneficial effects in context of jaundice seen on the serum enzymes particularly in the AST, SGOT, SGPT and total bilirubin clearance. Growing number of clinical studies reveal the importance of particular plant species and their active compounds, which serve as basis for further drug development.
More studies are required in order to improve the efficiency and specificity of jaundice treatment based on ethnopharmacological knowledge and to standardize the clinical procedures. The broad spectrum of biologically active compounds could allow additional applications beyond jaundice cure, like gastrointestinal disorders, blood pressure and so on. The high efficiency and low number of side effects by using plant constituents indicates the ethnopharmacological approach of jaundice treatment as a high priority for future research.
Author Contributions
DT, AM, EP, ZM, YL, and AA have written the first draft of the manuscript. AS, SN, LH, and JH revised and improved the first draft. All authors have seen and agreed on the finally submitted version of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
AA acknowledges the support by the Polish KNOW (Leading National Research Centre) Scientific Consortium “Healthy Animal—Safe Food,” decision of Ministry of Science and Higher Education No. 05-1/KNOW2/2015. AM acknowledges the support by UEFISCDI, Romania, project no. PNII-RU-TE-2014-4-1247 and ZM was supported by a postdoctoral research fellowship from the Universiti Sains Malaysia Research University Individual (RUI) grant (ref. no. 1001/PPSP/812151).
Abbreviations
- ACE
angiotensin converting enzyme
- Ach
acetylcholine
- ALP
alkaline phosphatase
- ALT
alanine transaminase
- AR
aldose reductase
- AspAT
aspartate aminotransferase
- AST
aspartate transaminase
- ATP
adenosine triphosphate
- AUC
area under the concentration time curve
- cAMP
cyclic adenosine monophosphate
- CaOX
calcium oxalate
- CCL4
carbon tetrachloride
- CED
Caenorhabditis elegans cell-death gene
- CGT
ceramide galactosyltransferase
- Cmax
maximum plasma concentration
- COX
cyclooxygenase
- CrMP
chromium mesoporphyrin
- CYP
cytochromes P450
- CYP2E1
Cytochrome P450 2E1
- D-Gal N
D-galactosamine
- DTD
DT-diaphorase
- ERK
extracellular signal–regulated kinase
- ET-NANB
enteric transmitted non-A non-B
- GABA
gamma-Aminobutyric acid (γ-Aminobutyric acid)
- GOT
glutamic oxaloacetic transaminase
- GPT
glutamic–pyruvic transaminase
- GSH
glutathione stimulation hormone
- GST
glutathione S-transferase
- HAV
hepatitis A virus
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HDV
hepatitis D virus/hepatitis delta virus
- HEV
hepatitis E virus
- HIV
human immunodeficiency virus
- HO 1
heme oxygenase 1
- ICE
interleukin-1beta-converting enzyme
- INH
isoniazid
- JNK
c-Jun N-terminal kinases
- LDH
lactate dehydrogenase
- LOX
lipoxygenase
- LPS
Lipopolysaccharide
- LR
renal clearance
- MAPK
mitogen-activated protein kinases
- MDA
malondialdehyde
- mRNA
messenger RNA
- NA
noradrenaline
- Na+ K+-ATPase
sodium potassium adenosine triphosphatase
- NANBH non-A
non-B hepatitis
- NK1
neurokinin 1 OTC over the counter PROD pentoxyresorufin O-dealkylase
- PZA
pyrazinamide RBC red blood cells
- RIF
rifampin
- ROS
reactive oxygen species
- RT
reverse transcriptase
- SGOT
serum glutamic oxaloacetic transaminase
- SnMP
tin mesoporphyrin
- SOD
superoxide dismutase
- t1/2β
elimination half life
- t-BHP
tert-butyl hydroperoxide
- TCM
Traditional Chinese medicine
- TK
tyrosine kinase tmax maximum time
- TNF-α
tumor necrosis factor- α
- UDPGT
uridine diphosphoglucuronyl transferase
- UGT
uridine 5′-diphospho-glucuronosyltransferase (UDP- glucuronosyltransferase)
- wh. pl.
whole plant
- WHO
World Health Organization
- WW II
World War II.
References
- Agarwal K., Dhir H., Sharma A., Talukder G. (1992). The efficacy of two species of Phyllanthus in counteracting nickel clastogenicity. Fitoterapia 63 49–54. [Google Scholar]
- Akbarsha M. A., Murugaian P. (2000). Aspects of the male reproductive toxicity/male antifertility property of andrographolide in albino rats: effect on the testis and the cauda epididymidal spermatozoa. Phytother. Res. 14 432–435. [DOI] [PubMed] [Google Scholar]
- Alcaraz M. J., Jiménez M. J. (1988). Flavonoids as anti-inflammatory agents. Fitoterapia 59 25–38. [Google Scholar]
- Al-Qarawi A. A., Abdel-Rahman H. A., El-Mougy S. A. (2001). Hepatoprotective activity of licorice in rat liver injury models. J. Herbs Spices Med. Plants 8 7–14. 10.1300/J044v08n01_02 [DOI] [Google Scholar]
- Anand K. K., Singh B., Saxena A. K., Chandan B. K., Gupta V. N. (1994). Hepatoprotective studies of a fraction from the fruits of Terminalia belerica Roxb. On experimental liver injury in rodents. Phytother. Res. 8 287–292. 10.1002/ptr.2650080507 [DOI] [Google Scholar]
- Angulo P., Patel T., Jorgensen R. A., Therneau T. M., Lindor K. D. (2000). Silymarin in the treatment of patients with primary biliary cirrhosis with a suboptimal response to ursodeoxycholic acid. Hepatology 32 897–900. 10.1053/jhep.2000.18663 [DOI] [PubMed] [Google Scholar]
- Arase Y., Ikeda K., Murashima N., Chayama K., Tsubota A., Koida I., et al. (1997). The long term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer 79 1494–1500. [DOI] [PubMed] [Google Scholar]
- Arseculeratne S. N., Gunatilaka A. A. L., Panabokke R. G. (1985). Studies on medicinal plants of Sri Lanka. Part 14: toxicity of some traditional medicinal herbs. J. Ethnopharmacol. 13 323–335. 10.1016/0378-8741(85)90078-9 [DOI] [PubMed] [Google Scholar]
- Asl M. N., Hosseinzadeh H. (2008). Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother. Res. 22 709–724. 10.1002/ptr.2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann T. L., Ghia F., Torssell K. B. G. (1993). Lignans and lactones from Phyllanthus anisolobus. Phytochemistry 33 189–191. 10.1016/0031-9422(93)85420-V [DOI] [Google Scholar]
- Bagalkotkar G., Sagineedu S. R., Saad M. S., Stanslas J. (2006). Phytochemicals from Phyllanthus niruri Linn. and their pharmacological properties: a review. J. Pharm. Pharmacol. 58 1559–1570. 10.1211/jpp.58.12.0001 [DOI] [PubMed] [Google Scholar]
- Barzaghi N., Crema F., Gatti G., Pifferi G., Perucca E. (1990). Pharmacokinetic studies on IdB 1016, a silybin-phosphatidylcholine complex, in healthy human subjects. Eur. J. Drug Metab. Pharmacokinet. 15 333–338. 10.1007/BF03190223 [DOI] [PubMed] [Google Scholar]
- Beretz A., Anton R., Stoclet J. C. (1978). Flavonoid compounds are potent inhibitors of cyclic AMP phosphodiesterase. Experientia 34 1054–1055. 10.1007/BF01915343 [DOI] [PubMed] [Google Scholar]
- Blumenthal M., Goldberg A., Brinkmann J. (2000). Herbal Medicine, Expanded Commission E Monographs Cd-Rom. Austin, TX: American Botanical Council. [Google Scholar]
- Bunout D., Hirsch S., Petermann M., De La Maza M. P., Silva G., Kelly M., et al. (1992). Controlled study of the effect of silymarin on alcoholic liver disease. Rev. Med. Chil 120 1370–1375. [PubMed] [Google Scholar]
- Cai Y., Xu Y., Chan H. F., Fang X., He C., Chen M. (2016). Glycyrrhetinic acid mediated drug delivery carriers for hepatocellular carcinoma therapy. Mol. Pharm. 13 699–709. 10.1021/acs.molpharmaceut.5b00677 [DOI] [PubMed] [Google Scholar]
- Calabrese C., Berman S. H., Babish J. G., Ma X., Shinto L., Dorr M., et al. (2000). A phase I trial of andrographolide in HIV positive patients and normal volunteers. Phytother. Res. 14 333–338. [DOI] [PubMed] [Google Scholar]
- Calixto J. B., Santos A. R. S., Filho V. C., Yunes R. A. (1998). A review of the plants of the genus Phyllanthus: and therapeutic potential. Med. Res. Rev. 18 225–258. [DOI] [PubMed] [Google Scholar]
- Campos A. H., Schor N. (1999). Phyllanthus niruri inhibits calcium oxalate endocytosis by renal tubular cells: its role in urolithiasis. Nephron 81 393–397. 10.1159/000045322 [DOI] [PubMed] [Google Scholar]
- Chander R., Srivastava V., Tandon And J. S., Kapoor N. K. (1995). Antihepatotoxic activity of diterpenes of Andrographis paniculata (Kal-Megh) against Plasmodium berghei-induced hepatic damage in Mastomys natalensis. Int. J. Pharmacogn. 33 135–138. 10.3109/13880209509055213 [DOI] [Google Scholar]
- Chatterjee A., Pakrashi S. C. (1991). The Treatise on Indian Medicinal Plants, Vol. 1. New Delhi: Publications and Information Directorate, CSIR, 172. [Google Scholar]
- Choi M.-K., Kim H.-G., Han J.-M., Lee J.-S., Lee J. S., Chung S. H., et al. (2015). Hepatoprotective effect of Terminalia chebula against t-BHP-induced acute liver injury in C57/BL6 mice. Evid. Based Complement. Alternat. Med. 2015:517350 10.1155/2015/517350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra R. N., Nayar S. L., Chopra I. C., Asolkar L. V., Kakkar K. K., Chakre O. J. (1956). Glossary of Indian medicinal plants; [with] Supplement. New Delhi: Council of Scientific & Industrial Research. [Google Scholar]
- Choudhury B. R., Poddar M. K. (1984). Andrographolide and kalmegh (Andrographis paniculata) extract: in vivo and in vitro effect on hepatic lipid peroxidation. Methods Find. Exp. Clin. Pharmacol. 6 481–485. [PubMed] [Google Scholar]
- Chturvedi G. N., Tomar G. S., Tiwari S. K., Singh K. P. (1983). Clinical studies on Kalmegh (Andrographis paniculata nees) in infective hepatitis. Anc. Sci. Life 2 208–215. [PMC free article] [PubMed] [Google Scholar]
- Cruz T., Galvez J., Ocete M. A., Crespo M. E., Sanchez de Medina L.-H. F., Zarzuelo A. (1998). Oral administration of rutoside can ameliorate inflammatory bowel disease in rats. Life Sci. 62 687–695. 10.1016/S0024-3205(97)01164-8 [DOI] [PubMed] [Google Scholar]
- Cruz-Vega D., Verde-Star M. J., Salinas-Gonzalez N. R., Rosales-Hernandez B., Estrada-Garcia I., Mendez-Aragon P., et al. (2009). Review of pharmacological effects of Glycyrrhiza radix and its bioactive compounds. Zhongguo Zhong Yao Za Zhi. 22 557–559. [PubMed] [Google Scholar]
- Dafni A., Yaniv Z., Palevitch D. (1984). Ethnobotanical survey of medicinal plants in northern Israel. J. Ethnopharmacol. 10 295–310. 10.1016/0378-8741(84)90017-5 [DOI] [PubMed] [Google Scholar]
- Dastagir G., Rizvi M. A. (2016). Glycyrrhiza glabra L.(Liquorice). Pak. J. Pharm. Sci 29 1727–1733. [PubMed] [Google Scholar]
- Deng W. L., Nie R. J., Liu J. Y. (1982). Comparison of pharmacological effect of four andrographolides. Yaoxue Tongbao 17 195–198. [Google Scholar]
- Dhar M. L., Dhar M. M., Dhawan B. N., Mehrotra B. N., Roy C. (1968). Screening of Indian plants for biological activity: Part 1. Indian J. Exp. Biol. 6 232–247. [PubMed] [Google Scholar]
- Dhir H., Roy A. K., Sharma A., Talukder G. (1990). Protection afforded by aqueous extracts of Phyllanthus species against cytotoxicity induced by lead and aluminium salts. Phytother. Res. 4 172–176. 10.1002/ptr.2650040503 [DOI] [Google Scholar]
- Dias M. A., Campos A. H., Cechinel Filho V., Yunes R. A. C. J. (1995). Analysis of the mechanisms underlying the contractile response induced by the hydroalcoholic extract of Phyllanthus urinaria in the guinea-pig urinary bladder in-vitro. J. Pharm. Pharmacol. 47 846–851. 10.1111/j.2042-7158.1995.tb05752.x [DOI] [PubMed] [Google Scholar]
- Dixit S. P., Achar M. P., Thabrew M. R. (1982). Phyllanthus niruri (Bhumyamalaki) and jaundice in children. J. Natl. Integ. Med. Ass. 25 269–272. [Google Scholar]
- Duarte J., Perez-Vizcaino F., Zarzuelo A., Jimenez J., Tamargo J. (1993). Vasodilator effects of quercetin in isolated rat vascular smooth muscle. Eur. J. Pharmacol. 239 1–7. 10.1016/0014-2999(93)90968-N [DOI] [PubMed] [Google Scholar]
- Eerdunbayaer, Orabi M. A. A., Aoyama H., Kuroda T., Hatano T. (2014). Structures of new phenolics isolated from licorice, and the effectiveness of licorice phenolics on vancomycin-resistant Enterococci. Molecules 19 13027–13041. 10.3390/molecules190913027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein M. T., Espiner E. A., Donald R. A., Hughes H. (1977). Effect of eating liquorice on the renin-angiotensin aldosterone axis in normal subjects. Br. Med. J. 1 488–490. 10.1136/bmj.1.6059.488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. C. (2009). Trease and Evans’ Pharmacognosy. Amsterdam: Elsevier. [Google Scholar]
- Feher J., Deák G., Muzes G. (1989). Hepatoprotective activity of silymarin (Legalon) therapy in patients with chronic liver disease. Orv. Hetil. 130 2723–2727. [PubMed] [Google Scholar]
- Ferenci P., Dragosics B., Dittrich H., Frank H., Benda L., Lochs H., et al. (1989). Randomized controlled trial of silymarin treatment in patients with cirrhosis of the liver. J. Hepatol. 9 105–113. 10.1016/0168-8278(89)90083-4 [DOI] [PubMed] [Google Scholar]
- Filho V. C., Santos A. R. S., Campos R. O. P., Miguel O. G., Yunes R. A., Ferrari F., et al. (1996). Chemical and pharmacological studies of Phyllanthus caroliniensis in Mice. J. Pharm. Pharmacol. 48 1231–1236. 10.1111/j.2042-7158.1996.tb03928.x [DOI] [PubMed] [Google Scholar]
- Finney R. S. H., Somers G. F. (1958). The antiinflammatory activity of glycyrrhetinic acid and derivatives. J. Pharm. Pharmacol. 10 613–620. 10.1111/j.2042-7158.1958.tb10349.x [DOI] [PubMed] [Google Scholar]
- Fleming T. (1998). PDR for Herbal Medicines. Montvale, NJ: Medical Economics Company. [Google Scholar]
- Flora K., Hahn M., Rosen H., Benner K. (1998). Milk thistle (Silybum marianum) for the therapy of liver disease. Am. J. Gastroenterol. 93 139–143. 10.1111/j.1572-0241.1998.00139.x [DOI] [PubMed] [Google Scholar]
- Flora K. D., Rosen H. R., Benner K. G. (1996). The use of naturopathic remedies for chronic liver disease. Am. J. Gastroenterol. 91 2654–2655. [PubMed] [Google Scholar]
- Foo L. Y. (1995). Amariinic acid and related ellagitannins from Phyllanthus amarus. Phytochemistry 39 217–224. 10.1016/0031-9422(94)00836-I [DOI] [Google Scholar]
- Foo L. Y., Wong H. (1992). Phyllanthusiin D, an unusual hydrolysable tannin from Phyllanthus amarus. Phytochemistry 31 711–713. 10.1016/0031-9422(92)90071-W [DOI] [Google Scholar]
- Foster S. (1991). Milk thistle: Silybum marianum. Botanical Series, (305). Austin, TX: American Botanical Council. [Google Scholar]
- Fu B., Li S., Yu X., Yang P., Yu G., Feng R., et al. (2010). Chinese ecosystem research network: progress and perspectives. Ecol. Complex 7 225–233. 10.1016/j.ecocom.2010.02.007 [DOI] [Google Scholar]
- Fujita T., Sezik E., Tabata M., Yesilada E., Honda G., Takeda Y., et al. (1995). Traditional medicine in Turkey VII. Folk medicine in middle and west Black Sea regions. Econ. Bot. 49 406–422. 10.1007/BF02863092 [DOI] [Google Scholar]
- Fukai T., Tantai L., Nomura T. (1996). Isoprenoid-substituted flavonoids from Glycyrrhiza glabra. Phytochemistry 43 531–532. 10.1016/0031-9422(96)00307-X [DOI] [Google Scholar]
- Gálvez J., Cruz T., Crespo E., Ocete M. A., Lorente M. D., de Medina F. S., et al. (1997). Rutoside as mucosal protective in acetic acid-induced rat colitis. Planta Med. 63 409–414. 10.1055/s-2006-957723 [DOI] [PubMed] [Google Scholar]
- Gálvez J., Sánchez de Medina F., Jiménez J., Torres M. I., Fernández M. I., Núñez M. C., et al. (1995). Effect of quercitrin on lactose-induced chronic diarrhoea in rats. Planta Med. 61 302–306. 10.1055/s-2006-958088 [DOI] [PubMed] [Google Scholar]
- Gazak R., Walterova D., Kren V. (2007). Silybin and silymarin–new and emerging applications in medicine. Curr. Med. Chem. 14 315–338. 10.2174/092986707779941159 [DOI] [PubMed] [Google Scholar]
- Girach R. D., Aminuddin Siddiqui P. A., Khan S. A. (1994). Traditional plant remedies among the Kondh of district Dhenkanal (Orissa). Int. J. Pharmacogn. 32 274–283. 10.3109/13880209409083005 [DOI] [Google Scholar]
- Greive M. (1981). A Modern Herbal, Vol. 2 New York, NY: Dover Publications [Google Scholar]
- Gulati R. K., Agarwal S., Agrawal S. S. (1995). Hepatoprotective studies on Phyllanthus emblica Linn. and quercetin. Indian J. Exp. Biol. 33 261–268. [PubMed] [Google Scholar]
- Gupta S., Choudhry M. A., Yadava J. N. S., Srivastava V., Tandon J. S. (1990). Antidiarrhoeal activity of diterpenes of Andrographis paniculata (Kal-Megh) against Escherichia coli enterotoxin in in vivo models. Int. J. Crude Drug Res. 28 273–283. 10.3109/13880209009082833 [DOI] [Google Scholar]
- Handa S. S., Sharma A. (1990). Hepatoprotective activity of andrographolide from Andrographis paniculata against carbontetrachloride. Indian J. Med. Res. 92 276–283. [PubMed] [Google Scholar]
- Harnisch G., Stolze H. (1983). “Silybum marianum: mariendistel,” in Bewaehrte Flanzendrogen in Wissenschaft Und Medizin (Melsungen: Notamed Verlag), 203–215. [Google Scholar]
- Harrison P. M. (1977). Ferritin: an iron-storage molecule. Semin. Hematol. 14 55–70. [PubMed] [Google Scholar]
- Hidaka I., Hino K., Korenaga M., Gondo T., Nishina S., Ando M., et al. (2007). Stronger Neo-Minophagen CTM, a glycyrrhizin-containing preparation, protects liver against carbon tetrachloride-induced oxidative stress in transgenic mice expressing the hepatitis C virus polyprotein. Liver Int. 27 845–853. 10.1111/j.1478-3231.2007.01492.x [DOI] [PubMed] [Google Scholar]
- Hino K., Sainokami S., Shimoda K., Iino S., Wang Y., Okamoto H., et al. (1994). Genotypes and titers of hepatitis C virus for predicting response to interferon in patients with chronic hepatitis C. J. Med. Virol. 42 299–305. 10.1002/jmv.1890420318 [DOI] [PubMed] [Google Scholar]
- Hiramatsu N., Hayashi N., Katayama K., Mochizuki K., Kawanishi Y., Kasahara A., et al. (1994). Immunohistochemical detection of Fas antigen in liver tissue of patients with chronic hepatitis C. Hepatology 19 1354–1359. 10.1002/hep.1840190606 [DOI] [PubMed] [Google Scholar]
- Hobbs C. (1987). Milk Thistle: The Liver Herb. Capitola, CA: Botanica Press. [Google Scholar]
- Huo H. Z., Wang B., Liang Y. K., Bao Y. Y., Gu Y. (2011). Hepatoprotective and antioxidant effects of licorice extract against CCl4-induced oxidative damage in rats. Int. J. Mol. Sci. 12 6529–6543. 10.3390/ijms12106529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain R. A., Dickey J. K., Rosser M. P., Matson J. A., Kozlowski M. R., Brittain R. J., et al. (1995). A novel class of non-peptidic endothelin antagonists isolated from the medicinal herb Phyllanthus niruri. J. Nat. Prod. 58 1515–1520. 10.1021/np50124a006 [DOI] [Google Scholar]
- Ikeda K., Arase Y., Kobayashi M., Saitoh S., Someya T., Hosaka T., et al. (2006). A long-term glycyrrhizin injection therapy reduces hepatocellular carcinogenesis rate in patients with interferon-resistant active chronic hepatitis C: a cohort study of 1249 patients. Dig. Dis. Sci. 51 603–609. 10.1007/s10620-006-3177-0 [DOI] [PubMed] [Google Scholar]
- Ikeda K., Saitoh S., Koida I., Arase Y., Tsubota A., Chayama K., et al. (1993). A multivariate analysis of risk factors for hepatocellular carcinogenesis: a prospective observation of 795 patients with viral and alcoholic cirrhosis. Hepatology 18 47–53. 10.1002/hep.1840180109 [DOI] [PubMed] [Google Scholar]
- Iruretagoyena M. I., Tobar J. A., González P. A., Sepúlveda S. E., Figueroa C. A., Burgos R. A., et al. (2004). Andrographolide interferes with T cell activation and reduces experimental autoimmune encephalomyelitis in the mouse. J. Pharm. Exp. Ther. 312 366–372. 10.1124/jpet.104.072512 [DOI] [PubMed] [Google Scholar]
- Ishikawa S., Saito T. (1980). The effect of glycyrrhetinic acid on the action of aldosterone in stimulating sodium transport in frog skin. Endocrinol. Jpn. 27 697–701. 10.1507/endocrj1954.27.697 [DOI] [PubMed] [Google Scholar]
- Ishimaru K., Yoshimatsu K., Yamakawa T., Kamada H., Shimomura K. (1992). Phenolic constituents in tissue cultures of Phyllanthus niruri. Phytochemistry 31 2015–2018. 10.1016/0031-9422(92)80352-F [DOI] [Google Scholar]
- Jarukamjorn K., Don-in K., Makejaruskul C., Laha T., Daodee S., Pearaksa P., et al. (2006). Impact of Andrographis paniculata crude extract on mouse hepatic cytochrome P450 enzymes. J. Ethnopharmacol. 105 464–467. 10.1016/j.jep.2005.11.024 [DOI] [PubMed] [Google Scholar]
- Jeong H. G., You H. J., Park S. J., Moon A. R., Chung Y. C., Kang S. K., et al. (2002). Hepatoprotective effects of 18β-glycyrrhetinic acid on carbon tetrachloride-induced liver injury: inhibition of cytochrome P450 2E1 expression. Pharmacol. Res. 46 221–227. 10.1016/S1043-6618(02)00121-4 [DOI] [PubMed] [Google Scholar]
- Juang L., Sheu S., Lin T. (2004). Determination of hydrolyzable tannins in the fruit of Terminalia chebula Retz. by high-performance liquid chromatography and capillary electrophoresis. J. Sep. Sci. 27 718–724. 10.1002/jssc.200401741 [DOI] [PubMed] [Google Scholar]
- Kapil A., Koul I. B., Banerjee S. K., Gupta B. D. (1993). Antihepatotoxic effects of major diterpenoid constituents of Andrographis paniculata. Biochem. Pharmacol. 46 182–185. 10.1016/0006-2952(93)90364-3 [DOI] [PubMed] [Google Scholar]
- Ketterer B. (1988). Protective role of glutathione and glutathione transferases in mutagenesis and carcinogenesis. Mutat. Res. 202 343–361. 10.1016/0027-5107(88)90197-2 [DOI] [PubMed] [Google Scholar]
- Khatoon S., Rai V., Rawat A. K. S., Mehrotra S. (2006). Comparative pharmacognostic studies of three Phyllanthus species. J. Ethnopharmacol. 104 79–86. 10.1016/j.jep.2005.08.048 [DOI] [PubMed] [Google Scholar]
- Kimura M., Watanabe H., Abo T. (1992). Selective activation of extrathymic T cells in the liver by glycyrrhizin. Biotherapy 5 167–176. 10.1007/BF02171049 [DOI] [PubMed] [Google Scholar]
- Kitagawa I., Chen W. Z., Hori K., Harada E., Yasuda N., Yoshikawa M., et al. (1994). Chemical studies of Chinese licorice-roots. I. elucidation of five new flavonoid constituents from the roots of Glycyrrhiza glabra L. collected in Xinjiang. Chem. Pharm. Bull. 42 1056–1062. 10.1248/cpb.42.1056 [DOI] [PubMed] [Google Scholar]
- Kokate C. K., Purohit A. P., Gokhale S. B. (2003). Test Book of Pharmacognosy. Pune: Nirali Prakashan. [Google Scholar]
- Kumar R. A., Sridevi K., Kumar N. V., Nanduri S., Rajagopal S. (2004). Anticancer and immunostimulatory compounds from Andrographis paniculata. J. Ethnopharmacol. 92 291–295. 10.1016/j.jep.2004.03.004 [DOI] [PubMed] [Google Scholar]
- Kusirisin W., Srichairatanakool S., Lerttrakarnnon P., Lailerd N., Suttajit M., Jaikang C., et al. (2009). Antioxidative activity, polyphenolic content and anti-glycation effect of some Thai medicinal plants traditionally used in diabetic patients. Med. Chem. 5 139–147. 10.2174/157340609787582918 [DOI] [PubMed] [Google Scholar]
- Lau G. K., Tsiang M., Hou J., Yuen S., Carman W. F., Zhang L., et al. (2000). Combination therapy with lamivudine and famciclovir for chronic hepatitis B–infected Chinese patients: a viral dynamics study. Hepatology 32 394–399. 10.1053/jhep.2000.9143 [DOI] [PubMed] [Google Scholar]
- Lee C. D., Ott M., Thyagarajan S. P., Shafritz D. A., Burk R. D., Gupta S. (1996). Phyllanthus amarus down-regulates hepatitis B virus mRNA transcription and replication. Eur. J. Clin. Invest. 26 1069–1076. 10.1046/j.1365-2362.1996.410595.x [DOI] [PubMed] [Google Scholar]
- Lee H.-S., Won N. H., Kim K. H., Lee H., Jun W., Lee K.-W. (2005). Antioxidant effects of aqueous extract of Terminalia chebula in vivo and in vitro. Biol. Pharm. Bull. 28 1639–1644. 10.1248/bpb.28.1639 [DOI] [PubMed] [Google Scholar]
- Lee J. R., Park S. J., Lee H.-S., Jee S. Y., Seo J., Kwon Y. K., et al. (2009). Hepatoprotective activity of licorice water extract against cadmium-induced toxicity in rats. J. Evid. Based Complement. Altern. Med. 6 195–201. 10.1093/ecam/nem078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Hyun P., Kim S., Kim K., Lee S., Kim B., et al. (2005). Suppression of the onset and progression of collagen-induced arthritis by chebulagic acid screened from a natural product library. Arthritis Rheum. 52 345–353. 10.1002/art.20715 [DOI] [PubMed] [Google Scholar]
- Leng-Peschlow E. (1994). Alcohol-related liver diseases-use of Legalon®. Z. Klin. Med. 2 22–27. [Google Scholar]
- Li Z., Li Q., Jiang X., Zhang K., Guan R. (2014). Isolation and preparation of gallic acid from Terminalia chebula Retz. with high-speed counter-current chromatography. Se Pu 32 1404–1408. [DOI] [PubMed] [Google Scholar]
- Lin C. N., Tome W. P. (1988). Antihepatotoxic principles of Sambucus formosana. Planta Med. 54 223–224. 10.1055/s-2006-962410 [DOI] [PubMed] [Google Scholar]
- Lin G., Nnane I. P., Cheng T.-Y. (1999). The effects of pretreatment with glycyrrhizin and glycyrrhetinic acid on the retrorsine-induced hepatotoxicity in rats. Toxicon 37 1259–1270. 10.1016/S0041-0101(98)00263-3 [DOI] [PubMed] [Google Scholar]
- Lindahl M., Tagesson C. (1993). Selective inhibition of group II phospholipase A2 by quercetin. Inflammation 17 573–582. 10.1007/BF00914195 [DOI] [PubMed] [Google Scholar]
- Loguercio C., Festi D. (2011). Silybin and the liver: from basic research to clinical practice. World J. Gastroenterol. 17 2288–2301. 10.3748/wjg.v17.i18.2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luper S. (1998). A review of plants used in the treatment of liver disease: part 1. Altern. Med. Rev. 3 410–421. [PubMed] [Google Scholar]
- Mabberley D. J. (2008). Mabberley’s Plant-book: A Portable Dictionary of Plants, Their Classification and Uses. Cambridge: Cambridge University Press. [Google Scholar]
- Magliulo E., Gagliardi B., Fiori G. P. (1978). Results of a double blind study on the effect of silymarin in the treatment of acute viral hepatitis, carried out at two medical centres (author’s transl). Med. Klin. 73 1060–1065. [PubMed] [Google Scholar]
- Mao X., Wu L.-F., Guo H.-L., Chen W.-J., Cui Y.-P., Qi Q., et al. (2016). The genus Phyllanthus: an ethnopharmacological, phytochemical, and pharmacological review. J. Evid. Based Complement. Altern. Med. 2016:7584952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A. (1994). Glutathione, ascorbate, and cellular protection. Cancer Res. 54(7 Suppl.), 1969s–1975s. [PubMed] [Google Scholar]
- Meyer H. S., Eldredge J. D., Hogan R. (1999). Herbal medicines. The complete german commission e monographs: therapeutic guide to herbal medicines. JAMA 281 1852–1853. 10.1001/jama.281.19.1852-JBK0519-2-1 [DOI] [Google Scholar]
- Mhaskar K. S., Blatter E., Caius J. F. (2000). Kirtikar and Basu’s Illustrated Indian Medicinal Plants: Their Usage in Ayurveda and Unani Medicines. New Delhi: Sri Satguru Publications. [Google Scholar]
- Miadonna A., Tedeschi A., Leggieri E., Lorini M., Froldi M., Zanussi C. (1987). Effects of silybin on histamine release from human basophil leucocytes. Br. J. Clin. Pharmacol. 24 747–752. 10.1111/j.1365-2125.1987.tb03241.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita E., Hayashi N., Iio S., Takehara T., Hijioka T., Kasahara A., et al. (1994). Role of fas ligand in apoptosis induced by hepatitis C virus infection. Biochem. Biophys. Res. Commun. 204 468–474. 10.1006/bbrc.1994.2483 [DOI] [PubMed] [Google Scholar]
- Miyakawa Y., Iino S. (2001). Toward prevention of hepatocellular carcinoma developing in chronic hepatitis C. J. Gastroenterol. Hepatol. 16 711–714. 10.1046/j.1440-1746.2001.02543.x [DOI] [PubMed] [Google Scholar]
- Morales M. A., Lozoya X. (1994). Calcium-antagonist effects of quercetin on aortic smooth muscle. Planta Med. 60 313–317. 10.1055/s-2006-959491 [DOI] [PubMed] [Google Scholar]
- Morazzoni P., Bombardelli E. (1995). Silybum marianum (Carduus marianus). Fitoterapia 66 3–42. [Google Scholar]
- Morton J. F. (1981). Atlas of Medicinal Plants of Middle America: Bahamas to Yucatan. Springfield: Charles C. Thomas, 458–462. [Google Scholar]
- Mourelle M., Muriel P., Favari L., Franco T. (1989). Prevention of CCl4-induced liver cirrhosis by silymarin. Fundam. Clin. Pharmacol. 3 183–191. 10.1111/j.1472-8206.1989.tb00449.x [DOI] [PubMed] [Google Scholar]
- Naik A. D., Juvekar A. R. (2003). Effects of alkaloidal extract of Phyllanthus niruri on HIV replication. Indian J. Med. Sci. 57 387. [PubMed] [Google Scholar]
- Nakamura T., Fujii T., Ichihara A. (1985). Enzyme leakage due to change of membrane permeability of primary cultured rat hepatocytes treated with various hepatotoxins and its prevention by glycyrrhizin. Cell Biol. Toxicol. 1 285–295. 10.1007/BF00118193 [DOI] [PubMed] [Google Scholar]
- Nanduri S., Nyavanandi V. K., Thunuguntla S. S. R., Kasu S., Pallerla M. K., Ram P. S., et al. (2004). Synthesis and structure-activity relationships of andrographolide analogues as novel cytotoxic agents. Bioorg. Med. Chem. Lett. 14 4711–4717. 10.1016/j.bmcl.2004.06.090 [DOI] [PubMed] [Google Scholar]
- Nomura T., Fukai T., Akiyama T. (2002). Chemistry of phenolic compounds of licorice (Glycyrrhiza species) and their estrogenic and cytotoxic activities. Pure Appl. Chem. 74 1199–1206. 10.1351/pac200274071199 [DOI] [Google Scholar]
- Nose M., Ito M., Kamimura K., Shimizu M., Ogihara Y. (1994). A comparison of the antihepatotoxic activity between glycyrrhizin and glycyrrhetinic acid. Planta Med. 60 136–139. 10.1055/s-2006-959435 [DOI] [PubMed] [Google Scholar]
- Ody P. (2000). The Complete Guide Medicinal Herbal. London: Dorling Kindersley. [Google Scholar]
- Ogata T., Higuchi H., Mochida S., Matsumoto H., Kato A., Endo T., et al. (1992). HIV-1 reverse transcriptase inhibitor from Phyllanthus niruri. AIDS Res. Hum. Retroviruses 8 1937–1944. 10.1089/aid.1992.8.1937 [DOI] [PubMed] [Google Scholar]
- Ohno H., Miyoshi S., Araho D., Kanamoto T., Terakubo S., Nakashima H., et al. (2014). Efficient utilization of licorice root by alkaline extraction. In Vivo 28 785–794. [PubMed] [Google Scholar]
- Ohuchi K., Tsurufuji A. (1982). A study of the anti-inflammatory mechanism of glycyrrhizin. Mino. Med. Rev. 27 188–193. [Google Scholar]
- Oliver-Bever B. (1983). Medicinal plants in tropical west africa III. Anti-infection therapy with higher plants. J. Ethnopharmacol. 9 1–83. 10.1016/0378-8741(83)90028-4 [DOI] [PubMed] [Google Scholar]
- Owoyele B. V., Olaleye S. B., Oke J. M., Elegbe R. A. (2001). Anti-Inflammatory and analgesic activities of leaf extracts of Landolphia owariensis. Afr. J. Biomed. Res. 4 131–133. [Google Scholar]
- Padmalatha K., Jayaram K., Raju N. L., Prasad M. N. V., Arora R. (2009). Ethnopharmacological and biotechnological significance of Vitex. Biorem. Biodiv. Bioavail. 3 6–14. 10.3329/bjsir.v43i3.1149 [DOI] [Google Scholar]
- Panunto W., Jaijoy K., Lerdvuthisopon N., Lertprasertsuke N., Jiruntanat N., Soonthornchareonnon N., et al. (2010). Acute and chronic toxicity studies of the water extract from dried fruits of Terminalia chebula Rezt. in rats. Int. J. Appl. Res. Nat. Prod. 3 36–43. [Google Scholar]
- Parés A., Planas R., Torres M., Caballería J., Viver J. M., Acero D., et al. (1998). Effects of silymarin in alcoholic patients with cirrhosis of the liver: results of a controlled, double-blind, randomized and multicenter trial. J. Hepatol. 28 615–621. 10.1016/S0168-8278(98)80285-7 [DOI] [PubMed] [Google Scholar]
- Pathak D., Pathak K., Singla A. K. (1991). Flavonoids as medicinal agents-recent advances. Fitoterapia 62 371–389. [Google Scholar]
- Perry L. M., Metzger J. (1980). Medicinal Plants of East and Southeast Asia, Attributed Properties and Uses. Cambridge, MA: MIT Press, 149–151. [Google Scholar]
- Pettit G. R., Cragg G. M., Gust D., Brown P., Schmidt J. M. (1982a). The structures of phyllanthostatin 1 and phyllanthoside from the Central American tree Phyllanthus acuminatus Vahl. Can. J. Chem. 60 939–941. [Google Scholar]
- Pettit G. R., Cragg G. M., Herald D. L., Schmidt J. M., Lohavanijaya P. (1982b). Isolation and structure of combretastatin. Can. J. Chem. 60 1374–1376. 10.1139/v82-202 [DOI] [Google Scholar]
- Piva R., Penolazzi L., Borgatti M., Lampronti I., Lambertini E., Torreggiani E., et al. (2009). Apoptosis of human primary osteoclasts treated with molecules targeting nuclear factor-κB. Ann. N. Y. Acad. Sci. 1171 448–456. 10.1111/j.1749-6632.2009.04906.x [DOI] [PubMed] [Google Scholar]
- Polya G. M., Wang B. H., Foo L. Y. (1995). Inhibition of signal-regulated protein kinases by plant-derived hydrolysable tannins. Phytochemistry 38 307–314. 10.1016/0031-9422(94)00547-7 [DOI] [PubMed] [Google Scholar]
- Pradhan S. C., Girish C. (2006). Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian J. Med. Res. 124 491–504. [PubMed] [Google Scholar]
- Prakash A., Satyan K. S., Wahi S. P., Singh R. P. (1995). Comparative hepatoprotective activity of three Phyllanthus species, P. urinaria, P. niruri and P. simplex, on carbon tetrachloride induced liver injury in the rat. Phytother. Res. 9 594–596. 10.1002/ptr.2650090813 [DOI] [Google Scholar]
- Pramyothin P., Udomuksorn W., Poungshompoo S., Chaichantipyuth C. (1994). Hepatoprotective effect of Andrographis paniculata and its constituent, andrographolide, on ethanol hepatotoxicity in rats. Asia Pac. J. Pharmacol. 9 73–78. [Google Scholar]
- Puri A., Saxena R., Saxena R. P., Saxena K. C., Srivastava V., Tandon J. S. (1993). Immunostimulant agents from Andrographis paniculata. J. Nat. Prod. 56 995–999. 10.1021/np50097a002 [DOI] [PubMed] [Google Scholar]
- Qian-Cutrone J., Huang S., Trimble J., Li H., Lin P. F., Alam M., et al. (1996). Niruriside, a new HIV REV/RRE binding inhibitor from Phyllanthus niruri. J. Nat. Prod. 59 196–199. 10.1021/np9600560 [DOI] [PubMed] [Google Scholar]
- Rajurkar N. S., Pardeshi B. M. (1997). Analysis of some herbal plants from India used in the control of diabetes mellitus by NAA and AAS techniques. Appl. Radiat. Isot. 48 1059–1062. 10.1016/S0969-8043(97)00103-6 [DOI] [PubMed] [Google Scholar]
- Ramellini G., Meldolesi J. (1974). Stabilization of isolated rat liver plasma membranes by treatment in vitro with silymarin. Arzneimittelforschung 24 806–808. [PubMed] [Google Scholar]
- Raphael T. J., Kuttan G. (2003). Effect of naturally occurring triterpenoids glycyrrhizic acid, ursolic acid, oleanolic acid and nomilin on the immune system. Phytomedicine 10 483–489. 10.1078/094471103322331421 [DOI] [PubMed] [Google Scholar]
- Row L. R., Srinivasulu C., Smith M., Rao G. S. R. S. (1966). Crystalline constituents of euphorbiaceae-V. Tetrahedron 22 2899–2908. 10.1016/S0040-4020(01)99083-0 [DOI] [Google Scholar]
- Rush G. F., Gorski J. R., Ripple M. G., Sowinski J., Bugelski P., Hewitt W. R. (1985). Organic hydroperoxide-induced lipid peroxidation and cell death in isolated hepatocytes. Toxicol. Appl. Pharmacol. 78 473–483. 10.1016/0041-008X(85)90255-8 [DOI] [PubMed] [Google Scholar]
- Sabouri Ghannad M., Mohammadi A., Safiallahy S., Faradmal J., Azizi M., Ahmadvand Z. (2014). The effect of aqueous extract of Glycyrrhiza glabra on herpes simplex virus 1. Jundishapur J. Microbiol. 7:e11616 10.5812/jjm.11616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmi H., Sarna S. (1982). Effect of silymarin on chemical, functional, and morphological alterations of the liver: a double-blind controlled study. Scand. J. Gastroenterol. 17 517–521. 10.3109/00365528209182242 [DOI] [PubMed] [Google Scholar]
- Sanchez de Medina F., Vera B., Galvez J., Zarzuelo A. (2002). Effect of quercitrin on the early stages of hapten induced colonic inflammation in the rat. Life Sci. 70 3097–3108. 10.1016/S0024-3205(02)01568-0 [DOI] [PubMed] [Google Scholar]
- Santos A. R., Filho V. C., Niero R., Viana A. M., Moreno F. N., Campos M. M., et al. (1994). Analgesic effects of callus culture extracts from selected species of Phyllanthus in mice. J. Pharm. Pharmacol. 46 755–759. 10.1111/j.2042-7158.1994.tb03897.x [DOI] [PubMed] [Google Scholar]
- Saraswat B., Visen P. K. S., Patnaik G. K., Dhawan B. N. (1995). Effect of andrographolide against galactosamine-induced hepatotoxicity. Fitoterapia 66 415–420. [Google Scholar]
- Sarkar R., Hazra B., Mandal N. (2012). Reducing power and iron chelating property of Terminalia chebula (Retz.) alleviates iron induced liver toxicity in mice. BMC Complement. Altern. Med. 12:144 10.1186/1472-6882-12-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satomi Y., Nishino H., Shibata S. (2005). Glycyrrhetinic acid and related compounds induce G1 arrest and apoptosis in human hepatocellular carcinoma HepG2. Anticancer Res. 25 4043–4047. [PubMed] [Google Scholar]
- Schuppan D., Jia J., Brinkhaus B., Hahn E. G. (1999). Herbal products for liver diseases: a therapeutic challenge for the new millennium. Hepatology 30 1099–1104. 10.1002/hep.510300437 [DOI] [PubMed] [Google Scholar]
- Sharma A., Rathore H. S. (2010). Prevention of acetaminophen induced hepatorenal toxicity in mice with fruits of Terminalia chebula (Myrobalan). Thai. J. Toxicol. 25 144–153. [Google Scholar]
- Sharma A., Singh R. T., Sehgal V., Handa S. S. (1991). Antihepatotoxic activity of some plants used in herbal formulations. Fitoterapia 62 131–138. 10.1016/j.fitote.2015.01.001 [DOI] [Google Scholar]
- Shebl R. I., Amin M. A., Emad-Eldin A., Bin Dajem S. M., Mostafa A. S., Ibrahim E. H., et al. (2012). Antiviral activity of liquorice powder extract against varicella zoster virus isolated from Egyptian patients. Chang Gung Med. J. 35 231–239. [DOI] [PubMed] [Google Scholar]
- Shen Y.-C., Chen C.-F., Chiou W.-F. (2002). Andrographolide prevents oxygen radical production by human neutrophils: possible mechanism(s) involved in its anti-inflammatory effect. Br. J. Pharmacol. 135 399–406. 10.1038/sj.bjp.0704493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S. (2000). A drug over the millennia: pharmacognosy, chemistry, and pharmacology of licorice. J. Pharm. Soc. Jpn. 120 849–862. 10.1248/yakushi1947.120.10_849 [DOI] [PubMed] [Google Scholar]
- Shiki Y., Shirai K., Saito Y., Yoshida S. H. O., Mori Y., Wakashin M. (1992). Effect of glycyrrhizin on lysis of hepatocyte membranes induced by anti-liver cell membrane antibody. J. Gastroenterol. Hepatol. 7 12–16. 10.1111/j.1440-1746.1992.tb00927.x [DOI] [PubMed] [Google Scholar]
- Shimizu M., Horie S., Terashima S., Ueno H., Hayashi T., Arisawa M., et al. (1989). Studies on aldose reductase inhibitors from natural products. II. Active components of a Paraguayan crude drug “Para-parai mi,” Phyllanthus niruri. Chem. Pharm. Bull. 37 2531–2532. 10.1248/cpb.37.2531 [DOI] [PubMed] [Google Scholar]
- Shisheva A., Shechter Y. (1992). Quercetin selectively inhibits insulin receptor function in vitro and the bioresponses of insulin and insulinomimetic agents in rat adipocytes. Biochemistry 31 8059–8063. [DOI] [PubMed] [Google Scholar]
- Šimanek V., Kren V., Ulrichová J., Vicar J., Cvak L. (2000). Silymarin: what is in the name? An appeal for a change of editorial policy. Hepatology 32 442–444. 10.1021/bi00149a041 [DOI] [PubMed] [Google Scholar]
- Singh R. P., Banerjee S., Rao A. R. (2001). Modulatory influence of Andrographis paniculata on mouse hepatic and extrahepatic carcinogen metabolizing enzymes and antioxidant status. Phytother. Res. 15 382–390. 10.1053/jhep.2000.9770 [DOI] [PubMed] [Google Scholar]
- Srividya N., Periwal S. (1995). Diuretic, hypotensive and hypoglycaemic effect of Phyllanthus amarus. Indian J. Exp. Biol. 33 861–864. 10.1002/ptr.730 [DOI] [PubMed] [Google Scholar]
- Suolinna E. M., Lang D. R., Racker E. (1974). Quercetin, an artificial regulator of the high aerobic glycolysis of tumor cells. J. Natl. Cancer Inst. 53 1515–1519. [DOI] [PubMed] [Google Scholar]
- Suzuki H. (1983). Effects of glycyrrhizin on biochemical tests in patients with chronic hepatitis. Double Blind Trial Asian Med. J. 26 423–438. 10.1093/jnci/53.5.1515 [DOI] [Google Scholar]
- Suzuki H., Ohta Y., Takino T., Fujisawa K., Hirayama C., Shimizu N., et al. (1977). The therapeutic effects of stronger neo minophagen C for chronic hepatitis. Igaku No Ayumi 102 562–568. [Google Scholar]
- Syamasundar K. V., Singh B., Thakur R. S., Husain A., Kiso Y., Hikino H. (1985). Antihepatotoxic principles of Phyllanthus niruri herbs. J. Ethnopharmacol. 14 41–44. [DOI] [PubMed] [Google Scholar]
- Takahara T., Watanabe A., Shiraki K. (1994). Effects of glycyrrhizin on hepatitis B surface antigen: a biochemical and morphological study. J. Hepatol. 21 601–609. 10.1016/S0168-8278(94)80108-8 [DOI] [PubMed] [Google Scholar]
- Tanaka N., Yamamura Y., Santa T., Kotaki H., Uchino K., Sawada Y., et al. (1993). Pharmacokinetic profiles of glycyrrhizin in patients with chronic hepatitis. Biopharm. Drug Dispos. 14 609–614. 10.1016/S0168-8278(94)80108-8 [DOI] [PubMed] [Google Scholar]
- Tănăsescu C., Petrea S., Băldescu R., Macarie E., Chiriloiu C., Purice S. (1987). Use of the Romanian product Silimarina in the treatment of chronic liver diseases. Med. Interne 26 311–322. 10.1002/bdd.2510140707 [DOI] [PubMed] [Google Scholar]
- Tandon A., Tandon B. N., Bhujwala R. A. (2001). Treatment of subacute hepatitis with lamivudine and intravenous glycyrrhizin: a pilot study. Hepatol. Res. 20 1–8. 10.1016/S1386-6346(00)00123-6 [DOI] [PubMed] [Google Scholar]
- Tandon A., Tandon B. N., Bhujwala R. A. (2002). Clinical spectrum of acute sporadic hepatitis E and possible benefit of glycyrrhizin therapy. Hepatol. Res. 23 55–61. 10.1016/S1386-6346(01)00155-3 [DOI] [PubMed] [Google Scholar]
- Tasaduq S. A., Singh K., Sethi S., Sharma S. C., Bedi K. L., Singh J., et al. (2003). Hepatocurative and antioxidant profile of HP-1, a polyherbal phytomedicine. Hum. Exp. Toxicol. 22 639–645. 10.1191/0960327103ht406oa [DOI] [PubMed] [Google Scholar]
- Tasduq S. A., Singh K., Satti N. K., Gupta D. K., Suri K. A., Johri R. K. (2006). Terminalia chebula (fruit) prevents liver toxicity caused by sub-chronic administration of rifampicin, isoniazid and pyrazinamide in combination. Hum. Exp. Toxicol. 25 111–118. 10.1191/0960327106ht601oa [DOI] [PubMed] [Google Scholar]
- Thai Pharmacopoeia Committee (1995). Thai herbal pharmacopoeia. Depart. Med. Sci. 1 51–56. [Google Scholar]
- Thyagarajan S. P., Thirunalasundari T., Subramanian S., Venkateswaran P. S., Blumberg B. S. (1988). Effect of phyllanthus amarus on chronic carriers of hepatitis b virus. Lancet 332 764–766. 10.1016/S0140-6736(88)92416-6 [DOI] [PubMed] [Google Scholar]
- Trivedi N., Rawal U. M. (2000). Hepatoprotective and toxicological evaluation of Andrographis paniculata on severe liver damage. Ind. J. Pharmacol. 32 288–293. [Google Scholar]
- Uesato S., Kitagawa Y., Kamishimoto M., Kumagai A., Hori H., Nagasawa H. (2001). Inhibition of green tea catechins against the growth of cancerous human colon and hepatic epithelial cells. Cancer Lett. 170 41–44. 10.1016/S0304-3835(01)00571-7 [DOI] [PubMed] [Google Scholar]
- Ullah M., Khan M. U., Mahmood A., Malik R. N., Hussain M., Wazir S. M., et al. (2013). An ethnobotanical survey of indigenous medicinal plants in Wana district south Waziristan agency. Pak. J. Ethnopharmacol. 150 918–924. 10.1016/j.jep.2013.09.032 [DOI] [PubMed] [Google Scholar]
- Ulmann A., Menard J., Corvol P. (1975). Binding of glycyrrhetinic acid to kidney mineralocorticoid and glucocorticoid receptors. Endocrinology 97 46–51. 10.1210/endo-97-1-46 [DOI] [PubMed] [Google Scholar]
- Umarani D., Devaki T., Govindaraju P., Shanmugasundaram K. R. (1985). Ethanol induced metabolic alterations and the effect of Phyllanthus niruri in their reversal. Anc. Sci. Life 4 174–180. [PMC free article] [PubMed] [Google Scholar]
- Unander D. W., Blumberg B. S. (1991). In vitro activity of Phyllanthus (Euphorbiaceae) species against the DNA polymerase of hepatitis viruses: effects of growing environment and inter- and intra-specific differences. Econ. Bot. 45 225–242. 10.1007/BF02862050 [DOI] [Google Scholar]
- Unander D. W., Webster G. L., Blumberg B. S. (1990). Records of usage or assays in Phyllanthus (Euphorbiaceae) I. Subgenera Isocladus, Kirganelia, Cicca and Emblica. J. Ethnopharmacol. 30 233–264. 10.1016/0378-8741(90)90105-3 [DOI] [PubMed] [Google Scholar]
- Unander D. W., Webster G. L., Blumberg B. S. (1991). Uses and bioassays in Phyllanthus (Euphorbiaceae): a compilation. II. The subgenus Phyllanthus. J. Ethnopharmacol. 34 97–133. 10.1016/0378-8741(91)90029-D [DOI] [PubMed] [Google Scholar]
- Unander D. W., Webster G. L., Blumberg B. S. (1992). Usage and bioassays in Phyllanthus (Euphorbiaceae): a compilation III. The subgenera Eriococcus, Conami, Gomphidium, Botryanthus, Xylophylla and Phyllanthodendron, and a complete list of the species cited in the three-part series. J. Ethnopharmacol. 36 103–112. 10.1016/0378-8741(92)90009-G [DOI] [PubMed] [Google Scholar]
- Unander D. W., Webster G. L., Blumberg B. S. (1995). Usage and bioassays in Phyllanthus (Euphorbiaceae). IV. Clustering of antiviral uses and other effects. J. Ethnopharmacol. 45 1–18. 10.1016/0378-8741(94)01189-7 [DOI] [PubMed] [Google Scholar]
- Utsumi K. (1984). Action of glycyrrhizin on biomembrane [in Japanese]. Proc. Symp. Liver Glycyrrhizin 104. [Google Scholar]
- van Rossum T. G., Vulto A. G., Hop W. C., Brouwer J. T., Niesters H. G., Schalm S. W. (1999). Intravenous glycyrrhizin for the treatment of chronic hepatitis C: a double-blind, randomized, placebo-controlled phase I/II trial. J. Gastroenterol. Hepatol. 14 1093–1099. 10.1046/j.1440-1746.1999.02008.x [DOI] [PubMed] [Google Scholar]
- van Rossum T. G., Vulto A. G., Hop W. C., Schalm S. W. (2001). Glycyrrhizin-induced reduction of ALT in European patients with chronic hepatitis C. Am. J. Gastroenterol. 96 2432–2437. 10.1111/j.1572-0241.2001.04049.x [DOI] [PubMed] [Google Scholar]
- Varshney I. P., Jain D. C., Srivastava H. C. (1983). Study of saponins from Glycyrrhiza glabra root. Int. J. Crude Drug Res. 21 169–172. 10.3109/13880208309070637 [DOI] [Google Scholar]
- Veldt B. J., Hansen B. E., Ikeda K., Verhey E., Suzuki H., Schalm S. W. (2006). Long-term clinical outcome and effect of glycyrrhizin in 1093 chronic hepatitis C patients with non-response or relapse to interferon. Scand. J. Gastroenterol. 41 1087–1094. 10.1080/00365520600641365 [DOI] [PubMed] [Google Scholar]
- Venkateswaran P. S., Millman I., Blumberg B. S. (1987). Effects of an extract from Phyllanthus niruri on hepatitis B and woodchuck hepatitis viruses: in vitro and in vivo studies. Proc. Natl. Acad. Sci. U.S.A. 84 274–278. 10.1073/pnas.84.1.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visen P. K. S., Shukia B., Patnaik G. K., Dhawan B. N. (1993). Andrographolide protects rat hepatocytes against paracetamol-induced damage. J. Ethnopharmacol. 40 131–136. 10.1016/0378-8741(93)90058-D [DOI] [PubMed] [Google Scholar]
- Wagner H., Diesel P., Seitz M. (1974). The chemistry and analysis of silymarin from Silybum marianum Gaertn. Arzneimittelforschung 24 466–471. [PubMed] [Google Scholar]
- Wakamatsu T., Nakahashi Y., Hachimine D., Seki T., Okazaki K. (2007). The combination of glycyrrhizin and lamivudine can reverse the cisplatin resistance in hepatocellular carcinoma cells through inhibition of multidrug resistance-associated proteins. Int. J. Oncol. 31 1465–1472. 10.3892/ijo.31.6.1465 [DOI] [PubMed] [Google Scholar]
- Wang B. E. (2000). Treatment of chronic liver diseases with traditional Chinese medicine. J. Gastroenterol. Hepatol. 15(Suppl.), E67–E70. 10.1046/j.1440-1746.2000.02100.x [DOI] [PubMed] [Google Scholar]
- Wang L., Yang R., Yuan B., Liu Y., Liu C. (2015). The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb. Acta Pharm. Sin. B 5 310–315. 10.1016/j.apsb.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Cheng H., Li Y., Meng L., Zhao G., Mai K. (1995). Herbs of the genus Phyllanthus in the treatment of chronic hepatitis B: observations with three preparations from different geographic sites. J. Lab. Clin. Med. 126 350–352. [PubMed] [Google Scholar]
- Wang Y., Kong L., Chen Y. (2005). Behavioural and biochemical effects of fractions prepared from Banxia houpu decoction in depression models in mice. Phytother. Res. 19 526–529. 10.1002/ptr.1697 [DOI] [PubMed] [Google Scholar]
- Wang Z., Okamoto M., Kurosaki Y., Nakayama T., Kimura T. (1996). Pharmacokinetics of glycyrrhizin in rats with D-galactosamine-induced hepatic disease. Biol. Pharm. Bull. 19 901–904. 10.1248/bpb.19.901 [DOI] [PubMed] [Google Scholar]
- Watari N. (1973). An electronmicroscopic study of the effects of glycyrrhizin on experimentally injured liver. Mini Med. Rev. 11 12–17. [Google Scholar]
- Wellington K., Jarvis B. (2001). Silymarin: a review of its clinical properties in the management of hepatic disorders. BioDrugs 15 465–489. 10.2165/00063030-200115070-00005 [DOI] [PubMed] [Google Scholar]
- WHO (1999). WHO Monographs on Selected Medicinal Plants, Vol. 2 Geneva: World Health Organization. [Google Scholar]
- Williamson E. M. (2003). “Liquorice,” in Potter’s Cyclopedia of Herbal Medicines. ed. Daniels C. W. (Saffron Walden: C. W. Daniels; ), 269–271. [Google Scholar]
- Wong W., Wu P., Yuen M., Cheng C., Yew W., Wong P., et al. (2000). Antituberculosis drug-related liver dysfunction in chronic hepatitis B infection. Hepatology 31 201–206. 10.1002/hep.510310129 [DOI] [PubMed] [Google Scholar]
- Xu H. (1986). Oriental materia medica: a concise guide. New Canaan, CT: Keats Publishing. [Google Scholar]
- Yamamoto S., Maekawa Y., Imamura M., Hisajima T. (1958). Treatment of hepatitis with the antiallergic drug, Stronger Neo-Minophagen C. Clin. Med. Pediatr. 13:73. [Google Scholar]
- Yamamura Y., Tanaka N., Santa T., Kotaki H., Aikawa T., Uchino K., et al. (1995). The relationship between pharmacokinetic behaviour of glycyrrhizin and hepatic function in patients with acute hepatitis and liver cirrhosis. Biopharm. Drug Dispos. 16 13–21. 10.1002/bdd.2510160103 [DOI] [PubMed] [Google Scholar]
- Yang R., Yuan B.-C., Ma Y.-S., Zhou S., Liu Y. (2016). The anti-inflammatory activity of licorice, a widely used Chinese Herb. Pharm. Biol. 55 5–18. 10.1080/13880209.2016.1225775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnell E. (1997). Botanical medicine for cystitis. J. Altern. Complement. Med. 3 269–275. 10.1089/act.1997.3.269 [DOI] [Google Scholar]
- Yeasmin T., Akhter Q. S., Tasnim M., Jahan S. (2016). Hepatoprotective effect of Terminalia chebula (Haritaki) on serum bilirubin in paracetamol induced liver damage in wister albino rats. Dinajpur. Med. Col. J. 9 78–83. [Google Scholar]
- Yeh S. F., Hong C. Y., Huang Y. L., Liu T. Y., Choo K. B., Chou C. K. (1993). Effect of an extract from Phyllanthus amarus on hepatitis B surface antigen gene expression in human hepatoma cells. Antiviral Res. 20 185–192. 10.1016/0166-3542(93)90019-F [DOI] [PubMed] [Google Scholar]
- Yoshikawa M., Matsui Y., Kawamoto H., Umemoto N., Oku K., Koizumi M., et al. (1997). Effects of glycyrrhizin on immune-mediated cytotoxicity. J. Gastroenterol. Hepatol. 12 243–248. 10.1111/j.1440-1746.1997.tb00416.x [DOI] [PubMed] [Google Scholar]
- Yu J.-Y., Ha J. Y., Kim K.-M., Jung Y.-S., Jung J.-C., Oh S. (2015). Anti-Inflammatory activities of licorice extract and its active compounds, glycyrrhizic acid, liquiritin and liquiritigenin, in BV2 cells and mice liver. Molecules 20 13041–13054. 10.3390/molecules200713041 [DOI] [PMC free article] [PubMed] [Google Scholar]