Abstract
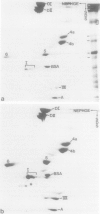
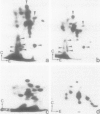
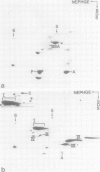
Isolated desmosomes from bovine epidermis contain two major polypeptides of mol. wts. 75 000 (D6) and 83 000 (D5) which, like the desmoplakins of mol. wt. greater than 200 000, are associated with the insoluble desmosomal plaque structure. We have characterized these two polypeptides and examined their significance by peptide map comparisons and translation of bovine epidermal mRNA in vitro. Polypeptide D5 is different from polypeptide D6 by its apparent mol. wt., its isoelectric pH (approximately 6.35, whereas D6 is a basic polypeptide isoelectric at pH approximately 8.5) and its peptide map. By all these criteria desmosomal polypeptides D5 and D6 are also different from cytokeratins, desmoplakins and the glycosylated desmosomal proteins. Both polypeptides are synthesized from different mRNAs separable by gel electrophoresis on agarose: mRNA coding for polypeptide D5 is approximately 3500 nucleotides long, that for D6 is significantly shorter (estimated to 3050 nucleotides), and both contain relatively large proportions of non-coding sequences. The translational products of these mRNAs co-migrate, on two-dimensional gel electrophoresis, with the specific polypeptides from bovine epidermis, indicating that they are genuine polypeptides and are not the result of considerable post-translational processing or modification of precursor molecules. The cell and tissue distribution of these two cytoskeletal proteins and possible functions are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Cohen S. M., Gorbsky G., Steinberg M. S. Immunochemical characterization of related families of glycoproteins in desmosomes. J Biol Chem. 1983 Feb 25;258(4):2621–2627. [PubMed] [Google Scholar]
- Colaco C. A., Evans W. H. A biochemical dissection of the cardiac intercalated disk: isolation of subcellular fractions containing fascia adherentes and gap junctions. J Cell Sci. 1981 Dec;52:313–325. doi: 10.1242/jcs.52.1.313. [DOI] [PubMed] [Google Scholar]
- Cowin P., Garrod D. R. Antibodies to epithelial desmosomes show wide tissue and species cross-reactivity. Nature. 1983 Mar 10;302(5904):148–150. doi: 10.1038/302148a0. [DOI] [PubMed] [Google Scholar]
- Dodemont H. J., Soriano P., Quax W. J., Ramaekers F., Lenstra J. A., Groenen M. A., Bernardi G., Bloemendal H. The genes coding for the cytoskeletal proteins actin and vimentin in warm-blooded vertebrates. EMBO J. 1982;1(2):167–171. doi: 10.1002/j.1460-2075.1982.tb01142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drochmans P., Freudenstein C., Wanson J. C., Laurent L., Keenan T. W., Stadler J., Leloup R., Franke W. W. Structure and biochemical composition of desmosomes and tonofilaments isolated from calf muzzle epidermis. J Cell Biol. 1978 Nov;79(2 Pt 1):427–443. doi: 10.1083/jcb.79.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Pickett R. A., 2nd, Hampton J., Lerner R. A. Radioiodination of proteins in single polyacrylamide gel slices. Tryptic peptide analysis of all the major members of complex multicomponent systems using microgram quantities of total protein. J Biol Chem. 1977 Sep 25;252(18):6510–6515. [PubMed] [Google Scholar]
- Evans W. H., Gurd J. W. Preparation and properties of nexuses and lipid-enriched vesicles from mouse liver plasma membranes. Biochem J. 1972 Jul;128(3):691–700. doi: 10.1042/bj1280691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Moll R., Mueller H., Schmid E., Kuhn C., Krepler R., Artlieb U., Denk H. Immunocytochemical identification of epithelium-derived human tumors with antibodies to desmosomal plaque proteins. Proc Natl Acad Sci U S A. 1983 Jan;80(2):543–547. doi: 10.1073/pnas.80.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Moll R., Schiller D. L., Schmid E., Kartenbeck J., Mueller H. Desmoplakins of epithelial and myocardial desmosomes are immunologically and biochemically related. Differentiation. 1982;23(2):115–127. doi: 10.1111/j.1432-0436.1982.tb01274.x. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Scheer U., Krohne G., Jarasch E. D. The nuclear envelope and the architecture of the nuclear periphery. J Cell Biol. 1981 Dec;91(3 Pt 2):39s–50s. doi: 10.1083/jcb.91.3.39s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Schiller D. L., Moll R., Winter S., Schmid E., Engelbrecht I., Denk H., Krepler R., Platzer B. Diversity of cytokeratins. Differentiation specific expression of cytokeratin polypeptides in epithelial cells and tissues. J Mol Biol. 1981 Dec 25;153(4):933–959. doi: 10.1016/0022-2836(81)90460-5. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Schiller D. L., Winter S., Jarasch E. D., Moll R., Denk H., Jackson B. W., Illmensee K. Differentiation-related patterns of expression of proteins of intermediate-size filaments in tissues and cultured cells. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):431–453. doi: 10.1101/sqb.1982.046.01.041. [DOI] [PubMed] [Google Scholar]
- Freudenstein C., Keenan T. W., Eigel W. N., Sasaki M., Stadler J., Franke W. W. Preparation and characterization of the inner coat material associated with fat globule membranes from bovine and human milk. Exp Cell Res. 1979 Feb;118(2):277–294. doi: 10.1016/0014-4827(79)90153-8. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Green H. Multiple keratins of cultured human epidermal cells are translated from different mRNA molecules. Cell. 1979 Jul;17(3):573–582. doi: 10.1016/0092-8674(79)90265-4. [DOI] [PubMed] [Google Scholar]
- Geisler N., Weber K. Comparison of the proteins of two immunologically distinct intermediate-sized filaments by amino acid sequence analysis: desmin and vimentin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4120–4123. doi: 10.1073/pnas.78.7.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerace L., Blobel G. Nuclear lamina and the structural organization of the nuclear envelope. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):967–978. doi: 10.1101/sqb.1982.046.01.090. [DOI] [PubMed] [Google Scholar]
- Gerace L., Blobel G. The nuclear envelope lamina is reversibly depolymerized during mitosis. Cell. 1980 Jan;19(1):277–287. doi: 10.1016/0092-8674(80)90409-2. [DOI] [PubMed] [Google Scholar]
- Goodenough D. A., Stoeckenius W. The isolation of mouse hepatocyte gap junctions. Preliminary chemical characterization and x-ray diffraction. J Cell Biol. 1972 Sep;54(3):646–656. doi: 10.1083/jcb.54.3.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbsky G., Steinberg M. S. Isolation of the intercellular glycoproteins of desmosomes. J Cell Biol. 1981 Jul;90(1):243–248. doi: 10.1083/jcb.90.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanukoglu I., Fuchs E. The cDNA sequence of a human epidermal keratin: divergence of sequence but conservation of structure among intermediate filament proteins. Cell. 1982 Nov;31(1):243–252. doi: 10.1016/0092-8674(82)90424-x. [DOI] [PubMed] [Google Scholar]
- Hertzberg E. L., Gilula N. B. Liver gap junctions and lens fiber junctions: comparative analysis and calmodulin interaction. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):639–645. doi: 10.1101/sqb.1982.046.01.060. [DOI] [PubMed] [Google Scholar]
- Hunter T., Garrels J. I. Characterization of the mRNAs for alpha-, beta- and gamma-actin. Cell. 1977 Nov;12(3):767–781. doi: 10.1016/0092-8674(77)90276-8. [DOI] [PubMed] [Google Scholar]
- Jarasch E. D., Grund C., Bruder G., Heid H. W., Keenan T. W., Franke W. W. Localization of xanthine oxidase in mammary-gland epithelium and capillary endothelium. Cell. 1981 Jul;25(1):67–82. doi: 10.1016/0092-8674(81)90232-4. [DOI] [PubMed] [Google Scholar]
- Kartenbeck J., Franke W. W., Moser J. G., Stoffels U. Specific attachment of desmin filaments to desmosomal plaques in cardiac myocytes. EMBO J. 1983;2(5):735–742. doi: 10.1002/j.1460-2075.1983.tb01493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katcoff D., Nudel U., Zevin-Sonkin D., Carmon Y., Shani M., Lehrach H., Frischauf A. M., Yaffe D. Construction of recombinant plasmids containing rat muscle actin and myosin light chain DNA sequences. Proc Natl Acad Sci U S A. 1980 Feb;77(2):960–964. doi: 10.1073/pnas.77.2.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis T. E., Geiger B., Schmid E., Jorcano J. L., Franke W. W. De novo synthesis and specific assembly of keratin filaments in nonepithelial cells after microinjection of mRNA for epidermal keratin. Cell. 1983 Apr;32(4):1125–1137. doi: 10.1016/0092-8674(83)90296-9. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments: a chemically heterogeneous, developmentally regulated class of proteins. Annu Rev Biochem. 1982;51:219–250. doi: 10.1146/annurev.bi.51.070182.001251. [DOI] [PubMed] [Google Scholar]
- Magin T. M., Jorcano J. L., Franke W. W. Translational products of mRNAs coding for non-epidermal cytokeratins. EMBO J. 1983;2(8):1387–1392. doi: 10.1002/j.1460-2075.1983.tb01596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minty A. J., Caravatti M., Robert B., Cohen A., Daubas P., Weydert A., Gros F., Buckingham M. E. Mouse actin messenger RNAs. Construction and characterization of a recombinant plasmid molecule containing a complementary DNA transcript of mouse alpha-actin mRNA. J Biol Chem. 1981 Jan 25;256(2):1008–1014. [PubMed] [Google Scholar]
- Osborn M., Geisler N., Shaw G., Sharp G., Weber K. Intermediate filaments. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):413–429. doi: 10.1101/sqb.1982.046.01.040. [DOI] [PubMed] [Google Scholar]
- Paul D. L., Goodenough D. A. In vitro synthesis and membrane insertion of bovine MP26, an integral protein from lens fiber plasma membrane. J Cell Biol. 1983 Mar;96(3):633–638. doi: 10.1083/jcb.96.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quax-Jeuken Y. E., Quax W. J., Bloemendal H. Primary and secondary structure of hamster vimentin predicted from the nucleotide sequence. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3548–3552. doi: 10.1073/pnas.80.12.3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel J. P., Nicholson B. J., Yancey S. B. Partial sequence and turnover of rat liver gap junction protein. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):633–637. doi: 10.1101/sqb.1982.046.01.059. [DOI] [PubMed] [Google Scholar]
- Roop D. R., Hawley-Nelson P., Cheng C. K., Yuspa S. H. Keratin gene expression in mouse epidermis and cultured epidermal cells. Proc Natl Acad Sci U S A. 1983 Feb;80(3):716–720. doi: 10.1073/pnas.80.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D. L., Franke W. W., Geiger B. A subfamily of relatively large and basic cytokeratin polypeptides as defined by peptide mapping is represented by one or several polypeptides in epithelial cells. EMBO J. 1982;1(6):761–769. doi: 10.1002/j.1460-2075.1982.tb01243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerrow C. J., Matoltsy A. G. Chemical characterization of isolated epidermal desmosomes. J Cell Biol. 1974 Nov;63(2 Pt 1):524–530. doi: 10.1083/jcb.63.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerrow C. J., Matoltsy A. G. Isolation of epidermal desmosomes. J Cell Biol. 1974 Nov;63(2 Pt 1):515–523. doi: 10.1083/jcb.63.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Rice R. H., Roop D. R., Trus B. L., Steven A. C. Complete amino acid sequence of a mouse epidermal keratin subunit and implications for the structure of intermediate filaments. Nature. 1983 Apr 28;302(5911):794–800. doi: 10.1038/302794a0. [DOI] [PubMed] [Google Scholar]
- Steinert P., Idler W., Aynardi-Whitman M., Zackroff R., Goldman R. D. Heterogeneity of intermediate filaments assembled in vitro. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):465–474. doi: 10.1101/sqb.1982.046.01.043. [DOI] [PubMed] [Google Scholar]
- Yamasaki H., Enomoto T., Martel N., Shiba Y., Kanno Y. Tumour promoter-mediated reversible inhibition of cell-cell communication (electrical coupling). Relationship with phorbol ester binding and de novo macromolecule synthesis. Exp Cell Res. 1983 Jul;146(2):297–308. doi: 10.1016/0014-4827(83)90132-5. [DOI] [PubMed] [Google Scholar]
- Zehner Z. E., Paterson B. M. Characterization of the chicken vimentin gene: single copy gene producing multiple mRNAs. Proc Natl Acad Sci U S A. 1983 Feb;80(4):911–915. doi: 10.1073/pnas.80.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]