Abstract
Eosinophilic esophagitis (EoE) is a clinicopathological diagnosis seen in children as well as adults. Growing evidence suggests that EoE is strongly associated with atopic disorders. Presenting symptoms differ in children and adults and it is not known whether atopic features vary by age. This study was designed to compare atopic features and allergic sensitization between children and adults with EoE. We conducted a retrospective analysis of demographic and clinical data from 50 children (aged 2–18 years) and 50 adults (aged 21–75 years) with a biopsy-proven diagnosis of EoE referred to our allergy clinic. Data regarding patient characteristics, history of atopic diseases, and allergy test results were collected for analysis. The majority of children and adults were white and male patients. When compared with adults, a higher percentage of children had a history of asthma (52% versus 24%; p < 0.05). There was no statistically significant difference between adults and children regarding history of allergic rhinitis, atopic dermatitis, immunoglobulin E–mediated food allergy, and family history of atopy. There was no statistically significant difference between children and adults regarding immediate-type sensitization to foods and aeroallergens. Compared with adults, a higher percentage of children showed a positive reaction to one or more foods on patch testing (62% versus 31%; p = 0.01). A high prevalence of comorbid atopic diseases and sensitizations to food and environmental allergens was seen in both children and adults. Children had a significantly higher rate of asthma and positive patch test to foods compared with adults.
Keywords: Adult, allergic rhinitis, asthma, atopic dermatitis, atopy, children, eosinophilic esophagitis, food allergy, patch test, prick test
Eosinophilic esophagitis (EoE) is a chronic inflammatory process and a clinicopathological disease characterized by esophageal dysfunction and eosinophilic infiltration.1–3 Clinical symptoms are variable and differ by age.1,4 EoE has become an increasingly recognized condition in both children and adults since 1990.5–8 It is estimated to affect up to 1 in 2500 individuals.9 Worldwide, the incidence rates range between 0.7 and 10 per 100,000 person years and the prevalence rates range from 0.2 to 55 per 100,000 persons.6,7,10 EoE occurs in most racial and ethnic groups, but through observational studies EoE appears to disproportionally affect non-Hispanic white subjects with an increased male-to-female ratio.1,7,11
Patients with EoE often have a concurrent or history of atopic diseases as well as a family history of atopy. Atopic diseases such as food allergy, atopic dermatitis, allergic rhinitis, allergic conjunctivitis, and asthma have been reported in the majority of patients with EoE (50–80%).2 Association with atopy has been reported in both children and adults.9,12,13
In addition to a clinical history of allergic diseases, antigen sensitizations based on skin-prick testing (SPT), serum antigen–specific immunoglobulin (IgE), and atopy patch testing (APT) have also been reported in EoE patients.2,14–16 In a study of 45 children with EoE, younger patients showed more IgE and APT to foods and older patients showed greater IgE sensitization to inhalant allergens.14 In children with EoE the overall prevalence of food and inhalant sensitization has been up to 80%.15 In a cohort of 50 adults with EoE, more patients had sensitizations to aeroallergens alone than foods alone.16 In both children and adults, sensitizations to food allergens are most commonly seen to milk, soybean, wheat, egg, and nuts.15–20 In adults, sensitizations were also commonly seen to tomato, carrots, and onions.21
Animal models have shown a potential role for aeroallergens eliciting EoE using dust mites, cockroaches, and Aspergillus.22,23 Rayapudi et al. found in 236 children, 38% had sensitizations to indoor allergens, particularly cockroach and dust mite.23 Gonsalves et al. studied 50 adults and 30% had isolated positive SPT to aeroallergens, most commonly to pollen and dust mite.16
The pathogenesis of EoE is still not entirely clear. A growing body of evidence suggests that the disease is most likely a combination of both IgE and non-IgE mechanisms.24,25 Cytokines such as interleukin (IL)-4, IL-5, and IL-13, as well as mast cells, play an important role in atopic conditions and the migration of eosinophils to the esophagus. Among the Th2 cytokines, IL-13 is thought to play a unique role because it can generate EoE in mice and blockade of IL-13 in mice has led to reduced esophageal inflammation.26,27 Several studies have shown a positive correlation between eotaxin 3, IL-5, and IL-13 messenger RNA (mRNA) expression in esophageal tissue and disease activity.28 Food antigen sensitization is thought to be involved in the pathogenesis based on improvement and/or resolution of symptoms and pathological findings with dietary therapies.29 It has been postulated that in EoE, a type IV (Th1 mediated) delayed allergic response also plays a role.30 Therefore, food patch testing may be helpful in identifying foods related to the delayed hypersensitivity response.31
Because clinical presentations vary in children and adults, we sought to investigate if atopic features are different. Through our data, we examine the presence of atopy and allergic sensitization in children and adults with EoE.
METHODS
Chart Review Methodology
The study was approved by the Institutional Review Board at Pennsylvania State University.
We performed a retrospective chart review of the electronic medical records from 50 children (aged 2–18 years) and 50 adults (aged 21–75 years) randomly selected from the 180 patients referred to our hospital-based allergy clinic at Hershey Medical Center between 2006 and 2012. Patients had a physician diagnosis of EoE based on their clinical presentations and endoscopic biopsy showing 15 or more eosinophils per high power field despite a trial of a proton pump inhibitor for at least 8 weeks to exclude gastroesophageal reflux disease. Medical records were reviewed for data on demographics; personal history of asthma, allergic rhinitis, IgE-mediated food allergy, and atopic dermatitis; family history of atopy; and allergy test results (SPT and serum-specific IgE levels to food and aeroallergens and APT for foods). SPT is our preferred method of measuring IgE sensitization because of its high sensitivity. Some patients had serum-specific IgE levels drawn before their initial visit to our clinic. We performed serum-specific IgE levels for patients on antihistamines at the time of the office visit as well as for parental preference. APT was performed on 71 patients. APT was not performed if patients or their parents refused.
Definitions
Serum in vitro assays of specific IgE > 0.35 kU/L (ImmunoCaP Phadia AB, Portage, MI) and SPT with a wheal size of 3 mm greater than the negative control were considered positive testing and an indication of sensitization to foods and/or aeroallergens. SPT was performed by puncturing the forearm or back with a bifurcated needle (ALK, Horsholm, Denmark) and introducing a purified commercial allergen preparation (Greer Laboratories, Lenoir, NC) to be absorbed through the skin. Reactions were recorded by measuring the wheal and flare at 20 minutes. APT was performed using Finn Chambers (Allerderm Laboratories, Inc., Petaluma, CA). APT results were interpreted at 72 hours. APT showing palpable erythema, papules, or pustules was considered positive testing and an indication of a delayed hypersensitivity. APT included the following panel of foods: rice, barley, wheat, oat, potato, soy, peanut, corn, apple, banana, peaches, carrot, green beans, sweet peas, beef, chicken, turkey, ham, egg, and milk. Each patient did not receive the same panel of food or environmental allergens for SPT or serum-specific IgE testing. Personal history of asthma, allergic rhinitis, atopic dermatitis, and IgE-mediated food allergy was considered positive if documented as such in the medical history section of the medical record. Family history of atopy was considered positive if there was a first-degree relative with a history of asthma, allergic rhinitis, atopic dermatitis, or food allergy documented in the medical record.
Statistical Analysis
All statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, NC). Categorical variables were summarized with proportions and continuous variables were summarized with means, medians, and standard deviations. A chi-square test was used to compare the group proportions, and an exact test was used as needed when cell counts were too small to meet the assumptions of the chi-square test. A value of p < 0.05 was considered significant.
RESULTS
Patient Characteristics
A total of 100 patients were included in this study. Participant characteristics are listed in Table 1. Of the 50 children, the majority were white (78%) and male (72%) subjects, with the mean age at diagnosis being 8 years (Table 1). Among the 50 adults, most of the patients (92%) were white with 60% male subjects. The mean age at diagnosis was 40 years (Table 1).
Table 1.
Characteristics of the study participants
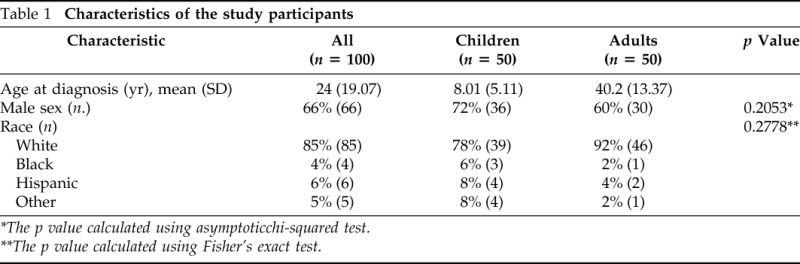
*The p value calculated using asymptoticchi-squared test.
**The p value calculated using Fisher's exact test.
History of Concurrent Atopy
Coexisting allergic diseases were common in both children and adults. Cumulatively, among all 100 patients, allergic rhinitis was concomitant in 65%, asthma in 38%, atopic dermatitis in 17%, IgE-mediated food allergy in 22%, and family history of atopy in 62%. When compared with adults, a higher percentage of children had a history of asthma (52% versus 24%; p < 0.05; Figure 1), which was statistically significant. There was no statistically significant difference between adults and children regarding history of allergic rhinitis, atopic dermatitis, IgE-mediated food allergy, and family history of atopy in general (Fig. 1).
Figure 1.
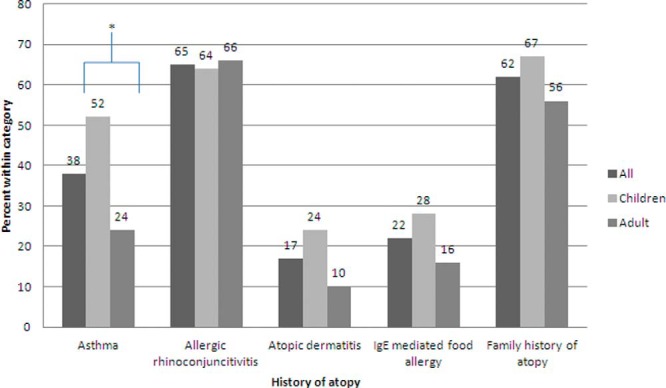
History of atopy in children and adults with eosinophilic esophagitis (EoE). Each bar represents the percentage of participants, all (n = 100), children (n = 46), and adults (n = 45), with a positive history for that particular atopic disease except for the family history group where data for 4 children and 5 adults is missing (*p < 0.05).
Immediate Hypersensitivity to Foods
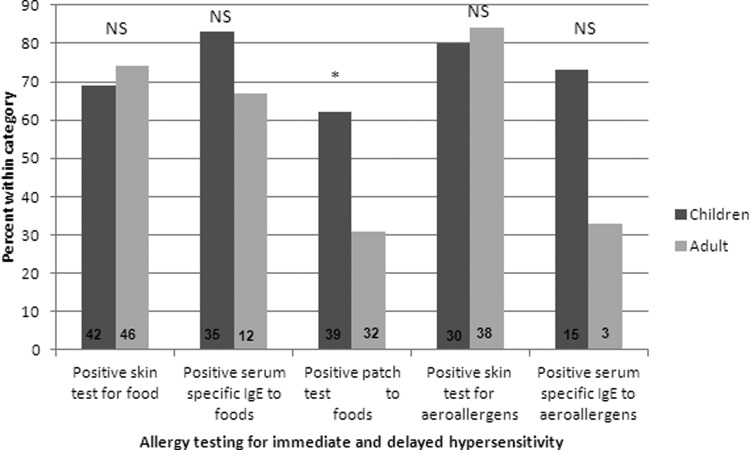
Of the 100 patients, 42 (84%) children and 46 (92%) adults had SPT performed to foods. Among children, 29 (69%) patients, and in adults, 34 (74%) patients showed sensitization to one or more foods (p = 0.61). In children, the three most common sensitizations were to peanut, tree nut, milk, and soy (Table 2). In adults, the three most common sensitizations were to tree nut, shellfish, soy, and fruits (Table 3).
Table 2.
Most common sensitizations found in immediate and delayed hypersensitivity testing for food and aeroallergens in children

Percentages are percentages of positive tests.
IgE = immunoglobulin E.
Table 3.
Most common sensitizations found in immediate and delayed hypersensitivity testing for food and aeroallergens in adults

Percentages are percentages of positive tests.
IgE = immunoglobulin E.
Thirty-five children and 12 adults had food-specific IgE levels measured. Twenty-nine (83%) children and eight (67%) adults had one or more food-specific IgE level of >0.35 kU/L (p = 0.41). In children, the three most common sensitizations were to milk, peanut, and egg (Table 2). In adults, the three most common sensitizations were to wheat, egg, and fruit (Table 3). Twenty-seven children and eight adults had both SPT and serum-specific IgE for food performed. Looking at both SPT and food-specific IgE levels there was no statistically significant difference between children and adults regarding immediate-type sensitization to foods.
Delayed Hypersensitivity to Foods
Of the 100 patients, 39 children and 32 adults had APT performed to foods. Children had a significantly higher number of positive reactions to APT (24 [62%] children versus 10 [31%] adults; p = 0.01; Fig. 2). In children, the most common foods were vegetables, meats, milk, and soy (Table 2). In adults, the most common foods were vegetables, potato, and milk (Table 3).
Figure 2.
Allergy testing in children and adults with eosinophilic esophagitis (EoE). Each bar represents the percentage of participants with positive testing for that category. Numbers within the bars represent the n of that group (*p < 0.05; NS = p > 0.05).
Immediate Hypersensitivity to Aeroallergens
Of the 100 patients, 30 children and 38 adults had SPT to aeroallergens. Twenty-four (80%) children and 32 (84%) adults showed sensitizations to one or more aeroallergen (p = 0.65). In children, the most common sensitizations were to trees, dust mite, cat, and mold (Table 2). In adults, the most common sensitizations were to dust mite, trees, and grass (Table 3).
Fifteen children and three adults had aeroallergen-specific IgE levels measured. Eleven (73%) children and one (33%) adult showed sensitizations to one or more aeroallergens (p = 0.51). In children, the most common sensitizations were to dog, cat, and dust mite (Table 2). The one adult patient showed sensitizations to cat, dog, and weed pollens (Table 3). A total of six patients had both SPT and serum-specific IgE for aeroallergens performed. When looking at both SPT and aeroallergen-specific IgE levels there was no statistically significant difference between children and adults regarding immediate-type sensitization to aeroallergens.
DISCUSSION
EoE is commonly associated with atopy. The disease has been increasingly diagnosed in both children and adults with variable clinical features. We follow a growing number of patients in both age groups and sought to investigate whether different atopic features present in the two age groups by examining atopic history and sensitization to aeroallergens and foods.
Regarding history of atopy, we found that, cumulatively, among children and adults, there was a large percentage of concomitant allergic disease. Coexisting allergic rhinitis (65%) was the most common and atopic dermatitis (17%) was the least common. When we separated children and adults, there was a higher percentage of children with a history of asthma, atopic dermatitis, and IgE-mediated food allergy. This difference was only statistically significant for asthma (Figure 1). It is possible that we saw a greater percentage of children with concomitant allergic disease because sensitizations to food and inhalant allergens start early in life and symptoms can improve with age. The higher percentage of children with a history of asthma may be related to overdiagnosis of asthma in this age group, because making a reliable diagnosis of asthma among preschoolers with recurrent wheeze remains difficult.32 It is well known that not all children who wheeze have asthma.33,34 It is also possible that we see a statistically significant difference for asthma, as opposed to other atopic disorders, because it has been proposed that an interrelation exists between eosinophilic digestive disease and allergic bronchial asthma in terms of similarity in pathogenesis.35
The majority of our patients had sensitizations to food allergens, aeroallergens, or both, as identified by SPT and/or ImmunoCap IgE assay. The most common foods found correlate with the existing literature. Previous studies have shown that in children and adults EoE may be driven by food antigens and we found similar rates of IgE sensitization to foods in both populations.4 IgE sensitization to aeroallergens was similar between children and adults and we found no statistically significant difference between the two groups. We had a very small number of adults that had an ImmunoCap IgE assay performed for aeroallergens, which may have affected our ability to detect a difference. We found mold and dust mites among the most common sensitizations, as previously reported.
Our study also compared the results of APT among children and adults, which can be a measure of non–IgE-mediated sensitization to foods. In a previous study with a smaller number of patients, we found that children tended to have higher rates of positive results to APT, although this did not reach statistical significance.36 In this study, we found that compared with adults, children had a statistically significant higher number of positive APT to foods. Molina-Infante et al. also reported a low rate of positive APT to foods in their cohort of 22 adults with EoE.37 Previous studies have shown both very low and very high positivity rates of APT in children with EoE.38–40 The variance in the previously reported literature suggests that age is not a sufficient explanation for the difference between children and adults.
One strength of this study is that the children and adults had a physician-documented diagnosis of EoE documented by esophageal biopsies after being treated for gastroesophageal reflux disease. Also, we included both children and adults in our analysis, whereas most studies focus on only one age group. Limitations of this study include the retrospective nature and the variation in testing for food and environmental allergen sensitization among our patients. The majority of children and adults received SPT and APT; however, a smaller number had serum-specific IgE measured for food and aeroallergens. SPT is our preferred method of measuring IgE sensitization because of its high sensitivity. The inconsistency in testing affected the size of the groups analyzed for sensitization, which may mask other significant atopic differences between children and adults. The majority of the patients being white, which reflects the patient population in the area, is in concordance with the current literature showing that EoE disproportionally affects non-Hispanic white subjects.1,7,11
In accordance with the literature, our findings illustrate a high prevalence of comorbid atopic diseases and sensitizations to food and environmental allergens in both children and adults. However, we found that children had a significantly higher rate of asthma and positive patch test to foods compared with adults. There was no significant difference between children and adults regarding rates of IgE sensitization to foods and aeroallergens.
ACKNOWLEDGMENTS
The authors thank Shenil Shah, M.D., for his contribution to the database creation and data collection.
Footnotes
Presented in a poster presentation at the meeting of the American Academy of Allergy Asthma and Immunology, February 25, 2013, San Antonio, Texas
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J Allergy Clin Immunol 128:3–20.e6, 2011. [DOI] [PubMed] [Google Scholar]
- 2. Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: A systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology 133:1342–1363, 2007. [DOI] [PubMed] [Google Scholar]
- 3. Greenhawt M, Aceves SS, Spergel JM, Rothenberg ME. The management of eosinophilic esophagitis. J Allergy Clin Immunol Pract 1:332–340, 2013. [DOI] [PubMed] [Google Scholar]
- 4. Straumann A, Aceves SS, Blanchard C, et al. Pediatric and adult eosinophilic esophagitis: Similarities and differences. Allergy 67:477–490, 2012. [DOI] [PubMed] [Google Scholar]
- 5. DeBrosse CW, Collins MH, Buckmeier Butz BK, et al. Identification, epidemiology, and chronicity of pediatric esophageal eosinophilia, 1982–1999. J Allergy Clin Immunol 126:112–119, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hruz P, Straumann A, Bussmann C, et al. Escalating incidence of eosinophilic esophagitis: A 20-year prospective, population-based study in Olten County, Switzerland. J Allergy Clin Immunol 128:1349–1350, 2011. [DOI] [PubMed] [Google Scholar]
- 7. Prasad GA, Alexander JA, Schleck CD, et al. Epidemiology of eosinophilic esophagitis over three decades in Olmsted County, Minnesota. Clin Gastroenterol Hepatol 7:1055–1061, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Straumann A, Simon HU. Eosinophilic esophagitis: Escalating epidemiology? J Allergy Clin Immunol 115:418–419, 2005. [DOI] [PubMed] [Google Scholar]
- 9. Straumann A. Clinical evaluation of the adult who has eosinophilic esophagitis. Immunol Allergy Clin North Am 29:11–18, vii, 2009. [DOI] [PubMed] [Google Scholar]
- 10. Soon IS, Butzner JD, Kaplan GG, Debruyn JC. Incidence and prevalence of eosinophilic esophagitis in children. J Pediatr Gastroenterol Nutr 57:72–80, 2013. [DOI] [PubMed] [Google Scholar]
- 11. Dalby K, Nielsen RG, Kruse-Andersen S, et al. Eosinophilic esophagitis in infants and children in the region of southern Denmark: A prospective study of prevalence and clinical presentation. J Pediatr Gastroenterol Nutr 51:280–282, 2010. [DOI] [PubMed] [Google Scholar]
- 12. Otteson TD, Mantle BA, Casselbrant ML, Goyal A. The otolaryngologic manifestations in children with eosinophilic esophagitis. Int J Pediatr Otorhinolaryngol 76:116–119, 2012. [DOI] [PubMed] [Google Scholar]
- 13. Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic esophagitis. N Engl J Med 351:940–941, 2004. [DOI] [PubMed] [Google Scholar]
- 14. Sugnanam KK, Collins JT, Smith PK, et al. Dichotomy of food and inhalant allergen sensitization in eosinophilic esophagitis. Allergy 62:1257–1260, 2007. [DOI] [PubMed] [Google Scholar]
- 15. Erwin EA, James HR, Gutekunst HM, et al. Serum IgE measurement and detection of food allergy in pediatric patients with eosinophilic esophagitis. Ann Allergy Asthma Immunol 104:496–502, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gonsalves N, Yang GY, Doerfler B, et al. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology 142:1451–1459.e1, 2012. [DOI] [PubMed] [Google Scholar]
- 17. Simon D, Marti H, Heer P, et al. Eosinophilic esophagitis is frequently associated with IgE-mediated allergic airway diseases. J Allergy Clin Immunol 115:1090–1092, 2005. [DOI] [PubMed] [Google Scholar]
- 18. Lucendo AJ, Arias Á, González-Cervera J, et al. Empiric 6-food elimination diet induced and maintained prolonged remission in patients with adult eosinophilic esophagitis: A prospective study on the food cause of the disease. J Allergy Clin Immunol 131:797–804, 2013. [DOI] [PubMed] [Google Scholar]
- 19. Spergel JM, Brown-Whitehorn TF, Cianferoni A, et al. Identification of causative foods in children with eosinophilic esophagitis treated with an elimination diet. J Allergy Clin Immunol 130:461–467.e5, 2012. [DOI] [PubMed] [Google Scholar]
- 20. Kagalwalla AF, Shah A, Li BU, et al. Identification of specific foods responsible for inflammation in children with eosinophilic esophagitis successfully treated with empiric elimination diet. J Pediatr Gastroenterol Nutr 53:145–149, 2011. [DOI] [PubMed] [Google Scholar]
- 21. Roy-Ghanta S, Larosa DF, Katzka DA. Atopic characteristics of adult patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 6:531–535, 2008. [DOI] [PubMed] [Google Scholar]
- 22. Mishra A, Hogan SP, Brandt EB, Rothenberg ME. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest 107:83–90, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rayapudi M, Mavi P, Zhu X, et al. Indoor insect allergens are potent inducers of experimental eosinophilic esophagitis in mice. J Leukoc Biol 88:337–346, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jyonouchi S, Smith CL, Saretta F, et al. Invariant natural killer T cells in children with eosinophilic esophagitis. Clin Exp Allergy 44:58–68, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mishra A. Mechanism of eosinophilic esophagitis. Immunol Allergy Clin North Am 29:29–40, viii, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mishra A, Rothenberg ME. Intratracheal IL-13 induces eosinophilic esophagitis by an IL-5, eotaxin-1, and STAT6-dependent mechanism. Gastroenterology 125:1419–1427, 2003. [DOI] [PubMed] [Google Scholar]
- 27. Blanchard C, Mishra A, Saito-Akei H, et al. Inhibition of human interleukin-13-induced respiratory and esophageal inflammation by anti-human-interleukin-13 antibody (CAT-354). Clin Exp Allergy 35:1096–1103, 2005. [DOI] [PubMed] [Google Scholar]
- 28. Bhardwaj N, Ghaffari G. Biomarkers for eosinophilic esophagitis: A review. Ann Allergy Asthma Immunol 109:155–159, 2012. [DOI] [PubMed] [Google Scholar]
- 29. Reddy V, Ghaffari G. Eosinophilic esophagitis: Review of nonsurgical treatment modalities. Allergy Asthma Proc 34:421–426, 2013. [DOI] [PubMed] [Google Scholar]
- 30. Markowitz JE, Liacouras CA. Eosinophilic esophagitis. Gastroenterol Clin North Am 32:949–966, 2003. [DOI] [PubMed] [Google Scholar]
- 31. Blatman KH, Ditto AM. Chapter 26: Eosinophilic esophagitis. Allergy Asthma Proc 33(suppl 1):S88–S90, 2012. [DOI] [PubMed] [Google Scholar]
- 32. Fouzas S, Brand PL. Predicting persistence of asthma in preschool wheezers: Crystal balls or muddy waters? Paediatr Respir Rev 14:46–52, 2013. [DOI] [PubMed] [Google Scholar]
- 33. Martinez FD, Wright AL, Taussig LM, et al. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J med 332:133–138, 1995. [DOI] [PubMed] [Google Scholar]
- 34. Morgan WJ, Stern DA, Sherrill DL, et al. Outcome of asthma and wheezing in the first 6 years of life: Follow-up through adolescence. Am J Respir Crit Care Med 172:1253–1258, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yakoot M. Eosinophilic digestive disease (EDD) and allergic bronchial asthma; two diseases or expression of one disease in two systems? Ital J Pediatr 37:18, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Prematta T, Kunselman A, Ghaffari G. Comparison of food and aeroallergen sensitivity between adults and children with eosinophilic esophagitis. J Aller Ther S3:001, 2011. [Google Scholar]
- 37. Molina-Infante J, Martin-Noguerol E, Alvarado-Arenas M, et al. Selective elimination diet based on skin testing has suboptimal efficacy for adult eosinophilic esophagitis. J Allergy Clin Immunol 130:1200–1202, 2012. [DOI] [PubMed] [Google Scholar]
- 38. Rizo Pascual JM, De La Hoz Caballer B, Redondo Verge C, et al. Allergy assessment in children with eosinophilic esophagitis. J Investig Allergol Clin Immunol 21:59–65, 2011. [PubMed] [Google Scholar]
- 39. Assa'ad AH, Putnam PE, Collins MH, et al. Pediatric patients with eosinophilic esophagitis: An 8-year follow-up. J Allergy Clin Immunol 119:731–738, 2007. [DOI] [PubMed] [Google Scholar]
- 40. Paquet B, Bégin P, Paradis L, et al. Variable yield of allergy patch testing in children with eosinophilic esophagitis. J Allergy Clin Immunol 131:613, 2013. [DOI] [PubMed] [Google Scholar]