Abstract
Background: Levels of breath methane, together with breath hydrogen, are determined by means of repeated collections of both, following ingestion of a carbohydrate substrate, at 15–20 minutes intervals, until 10 samples have been obtained. The frequent sampling is required to capture a rise of hydrogen emissions, which typically occur later in the test: in contrast, methane levels are typically elevated at baseline. If methane emissions represent the principal objective of the test, a spot methane test (i.e. a single-time-point sample taken after an overnight fast without administration of substrate) may be sufficient.
Methods: We analysed 10-sample lactulose breath test data from 11 674 consecutive unique subjects who submitted samples to Commonwealth Laboratories (Salem, MA, USA) from sites in all of the states of the USA over a one-year period. The North American Consensus (NAC) guidelines criteria for breath testing served as a reference standard.
Results: The overall prevalence of methane-positive subjects (by NAC criteria) was 20.4%, based on corrected methane results, and 18.9% based on raw data. In our USA dataset, the optimal cut-off level to maximize sensitivity and specificity was ≥4 ppm CH4, 94.5% [confidence interval (CI): 93.5–95.4%] and 95.0% (CI: 94.6–95.5%), respectively. The use of a correction factor (CF) (5% CO2 as numerator) led to reclassifications CH4-high to CH4-low in 0.7 % and CH4-low to CH4-high in 2.1%.
Conclusions: A cut-off value for methane at baseline of either ≥4 ppm, as in our USA dataset, or ≥ 5 ppm, as described in a single institution study, are both highly accurate in identifying subjects at baseline that would be diagnosed as ‘methane-positive’ in a 10-sample lactulose breath test for small intestinal bacterial overgrowth.
Keywords: breath tests, spot-methane breath test, diagnostic test, small intestinal bacterial overgrowth
Study highlights
Methane produced in the intestinal tract is conventionally measured together with hydrogen in a multi-sample breath test that requires a carbohydrate substrate, which makes the test difficult to perform.
Here we analysed a dataset of more than 11 000 subjects—collected over 1 year from across the United States—and show that for methane- but not hydrogen determinations, a spot test after an overnight fast can be substituted for the full breath test and suggest a cut-off.
The presence of hydrogenotrophic micro-organisms, such as methanogens, lowers hydrogen emissions, and the ecological relationship between the two gases can clearly be shown in the average measurements of producers of none, one, or both of the gases.
We examined the impact of a widely used correction factor (CF) based on measured CO2 on reported breath test results. While its impact is negligible overall, the application of a CF may be problematic in individual cases.
Introduction
The production of methane by intestinal methanogens has recently received increased attention. Breath methane levels show a good correlation with qPCR of methanogens in stool [1, 2] and are established as relevant in the diagnosis of small intestinal bacterial overgrowth (SIBO) [3]. In addition, increased methane production by intestinal methanogens has also been associated with constipation [4], constipation-predominant irritable bowel syndrome (IBS-C) [5], obesity [6], decreased weight loss after bariatric surgery [7], multiple sclerosis [8], and other conditions [3]. Furthermore, data from both academic and commercial trials are now becoming available, which show an association with the suppression of methane production and improvements in biomarkers or clinical symptoms [2, 9, 10].
The development of a simplified approach to methane breath testing as a stand-alone test appears to be of great practical interest, because the current methodology—as detailed below—is cumbersome. Breath methane measurements are traditionally obtained in conjunction with hydrogen determinations to detect SIBO, and one popular test is the 10-sample lactulose breath test (LBT). After ingesting a substrate packet containing lactulose, the patient provides breath samples every 20 minutes over a 3-hour period. There is a 24-hour preparation period before taking the test; the first 12 hours require a specific diet and the last 12 hours require complete fasting [11]. A substrate-administering test such as LBT is required to measure hydrogen production—which may be undetectable at baseline but is significantly increased after a substrate load of lactulose, lactose, glucose, etc. has been given—and the substrate is metabolized by intestinal bacteria over time. In contrast, breath methane can nearly always be detected at baseline and subsequent increases are more modest than with hydrogen as time passes [12]. If methane alone is at issue—as is increasingly the case—the elaborate procedure described above may not be needed. A single ‘spot’ methane measurement (i.e. a single-time-point sample taken after an overnight fast without substrate administration) may be sufficient to identify subjects who are considered ‘methane positive’ by NAC criteria.
This question has been addressed by Rezaie et al., who showed that in a single institution sample of 12 183 consecutive subjects in Los Angeles, CA, USA, a cut-off for breath methane of ≥ 5 ppm at baseline resulted in a sensitivity, specificity, positive predictive value and negative predictive value of 96.1%, 99.7%, 98.5% and 99.3%, respectively, when compared to the reference standard [13]. Rezaie et al. used the new NAC guidelines as reference standard, which considers a methane level of ≥ 10 ppm at any time during administration of a traditional multiple sample breath test with substrate administration as 'methane positive' [14].
Based on these encouraging data, we examined the Commonwealth Laboratories' (Salem, MA, USA) database of consecutive multiple-sample, substrate-administering breath tests, to see whether a single methane breath test at baseline is sufficient to classify subjects into low- and high-methane emitters. Our test relies on a different breath collection technique but is generally comparable with the methodology employed by Rezaie et al. In contrast to point-of care testing by Rezaie etal., our samples were sent in for analysis from numerous sites throughout the United States. In addition, we examined our database for the influence of a widely used correction factor that tries to correct for sample contamination with room air.
Materials and Methods
We identified 11 675 consecutive, unique subjects who underwent breath testing for SIBO with lactulose as substrate by Commonwealth Laboratories from October 2014 to September 2015. The research was determined to be Institutional Review Board (IRB)-exempt by the New England IRB (Newton, MA, USA). In addition to the determination of an optimal cut-off value for the spot-methane breath test, we examined how the application, to the raw data, of a frequently used correction factor influences the classification outcome for methane and hydrogen. De-identified patient-level data were cleaned by excluding repeat tests from the same subjects. The NAC criteria were used for classification: any methane result ≥10 ppm during breath testing is ‘methane-positive’; a rise of ≥ 20 ppm of hydrogen over baseline by 90 minutes indicates SIBO [14]. We then compared methane and hydrogen high- and low-producer classifications made using the raw data with ‘normalized’ data, obtained after multiplication with a CF.
Clinical laboratory methods
Unlike decentralized breath tests performed in a physician’s office, Commonwealth Laboratories uses a central clinical testing laboratory that receives and analyses breath samples from various clinics throughout the country. Breath testing is performed using standardized instructions, sent together with breath collection materials to the requestor. After inhaling normally, patients exhale normally through a drinking straw into a test tube for 2–5 seconds until they observe condensation on the test tube wall. The straw is then removed, and the test tube securely closed with a screw cap and sent by courier to Commonwealth Laboratories for analysis [15]. In contrast, Rezai et al. used on-site QuinTron Instrument Company (Milwaukee, WI, USA) equipment, where the first 500 mL of expired air (dead space) are discarded and exhaled air is subsequently diverted into a foil bag [12]. Both methods aim to obtain an alveolar sample.
The Commonwealth analyses were conducted with the following gas chromatographs: Agilent Technologies Gas Chromatograph 7890B (Agilent Technologies, Santa Clara, CA, USA), QuinTron Microlyzer SC (Milwaukee, WI, USA), SRI 8610C (SRI Instruments, Torrance, CA, USA), with 13.8%, 21.0% and 65.2% of the samples analysed, respectively, by the equipment. A direct instrument-to-instrument comparison was not done and formal hypothesis testing is therefore not appropriate, but the results of this study are consistent with a comparable analytic performance (Table 1).
Table 1.
Comparison of the methane- (CH4) and hydrogen- (H2) positive rates obtained in this study with different types of instrument
| Instrument | CH4 |
H2 |
||
|---|---|---|---|---|
| Raw | Corrected | Raw | Corrected | |
| Agilent Gas Chromatograph | 17.5% | 19.8% | 32.9% | 34.0% |
| Quintron Microlyzer SC | 19.2% | 20.0% | 27.2% | 27.2% |
| SRI 8610C | 19.1% | 20.8% | 32.1% | 37.8% |
The QuinTron Microlyzer SC has a self-correcting feature that automatically applies a CF; however, this option was not used, i.e. raw results were recorded and correction was made subsequently. In addition, a CTC autosampler (CTC Analytics AG, Zwingen, Switzerland) and Chemstation software (Agilent) were part of the analysis workflow. The breath tests that Commonwealth offers are classified under 'System, Breath Measurements' within Clinical Chemistry (FDA Product Code NRH, Regulation Number 862.1820). All instrumentation was validated by following internal operating procedures that were created using guidance documents (Clinical Laboratory Improvement Amendments 42 CFR 493 and Massachusetts Department of Public Health 105 CMR 108), which assist in establishing laboratory quality standards.
Statistical methods
Depending on patient co-operation, collection method, and analysis work flow, collected air may not represent an alveolar sample: air from the anatomical dead space or room air may enter the patient’s breath sample. In 1979 Niu et al. [16], suggested a CF that normalizes the breath sample for the degree of departure from an alveolar sample. Some manufacturers of table-top breath analysers build the CF into the internal logic of the instrument; in contrast, we obtained both raw values and corrected values in our analysis. The assumptions for the CF are as follows: -
Patients exhale a constant amount of CO2. Niu et al. assumed that the percentage of CO2 in an optimal breath sample without room air admixture is 5.0%, and we adhere to this convention, others use 5.5%.
There are only trace amounts of CO2 in room air.
The CF is 5.0% divided by the measured CO2 in the actual breath sample.
Corrected hydrogen and methane (CH4) values are the raw measurements multiplied by the CF; for example, normalized [CH4] = observed [CH4] × (5% / observed % CO2).
CFs that are too high—either for an individual sample or as an average for the laboratory or testing station—raise concerns about faulty collection techniques, although acceptable limits have not been published and removal of outliers is therefore problematic. We analysed the degree to which CF influenced the final adjudication on whether a patient was considered methane- or hydrogen-positive.
SAS 9.4 software (SAS Institute, Cary, NC, USA) was used for the statistical analysis. A single individual was excluded from the analysis because the measured CO2 value in the sample would have resulted in a CF of 50, resulting in meaningless corrected methane and hydrogen values. Commonly used descriptive statistics (mean, standard deviation, standard error, and confidence interval) were calculated. No hypothesis testing was performed. Data from Rezaie et al. were extracted from the abstract of the American College of Gastroenterology meeting in 2015 [13]; a full publication was not available at the time of this writing. Similarly to Rezaie et al., the new NAC guidelines were used as reference standard. The methane-positive or -negative patients were classified according to the patient’s baseline methane measurement ≥ or < the cut-off point, respectively. The cut-off points of 1-10 ppm were used to evaluate the sensitivity, specificity, positive predictive value and negative predictive value. A receiver operating characteristic (ROC) curve was not generated, as the diagram would have been relatively uninformative without the ROC of Rezaie et al., which is not available for comparison: instead, sensitivities and specificities obtained in both studies were compared over the available range of interest (3–10 ppm).
Results
Samples from 50 states and the District of Columbia were available for analysis. The overall prevalence of methane-positive subjects (by NAC criteria) was 20.4% based on corrected methane results and 18.9% based on raw data. The overall prevalences of hydrogen-positive subjects, corrected and raw, were 35.0% and 31.2%, respectively. Using corrected results, 5.5% of subjects were simultaneously methane- and hydrogen-positive and 44.5% were either positive for hydrogen or methane, but not both. Using raw data, 4.3% of subjects were both methane- and hydrogen-positive and 41.5% were either positive for methane or positive for hydrogen, but not both. The relationships are depicted in a Venn diagram in Figure 1.
Figure 1.
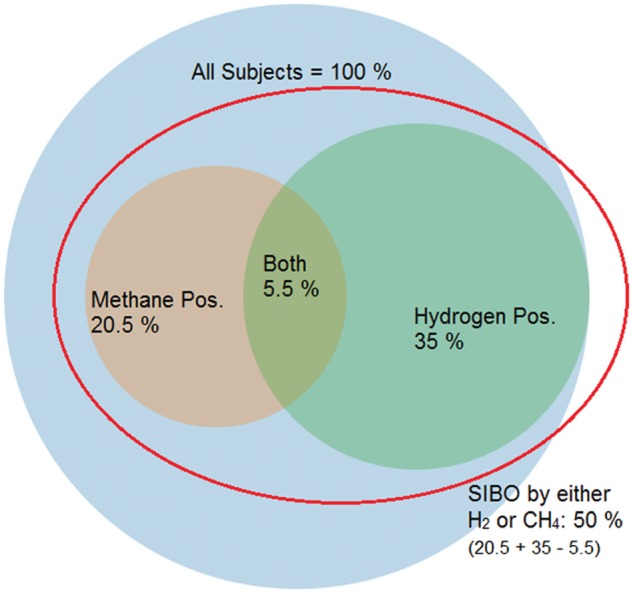
Using corrected results, in the overall sample, only 5.5% of subjects are positive for both hydrogen and methane (based on the 10 sample lactulose SIBO test). Put differently, of those who tested hydrogen-positive, 15.7% were also methane-positive, and of those who tested methane-positive, 27% were also hydrogen-positive.
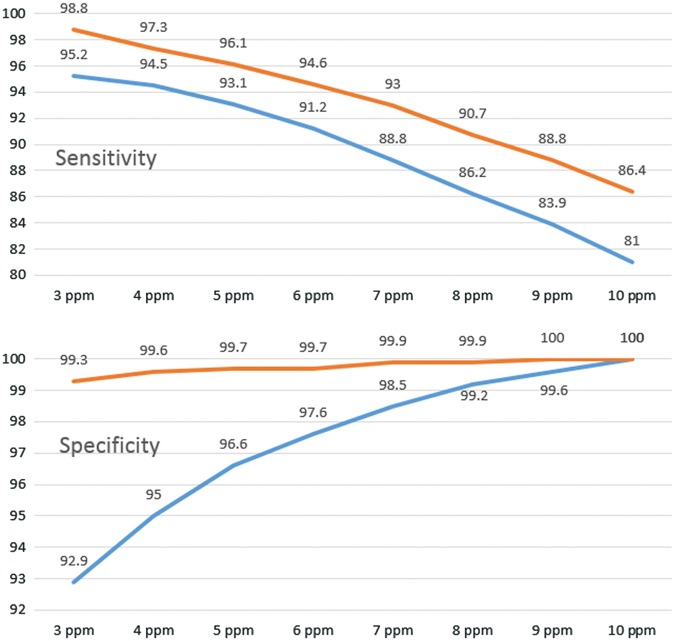
Our data were similar to the single-institution results of Rezaie et al. In our national dataset, employing the Commonwealth Laboratories' methodology, the optimal cut-off point to maximize sensitivity and specificity was ≥4 ppm CH4 (94.5% and 95.0%, respectively) with a minimal difference to the previously proposed ≥5 ppm as cut-off (Table 2). Figure 2 shows that the sensitivities of both studies follow a parallel course, decreasing with increasing cut-off values. Our USA sample showed the expected reversed course for specificity, i.e. increasing specificity with higher cut-off values, while the curve from the Los Angeles sample was relatively flat, with higher reported specificities along the spectrum of examined cut-off values.
Table 2.
Comparison of the sensitivity and specificity based on CH4 cut-off when a baseline methane measurement (new test) is compared to the results of the full 10-sample lactulose breath test (reference test). United States of America (USA, results of this study) vs. Los Angeles (LA, Rezaie et al. [13])
| CH4 cut-off ≥ | Sensitivity (CI), % |
Specificity (CI) , % |
PPV (CI) , % |
NPV (CI) , % |
||||
|---|---|---|---|---|---|---|---|---|
| USA | LA | USA | LA | USA | LA | USA | LA | |
| 3 ppm | 95.2 (94.2–96.0) | 98.8 (98.2–99.3) | 92.9 (92.4–93.4) | 99.3 (99.1–99.4) | 77.6 (76.0–79.1) | 96.0 (95.1–96.9) | 98.7 (98.4–98.9) | 99.8 (99.7–99.9) |
| 4 ppm | 94.5 (93.5–95.4) | 97.3 (96.4–97.9) | 95.0 (94.6–95.5) | 99.6 (99.4–99.7) | 83.0 (81.5–84.4) | 97.7 (96.9–98.3) | 98.5 (98.3–98.8) | 99.5 (99.3–99.6) |
| 5 ppm | 93.1 (92.0–94.1) | 96.1 (95.1–96.9) | 96.6 (96.2–96.9) | 99.7 (99.6–99.8) | 87.5 (86.1–88.7) | 98.5 (97.8–99.0) | 98.2 (97.9–98.5) | 99.3 (99.1–99.4) |
| 6 ppm | 91.2 (90.0–92.3) | 94.6 (93.4–95.5) | 97.6 (97.2– 97.9) | 99.7 (99.6–99.8) | 90.6 (89.4–91.7) | 99.1 (98.5–99.5) | 97.7 (97.4–98.0) | 99.0 (98.8–99.2) |
| 7 ppm | 88.8 (87.4–90.0) | 93 (91.8–94.1) | 98.5 (98.2–98.7) | 99.9 (99.8–99.9) | 93.8 (92.7–94.7) | 99.3 (98.7–99.6) | 97.1 (96.8-97.5) | 98.7 (98.5–98.9) |
| 8 ppm | 86.2 (84.7–87.5) | 90.7 (89.3–92) | 99.2 (99.0–99.3) | 99.9 (99.9–100) | 96.4 (95.5–97.1) | 99.7 (99.2–99.9) | 96.5 (96.1–96.9) | 98.3 (98.1–98.6) |
| 9 ppm | 83.9 (82.4–85.4) | 88.8 (87.3–90.2) | 99.6 (99.5–99.7) | 100 (99.9–100) | 98.2 (97.6–98.2) | 99.9 (99.6–100) | 96.0 (95.6–96.4) | 98 (97.7–98.2) |
| 10 ppm | 81.0 (79.4–82.6) | 86.4 (84.8–87.9) | 100 | 100 | 100 (99.8–100) | 100 (99.8–100) | 95.3 (94.9–95.8) | 97.6 (97.3–97.8) |
The prevalence of methane positive subjects was 20.4% in the USA Sample (this study) and 15.5% in Los Angeles (Rezaie et al.).
CI = confidence interval; PPV = positive predictive value; NPV = negative predictive value.
Figure 2.
Correlation of sensitivity and specificity with increasing cut-off values from 3 ppm to 10 ppm. The Los Angeles dataset is in orange, the USA dataset in blue.
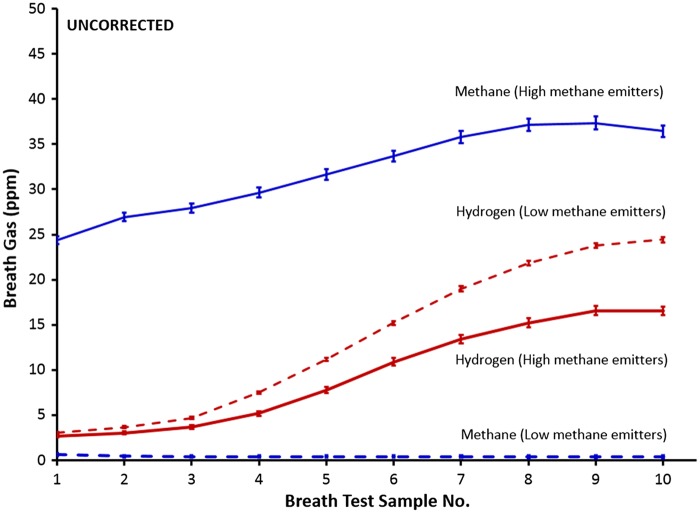
Figure 3 shows the time course of the average hydrogen- and methane production in subjects who were either high- or low-level methane producers, based on the NAC reference standard, over 10 samples (numbered from 1 to 10) spaced 20 minutes apart. Importantly, a correction factor was not applied. The line is flat for subjects who are ‘low-methane’, i.e. in subjects where methane measurements never reached 10 ppm. In comparison, there was a modest rise of the average methane measurement from approximately 25 ppm to 35 ppm for high-methane subjects, i.e. subjects who reached 10 ppm at some point during the 10-sample test. This contrasted with the substantial rise in hydrogen level between Sample 1 (approximately 3 ppm) and Sample 10 (approximately 25 ppm) in those who reached 20 ppm above baseline any time during the test (hydrogen-positive) in subjects who were classified as ‘low methane’. Interestingly, the hydrogen measurements of hydrogen-positive subjects who were also methane-positive is significantly lower (P ≤ 0.05) than the hydrogen measurements for the subjects who were hydrogen-positive but methane-negative, but still showed a similar rise over time.
Figure 3.
Time course of the average hydrogen- (red) and methane production (blue) in subjects who were either high- or low-level methane emitters, based on the reference standard (North American Consensus) (mean ± standard error) over 10 samples spaced 20 minutes apart (numbered from 1 to 10). No correction factor was applied.
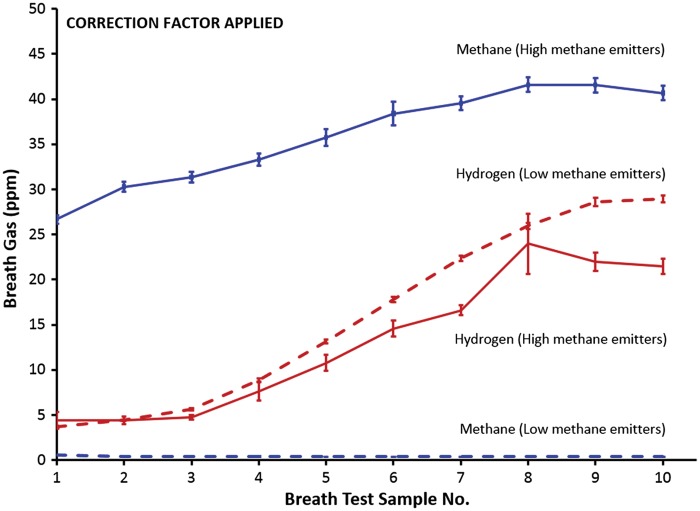
Figure 4 applies a similar analysis to that in Figure 3, but the averages of corrected methane and hydrogen measurements were used. One extreme ‘outlier’ was removed from the analysis. The results were broadly similar; however, the curve did not appear as smooth as in Figure 3. The removal of further outliers could have made it more closely resemble Figure 3 but this would quickly have become subjective and was not done.
Figure 4.
The application of a correction factor leads to a less smooth appearance of the curves than in Figure 3; larger error bars and less separation of the curves ‘mean hydrogen values for high-methane emitters’ (red solid line) and ‘mean hydrogen values for low-methane emitters’ (red dashed line). One extreme outlier was removed.
Regardless, the observed differences had a negligible effect on the classification of subjects. The use of a CF (5% CO2 as numerator) led to reclassifications per reference standard CH4-High to CH4-low in 0.7 % of the patients and CH4-Low to CH4-high in 2.1% of the patients.
Discussion
Early studies evaluating breath methane as a potential marker for intestinal methane production in the general population, proposed a criterion for methane positivity that was based on the ability to detect breath methane at a level at least 1 ppm higher than the atmospheric methane level of approximately 1.8 ppm. Specifically, if a test subject had a breath methane level ≥3 ppm on a breath sample taken after an overnight fast, he or she was considered methane-positive [17]. Here we follow the approach taken by Rezaie et al. (Los Angeles, CA), who determined a cut-off level for a positive result of a spot-methane breath test based on its ability to predict a positive result on a multi-sample LBT for SIBO [13] .
The results of the Los Angles group and our results (USA dataset) are largely comparable. The prevalence of high-methane subjects was 15.5% in the Los Angeles sample and 20.4% in our USA sample. In our dataset, the optimal cut-off point that maximizes sensitivity and specificity for a spot-methane breath test after an overnight fast was ≥4 ppm, Rezaie at al chose a cut-off of ≥ 5 ppm. Given the closeness of the results, we feel comfortable in proposing a cut-off of ≥ 5 ppm as a consensus. In this case, i.e. using ≥5 ppm as cut-off, we obtain a sensitivity and specificity of 93.1% (CI: 92.0–94.1%) and 96.6% (CI: 96.2–96.9%), respectively, compared with 96.1% (CI: 95.1–96.9%) and 99.7% (CI: 99.6–99.8%), given by Rezaie et al. Interesting to note are the high specificity data obtained by the Los Angeles group, which is fairly uniform over the range of examined cut-off values while, as expected, our specificities decline with increasing sensitivity.
It is well known that, even when tests are evaluated in a study of adequate quality—for example, using consecutive patients—similar methodology and the same reference standard, performance of a diagnostic test in one setting may vary significantly from the results reported elsewhere, in part because, even if the overall disease prevalence is similar, sub-groups are different [18]. Here, however, we are dealing only with minimal differences. A cut-off of ≥ 5 ppm for a spot-methane breath test appears to hold up well in a large dataset obtained from a national sample. In other words, obtaining a single-time-point breath test sample after an overnight fast seems to be sufficient to classify subjects or patients into low- and high-methane producers. If methane is the issue—and hydrogen not—a full 10-sample LBT does not appear to be warranted.
Like others [12], we advocate avoiding using the terms ‘methane-negative’ and ‘methane-positive’ and prefer the more neutral ‘low methane' and ‘high methane' for breath test results. It has been suggested that methanogens are almost universally present in the human intestine [19], and recently methane could be detected in all breath samples of 112 healthy volunteers using high-sensitivity equipment and isotopic measurements when compared with inhaled air [20].
The impact of the use of a CF on breath test results has received little or no attention in the literature. It is well established that fasting- and peak breath hydrogen concentrations after lactulose ingestion tend to be lower in methanogenic patients, and this difference may be related to the fact that methanogens consume four molecules of hydrogen for every molecule of methane produced [12]. This is what we found in our dataset of uncorrected breath hydrogen data: the hydrogen measurements of hydrogen-positive subjects who were also methane-positive, to use the traditional nomenclature, were significantly lower (P ≤ 0.05) than the hydrogen measurements for the subjects who were hydrogen-positive but methane-negative. Surprisingly, this relationship was somewhat weaker when corrected hydrogen and methane values were used.
We think that the analysis of data points that were twice corrected (both for methane and hydrogen) may lead to results that reflect the data manipulation, rather than true biology, especially when data are not cleaned for outliers; for example, a measured CO2 value of 0.1% in the sample would lead to a CF of 5/0.1 = 50. We removed one extreme outlier, but did not examine for others, as this would introduce a degree of subjectivity, which we wanted to avoid. While the impact of the use of the CF in clinical practice and this study seems to be small, our results suggest that its use can be problematic in some scenarios. Further work examining the impact of the CF on breath test results appears worthwhile. In the interim, we recommend that scientific studies report both raw and corrected values, and an analysis based on both, together with classification changes.
We do not advocate the abandonment of the traditional hydrogen and methane multiple sample breath tests: for many clinical indications, it remains indispensable and there is great value in quantifying both gases simultaneously. However, for narrower purposes—for example, when these results have previously been obtained, or the interest is focused on methane alone, and the administration of the full test is too burdensome—the spot-methane breath test with a cut-off of ≥ 5 ppm appears to be a viable alternative. A spot-methane breath test also offers economic advantages. Costing of laboratory tests is a complex topic; while it is likely that a single methane breath test would be offered at a lower price than the whole 10-sample LBT test, cost savings are not linear as they comprise fixed and variable costs, which depend in part on the volume of tests performed, the ability to do batch testing, automation of data flow, quality control measures, and many other factors [21] . Much greater savings are expected to accrue from savings in patient time. A spot test could be done first thing in the morning, before going to work.
Lastly it should be mentioned that our analysis and the one conducted by Rezaie et al. [13] are founded on a consensus statement [14], which is based on previously demonstrated correlations between quantitative bacterial small bowel cultures obtained to evaluate for suspected SIBO and breath test results. Methane in the breath is not universally accepted by clinicians as a diagnostic test for SIBO, as specifics with regard to the timing and magnitude of increase in breath methane excretion that constitutes SIBO remain largely unvalidated [22]; however, methane measurements are increasingly obtained to address other clinical questions and there are few outcome- or intervention studies that provide guidance on ‘clinical meaningfulness’ of a classification into high- and low-methane emitters in these novel scenarios. The ideal cut-off may therefore change as more data become available. However, in the interim, our proposed cut-off of ≥ 5 ppm predicts patients who would be found to have SIBO by methane criteria with a high degree of accuracy.
Breath tests are uniquely positioned to give a low-cost, non-invasive, easily obtainable metabolic snapshot of how the gut microbiome ‘lives and breathes’, and their relevance will certainly increase as we are now entering an era of microbiome manipulation.
Conflict of interest statement: none declared
Disclosure
The study was determined to be IRB-exempt by the New England Institutional Review Board, Needham Heights, MA, USA.
Meeting Presentation
This research was in part presented at the 2016 American College of Gastroenterology Annual Meeting in Las Vegas, NV, and received a Presidential Poster award.
References
- 1. Ghoshal U, Shukla R, Srivastava D. et al. Irritable Bowel Syndrome, Particularly the Constipation-Predominant Form, Involves an Increase in Methanobrevibacter smithii, Which Is Associated with Higher Methane Production. Gut Liver 2016;10:932–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mathur R, Chua KS, Mamelak M. et al. Metabolic effects of eradicating breath methane using antibiotics in prediabetic subjects with obesity. Obesity 2016;24:576–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Lacy Costello BP, Ledochowski M, Ratcliffe NM.. The importance of methane breath testing: a review. J Breath Res 2013;7:024001. [DOI] [PubMed] [Google Scholar]
- 4. Kunkel D, Basseri RJ, Makhani MD. et al. Methane on breath testing is associated with constipation: a systematic review and meta-analysis. Dig Dis Sci 2011;56:1612–18. [DOI] [PubMed] [Google Scholar]
- 5. Kim G, Deepinder F, Morales W. et al. Methanobrevibacter smithii is the predominant methanogen in patients with constipation-predominant IBS and methane on breath. Dig Dis Sci 2012;57:3213–18. [DOI] [PubMed] [Google Scholar]
- 6. Mathur R, Amichai M, Chua KS. et al. Methane and hydrogen positivity on breath test is associated with greater body mass index and body fat. J Clin Endocrinol Metab 2013. ;98:E698–E702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mathur R, Mundi MS, Chua KS. et al. Intestinal methane production is associated with decreased weight loss following bariatric surgery. Obes Res Clin Pract 2016;10:728–33. [DOI] [PubMed] [Google Scholar]
- 8. Jangi S, Gandhi R, Cox LM. et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun 2016;7:12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pimentel M, Chatterjee S, Chow EJ. et al. Neomycin improves constipation-predominant irritable bowel syndrome in a fashion that is dependent on the presence of methane gas: subanalysis of a double-blind randomized controlled study. Dig Dis Sci 2006;51:1297–301. [DOI] [PubMed] [Google Scholar]
- 10. Gottlieb K, Wacher V, Sliman J. et al. Su1210 SYN-010, a Proprietary Modified-Release Formulation of Lovastatin Lactone, Lowered Breath Methane and Improved Stool Frequency in Patients With IBS-C: Results of a Multi-Center Randomized Double-Blind Placebo-Controlled Phase 2a Trial. Gastroenterology 2016;150:S496–97. [Google Scholar]
- 11. Commonwealth Labs - Hydrogen Breath Testing [Internet]. [cited 2016. Aug 25]. Available from: http://www.hydrogenbreathtesting.com/our-tests/our-tests-sibo.html
- 12. Levitt MD, Furne JK, Kuskowski M. et al. Stability of human methanogenic flora over 35 years and a review of insights obtained from breath methane measurements. Clin Gastroenterol Hepatol 2006;4:123–29. [DOI] [PubMed] [Google Scholar]
- 13. Rezaie A,, Chang B,, Chua KS. et al. Accurate identification of excessive methane gas producers by a single fasting measurement of exhaled methane: a large-scale database analysis. Am J Gastroenterol 2015;110:S759–60. [Google Scholar]
- 14. Rezaie A, Buresi M, Lembo A. et al. 450 Hydrogen- and Methane- Based Breath Testing (BT) in Gastrointestinal (GI) Disorders: Report of the North American Consensus Meeting. Gastroenterology 2016;150:S97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Small Intestinal Bacterial Overgrowth (SIBO) 10-Tube Breath Test Instructions [Internet]. [cited 2016 Aug 30]. Available from: http://hydrogenbreathtesting.com/downloads/F-125_RevE_SIBO_10_Instruction_Booklet.pdf
- 16. Niu HC, Schoeller D, Klein P.. Improved gas chromatographic quantitation of breath hydrogen by normalization to respiratory carbon dioxide. J Lab Clin Med 1979;94:755–63. [PubMed] [Google Scholar]
- 17. Bond JH, Engel RR, Levitt MD.. Factors influencing pulmonary methane excretion in man an indirect method of studying the in situ metabolism of the methane-producing colonic bacteria. J Exp Med 1971;133:572–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Irwig L. Evidence base of clinical diagnosis: Designing studies to ensure that estimates of test accuracy are transferable. BMJ 2002;324:669–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dridi B, Henry M, El Khéchine A. et al. High prevalence of Methanobrevibacter smithii and Methanosphaera stadtmanae detected in the human gut using an improved DNA detection protocol. PLoS ONE 2009;4:e7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Keppler F, Schiller A, Ehehalt R. et al. Stable isotope and high precision concentration measurements confirm that all humans produce and exhale methane. J Breath Res 2016;10:016003. [DOI] [PubMed] [Google Scholar]
- 21. Broughton PM, Woodford FP.. Benefits of costing in the clinical laboratory. J Clin Pathol 1983;36:1028–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saad RJ, Chey WD.. Breath testing for small intestinal bacterial overgrowth: maximizing test accuracy. Clin Gastroenterol Hepatol 2014;12:1964–72. [DOI] [PubMed] [Google Scholar]