Abstract
Background and aims. Cholangiocarcinoma is a rare but devastating malignancy associated with a poor prognosis and a high mortality rate. With the recent advances in detection and treatment, it is unclear if the incidence and outcomes of cholangiocarcinoma are improving in the United States. The aim of this study was to evaluate the trends in the incidence, costs and mortality rates of cholangiocarcinoma- related hospital admissions in the USA.
Methods. We utilized the National Inpatient Sample Database (NIS) from 1997–2012 for all patients in whom cholangiocarcinoma (ICD-9 code 155.1, 156) was the principal discharge diagnosis. The temporal trends in the number of hospital admissions, length of stay and, hospitalization costs along with mortality rates over the study period were determined by using regression analysis for trends.
Results. There was a significant increase in the number of hospital admissions for cholangiocarcinoma as the principal diagnosis from 1997 to 2012 (10 357 vs 11 970, P<0.001). The mean length of stay for cholangiocarcinoma decreased by 17 % between 1997 and 2012 from 9.5 days to 7.9 days (P<0.001). However, during the same period, the mean hospital charges per patient (adjusted for inflation) increased 113.25% from $36 460 in 1997 to $77 753 in 2012. The in-hospital mortality rate decreased from 9.3% in 1997 to 6.4% in 2012 (P<0.001).
Conclusions. There was a significant increase in the number of hospital admissions and associated costs from cholangiocarcinoma in the USA between 1997 and 2012. However, this was accompanied by a decrease in the inpatient mortality rates from cholangiocarcinoma.
Keywords: cholangiocarcinoma, inpatient admissions, hospital charges, in-hospital mortality, epidemiology
Introduction
Cholangiocarcinoma is a rare but devastating cancer, accounting for approximately 3% of all gastrointestinal cancers [1]. It is usually diagnosed at an advanced stage, with a 5-year survival rate of only about 10% [2,3]. Chronic inflammation of the bile ducts is an important risk factor for cholangiocarcinoma. Cholangiocarcinoma is classified, based on location, into intrahepatic and extrahepatic types (includes gall bladder). The majority of cholangiocarcinoma cases (about 90%) are extrahepatic in location. Incidence of cholangiocarcinoma is very high in certain regions of the world such as Thailand, China and Southeast Asia [4]. Colonization with liver flukes Opisthorchis viverrini (Thailand, Laos PDR and Vietnam) and Clonorchis sinensis (China, Taiwan, eastern Russia, Korea and Vietnam) causes chronic inflammation and is a risk factor for the development of cholangiocarcinoma [4–6].
In the western world, primary sclerosing cholangitis (PSC) is the most common predisposing risk factor for development of cholangiocarcinoma. Lifetime incidence of cholangiocarcinoma among PSC patients is about 5–10% [7]. Patients with other chronic liver diseases such as chronic viral hepatitis, alcoholic liver disease or cirrhosis from other causes are also at increased risk of cholangiocarcinoma [3,8–11]. Data from the Surveillance, Epidemiology, and End Results (SEER) program and the US Division of Vital Statistics databases between 1973 and 1997 indicated increasing incidence and mortality rates from intrahepatic cholangiocarcinoma [12]. However, the most recent epidemiologic data suggest decreasing incidence and mortality rates of extrahepatic cholangiocarcinoma in the USA [13–15].
There is ambiguity in the literature about the actual incidence and mortality rates associated with cholangiocarcinoma in the USA. There is also lack of data regarding in-patient hospitalizations and mortality rates related to cholangiocarcinoma. Hence, the purpose of this study was to assess the frequency of hospital admissions with a principal diagnosis of cholangiocarcinoma and associated mortality rates in the USA from 1997 to 2012. We also assessed the potential influence of patient and hospital characteristics on health-related outcomes in these patients.
Methods
We used the Nationwide Inpatient Sample (NIS) to obtain a population- based estimate of national trends. The NIS is part of the Healthcare Cost and Utilization Project (HCUP) sponsored by the Agency for Healthcare Research and Quality in Rockville, MD. The NIS is the largest publicly available all-payer inpatient care database in the USA. It was designed to approximate a 20% sample of nonfederal hospitals and stratified according to geographic region, ownership, location, teaching status and number of beds . The 1997 NIS is drawn from 22 states and contains information on all in-patient stays from more than 1000 hospitals, totaling about 7.1 million records. The 2012 NIS contains discharge data from more than 4000 hospitals in 45 states totaling about 8 million records. This large database is an excellent representative sample of the general US population and is useful for analyzing healthcare utilization, access, charges, quality and outcomes[16–18]. The NIS database provides only administrative data for analysis. Patient- specific clinical data (i.e. laboratory tests, procedures) are not available.
To identify cases of cholangiocarcinoma, we queried the NIS database in order to recover hospital data on all discharge diagnoses with a primary ICD-9-CM diagnosis code of 155.1 (malignant neoplasm of intrahepatic bile ducts) and 156 (malignant neoplasm of gall bladder including extrahepatic ducts, ampulla of Vater, unspecified sites). According to the HCUPnet, principal diagnosis is defined as ‘the condition established after study to be chiefly responsible for occasioning the admission of the patient to the hospital for care.’ The principal diagnosis is always the reason for admission (definition according to the Uniform Bill (UB-92). Therefore, we believe that we captured data regarding hospitalizations due to cholangiocarcinoma.
The query parameters were configured for the period 1997–2012. NIS data are available from 1988 to 2012; however, there was a change in the NIS dataset in 1997 to include details of the patient and hospital characteristics and allow in-depth analysis of trends over time. Therefore, this particular period was chosen for our study.
Variables recorded
Patient demographics recorded included age and sex. Hospital characteristics recorded were region (northeast, midwest, south and west), location (metropolitan vs non-metropolitan area) and size (small, medium and large). As per the HCUPnet definitions, metropolitan areas are those with a population of at least 50 000 people. Areas with population less than that are the non-metropolitan areas. The definition of bed size varied according to the hospital location and teaching status. The range for small hospitals was 1–299 beds. The bed-size range for medium hospitals was 50–499, and the bed-size range for large hospitals was 100–500 or more. We also looked at the payer status for all admissions. ‘Hospital charges’ is defined as the amount the hospital charged for the entire hospital stay. It does not include professional (physician) fees. ‘Aggregate charges’ or the ‘national bill’ is defined as the sum of all charges for all hospital stays in the USA. ‘Length of stay’ is defined as the number of nights the patient remained in the hospital for this stay.
Statistical analysis
Trends for the annual point estimates of the frequency of cholangiocarcinoma for the data sample were plotted and analyzed. The annual frequency of discharges with cholangiocarcinoma was computed by dividing the number of discharges with cholangiocarcinoma listed in the NIS database in a given year by the total number of all discharges listed in the NIS for the same year. All analyses were performed using SAS (version 9.4, The SAS Institute, Cary, NC).
In addition to the percentages available adjacent to the data in the tables, the frequency per 100 000 admissions for each categorical variable was also calculated. These numbers represent the density of patients diagnosed with cholangiocarcinoma compared with the total number of hospital discharges per category. Each frequency was calculated by dividing the number of patients with cholangiocarcinoma by the total discharges in a specific categorical variable for each year and multiplying the number by 100 000. We viewed the counts as arising from a Poisson distribution and the total discharges as an offset yielding Poisson rates that have been compared over time using Poisson regression. This yields relative rates (RRs) and 95% confidential intervals (CIs) that express the ratio of rate per 100 000 in 2012 to that in 1997. These values differ from the percentages, which describe each category exclusively for either patients with cholangiocarcinoma or total discharges. The percentages distinguished differences among the variables for each specific year, whereas the frequencies were vital for comparing trends from 1997 to 2012, especially for age group and region.
Results
Number and cost of cholangiocarcinoma discharges
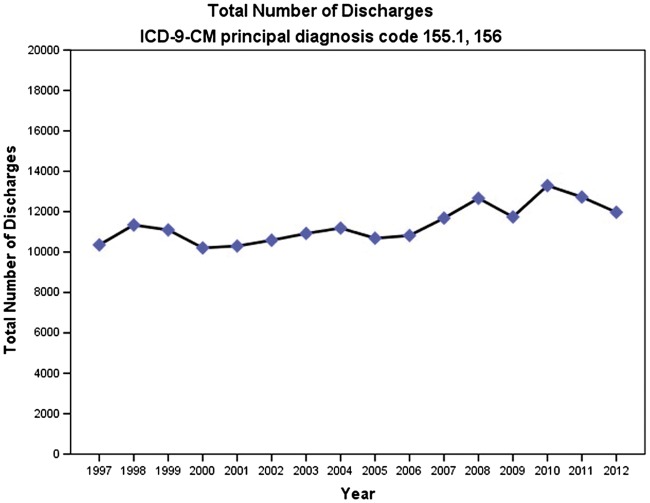
From 1997 to 2012, the total number of hospital admissions with a principal diagnosis of cholangiocarcinoma increased by 15.57% from 10 357 to 11 970 (P<0.001) (Figure 1). The number of hospital admissions with cholangiocarcinoma either as a primary or a secondary diagnosis increased from 19 876 to 31 050 during this period (P<0.001). The frequency of hospital discharges for cholangiocarcinoma as a principal diagnosis increased from 31.17/100 000 discharges to 42.16/100 000 discharges (RR = 1.35 (1.32–1.39); P<0.001). The average length of hospital stay for patients with cholangiocarcinoma decreased between 1997 and 2012 from 9.5 days to 7.9 days (P<0.001).
Figure 1.
Total number of hospital discharges with principal diagnosis of cholangiocarcinoma
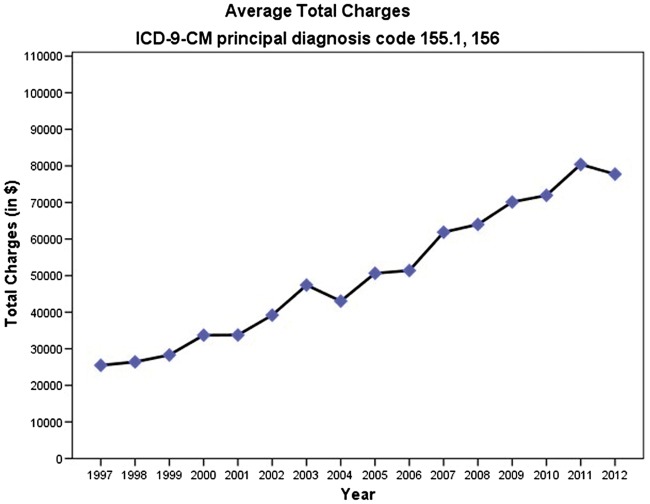
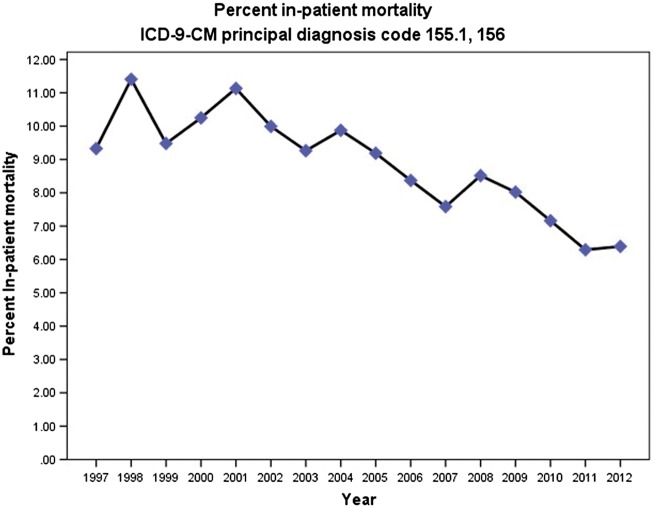
The aggregate costs of hospitalizations where cholangiocarcinoma was the primary discharge diagnosis increased 244.29% from $146 112 904 in 1997 (adjusted for inflation) to $503 051 348 in 2012. Despite the decrease in the average length of stay, the mean total charges for cholangiocarcinoma-related hospital admissions increased substantially between 1997 and 2012. Mean hospital charges per patient increased 113.25% from $36 460 in 1997 (adjusted for inflation) to $77 753 in 2012 (p<0.001) (Figure 2). The portion of the national bill (total aggregate charges for cholangiocarcinoma/total national bill) for cholangiocarcinoma discharges increased from 0.027% in 1997 to 0.084% in 2012. However, the in-hospital mortality rate decreased from 9.3% in 1997 to 6.4% in 2012 (P<0.001) (Figure 3).
Figure 2.
Average total hospital charges per hospitalization due to cholangiocarcinoma
Figure 3.
Frequency of in-hospital deaths for hospitalizations with principal diagnosis of cholangiocarcinoma
Cholangiocarcinoma discharges by patient characteristics
Patients in the age group of 65–84 years had the highest rate of discharge for cholangiocarcinoma both in 1997 and 2012 (Table 1). The frequency of discharge rates increased marginally in the 1–17 year (RR = 1.39 (1.21–1.58); P<0.001), 18–44 (RR = 1.06 (1.01-1.12); P=0.212) and the 65–84 year (RR = 1.05 (1.02–1.09); P=0.003) age groups. Surprisingly, the >85-year age group saw a decline in the frequency of cholangiocarcinoma (RR = 0.76 (0.7–0.82); P<0.001). The frequency of cholangiocarcinoma increased in men from 34.09/100 000 discharges in 1997 to 42.85/100 000 discharges in 2012 (RR = 1.26 (1.21–1.3); P<0.001). This increase in frequency was more pronounced in women: 29.14/100 000 discharges in 1997 to 41.55/100 000 discharges in 2012 (RR = 1.43 (1.38–1.48); P<0.001), almost equaling that of men.
Table 1.
Number and frequency of discharges with cholangiocarcinoma by patient and hospital characteristics in 1997 and 2012
| Category | 1997 Cholangiocarcinoma (N,%) | 2012 Cholangiocarcinoma (N,%) | 1997 Total (N,%) | 2012 Total (N,%) | Cholangiocarcinoma per 100 000 admissions in 1997 | Cholangiocarcinoma per 100 000 admissions in 2012 |
|---|---|---|---|---|---|---|
| All discharges | 10 357(100) | 11 970(100) | 33 230 554(100) | 28 391 049(100) | 31.17 | 42.16 |
| Age group (years) | ||||||
| 1–17 | * | * | 1 766 699(5) | 1 376 026(5) | * | * |
| 18–44 | 510(5) | 385(3) | 9 074 102(27) | 4 943 137(17) | 5.62 | 7.79 |
| 45–64 | 2523(24) | 3870(32) | 6 226 540(19) | 9 003 812(32) | 40.51 | 42.98 |
| 65–84 | 6030(58) | 6410(54) | 9 644 446(29) | 9 724 120(34) | 62.52 | 65.92 |
| 85+ | 1294(13) | 1305(11) | 2 245 807(7) | 2 980 584(11) | 57.62 | 43.78 |
| Sex | ||||||
| Male | 4648(45) | 5750(48) | 13 632 763(41) | 13 418 972(47) | 34.09 | 42.85 |
| Female | 5709(55) | 6220(52) | 19 593 669(59) | 14 969 687(53) | 29.14 | 41.55 |
| Payer | ||||||
| Medicare | 6373(62) | 7125(60) | 12 070 265(36) | 14 227 410(50) | 52.80 | 50.08 |
| Medicaid | 647(6) | 880(7) | 5 448 491(16) | 3 926 823(14) | 11.88 | 22.41 |
| Private insurance | 2795(27) | 3165(26) | 12 814 864(39) | 7 360 684(26) | 21.81 | 43.00 |
| Uninsured | 251(2) | 394(3) | 1 615 542(5) | 1 779 681(6) | 15.54 | 22.19 |
| Other | 275(3) | 360(3) | 1 201 195(4) | 1 026 506(4) | 22.93 | 35.07 |
| Income | ||||||
| Low | * | 3085(26) | * | 8 646 773(30) | * | 35.68 |
| Not low | * | 8590(72) | * | 19 070 345(67) | 45.04 | |
| Owner | ||||||
| Government | 1363(13) | 1860(16) | 4 707 979(14) | 3 390 284(12) | 28.95 | 54.86 |
| Private, not-for-profit | 7811(75) | 9110(76) | 23 924 271(72) | 20 916 148(74) | 32.65 | 43.55 |
| Private, for-profit | 1157(11) | 1000(8) | 4 464 111(13) | 4 084 618(14) | 25.92 | 24.48 |
| Location | ||||||
| Non-metropolitan | 1007(10) | 650(5) | 5 165 442(16) | 3 273 738(12) | 19.50 | 19.86 |
| Metropolitan | 9323(90) | 11 320(95) | 27 930 919(84) | 25 117 311(88) | 33.38 | 45.07 |
| Bed size | ||||||
| Small (1–299) | 1542(15) | 1175(10) | 5 284 288(16) | 4 118 586(15) | 29.18 | 28.53 |
| Medium (50–499) | 2999(29) | 2590(22) | 11 047 425(33) | 7 416 819(26) | 27.15 | 34.92 |
| Large (100–500+) | 5790(56) | 8205(69) | 16 764 648(51) | 16 855 643(59) | 34.54 | 48.68 |
| Region | ||||||
| Northeast | 2130(20.5) | 2900(24) | 6 784 405(20) | 5 652 002(20) | 31.40 | 51.31 |
| Midwest | 2507(24) | 2620(22) | 7 740 346(23) | 6 525 448(23) | 32.39 | 40.15 |
| South | 3890(37.5) | 4155(35) | 12 373 424(37) | 11 019 844(39) | 31.44 | 37.70 |
| West | 1829(18) | 2295(19) | 6 332 379(19) | 5 193 755(18) | 28.89 | 44.19 |
The relative frequency of cholangiocarcinoma was the highest for Medicare in both 1997 and 2012 among all payer statuses. However, it declined from 52.8/100 000 in 1997 to 50.08/100 000 discharges in 2012 (RR = 0.95 (0.92–0.98); P=0.002). On the contrary, the frequency of cholangiocarcinoma increased for all other forms of payment over the study period. The relative frequency of patients with cholangiocarcinoma almost doubled in the Medicaid group from 11.88/100 000 in 1997 to 22.41/100 000 discharges in 2012 (RR = 1.89 (1.7–2.09); P<0.001) as well as in the private insurance group from 21.81/100 000 in 1997 to 43/100 000 discharges in 2012 (RR = 1.97 (1.87–2.08); P<0.001).
Cholangiocarcinoma discharges by hospital characteristics
Metropolitan areas had higher frequencies of cholangiocarcinoma discharges than non-metropolitan areas both in 1997 and 2012 (Table 1). The frequency of discharges for metropolitan areas increased from 33.38/100 000 in 1997 to 45.07/100 000 in 2012 (RR = 1.35 (1.31–1.39); P<0.001). For non-metropolitan areas, the frequency of discharges remained similar. Patients with cholangiocarcinoma were more likely to be diagnosed in a large hospital with a slight increase in number of cases (RR = 1.41 (1.36–1.46); P<0.001). Medium-sized hospitals also had a marginal increase in cases with cholangiocarcinoma, whereas the frequency remained unchanged for small hospitals (RR = 1.29 (1.22–1.36); P<0.001).
The south had the highest absolute number of both cholangiocarcinoma discharges and total discharges in 1997 as well as in 2012. Although the increase in frequency of cholangiocarcinoma was seen in all US regions, it was more marked in the northeast (RR = 1.63 (1.55–1.73); P<0.001) and the west (RR = 1.53 (1.44–1.63); P<0.001). The increase in frequency in the other regions was less steep but still statistically significant (P<0.001).
Discussion
This study looked at the incidence and outcomes of cholangiocarcinoma in the USA from a nationwide population-based in-patient database in the last 16 years (1997–2012). There was a significant increase in the frequency of cholangiocarcinoma-related hospital admissions and associated costs despite a decrease in the hospital length of stay. The in-patient mortality rates also decreased during the study period.
During the years 1997 and 2012, the frequency of cholangiocarcinoma-related discharges as a primary diagnosis increased by 15.57%. During the same time period, the primary or secondary diagnosis of cholangiocarcinoma increased by as much as 56.22%. The rise in incidence of cholangiocarcinoma could be attributed to an increase in its risk factors such as obesity [19], alcoholic cirrhosis [20], metabolic syndrome [21] and primary sclerosing cholangitis [22]. Increasing use and improved quality of cross-sectional imaging modalities such as magnetic resonance imaging (MRI) and computed tomography (CT) may have contributed to this trend. A study assessing the use of cross-sectional imaging studies in a large health plan found that imaging with CT doubled, and imaging with MRI tripled during a period from 1997 to 2006 [23]. That study also found that cross-sectional studies added to existing studies instead of replacing them and that the annual per enrollee cost of radiological imaging more than doubled [23].
Although the average length of hospital stay during the study period decreased from 9.5 days to 7.9 days, the average total charges per patient, adjusted for inflation increased by 113.26%. Likewise, the overall percentage of total hospital costs associated with diagnosis of cholangiocarcinoma more than tripled during this time period from 0.027% to 0.084%. The increase in cost per patient may be due to increasing use of cross-sectional imaging and interventional procedures such as endoscopic retrograde cholangiopancreatography (ERCP) and percutaneous transhepatic approaches for biliary drainage and tissue acquisition along with broadened criteria for resectability [5]. Liver transplantation with neo-adjuvant radiation and chemotherapy is now an approved method of treatment in select cases of hilar cholangiocarcinoma [24]. Although the numbers are small, the high costs associated with liver transplantation might have played a role in overall increased costs. During the study period, there was a decreasing trend in hospital mortality rates. This might be due to improvements in early diagnosis, efficacy of treatments or the possibility of these patients being discharged to nursing homes and hospice care centers. These details could not be obtained because the database was administrative rather than clinical.
The 65–84 year age group had the highest rate of hospital admissions for cholangiocarcinoma both in 1997 and 2012. This finding is consistent with other epidemiologic reports that the typical age of presentation for cholangiocarcinoma is the seventh decade of life [14]. Interestingly, the >85 year age group in this study showed a decline in the frequency of cholangiocarcinoma. Whether this trend is due to different risk factors in this age group or due to survival bias is not known. Although an increased incidence of cholangiocarcinoma was noted in both sexes, it was more pronounced in women. There is no convincing evidence to suggest disproportionate increase in risk factors such as PSC, hepatitis C, choledochal cysts or chronic liver disease in women. Hence, this finding needs further study.
Payer statistics for cholangiocarcinoma remained largely unchanged over the 16 years studied. Medicare is the primary health insurer for 97% of the US population aged ≥65 years [25]. Consistent with epidemiology of cholangiocarcinoma being a disease of the elderly population, more than 60% of the patients belonged to the Medicare group. Even as the total discharges for patients with private insurance decreased from 1997 to 2012, the frequency of cholangiocarcinoma increased from 21.8/100 000 discharges to 43/100 000 discharges. This may be due to the fact that patients with private insurance are more likely to seek earlier treatment.
Since cholangiocarcinoma is a very rare malignancy, trained specialists and resources required for diagnosis and management will likely be concentrated in a few large major medical centers. This was confirmed by our findings that the frequency of cholangiocarcinoma in large hospitals increased by 40.94%, along with a decline in the frequency among small hospitals over the last 16 years. In addition, the frequency also increased in metropolitan areas but remained unchanged in non-metropolitan areas. Possible explanation is due to dense population in metropolitan areas and also due to patients being referred to large metropolitan hospitals with the appropriate resources and expertise to manage cholangiocarcinoma.
The design of this study and the nature of the NIS dataset include some important limitations. Since NIS is an administrative dataset, it is reflective of the coding practices of each healthcare institution. Importantly the data were not differentiated on the basis of the classification of cholangiocarcinoma as intrahepatic and extrahepatic but were instead recorded as a single entity. In addition, this dataset does not control for errors during data entry. Also, individual patient-specific clinical information, such as the procedures performed on the patient, was not obtainable. Future studies analyzing patient-specific trends and individual hospital coding practices may provide additional clarification about the information in this study. Importantly, the NIS dataset does not provide sufficient patient and hospital details to determine with certainty the factors leading to the rise in hospital discharges and their costs.
In conclusion, there was a significant increase in the frequency of cholangiocarcinoma-related hospitalizations and associated costs between 1997 and 2012. Cholangiocarcinoma is an escalating concern to the healthcare system in the USA. Further studies analyzing cost-effective diagnostic modalities, treatment strategies and preventive measures are necessary to reduce the burden of cholangiocarcinoma.
Author contributions
Vaibhav Wadhwa: study concept and design, acquisition of data, statistical analysis and interpretation of data, drafting of the manuscript; Yash Jobanputra: analysis and interpretation of data, drafting of the manuscript; Prashanthi Thota: study concept and design, drafting of the manuscript; K.V. Narayanan Menon: critical revision of the manuscript; Mansour Parsi: critical revision of the manuscript; Madhusudhan R Sanaka: study concept and design, drafting of the manuscript and critical revision of the manuscript.
Conflict of interest statement: none declared.
References
- 1. Rizvi S, Gores GJ.. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology 2013;145:1215–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Everhart JE, Ruhl CE.. Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterology 2009;136:1134–44. [DOI] [PubMed] [Google Scholar]
- 3. Tyson GL, El-Serag HB.. Risk factors for cholangiocarcinoma. Hepatology 2011;54:173–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sripa B, Pairojkul C.. Cholangiocarcinoma: lessons from Thailand. Curr Opin Gastroenterol 2008;24:349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Razumilava N, Gores GJ.. Cholangiocarcinoma. Lancet 2014;383:2168–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Watanapa P, Watanapa WB.. Liver fluke-associated cholangiocarcinoma. Br J Surg 2002;89:962–70. [DOI] [PubMed] [Google Scholar]
- 7. Razumilava N, Gores GJ.. Classification, diagnosis, and management of cholangiocarcinoma. Clin Gastroenterol Hepatol 2013;11:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kobayashi M, Ikeda K, Saitoh S, et al. Incidence of primary cholangiocellular carcinoma of the liver in japanese patients with hepatitis C virus-related cirrhosis. Cancer 2000;88:2471–7. [DOI] [PubMed] [Google Scholar]
- 9. Lee TY, Lee SS, Jung SW, et al. Hepatitis B virus infection and intrahepatic cholangiocarcinoma in Korea: a case-control study. Am J Gastroenterol 2008;103:1716–20. [DOI] [PubMed] [Google Scholar]
- 10. Shaib YH, El-Serag HB, Nooka AK, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a hospital-based case-control study. Am J Gastroenterol 2007;102:1016–21. [DOI] [PubMed] [Google Scholar]
- 11. Welzel TM, Graubard BI, El-Serag HB, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study. Clin Gastroenterol Hepatol 2007;5:1221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology 2001;33:1353–7. [DOI] [PubMed] [Google Scholar]
- 13. Khan SA, Emadossadaty S, Ladep NG, et al. Rising trends in cholangiocarcinoma: is the ICD classification system misleading us? J Hepatol 2012;56:848–54. [DOI] [PubMed] [Google Scholar]
- 14. Shaib Y, El-Serag HB.. The epidemiology of cholangiocarcinoma. Semin Liver Dis 2004;24:115–25. [DOI] [PubMed] [Google Scholar]
- 15. Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer 2002;2:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Healthcare cost and utilization project online (internet), Rockville, MD: Agency for healthcare research and quality. site]. Available at http://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed May 5, 2015.
- 17. Sethi S, Mikami S, Leclair J, et al. Inpatient burden of constipation in the United States: an analysis of national trends in the United States from 1997 to 2010. Am J Gastroenterol 2014;109:250–6. [DOI] [PubMed] [Google Scholar]
- 18. Sethi S, Wadhwa V, LeClair J, et al. In-patient discharge rates for the irritable bowel syndrome - an analysis of national trends in the United States from 1997 to 2010. Aliment Pharmacol Ther 2013;38:1338–46. [DOI] [PubMed] [Google Scholar]
- 19. Grainge MJ, West J, Solaymani-Dodaran M, et al. The antecedents of biliary cancer: a primary care case-control study in the United Kingdom. Br J Cancer 2009;100:178–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sorensen HT, Friis S, Olsen JH, et al. Risk of liver and other types of cancer in patients with cirrhosis: a nationwide cohort study in Denmark. Hepatology 1998;28:921–5. [DOI] [PubMed] [Google Scholar]
- 21. Welzel TM, Graubard BI, Zeuzem S, et al. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology 2011;54:463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Card TR, Solaymani-Dodaran M, West J.. Incidence and mortality of primary sclerosing cholangitis in the UK: a population-based cohort study. J Hepatol 2008;48:939–44. [DOI] [PubMed] [Google Scholar]
- 23. Smith-Bindman R, Miglioretti DL, Larson EB.. Rising use of diagnostic medical imaging in a large integrated health system. Health Aff (Millwood) 2008;27:1491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rea DJ, Heimbach JK, Rosen CB, et al. Liver Transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg 2005;242:451–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care 2002;40(8 Suppl):IV-3–18. [DOI] [PubMed] [Google Scholar]