Abstract
The key genetic component of methicillin resistance, the mecA determinant, is not native to Staphylococcus aureus. Thus, the evolution of methicillin-resistant S. aureus (MRSA) must have begun with the acquisition of the mecA determinant from an unknown heterologous source some time before the first reported appearance of MRSA isolates in clinical specimens in the U.K. and Denmark (in the early 1960s). We compared the genetic backgrounds and phenotypes of a group of methicillin-susceptible S. aureus (MSSA) isolates to the properties of MRSA strains isolated in Denmark and the U.K. during the same time period, and also to the genetic profiles of contemporary epidemic clones of MRSA. All early MRSA isolates resembled a large group of the early MSSA blood isolates in phenotypic and genetic properties, including phage group, antibiotype (resistance to penicillin, streptomycin, and tetracycline), pulsed-field gel electrophoresis pattern, and spaA type and multilocus sequence type, strongly suggesting that the early MSSA examined here represented the progeny of a strain that served as one of the first S. aureus recipients of the methicillin-resistance determinant in Europe. The genetic background of this group of early MSSA isolates was also very similar to that of the widely disseminated contemporary “Iberian clone” of MRSA, suggesting that genetic determinants present in early MSSA and essential for some aspects of the epidemicity and/or virulence of these strains may have been retained by this highly successful contemporary MRSA lineage.
One of the most serious contemporary challenges to the treatment of hospital-acquired infections worldwide is the appearance and global spread of methicillin (M)-resistant Staphylococcus aureus (MRSA), which carries a uniquely effective drug-resistance mechanism that can protect these pathogens against all members of the large β-lactam family of antibiotics. The first stage in the emergence of this devastatingly effective antibiotic-resistance mechanism must have been the acquisition of its key genetic determinant, the mec element, which is of unknown extraspecies origin. The number of times these foreign pieces of DNA have entered the species of S. aureus and the mechanism of their acquisition are not known at the present time.
The purpose of the studies described in this communication was to approach questions related to the origin and spread of MRSA by examining the genetic backgrounds of strains of M-susceptible Staphylococcus aureus (MSSA) recovered in the early 1960s, i.e., strains that may have been the original recipients of the mec element at the time when the first European isolates of MRSA were identified in Denmark (1, 2) and in the U.K. (3, 4).
Methods
Clinical Strains.
In Denmark, all positive blood-culture isolates of S. aureus identified since 1957 were preserved at the Statens Serum Institute in Denmark, where phage-typing and antibiotic-susceptibility profiles for the isolates were determined and relevant clinical data (place of hospitalization, isolation source) were recorded. All Danish isolates used in our study were from this collection. Four of the MRSA strains from the U.K. (ST61/6219, ST61/6421, ST63/458, and ST63/460) were obtained from the National Collection of Type Cultures (NCTC), London, U.K. The remaining three strains, 103895, 13136, and 6467, were kindly provided by J. Hamilton-Miller (Royal Free and University College Medical School, London; ref. 5).
Phenotypic Characterization.
The current international set of typing phages and two Danish experimental phages were used in phage typing (6–8). Antibiograms were determined as described (1).
Molecular Typing.
Macrorestriction profiles (SmaI∷PFGE) and restriction fragment-length polymorphisms of ClaI digests with mecA and Tn554 probes were done and pulsed-field gel electrophoresis (PFGE) types were assigned as described (1, 9–11). For MRSA strains, clonal types were identified by a combination of three sets of symbols standing for the particular ClaI-mecA polymorphism, the ClaI-Tn554 insertion pattern, and the SmaI-PFGE profile of the isolate, respectively. MSSA clonal lineages were characterized by their PFGE profiles. PFGE profiles were compared by visual inspection followed by computer analysis as described (1).
Methods used in spaA typing (12), multilocus sequence typing (MLST) (13), and DNA extraction with 4 M of guanidine thiocyanate were described (14). Primers used for spaA typing were as follows. spaAF1: GAC GAT CCT TCG GTG AGC, nucleotides 1096–1113; and spaAR1: CAG CAG TAG TGC CGT TTG C, nucleotides 1534–1516 (GenBank accession no. J01786) (15). For MLST, the primers adopted were the ones described (13), with the exception of arcCF2 (the forward primer for gene arcC), which was designed based on preliminary sequence data obtained from The Institute for Genomic Research (TIGR) web site (http://www.tigr.org) for strain COL of S. aureus (arcCF2, CCT TTA TTT GAT TCA CCA GCG). GeneSearch software (L. Krippahl, unpublished data) was used to identify and assign spaA types (12, 15–17). Sequences obtained in MLST were submitted to an Internet database, www.mlst.net, where an allelic profile was assigned (13). Allelic profiles were examined by computer analysis using STAR 0.9.1 (University of Oxford), followed by the construction of unweighted pair group method with arithmetic mean (UPGMA) trees.
Results
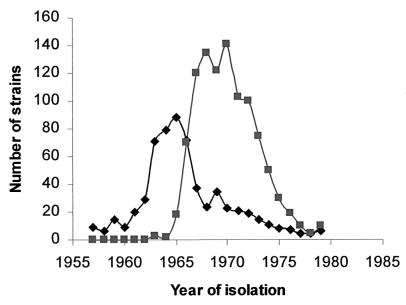
The preservation of all S. aureus blood isolates in Denmark since the early 1950s has allowed genetic analysis of the unique epidemiological scenario surrounding the emergence of MRSA in that country. (i) All but 9 of the 646 MRSA identified among a total of 3,704 S. aureus blood isolates collected between 1957 and 1970 showed a special multiresistance pattern that included (in addition to M) resistance to penicillin (P), streptomycin (S), tetracycline (T), and often to erythromycin (E) as well. (ii) The great majority of MRSA (616 of 646 or 95%) belonged to either phage group III (40 isolates) or the closely related 83A complex (576 isolates). These two phage types represented about half of all S. aureus blood isolates (including both MRSA and MSSA strains) identified in Denmark between 1957 and 1970. (iii) The same multiresistant antibiotype and phage group was also characteristic of the first MRSA isolates from the U.K. (3, 4). (iv) Interestingly, the P-S-T or P-S-T-E antibiotype also was identified in about 20% (627 strains) of the 3,058 MSSA strains collected during the same period, and the majority of these isolates (519 of 627 or 82%) also belonged to phage group III and/or the related 83A complex. (v) Yet another interesting feature of the epidemiology of early MRSA strains in Denmark was that the “wave” of MSSA strains with the P-S-T-(E) phenotype and belonging to phage groups III and or 83A preceded in time the appearance of the wave of MRSA strains with the same phenotype and phage types (Fig. 1).
Figure 1.
Sequential appearance of M-susceptible and M-resistant blood isolates of S. aureus belonging to phage group III and the related 83A complex (in Denmark). ■, P-S-T-M isolates; ♦, P-S-T isolates. The numbers plotted represent all S. aureus blood isolates identified in Denmark during the particular period.
These data strongly suggested that in our search for the original recipient of the mec gene complex we should focus on MSSA strains belonging to phage group/type III/83A with the P-S-T or P-S-T-E antibiotype. All but one of the 25 MSSA and all 24 MRSA strains selected for the genetic analysis shared this phage group and antibiotype.
Phage Types and Antibiotypes.
Of the 25 MSSA selected for genetic analysis, 16 were isolated during 1957–1964, and 9 between 1965 and 1968, i.e., shortly before and after the first MRSA were identified. Most MSSA (24 of 25) strains belonged to phage group III or to the 83A complex (a subdivision of phage group III; ref. 18). A single strain, E1410 (representing the 25th MSSA), was nontypable by the set of phages used. Of the 25 MSSA, 17 had the antibiotype P-S-T and the rest of the MSSA were P-S (2 isolates) or P (4 isolates); 2 strains were fully susceptible (Table 1).
Table 1.
Properties of historically early strains of MSSA
| Strain | Date | Origin* | Phage group | Clonal type (PFGE) | Antibiogram† | spaA type‡ | Allelic profile§ |
|---|---|---|---|---|---|---|---|
| E213 | 1957 | D ND | 83A | A19 | PST | YHGFMBQBLO | 3 3 1 1 4 4 16 |
| E298 | 1958 | D ND | 83A | A19 | PST | YHGFMBQBLO | |
| E306 | 1958 | D ND | 83A | A19 | PST | YHGFMBQBLO | 3 3 1 1 4 4 16 |
| E712 | 1959 | D 1 | 83A | A19 | PST | YO | |
| E803 | 1959 | D 2 | 83A | A22 | PST | YHGFMBQBLO | 3 3 1 1 4 4 16 |
| E1038 | 1960 | D 3 | 83A | A23 | PST | YHGFMBQBLO | |
| E1045 | 1960 | D 4 | 83A | A19 | PST | YHGFMBQBLO | |
| E1210 | 1961 | D 5 | 83A | A19 | PST | YHGFMBQBLO | |
| E1215 | 1961 | D 6 | 83A | A24 | PST | YHGFMBQBLO | 3 3 1 1 4 4 16 |
| E1404 | 1962 | D 7 | 83A | A19 | PST | YHGFMBQBLO | |
| E1600 | 1963 | D 9 | 83A | A19 | PST | YHGGFMBQBLO | |
| E1611 | 1963 | D 10 | 83A | A25 | PST | YHGFMBQBLO | |
| E1907 | 1964 | D 11 | 83A | A20 | PST | YHGFMBQBLO | 3 3 1 1 4 4 16 |
| E2251¶ | 1965 | D 14 | 83A | A19 | PST | YHGFMBQBLO | 3 3 1 1 4 4 16 |
| E2260¶ | 1965 | D 15 | 83A | A20 | PST | YHFGFMBQBLO | 3 3 1 1 4 4 16 |
| E2310¶ | 1965 | D 16 | 83A | A26 | PST | YHGFMBQBLO | |
| E2611¶ | 1966 | D 18 | 83A | A21 | PST | YHGFMBQBLO | |
| E228 | 1957 | D ND | 83A | f | PS | YHGFMBQBLO | 3 3 1 1 4 4 3 |
| E1410 | 1962 | D 8 | NT | g | PS | WGKAKAOMQ | 2 2 2 2 6 3 2 |
| E2104 | 1964 | D 4 | III | e1 | P | TJMBMDMGMK | 1 4 1 4 12 1 10 |
| E3001¶ | 1967 | D 7 | 83A | e2 | P | TJMBMDMMK | 1 4 1 4 12 1 10 |
| E3008¶ | 1967 | D 19 | III | A27 | P | YHGFMBQBLO | 3 3 1 1 4 4 3 |
| E2615¶ | 1966 | D 17 | III | d | P | UJFKBPE | |
| E3410¶ | 1968 | D 20 | 83A | d | Susceptible | UJFKBPE | 1 1 1 1 1 1 1 |
| E3445¶ | 1968 | D 7 | 83A | d | Susceptible | UJFKBPE |
ND, not determined; NT, nontypable.
Danish hospitals: D 1, Haderslev; D 2, Bispebjerg; D 3, Horsens; D 4, Blegdamshospitalet; D 5, Rigshospitalet; D 6, Frederiksberg; D 7, Aarhus; D 8, Sundby; D 9, Skive; D 10, Hjørring; D 11, Diakonissen; D 12, Gentofte; D 13, Varde; D 14, Kolding; D 15, Randers; D 16, Aalborg; D 17, Kalundborg; D 18, Holstebro; D 19, Kommunehospitalet; D 20, Næstved; D 21, Usserød; D 22, Fuglebakken. UK1, Carshalton, Surrey, U.K.; UK2, Colindale, U.K.
P, penicillin; S, streptomycin; T, tetracycline; M, methicillin; E, erythromycin; C, clindamycin.
Sequence types (ST) assigned as described (13).
Strains partially characterized in a previous study (1).
Of the total of 24 early MRSA, 17 originated in Denmark and 7 in the U.K. The 17 Danish MRSA were isolated between 1964 and 1970; 10 of the strains belonged to the phage complex 83A and 7 to phage group III. All 17 strains were resistant to P-S-T-M. The 7 British MRSA were isolated in 1960 and 1963; they belonged to phage group III and 6 of these strains had the P-S-T-M antibiotype. The MRSA isolates 10395 and 13136 represent the very first reported S. aureus isolates exhibiting resistance toward M (3). The remaining U.K. strains were recovered in the first recorded outbreak of nosocomial infection by MRSA between 1961 and 1962 in Queen Mary Hospital for Children (ref. 4; Table 2).
Table 2.
Properties of the historically early strains of MRSA
| Strain | Date | Origin* | Phage group | Clonal type (PFGE) | Antibiogram† | spaA type‡ | Allelic profile§ |
|---|---|---|---|---|---|---|---|
| 10395‖ | 1960 | UK2 | III | II∷nh∷A11 | PSTM | YHGFMBQBLO | 3 3 1 1 4 4 16 |
| 13136‖ | 1960 | UK2 | III | II∷nh∷A11 | PSTM | YHGFMBQBLO | 3 3 1 1 4 4 16 |
| ST61/6219** | 1961 | UK1 | III | II∷nh∷A11 | PSTM | YHFGFMBQBLO | 3 3 1 1 4 4 16 |
| ST61/6421** | 1961 | UK1 | III | II∷nh∷A11 | PSTM | YHFGFMBQBLO | 3 3 1 1 4 4 16 |
| 6467** | 1963 | UK1 | III | II∷nh∷A7 | PSTM | YHGFMBQBLO | 3 3 1 1 4 4 16 |
| ST63/458** | 1963 | UK1 | III | II∷nh∷A7 | PSTM | YHFGFMBQBLO | 3 3 1 1 4 4 16 |
| ST63/460** | 1963 | UK1 | III | II∷nh∷A11 | PSM | YHFGFMBQBLO | 3 3 1 1 4 4 16 |
| E2125¶ | 1964 | D 12 | 83A | II∷NH∷A1 | PSTM | YHFGFMBQBLO | 3 3 1 12 4 4 16 |
| E2265¶ | 1965 | D 5 | 83A | II∷nh∷A1 | PSTM | YHFGFMBQBLO | 3 3 1 12 4 4 16 |
| E2453¶ | 1965 | D 13 | 83A | II∷nh∷A1 | PSTM | YHFGFMBQBLO | 3 3 1 12 4 4 16 |
| E2600¶ | 1966 | D 19 | 83A | II∷nh∷A1 | PSTM | YHFGFMBQBLO | 3 3 1 12 4 4 16 |
| E2613¶ | 1966 | D 7 | 83A | II∷nh∷A1 | PSTM | YHFGFMBQBLO | 3 3 1 12 4 4 16 |
| E2614¶ | 1966 | D 7 | 83A | II∷nh∷A2 | PSTM | YHFGFMBQBLO | 3 3 1 12 4 4 16 |
| E3005¶ | 1967 | D 16 | 83A | II∷nh∷A2 | PSTM | YHFGFMBQBLO | 3 3 1 12 4 4 16 |
| E3018¶ | 1967 | D 19 | 83A | II∷nh∷A3 | PSTM | YHFGFMBQBLO | 3 3 1 12 4 4 16 |
| E3409¶ | 1967 | D 22 | 83A | II∷nh∷A1 | PSTM | YHFGFMBQBLO | 3 3 1 12 4 4 16 |
| E3411¶ | 1968 | D 11 | 83A | II∷nh∷A4 | PSTM | YHFGFMBQBLO | 3 3 1 12 4 4 16 |
| E4278¶ | 1969 | D 16 | III | II∷nh∷A11 | PSTM | YHFGFMBQBLO | 3 3 1 12 4 4 16 |
| E2213¶ | 1965 | D 11 | III | II∷DD∷A9 | PSTMEC | YHFGFMBQBLO | 3 3 1 12 4 4 16 |
| E2672¶ | 1966 | D 22 | III | II∷DD∷A6 | PSTMEC | YHFGFMBQBLO | 3 3 1 12 4 4 16 |
| E3015¶ | 1967 | D 11 | III | II∷DD∷A10 | PSTMEC | YHFGFMBQBLO | 3 3 1 12 4 4 16 |
| E3150¶ | 1967 | D 2 | III | II∷DD∷A11 | PSTMEC | YHFGFMBQBLO | 3 3 1 12 4 4 16 |
| E3520¶ | 1968 | D 21 | III | II∷zeta∷A7 | PSTMEC | YHGFMBQBLO | 3 3 1 1 4 4 16 |
| E3760¶ | 1968 | D 21 | III | II∷z∷A13 | PSTMEC | YHGFMBQBLO | 3 3 1 1 4 4 16 |
Macrorestriction (PFGE) Profiles.
Comparison of the PFGE patterns obtained after restriction of chromosomal DNA with SmaI for the 25 MSSA and 24 MRSA strains showed a remarkable uniformity for most of these historically early S. aureus isolates. All 17 MSSA isolates with the antibiotype P-S-T, and one with the antibiotype P, as well as all 24 MRSA isolates belonged to subtypes of a common PFGE pattern A. The remaining seven MSSA strains showed unique PFGE patterns d, f, g, and e (see Tables 1 and 2).
spaA Typing.
The spaA repeat sequences in the early MSSA and MRSA isolates were compared. The majority of the MSSA strains with antibiotype P-S-T (14 of 17 isolates) and one of the P-resistant MSSA strains (E3008) shared a uniform spaA repeat code, YHGFMBQBLO, corresponding to spaA type 1 (12). Of the remaining 2 MSSA with antibiotype P-S-T, one strain (E712) showed a complete deletion of the internal spaA sequences, YO, and strain E1600 had a duplication of the G repeat (YHGGFMBQBLO). An identical spaA type 1 was detected in the Danish MRSA strains E3520 and E3760, and in the U.K. strains 6467, 10395, and 13136. Each one of the rest of the 19 early MRSA strains shared a common spaA type 4, which only differed from type 1 present in most early MSSA by the duplication of a single F repeat that was inserted into the spaA sequence between the H and G repeats (YHFGFMBQBLO; see Tables 1 and 2).
The phage nontypable MSSA strain, E1410, the two MSSA strains with antibiotype P and PFGE pattern e (E2104 and E3001), and the three MSSA with PFGE pattern d (E2615, E3410, and E3445) each showed unique spaA types (see Table 1).
MLST.
A total of 37 early S. aureus isolates, 13 MSSA and 24 MRSA, also were compared by MLST. Each of the 7 MSSA analyzed with antibiotype P-S-T and spaA type 1 yielded a common 3, 3, 1, 1, 4, 4, 16 allelic profile for the 7 housekeeping genes arcC, aroE, glpF, gmk, pta, tpi, and yqiL (Table 1). An identical profile was detected in two Danish MRSA (E3520 and 3760) and in the seven MRSA strains isolated in the U.K. between 1960 and 1963. A very similar allelic profile, 3, 3, 1, 12, 4, 4, 16, differing only at the single locus gmk, was identified in the rest of the 15 MRSA sequenced (Table 2). Moreover, a similar profile was shown by two MSSA strains (E228 and E3008): allelic profile, 3, 3, 1, 1, 4, 4, 3, or type ST8 (see Table 1).
Completely different and unique MLS types were identified in the fully susceptible strain E3410 (type ST49), in the single-phage nontypable strain E1410 (type ST30) and in strain E2104 (type ST5) (Table 1). The MLS types ST8 and ST49 and 30 and 5 have been identified recently in a collection of S. aureus strains from the U.K. in 1997–1998 (13).
Early MSSA and MRSA and Contemporary MRSA Clones.
The early (“archaic”) MRSA isolates from Denmark and the U.K., which shared similar genetic backgrounds with early MSSA isolates, also were compared with the genetic profiles of several contemporary MRSA clones. Data in Table 3 show the remarkable similarities between properties of the archaic MRSA isolates and those of one of the most widely spread contemporary MRSA, the “Iberian” clone (19–21). Additional, close similarities became apparent between the genetic profile of the phage nontypable MSSA strain E1410 and “EMRSA 16”—another widely dispersed MRSA clone (22). The genetic background (PFGE pattern, spaA type, and MLS type) of the MSSA strains E2104 and 3001 was found to be very similar to the genetic background of two distinct MRSA lineages, the “Pediatric” clone (23) and the “New York” clone (refs. 24–26; see Table 3). Computer analysis of the MLST data followed by the construction of unweighted pair group method with arithmetic mean (UPGMA) trees (data not shown) confirmed the close genetic relatedness of the MRSA contemporary clones and their postulated evolutionary relatives (maximum genetic distance around 0.15).
Table 3.
Postulated evolutionary relationships among historically early isolates of MSSA, MRSA, and contemporary epidemic clones
| Strain | Clone | spaA type‡ | Allelic profile§ |
|---|---|---|---|
| E213 | MSSA | YHGFMBQBLO | 3 3 1 1 4 4 16 |
| ST61/6219 | Archaic | YHFGFMBQBLO | 3 3 1 1 4 4 16 |
| E2125 | Archaic | YHFGFMBQBLO | 3 3 1 12 4 4 16 |
| HPV107 | Iberian | YHFGFMBQBLO | 3 3 1 12 4 4 16 |
| PER34 | Iberian | YHGFMBQBLO | 3 3 1 1 4 4 16 |
| E2104 | MSSA | TJMBMDMGMK | 1 4 1 4 12 1 10 |
| E3001 | MSSA | TJMBMDMMK | 1 4 1 4 12 1 10 |
| HDE288 | Pediatric | TJMBDMGMK | 1 4 1 4 12 1 10 |
| BK2464 | New York | TJMBMDMGMK | 1 4 1 4 12 1 10 |
| E1410 | MSSA | WGKAKAOMQ | 2 2 2 2 6 3 2 |
| (Ref. 19) | EMRSA-16 | ND | 2 2 2 2 3 3 2‡‡ |
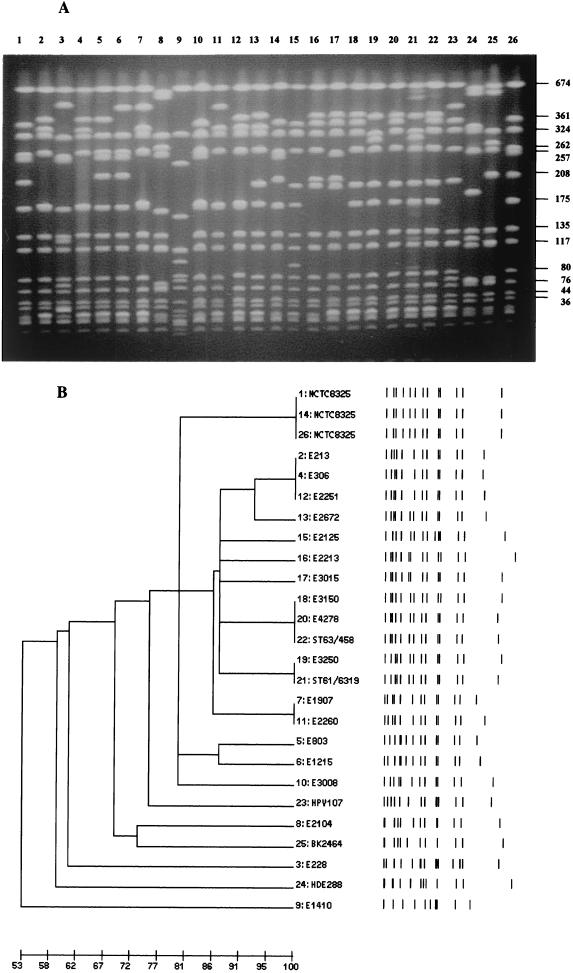
The PFGE patterns in Fig. 2A and the corresponding dendrogram (Fig. 2B) demonstrate the similarities between early MSSA and MRSA isolates and several contemporary MRSA clones such as the Iberian, Pediatric, and New York clones.
Figure 2.
(A) Comparison of PFGE patterns from historically early MSSA and MRSA isolates from Denmark and the U.K. and representatives of the Iberian, Pediatric, and New York clones of MRSA. Lanes 2–12 show early MSSA strains (Table 1). Lanes 13 and 15–22 show early MRSA strains (Table 2). Lanes 23–25 show contemporary MRSA (Table 3). Strains used in A are underlined in Tables 1, 2, and 3. Lane 2, E213; lane 3, E228; lane 4, E306; lane 5, E803; lane 6, E1215; lane 7, E1907; lane 8, E2104; lane 9, E1410; lane 10, E3008; lane 11, E2260; lane 12, E2251; lane 13, E2672; lane 15, E2125; lane 16, E2213; lane 17, E3015; lane 18, E3150; lane 19, E3520; lane 20, E4278; lane 21, ST61/6219; lane 22, ST63/458; lane 23, Iberian clone HPV107 (19); lane 24, Pediatric clone HDE288 (23); lane 25, New York clone BK2464 (24). Lanes 1, 14, and 26 show strain NCTC8325 used as molecular weight standard. (B) Computer-generated dendrogram of the PFGE patterns shown in A. Results are from analysis of similarity by Jacquard's coefficient; clustering was done by the minimum linkage method.
Discussion
The acquisition of M resistance has provided S. aureus with a mechanism that has made all members of the largest and most useful family of antimicrobial agents, the β-lactam antibiotics, obsolete as therapeutic agents against these bacteria. The emergence and global spread of MRSA may be viewed as a process of accelerated evolution (27), which took place in the contemporary clinical environment, condensed into a remarkably short time frame of 4 decades during which MRSA has emerged and spread to every corner of the world, driven by the selective pressure of immense quantities of antimicrobial agents introduced into the clinical environment. The starting point of this process is the entry of the resistance gene mecA and associated mec element into the species of S. aureus from a heterologous source. Although the exact timing of this event is not known, it is reasonable to assume that it is linked to the time of introduction of the penicillinase-resistant oxazolidine class of antibiotics, such as M, into therapy at the end of the 1950s and/or early 1960s. The very first MRSA in Europe was isolated in a British hospital in 1961 (3) and the first Danish blood isolates of MRSA were recovered in 1963 (2).
The early MRSA isolates may represent the progeny of MSSA that was the original recipient of the heterologous mec element. If this hypothesis is true, then one expects to identify the postulated recipient among MSSA strains originating in the same geographic area and same era. To test this hypothesis, we compared the genetic backgrounds of historically early MSSA as well as MRSA isolates from Denmark and from the U.K. recovered shortly before and after the introduction of M into clinical practice, i.e., around the year 1960.
The results of the experiments described in this communication confirm this hypothesis by demonstrating the striking similarities between MRSA strains and most of the MSSA isolated in Denmark and the U.K. during this era. Most early MSSA examined shared a common phage group and multiresistance (P-S-T) pattern with the early MRSA strains and showed close resemblance if not identity in genetic profiles. The simplest interpretation of these findings is that the largest group of the early MSSA characterized here indeed represented the progeny of an S. aureus strain that must have been one of the first recipients of the mec element during the evolutionary history of MRSA. The postulated heterologous donor of the mec element and its mode of introduction into S. aureus remain unknown at the present time.
The presence of resistance determinants to P, S, and T, and occasionally to E as well, in the earliest MRSA isolates most likely mirrors the sequential introduction of these different types of antimicrobial agents into therapeutic practice. Data from the records of the Danish Health Board indicate that the years of introduction of these antimicrobial agents were 1945/46 (P), 1948 (S), 1950 (T), 1953 (E), and 1960 (M) (8). The accumulation of multidrug-resistance traits in MRSA strains has remained the hallmark of antibiotic-resistant S. aureus throughout much of the subsequent history of this bacterial pathogen, most likely as the consequence of the preferential use of new antimicrobial agents against bacteria that were already resistant to all previously used therapeutic agents, thus presenting a scenario of built-in obsolescence for new antimicrobial agents (28). The introduction of radical and effective infection-control measures in Denmark in the 1970s caused a virtual disappearance of MRSA from Danish hospitals, including the archaic MRSA strains (29). The few MRSA isolates that were identified in 1986 in clinical specimens with properties of the archaic clone seem to have survived only in the unique and protected milieu of osteomyelitis (1).
Extending the genetic profiling (PFGE, spaA type, and MLS type) to some of the widely spread contemporary MRSA clones also has allowed us to identify among the historically early MSSA several strains with genetic backgrounds matching those of contemporary epidemic clones of MRSA. The most important one of these is the Iberian clone, which shares similar PFGE type, spaA type, and MLST pattern with the majority of early MSSA and MRSA and also carries a mec element similar in genetic structure to that of the mec element (type I SCCmec) identified in early MRSA by Hiramatsu and colleagues (30); the only difference being the insertion of linearized plasmid pUB110 in the case of the Iberian clone downstream of mecA (31).
Two of the early MSSA strains, E2104 and 3001, exhibiting resistance only to P, showed a PFGE pattern and spaA and MLS types that were very similar to the properties of two internationally spread MRSA: the Pediatric clone, which most often shows resistance only to β-lactam antibiotics (23), and another contemporary multiresistant MRSA widely spread in the northeastern part of the United States, the New York clone (25–26). Bacteria belonging to the New York clone carry a mec element that seems to correspond to what Hiramatsu and colleagues (30) named the type II SCCmec (ref. 30 and D.C.O., A.T., and H.d.L., unpublished data). The nature of the mec element in the Pediatric clone is currently under investigation.
A single isolate, strain E1410, among the 25 early MSSA, was nontypable by the phages used and was resistant to P-S, possessed a unique PFGE type g, and also showed spaA and MLS type, which was different from that shown in the rest of the 24 MSSA tested. A search through contemporary MRSA clones with the molecular typing techniques identified the MLS type seen in strain E1410, as typical of yet another major contemporary MRSA clone, the “EMRSA 16”, widely spread in hospitals in the U.K. (13). Interestingly, MSSA strains with the same genetic background are still frequent among MSSA isolates from the U.K. (13). Analysis of a representative of this clone suggests that it carries a type II SCCmec (30).
These observations indicate that MSSA strains ready to participate as recipients in the horizontal spread of mecA were already present among MSSA isolates recovered around the early 1960s. These strains may have remained in circulation to acquire various forms of the mec element and provide the genetic backgrounds for some of the contemporary MRSA clones.
The number of times the mec element entered the species of S. aureus from heterologous sources is not known and may be limited (11). Nevertheless, examination of MRSA isolates from the 1980s has demonstrated clearly the extensive horizontal spread of the mec gene throughout the entire genetic breath of S. aureus, possibly by homologous gene transfer (32). The role of such sporadic isolates in the epidemiology of MRSA is not clear.
Molecular epidemiological studies conducted in several countries since the late 1980s clearly indicate that the major factor responsible for the massive geographic spread of MRSA is the dissemination of a relatively few highly epidemic clones. The archaic MRSA carrying the P-S-T multiresistance traits and phage group 83A/III was clearly such a successful lineage, as judged by the rapid spread of this clone to many Danish as well as British hospitals within a few years of its appearance. The results described in this article indicate that one of the most successful contemporary MRSA clones, the Iberian MRSA, detected as the dominant MRSA in many hospitals in western and southern Europe and in at least one hospital in New York City (33), is a direct descendant of the archaic MRSA. These observations strongly suggest that the genetic background of this particular MRSA lineage, extending from MSSA identified in disease-causing strains since the late 1950s through the early MRSA isolates to one of the major contemporary MRSA clones, carries genetic traits essential for the superior epidemicity and virulence of these bacteria.
Acknowledgments
We thank Professor Jeremy M. T. Hamilton-Miller who kindly provided the 3 MRSA, originating from the U.K., that represent the first reported MRSA isolates. We also thank Dr. Richard B. Roberts (New York Hospital–Cornell Medical Center) who forward to us 4 MRSA strains, also from the U.K., that were isolated originally by Professor George T. Stewart (Univ. of Glasgow, Scotland) and obtained from the National Collection of Type Cultures, London, U.K. Partial support for this study was provided by project PRAXIS Grants XXI/2/2.2/SAU/1295/95 and P/SAU/14052/98 from Fundação para a Ciência e Tecnologia, Lisbon, Portugal (to H.d.L.) and by US Public Health Service National Institutes of Health Grant RO1 AI45738 (to A.T.). D.C.O. was supported by Fundação para a Ciência e Tecnologia Grant BD/4162/96, and M.I.C. was supported by Instituto de Tecnologia Química e Biológica/Universidade Nova de Lisboa Grant 022/BIC/2000. This publication made use of the MLST web site (http://www.mlst.net) developed by Dr. Man-Suen Chan, which was funded by the Wellcome Trust and sited at the Wellcome Trust Center for the Epidemiology of Infectious Disease, University of Oxford. Preliminary sequence data were obtained from the Institute for Genomic Research web site at http://www.tigr.org.
Abbreviations
- M
methicillin
- P
penicillin
- S
streptomycin
- T
tetracycline
- E
erythromycin
- MRSA
M-resistant S. aureus
- MSSA
M-susceptible S. aureus
- PFGE
pulsed-field gel electrophoresis
- MLST
multilocus sequence typing
References
- 1.de Lencastre H, Chung M, Westh H. Microb Drug Resist. 2000;6:1–10. doi: 10.1089/mdr.2000.6.1. [DOI] [PubMed] [Google Scholar]
- 2.Eriksen K R, Erichesen I. Acta Pathol Microbiol Scand Suppl. 1964;52:255–275. doi: 10.1111/apm.1964.62.2.255. [DOI] [PubMed] [Google Scholar]
- 3.Jevons M P. Br Med J. 1961;1:124–125. [Google Scholar]
- 4.Stewart G T, Holt R J. Br Med J. 1963;1:308–311. doi: 10.1136/bmj.1.5326.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamilton-Miller J M, T, Ramsay J. J Gen Microbiol. 1967;49:491–501. [Google Scholar]
- 6.Blair J E, Williams R E O. Bull W H O. 1961;24:771–784. [PMC free article] [PubMed] [Google Scholar]
- 7.Bülow P. APMIS Suppl. 1968;80:147–159. [Google Scholar]
- 8.Jessen O, Rosendal K, Bülow P, Faber V, Eriksen K R. N Engl J Med. 1969;281:627–635. doi: 10.1056/NEJM196909182811201. [DOI] [PubMed] [Google Scholar]
- 9.de Lencastre H, Couto I, Sanches I S, Melo-Cristino J, Torres-Pereira A, Tomasz A. Eur J Clin Microbiol Infect Dis. 1994;13:64–73. doi: 10.1007/BF02026129. [DOI] [PubMed] [Google Scholar]
- 10.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kreiswirth B, Kornblum J, Arbeit R D, Eisner W, Maslow J N, McGeer A, Low D E, NovicK R P. Science. 1993;259:227–230. doi: 10.1126/science.8093647. [DOI] [PubMed] [Google Scholar]
- 12.Shopsin B, Gomez M, Montgomery S O, Smith D H, Waddington M, Dodge D E, Bost D A, Riehman M, Naidich S, Kreiswirth B N. J Clin Microbiol. 1999;37:3556–3563. doi: 10.1128/jcm.37.11.3556-3563.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enright M C, Day N P, Davies C E, Peacock S J, Spratt B G. J Clin Microbiol. 2000;38:1008–1015. doi: 10.1128/jcm.38.3.1008-1015.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boom R, Sol C J, Salimans M M, Jansen C L, Wertheim-van Dillen P M, van der Noordaa J. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oliveira D C, Crisóstomo I, Santos-Sanches I, Major P, Alves C R, de Sousa M A, Konkoly-Thege M, de Lencastre H. J Clin Microbiol. 2001;39:574–580. doi: 10.1128/JCM.39.2.574-580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shopsin B, Gomez M, Waddington M, Riehman M, Kreiswirth B N. J Clin Microbiol. 2000;38:3453–3456. doi: 10.1128/jcm.38.9.3453-3456.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shopsin B, Mathema B, Zhao X, Martinez J, Kornblum J, Kreiswirth B N. Microb Drug Resist. 2000;6:239–244. doi: 10.1089/mdr.2000.6.239. [DOI] [PubMed] [Google Scholar]
- 18.Rosendal K, Bülow P. APMIS Suppl. 1971;79:377–384. doi: 10.1111/j.1699-0463.1971.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 19.Sanches I S, Ramirez M, Troni H, Abecassis M, Padua M, Tomasz A, de Lencastre H. J Clin Microbiol. 1995;33:1243–1246. doi: 10.1128/jcm.33.5.1243-1246.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dominguez M A, de Lencastre H, Linares J, Tomasz A. J Clin Microbiol. 1994;32:2081–2087. doi: 10.1128/jcm.32.9.2081-2087.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mato R, Santos Sanches I, Venditti M, Platt D J, Brown A, Chung M, de Lencastre H. Microb Drug Resist. 1998;4:107–112. doi: 10.1089/mdr.1998.4.107. [DOI] [PubMed] [Google Scholar]
- 22.Cox R A, Conquest C, Mallaghan C, Marples R R. J Hosp Infect. 1995;29:87–106. doi: 10.1016/0195-6701(95)90191-4. [DOI] [PubMed] [Google Scholar]
- 23.Sa-Leao R, Santos Sanches I, Dias D, Peres I, Barros R M, de Lencastre H. J Clin Microbiol. 1999;37:1913–1920. doi: 10.1128/jcm.37.6.1913-1920.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kreiswirth B N, Lutwick S M, Chapnick E K, Gradon J D, Lutwick L I, Sepkowitz D V, Eisner W, Levi M H. Microb Drug Resist. 1995;1:307–313. doi: 10.1089/mdr.1995.1.307. [DOI] [PubMed] [Google Scholar]
- 25.Roberts R B, de Lencastre A, Eisner W, Severina E P, Shopsin B, Kreiswirth B N, Tomasz A. J Infect Dis. 1998;178:164–171. doi: 10.1086/515610. [DOI] [PubMed] [Google Scholar]
- 26.Roberts R B, Chung M, de Lencastre H, Hargrave J, Tomasz A, Nicolau D P, John J F, Jr, Korzeniowski O Tri-State MRSA Collaborative Study Group. Microb Drug Resist. 2000;6:245–251. doi: 10.1089/mdr.2000.6.245. [DOI] [PubMed] [Google Scholar]
- 27.Tomasz A. Neth J Med. 1998;52:219–227. doi: 10.1016/s0300-2977(98)00035-7. [DOI] [PubMed] [Google Scholar]
- 28.Tomasz A. N Engl J Med. 1994;330:1247–1251. doi: 10.1056/NEJM199404283301725. [DOI] [PubMed] [Google Scholar]
- 29.Westh H, Jarlov J O, Kjersem H, Rosdahl V T. Clin Infect Dis. 1992;14:1186–1194. doi: 10.1093/clinids/14.6.1186. [DOI] [PubMed] [Google Scholar]
- 30.Ito T, Katayama Y, Asada K, Mori N, Tsutsumimoto K, Tiensasitorn C, Hiramatsu K. Antimicrob Agents Chemother. 2001;45:1323–1336. doi: 10.1128/AAC.45.5.1323-1336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliveira D C, Wu S W, de Lencastre H. Antimicrob Agents Chemother. 2000;44:1906–1910. doi: 10.1128/aac.44.7.1906-1910.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Musser J M, Kapur V. J Clin Microbiol. 1992;30:2058–2063. doi: 10.1128/jcm.30.8.2058-2063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts R B, Tennenberg A M, Eisner W, Hargrave J, Drusin L M, Yurt R, Kreiswirth B N. Microb Drug Resist. 1998;4:175–183. doi: 10.1089/mdr.1998.4.175. [DOI] [PubMed] [Google Scholar]