Abstract
Exposure of the lung to ionizing radiation that occurs in radiotherapy, as well as after accidental or intentional mass casualty incident can result in pulmonary fibrosis, which has few treatment options. Pulmonary fibrosis is characterized by an accumulation of extracellular matrix proteins that create scar tissue. Although the mechanisms leading to radiation-induced pulmonary fibrosis remain poorly understood, one frequent observation is the activation of the profibrotic cytokine transforming growth factor-beta (TGF-β). Our laboratory has shown that the metabolite lactate activates latent TGF-β by a reduction in extracellular pH. We recently demonstrated that lactate dehydrogenase-A (LDHA), the enzyme that produces lactate, is upregulated in patients with radiation-induced pulmonary fibrosis. Furthermore, genetic silencing of LDHA or pharmacologic inhibition using the LDHA inhibitor gossypol prevented radiation-induced extracellular matrix secretion in vitro through inhibition of TGF-β activation. In the current study, we hypothesized that LDHA inhibition in vivo prevents radiation-induced pulmonary fibrosis. To test this hypothesis, C57BL/6 mice received 5 Gy total-body irradiation plus 10 Gy thoracic irradiation from a 137Cs source to induce pulmonary fibrosis. Starting at 4 weeks postirradiation, mice were treated with 5 mg/kg of the LDHA inhibitor gossypol or vehicle daily until sacrifice at 26 weeks postirradiation. Exposure to radiation resulted in pulmonary fibrosis, characterized by an increase in collagen content, fibrosis area, extracellular matrix gene expression and TGF-β activation. Irradiated mice treated with gossypol had significantly reduced fibrosis outcomes, including reduced collagen content in the lungs, reduced expression of active TGF-β, LDHA and the transcription factor hypoxia-inducible factor-1 alpha (HIF-1α). These findings suggest that inhibition of LDHA protects against radiation-induced pulmonary fibrosis, and may be a novel therapeutic strategy for radiation-induced pulmonary fibrosis.
INTRODUCTION
Radiation-induced pulmonary fibrosis is a dose-limiting side effect of thoracic radiation therapy due to its associated morbidity and mortality (1). It is estimated that up to 20% of patients who receive radiation therapy for tumors in the thoracic region, including lung, lymph nodes and breast, will go on to develop pulmonary fibrosis (2, 3); however, there are few effective therapies for pulmonary fibrosis. Therefore, it is crucial to identify novel therapeutic targets and develop new therapies to combat pulmonary fibrosis.
Pulmonary fibrosis is characterized by an accumulation of extracellular matrix (ECM) proteins that ultimately compromise the lung’s ability to exchange oxygen. These ECM proteins are secreted by scar-forming myofibroblasts, which express markers of smooth muscle cells. Myofibroblasts arise from undifferentiated resident lung fibroblasts in response to profibrotic stimuli, including ionizing radiation, with lung fibroblasts differentiating into myofibroblasts and secreting extracellular matrix proteins that can contribute to fibrogenesis (4, 5). Therefore, inhibiting radiation-induced myofibroblast differentiation may be an important therapeutic approach in the prevention of radiation-induced extracellular matrix accumulation and fibrosis.
Our laboratory has identified the enzyme lactate dehydrogenase-A (LDHA) as a potential therapeutic target for inhibiting fibroblast to myofibroblast differentiation and extracellular matrix secretion. We recently reported that LDHA expression is highly upregulated in lung tissue from patients with radiation-induced pulmonary fibrosis, and is upregulated in primary human lung fibroblast cultures after ionizing irradiation (4). We also showed that lactate, the metabolic product of LDHA, is increased in patients with pulmonary fibrosis (6) and is secreted by lung fibroblasts in response to radiation, resulting in a reduction in extracellular pH in cell culture supernatants (4). Importantly, we demonstrated that by reducing extracellular pH, lactate is a novel activator of the profibrotic cytokine transforming growth factor beta (TGF-β) through a pH-dependent mechanism (6).
TGF-β is upregulated after exposure to radiation (7, 8), and is both necessary and sufficient for the development of pulmonary fibrosis (9–11). We have demonstrated that TGF-β induces expression of LDHA and lactate production in lung fibroblast cultures. Thus, we proposed a profibrotic feed-forward loop in which radiation upregulates LDHA, leading to increased lactate secretion, acidification of the extracellular space and ultimately activation of TGF-β to drive fibrosis.
In support of our hypothesized profibrotic feed-forward loop, we have previously demonstrated that genetic silencing or pharmacologic inhibition with the LDHA inhibitor gossypol in vitro can inhibit radiation-induced LDHA expression, lactate secretion, extracellular acidification and TGF-β activation in lung fibroblasts (4). Thus, in the current study, we examined whether inhibition of LDHA could inhibit radiation-induced pulmonary fibrosis in vivo. Our results demonstrate that the LDHA inhibitor gossypol is highly effective at inhibiting radiation-induced collagen accumulation and fibrosis, supporting the idea that LDHA is a potential therapeutic target for radiation-induced pulmonary fibrosis.
MATERIALS AND METHODS
Mice and Irradiations
All animal experiments were performed under supervision of the University of Rochester Committee on Animal Research. Male C57BL/6J 6–8-week-old mice were obtained from Jackson Laboratory (Bar Harbor, ME) and were housed five mice per cage. Mice were irradiated using a cesium-137 (137Cs) gamma-radiation source at approximately 154 cGy/min to achieve 5 Gy total-body irradiation plus 10 Gy thoracic irradiation. This protocol has been proposed as a model of accidental exposure (12). Thoracic irradiation was performed using a lead-shielded slit-beam collimator while animals were restrained in plastic jigs to shield the head, abdomen and limbs. Mice were sacrificed at week 26 postirradiation and lung tissue was collected. Ten mice were used per experimental group. No mortality from the radiation exposure was observed.
Gossypol Treatment
Gossypol (Sigma-Aldrich® LLC, St. Louis, MO) was administered by daily subcutaneous (s.c.) injection to mice starting at week 4 postirradiation at a dose of 5 mg/kg in 30 μl volume. Gossypol was prepared on a weekly basis in a solution of 60% dimethyl sulfoxide (DMSO)/40% phosphate buffered saline (PBS). Vehicle-treated animals were s.c. injected with equal volume per weight of 60% DMSO/40% PBS.
Hydroxyproline Assay
Hydroxyproline content was measured in right upper and lower lung lobes using a modified Woessner method as previously described (13). Lung tissue was homogenized in water and hydrolyzed overnight in 6 N HCl at 110°C. Samples were neutralized with NaOH, chloramine T reagent was added for 20 min, followed by inactivation with 3.15 N perchloric acid. Ehrlich’s solution was added, and samples were incubated for 20 min at 60°C. Absorbance was measured at 560 nm and a standard curve using purified hydroxyproline (Sigma-Aldrich) was used to extrapolate hydroxyproline content.
Lung Tissue Histologic Staining
The left lung lobe was inflated and fixed with formalin. Staining was performed on 5-μm-thick tissue sections. Trichrome staining for collagen fibers was performed as previously described elsewhere using Gomori Trichrome reagent (Richard-Allan Scientific™/Thermo Scientific™, Pittsburgh, PA) (13). Immunohistochemistry was performed as previously described using antibodies to active TGF-β (R&D Systems™, Minneapolis, MN) and HIF-1α (Novus Biologicals LLC, Littleton, CO). Staining for HIF-1α was performed using the manufacturers’ guidelines. For the active TGF-β stain, tissue sections were quenched with 3% H2O2 in methanol for 6 min, followed by blocking with 5% normal goat serum in 0.05% Tween in PBS (PBST). Primary antibody was prepared at a 1:100 dilution in 1% normal goat serum in PBST. To verify specificity for active and not latent TGF-β, active TGF-β antibody was preincubated with human recombinant TGF-β at a ratio of 5:1 TGF-β to antibody for 30 min prior to adding to slides. Primary antibody was incubated overnight, followed by washing and addition of a biotinylated secondary antibody (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) for 1 h. Streptavidin-HRP (Jackson ImmunoResearch) was added to slides for 15 min, followed by developing with NovaRED™ (Vector® Laboratories, Burlingame, CA). Slides were counterstained with hematoxylin (Biocare Medical, Concord, CA) and mounted.
Percentage Fibrosis Quantification
For irradiated mice (with or without gossypol), up to six step sections were made into the paraffin blocks at 150 μm intervals, and all step levels were trichrome stained and examined by microscopy. All slides were then digitized with a whole-slide scanner (Olympus VS110™; Olympus® America Inc., Melville, NY) and evaluated using cellSens Standard software version 1.15 (Olympus Soft Imaging Solutions, Munster, Germany). Briefly, total lung area was calculated by manually drawing regions of interest (ROIs) around the perimeter of each section of lung. Lesional area was next determined by manually drawing ROIs around lesions. For each mouse, total lesional area was calculated as a percentage of total lung area available for examination. ROIs were drawn by a pathologist who was blinded to the experimental conditions.
Quantitative Real-Time Polymerase Chain Reaction
Total RNA was isolated from mouse right middle lung lobes using TRIzol® reagent (Invitrogen™, Carlsbad, CA). Reverse transcription was performed using iScript™ cDNA synthesis kit (Bio-Rad® Laboratories Inc., Hercules, CA). Real-time polymerase chain reaction was performed using SsoAdvanced™ SYBR® Green (Bio-Rad). The following primer sequences were used: 18S: F:GCTTGCTCGCGCTT CCTTACCT R:TCACTGTACCGGCCGTGCGTA, Col1a1 F:CTGC TGGCAAAGATGGAG R:ACCAGGAAGACCCTGGAATC, Co-l3a1 F:AAATGGCATCCCAGGAG R:ATCTCGGCCAGGTTCTC, Fn1 F:TGCACGATGATATGGAGAGC R:TGGGTGTCACCTG ACTGAAC, TGF-β F:ATGTCACGGTTAGGGGCTC R:GGCT TGCATACTGTGCTGTATAG, HIF-1α F:GGGGAGGACGATG AACATCAA R:GGGTGGTTTCTTGTACCCACA, LDHA F:TGGC GACTCCAGTGTGCCTG R:AGGCACTGTCCACCTGCT.
Collagen Slot Blot
Right lung lobe homogenates were analyzed for native collagen using a slot blot manifold and immunodetection as previously described (14). Briefly, equal amounts of protein were applied to a PVDF membrane using a vacuum manifold (Amersham Biosciences, Piscataway, NJ). Membranes were probed with an antibody to collagen 1 (Santa Cruz Biotechnology® Inc., Dallas, TX) and a rabbit anti-goat secondary antibody (Jackson ImmunoResearch).
Western Blots
Western blots using right lung lobe homogenates were performed as previously described (15). PVDF membranes were probed using the following antibodies: fibronectin (Sigma-Aldrich) and β-tubulin (Abcam®, Cambridge, MA) and a goat anti-rabbit secondary antibody (Jackson ImmunoResearch). Densitometry was performed as previously described (15).
Statistical Analyses
All data are expressed as mean ± standard error. For the percentage fibrosis analysis, a nonparametric one-way analysis of variance (ANOVA) with a Dunn’s post test was used to establish statistical significance. For all other analyses, t test and ANOVA with Tukey post test were used to establish statistical significance using GraphPad Prism (GraphPad Software Inc., LaJolla, CA). Results were considered statistically significant at P < 0.05.
RESULTS
Gossypol Inhibits Radiation-Induced Pulmonary Fibrosis
We have previously reported elevated LDHA mRNA and protein expression in radiation-induced pulmonary fibrosis in humans and mice (4). To test the hypothesis that inhibiting LDHA activity will inhibit radiation-induced pulmonary fibrosis, mice were irradiated to induce fibrosis, then the LDHA inhibitor gossypol was administered to C57CL/6J mice starting at week 4 postirradiation and continuing until the day before sacrifice at week 26 postirradiation (Fig. 1A). The mice were sacrificed and the right lung lobes were removed and analyzed for collagen content by hydroxyproline assay. Gossypol alone does not cause lung injury or change expression of lung matrix proteins (data not shown). Radiation exposure resulted in a significant increase in hydroxyproline content (Fig. 1B). Gossypol significantly reduced hydroxyproline content to that consistent with control levels (Fig. 1B). Similarly, significant increases in collagen staining were observed in irradiated animals, as visualized with trichrome stain, compared to nonirradiated controls (Fig. 1C). However, gossypol-treated mice had little to no collagen staining and healthy lung architecture (Fig. 1C). To further quantitate the extent of fibrosis, we calculated the percentage area of fibrosis on trichrome-stained lung sections for each mouse. We sectioned through paraffin blocks, taking 5 μm sections every 150 μm, and ROIs were drawn around the entire lung section and the area of fibrotic lesions from the trichrome stain (Fig. 1D). No fibrosis was found in the nonirradiated, vehicle-treated mice, as expected. Irradiated and vehicle- treated mice showed areas of fibrosis that together averaged a 2% fibrosis area, which is consistent with the published literature on radiation-induced pulmonary fibrosis (9). Remarkably, we found no fibrosis at any level in any mice that were irradiated and received gossypol (Fig. 1D). Together, these results indicate that gossypol inhibited radiation-induced pulmonary fibrosis.
FIG. 1.
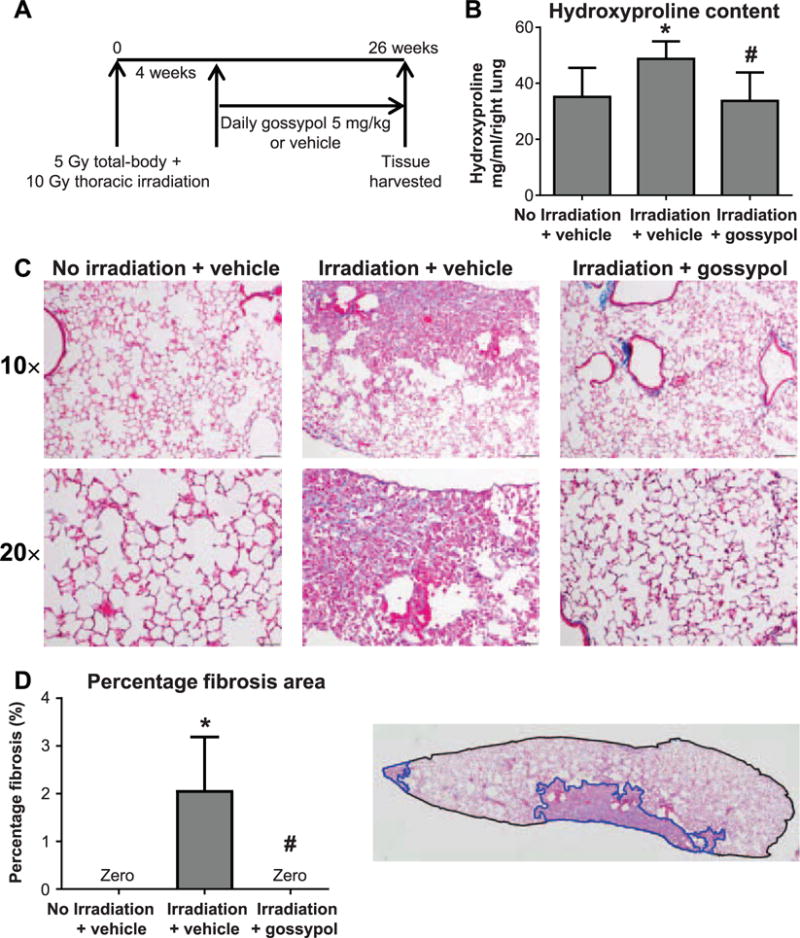
The LDHA inhibitor gossypol inhibits radiation-induced pulmonary fibrosis. Panel A: Mice received 5 Gy total-body irradiation plus 10 Gy thoracic ionizing irradiation from a 137Cs gamma radiation source and were treated with 5 mg/kg/day gossypol or vehicle beginning at week 4 postirradiation until week 26 postirradiation when lung tissue was harvested. Panel B: The right lung was analyzed for collagen content by hydroxyproline assay. Panel C: The left lung was formalin fixed, paraffin embedded and stained for collagen fibers using Gomori Trichrome. One representative mouse is shown from each treatment group. Images were taken at 10× and 20× magnification, and scale bars represent 100 μm. Panel D: Percentage fibrosis area was determined by drawing regions of interest (ROIs) around entire lung sections, and regions of fibrosis. One representative mouse section is shown; total lung section area is outlined in black, fibrotic lesions are outlined in blue. Total and fibrotic area were quantified as described in Materials and Methods. Data are shown as mean ± SEM for 5–6 sections per mouse and n = 7–10 mice per group. *P ≤ 0.05 compared to nonirradiated vehicle-treated controls; #P ≤ 0.05 compared to irradiated vehicle-treated mice (ANOVA).
Effects of Gossypol on Extracellular Matrix
Pulmonary fibrosis is characterized by an accumulation of extracellular matrix proteins. To examine whether gossypol prevented increases in extracellular matrix, we measured collagen and fibronectin mRNA levels in lung tissue by qRT-PCR. Irradiated vehicle-treated mice had increased levels of collagen 1, 3 and fibronectin mRNA expression levels compared to nonirradiated controls (Fig. 2A–C). In contrast, gossypol dampened radiation-induced increases in extracellular matrix mRNA (Fig. 2A–C). Collagen 1 mRNA levels were significantly reduced compared to that of vehicle-treated animals (Fig. 2A), while collagen 3 (Fig. 2B) and fibronectin mRNA levels (Fig. 2C) were also reduced, but not to statistically significant levels (P = 0.11 and P = 0.066, respectively).
FIG. 2.
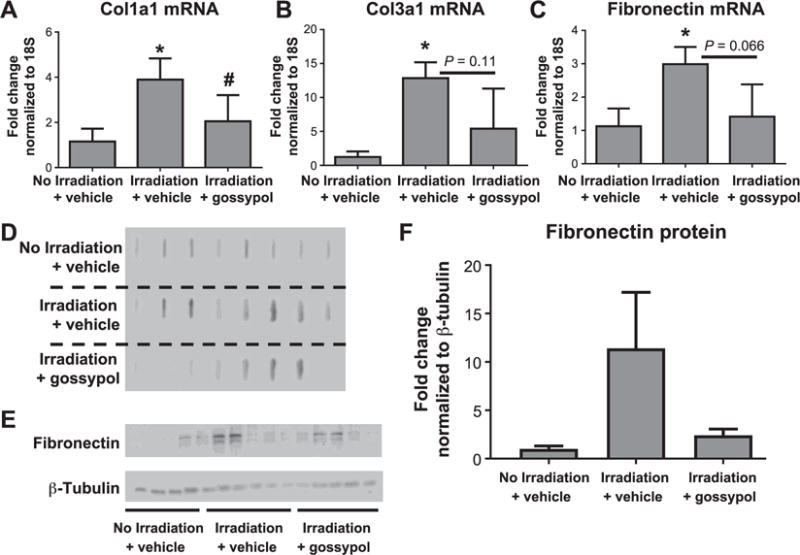
The effects of gossypol on extracellular matrix. Mice were irradiated and vehicle or gossypol treated as described in Materials and Methods. At week 26 postirradiation, the right middle lung lobe was collected for RNA isolation and mRNA expression analysis by qRT-PCR. mRNA quantification is expressed as fold change from nonirradiated controls normalized to 18S for col1a1 (panel A), col3a1 (panel B) and fibronectin (panel C). Total right lung homogenates were analyzed for native collagen using a slot blot (panel D), and for fibronectin by Western blot (panel E). Fibronectin protein was quantified using densitometry (panel F). Data are shown as mean ± SEM for n = 5–8 mice per group. *P ≤ 0.05 compared to nonirradiated vehicle-treated controls; #P ≤ 0.05 compared to irradiated vehicle-treated mice (ANOVA).
To determine if gossypol affected collagen protein expression, native collagen was examined using a slot blot and immunodetection. Compared to nonirradiated mice, the irradiated mice exhibited more intense bands for native collagen (Fig. 2D). However, only a few mice that were irradiated and treated with gossypol showed bands for collagen 1 (Fig. 2D). These data are consistent with our trichrome staining in Fig. 1. We also examined fibronectin protein expression by Western blot in right lung lobe homogenates. We observed very little expression for fibronectin in nonirradiated mice (Fig. 2E and F). Radiation increased fibronectin protein expression in some, but not all vehicle-treated mice (Fig. 2E and F). However, in irradiated vehicle-treated mice, there was visibly dampened expression of fibronectin. These data suggest that gossypol dampens expression of profibrotic extracellular matrix genes and protein.
Gossypol Inhibits Radiation-Induced TGF-β Expression and Activation
We previously demonstrated that lactate can activate latent TGF-β in a pH-dependent mechanism (6), and that gossypol inhibits radiation-induced increases in TGF-β bioactivity in primary human lung fibroblast cultures (4). To evaluate whether gossypol inhibits radiation-induced TGF-β expression in lung tissue, we measured levels of total TGF-β mRNA in lung homogenates. The irradiated mice had increased TGF-β mRNA expression compared to nonirradiated controls (Fig. 3A). However, irradiated mice treated with gossypol had decreased expression of TGF-β mRNA compared to vehicle-treated mice, which approached statistical significance (P = 0.065). Next, to differentiate between total and biologically active TGF-β expression in the lung, we stained lung tissue for active TGF-β using an antibody that detects the active TGF-β peptide. In nonirradiated mice, very little active TGF-β was detected in the lung, with only faint staining in the airways (Fig. 3B). Irradiated mice had large areas of diffuse staining for active TGF-β in areas of interstitial fibrosis, in alveolar macrophages and within alveolar septa (Fig. 3B). However, there was significantly reduced active TGF-β staining in irradiated mice treated with gossypol, which was localized mainly to the alveolar septa (Fig. 3B). To ensure that the TGF-β antibody was specific for the active, and not latent TGF-β, irradiated lung used in Fig. 4C was stained with antibody after a 30-min neutralization with human recombinant TGF-β prior to incubation on the slides. After neutralization, no positive staining was detected (Fig. 3B), suggesting that the antibody was properly neutralized and is specific for active and not latent TGF-β.
FIG. 3.
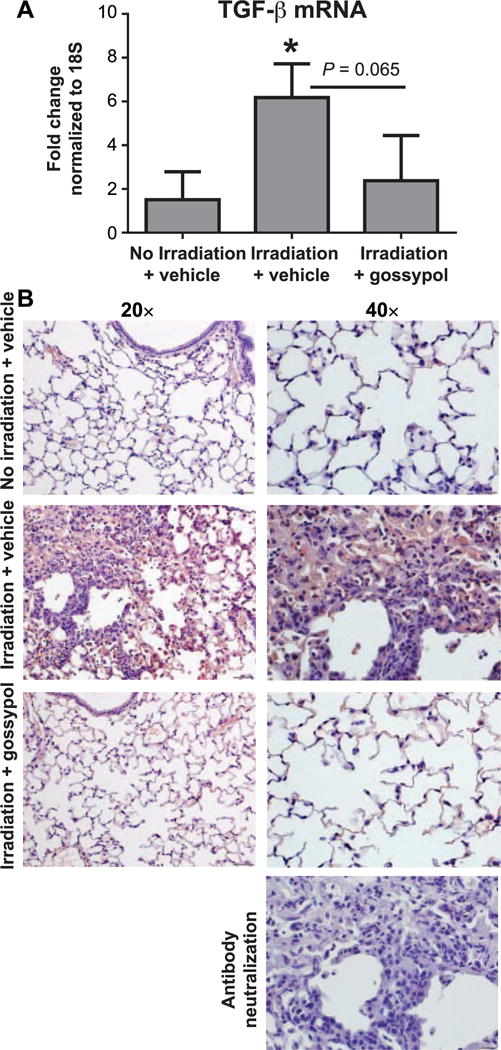
Gossypol dampens radiation-induced TGF-β expression and activation. Mice were irradiated and vehicle or gossypol treated as indicated in Materials and Methods. Whole lung tissue TGF-β mRNA was quantified using RT-PCR. Data is expressed as fold change compared to nonirradiated controls, normalized to 18S (panel A). Data are shown as mean ± SEM for n = 5–8 mice. *P ≤ 0.05 compared to nonirradiated vehicle-treated controls (ANOVA). Panel B: Lung tissue sections were stained with an antibody to active TGF-β (red) and counterstained with hematoxylin (blue) as described in Materials and Methods. One representative mouse is shown from each treatment group. For negative control, antibody neutralized with active human recombinant TGF-β was used at 30 min prior to staining irradiated lung tissue. Images were taken at 20× and 40× magnification. Scale bars represent 100 μm at 20× and 50 μm at 40×.
FIG. 4.
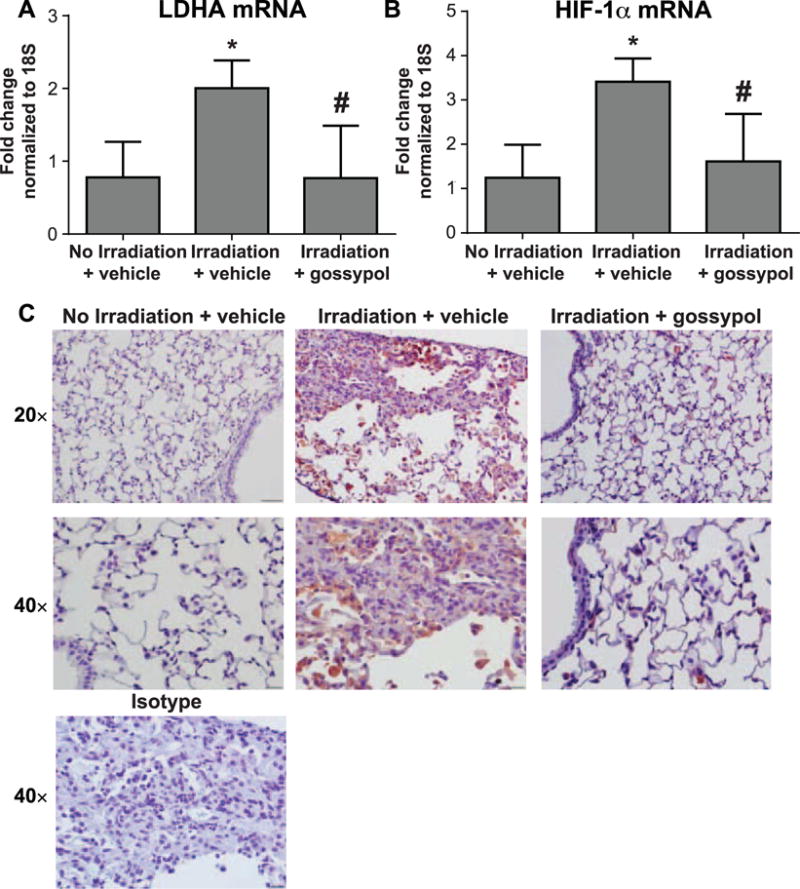
Gossypol disrupts the HIF-1α/LDHA axis. Mice were irradiated and vehicle or gossypol treated, described in Materials and Methods. Whole lung tissue LDHA and HIF-1α mRNA was quantified using RT-PCR. Data are expressed as fold change compared to nonirradiated controls normalized to 18S for LDHA (panel A) and HIF-1α (panel B). Data are displayed as mean ± SEM for n = 5–8 mice. *P ≤ 0.05 compared to nonirradiated vehicle-treated controls; #P ≤ 0.05 compared to irradiated vehicle-treated mice (ANOVA). Panel C: Lung tissue sections were stained with an antibody to HIF-1α (red) and counterstained with hematoxylin (blue) as described in Materials and Methods. One representative mouse is shown from each treatment group, plus an isotype-negative control stain. Images were taken at 20× and 40× magnification. Scale bars represent 100 μm at 20× and 50 μm at 40×.
Gossypol Disrupts the HIF-1α/LDHA Axis
HIF-1α is a transcription factor that has been shown to be activated after lung irradiation, and plays an important role in the development of fibrosis (16–18). HIF-1α is active during hypoxic conditions, and regulates LDHA at the transcriptional level (19). Therefore, we examined whether inhibition of LDHA with gossypol might be influencing the HIF-1α/LDHA axis. An upregulation of LDHA mRNA was observed in irradiated mice compared to nonirradiated controls (Fig. 4A). Gossypol treatment resulted in a significant decrease in LDHA mRNA compared to vehicle-treated mice (Fig. 4A). We also observed a significant increase in HIF-1α mRNA in irradiated mice (Fig. 4B). Gossypol significantly dampened radiation-induced HIF-1α mRNA levels (Fig. 4B). These data suggest that gossypol can disrupt expression of the transcription factor HIF-1α that regulates LDHA. In support of our mRNA data, immunostaining for HIF-1α in lung tissue from irradiated mice (Fig. 4C) was increased compared to nonirradiated controls (Fig. 4C), but was significantly reduced in lung tissue of irradiated mice treated with gossypol (Fig. 4C).
DISCUSSION
Radiation-induced lung injury is characterized by an acute pneumonitic phase occurring weeks to months postirradiation, followed by a delayed and chronic fibrotic phase occurring months to years later (3, 20). The pathogenic mechanisms involved in the late fibrotic phase are not fully understood, and few therapeutic options are available for patients. We have previously demonstrated that the enzyme LDHA and its metabolic product lactate are upregulated in human lung fibroblast cultures in response to irradiation in vitro and that inhibiting LDHA inhibits myofibroblast differentiation (4). In the current mouse model study, we demonstrated that gossypol, an LDHA activity inhibitor, inhibits radiation-induced pulmonary fibrosis in vivo, suggesting that inhibition of LDHA may be a novel therapy for radiation-induced pulmonary fibrosis. Gossypol was effective at inhibiting fibrotic lesions in the lung (Fig. 1), as well as major hallmarks of radiation-induced pulmonary fibrosis, including collagen accumulation (Fig. 1), extracellular matrix generation (Fig. 2) and TGF-β activation (Fig. 3).
We observed that 5 Gy total-body irradiation plus 10 Gy thoracic irradiation resulted in an average of 2% fibrosis area, based on quantification on the left lung lobe histology sections (Fig. 1). Compared to the finding in the literature, Puthawala et al. reported between 2 and 8% fibrosis in their studies using 14 Gy thoracic irradiation and 40–60% survival at 26 weeks (9). In contrast, we observed 100% survival at 26 weeks (data not shown). While the induction of fibrotic outcomes was mild, our study design allowed for reduced mortality while still modeling clinical presentation of fibrosis in humans. To ensure that we thoroughly examined lung tissue for evidence of fibrosis, we sectioned through the entire left lung, taking sections every 150 μm. In doing so, we observed fibrotic areas in irradiated, vehicle-treated mice, but no evidence of fibrosis in any irradiated, gossypol-treated mice. Therefore, we conclude that gossypol inhibited radiation-induced pulmonary fibrosis.
Gossypol is a potent LDHA inhibitor derived from cottonseed oil (21). It has been shown to have anti-cancer properties, including inhibitory effects on tumor growth in colorectal, mammary, breast, colon and melanoma cells (22–24). To our knowledge, only one other study has demonstrated antifibrotic potential of gossypol in vivo. In their published study, Chen et al. found that gossypol inhibited extracellular matrix generation and mRNA expression, and α-SMA expression in a model of liver fibrosis induced by a high fat diet (25). These data support our findings that gossypol has antifibrotic effects by inhibiting extracellular matrix formation (Figs. 1 and 2).
Based on our data, we propose a profibrotic feed-forward loop by which LDHA activity promotes fibrogenesis by activating latent TGF-β. Increased HIF-1α and LDHA activity after irradiation leads to increased production of lactate, which can acidify the extracellular space (4). Acidification leads to activation of latent TGF-β, a potent profibrotic cytokine (6). TGF-β then upregulates HIF-1α and LDHA, to perpetuate the cycle (6). In our study, we found increased HIF-1α and LDHA mRNA expression in irradiated lung tissue, but these increases were significantly suppressed in gossypol-treated mice (Fig. 4). These findings support our model; gossypol’s mode of action is to inhibit LDHA enzymatic activity, thus inhibiting perpetuation of the feed-forward loop, leading to decreased HIF-1α and LDHA at the transcriptional level.
The transcription factor HIF-1α regulates cellular metabolism by transcriptionally regulating glycolytic enzymes such as LDHA, which are classically activated during hypoxia (19, 26). Although it has been well documented that HIF-1α is activated in radiation-induced pulmonary fibrosis (16, 27), much focus has been directed on understanding HIF-1α and its associated oxidative damage in lung tissue after irradiation (3). To our knowledge, there are no other published studies that examine whether downstream glycolytic signaling pathways related to HIF-1α are also affected in radiation-induced fibrosis. Further studies are needed to determine if HIF-1α is directly regulating LDHA in response to radiation.
Interestingly, there are many similarities between cancer biology and emerging fibrosis biology related to cellular metabolism and glycolysis. Both cancer cells and differentiated myofibroblasts are highly proliferative, highly migratory and are resistant to apoptosis (28). Many of these phenotypes associated with cancer cells are dependent on a glycolytic phenotype (29). It was recently reported that metabolic reprograming is also a hallmark of idiopathic pulmonary fibrosis (IPF) (6, 30). Xie et al. reported that glycolytic reprograming was essential for TGF-β-induced myofibroblast differentiation (30). They found that inhibiting glycolysis with the phosphofructokinase inhibitor 3PO inhibited myofibroblast differentiation and pulmonary fibrosis in bleomycin and TGF-β overexpression models of pulmonary fibrosis (30). The new results here together with our previous findings suggest that glycolytic reprograming may also be a hallmark of radiation-induced pulmonary fibrosis (4), and indicate that targeting similar pathways may provide additional potential therapies for IPF and radiation-induced pulmonary fibrosis through effects on glycolytic metabolism.
The profibrotic cytokine TGF-β plays an essential role in the development of pulmonary fibrosis and causes myofibroblast differentiation (31). To elicit biological effects, TGF-β must be cleaved from a latency-associated peptide to become activated (32, 33). We have previously demonstrated that lactate activates latent TGF-β in a pH-dependent manner (6). Therefore, inhibiting lactate production by targeting LDHA may be an effective strategy to inhibit TGF-β activation. Herein we demonstrate that gossypol inhibits both total production and activation of TGF-β in vivo after irradiation (Fig. 3). This may be a major mechanism by which gossypol is antifibrotic.
While many studies have demonstrated that targeting TGF-β itself can inhibit pulmonary fibrosis (9, 34, 35), direct neutralization of TGF-β presents many challenges and concerns. TGF-β is essential for wound healing and cell proliferation, and plays an important role in modulating immune responses (36). Therefore, inhibiting TGF-β directly may have too many negative side effects. However, interfering with upstream mechanisms that simply promote ongoing activation of TGF-β during fibrogenesis may prove to have fewer undesired effects. Gossypol does not directly target TGF-β, but instead our data suggests that it may be decreasing TGF-β activation by interrupting the profibrotic feed-forward loop exerted by LDHA and lactate.
Our findings have clinical relevance considering the delay of administration of gossypol (4 weeks postirradiation) and suggests its utility as a countermeasure in the event of accidental or clinical exposure. Furthermore, given the degree of effectiveness of gossypol in this study, it is possible that delaying treatment, such as past the onset of the pneumonitic phase (around 8 weeks), could also prove to be successful at preventing fibrosis; we plan to test this hypothesis in future studies. To this end, we may also perform future studies to test the effect of gossypol side-by-side with other drugs that can mitigate pulmonary fibrosis, such as nintedanib and pirfenidone, two drugs recently approved by the FDA for treatment in idiopathic pulmonary fibrosis. Given that the lung is a dose-limiting organ for radiotherapy, prophylactic treatment with an antifibrotic agent such as gossypol potentially allows for higher radiation doses as part of thoracic cancer therapy, thereby increasing the probability of tumor eradication while sparing healthy lung tissue. Together, our results demonstrate that gossypol may be a novel therapeutic strategy for radiation-induced pulmonary fibrosis.
Acknowledgments
This research was supported in part by the National Institutes of Health (NHLBI grant nos. R01HL127001, T32HL066988 and NIEHS grant no. T32ES007026), a pilot grant awarded by the University of Rochester Center for Medical Countermeasures against Radiation (under NIH grant no. U19AI091036), the Greg Chandler and Guy F. Solimano Pulmonary Fibrosis Research Fund, a Davis Professorship and the Pulmonary Fibrosis Foundation. JLJ was supported by grant no. F31HL132453. RMK was supported in part by a Parker B. Francis Fellowship. The authors would like to acknowledge Wade Narrow for help with the hydroxyproline assay. The funders had no role in study design, data collection and analysis, decision to publish or manuscript preparation.
References
- 1.Oh Y-T, Noh OK, Jang H, Chun M, Park KJ, Park KJ, et al. The features of radiation induced lung fibrosis related with dosimetric parameters. Radiother Oncol. 2012;102:343–16. doi: 10.1016/j.radonc.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Camus P, Fanton A, Bonniaud P, Camus C, Foucher P. Interstitial lung disease induced by drugs and radiation. Respiration. 2004;71:301–26. doi: 10.1159/000079633. [DOI] [PubMed] [Google Scholar]
- 3.Marks LB, Yu X, Vujaskovic Z, Small W, Jr, Folz R, Anscher MS. Radiation-induced lung injury. Semin Radiat Oncol. 2003;13:333–45. doi: 10.1016/S1053-4296(03)00034-1. [DOI] [PubMed] [Google Scholar]
- 4.Judge JL, Owens KM, Pollock SJ, Woeller CF, Thatcher TH, Williams JP, et al. Ionizing radiation induces myofibroblast differentiation via lactate dehydrogenase. Am J Physiol Lung Cell Mol Physiol. 2015;309:L879–87. doi: 10.1152/ajplung.00153.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park S, Ahn JY, Lim MJ, Kim MH, Yun YS, Jeong G, et al. Sustained expression of NADPH oxidase 4 by p38 MAPK-Akt signaling potentiates radiation-induced differentiation of lung fibroblasts. J Mol Med (Berl) 2010;88:807–16. doi: 10.1007/s00109-010-0622-5. [DOI] [PubMed] [Google Scholar]
- 6.Kottmann RM, Kulkarni AA, Smolnycki KA, Lyda E, Dahanayake T, Salibi R, et al. Lactic acid is elevated in idiopathic pulmonary fibrosis and induces myofibroblast differentiation via pH-dependent activation of transforming growth factor-beta. Am J Respir Crit Care Med. 2012;186:740–51. doi: 10.1164/rccm.201201-0084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yi ES, Bedoya A, Lee H, Chin E, Saunders W, Kim SJ, et al. Radiation-induced lung injury in vivo: expression of transforming growth factor-beta precedes fibrosis. Inflammation. 1996;20:339–52. doi: 10.1007/BF01486737. [DOI] [PubMed] [Google Scholar]
- 8.Rube CE, Uthe D, Schmid KW, Richter KD, Wessel J, Schuck A, et al. Dose-dependent induction of transforming growth factor beta (TGF-beta) in the lung tissue of fibrosis-prone mice after thoracic irradiation. Int J Radiat Oncol Biol Phys. 2000;47:1033–42. doi: 10.1016/s0360-3016(00)00482-x. [DOI] [PubMed] [Google Scholar]
- 9.Puthawala K, Hadjiangelis N, Jacoby SC, Bayongan E, Zhao Z, Yang Z, et al. Inhibition of integrin alpha(v)beta6, an activator of latent transforming growth factor-beta, prevents radiation-induced lung fibrosis. Am J Respir Crit Care Med. 2008;177:82–90. doi: 10.1164/rccm.200706-806OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flechsig P, Dadrich M, Bickelhaupt S, Jenne J, Hauser K, Timke C, et al. LY2109761 attenuates radiation-induced pulmonary murine fibrosis via reversal of TGF-beta and BMP-associated proinflammatory and proangiogenic signals. Clin Cancer Res. 2012;18:3616–27. doi: 10.1158/1078-0432.CCR-11-2855. [DOI] [PubMed] [Google Scholar]
- 11.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induced prolonged severe fibrosis in rat lung. J Clin Investig. 1997;100:768–76. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams JP, Brown SL, Georges GE, Hauer-Jensen M, Hill RP, Huser AK, et al. Animal models for medical countermeasures to radiation exposure. Radiat Res. 2010;173:557–78. doi: 10.1667/RR1880.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lakatos HF, Burgess HA, Thatcher TH, Redonnet MR, Hernady E, Williams JP, et al. Oropharyngeal aspiration of a silica suspension produces a superior model of silicosis in the mouse when compared to intratracheal instillation. Exp Lung Res. 2006;32:181–99. doi: 10.1080/01902140600817465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehmann GM, Xi X, Kulkarni AA, Olsen KC, Pollock SJ, Baglole CJ, et al. The aryl hydrocarbon receptor ligand ITE inhibits TGFbeta1-induced human myofibroblast differentiation. Am J Pathol. 2011;178:1556–67. doi: 10.1016/j.ajpath.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kulkarni AA, Thatcher TH, Hsiao HM, Olsen KC, Kottmann RM, Morrissette J, et al. The triterpenoid CDDO-Me inhibits bleomycin-induced lung inflammation and fibrosis. PLoS One. 2013;8:e63798. doi: 10.1371/journal.pone.0063798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rabbani ZN, Mi J, Zhang Y, Delong M, Jackson IL, Fleckenstein K, et al. Hypoxia inducible factor 1alpha signaling in fractionated radiation-induced lung injury: role of oxidative stress and tissue hypoxia. Radiat Res. 2010;173:165–74. doi: 10.1667/RR1816.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins DF, Kimura K, Iwano M, Haase VH. Hypoxia-inducible factor signaling in the development of tissue fibrosis. Cell Cycle. 2008;7:1128–32. doi: 10.4161/cc.7.9.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim WY, Oh SH, Woo JK, Hong WK, Lee HY. Targeting heat shock protein 90 overrides the resistance of lung cancer cells by blocking radiation-induced stabilization of hypoxia-inducible factor-1alpha. Cancer Res. 2009;69:1624–32. doi: 10.1158/0008-5472.CAN-08-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Semenza GL, Jiang BH, Leung SW, Passantino R, Concordet JP, Maire P, et al. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem. 1996;271:32529–37. doi: 10.1074/jbc.271.51.32529. [DOI] [PubMed] [Google Scholar]
- 20.Tsoutsou PG, Koukourakis MI. Radiation pneumonitis and fibrosis: mechanisms underlying its pathogenesis and implications for future research. Int J Radiat Oncol Biol Phys. 2006;66:1281–93. doi: 10.1016/j.ijrobp.2006.08.058. [DOI] [PubMed] [Google Scholar]
- 21.Lee CY, Moon YS, Yuan JH, Chen AF. Enzyme inactivation and inhibition by gossypol. Mol Cell Biochem. 1982;47:65–70. doi: 10.1007/BF00234406. [DOI] [PubMed] [Google Scholar]
- 22.Gilbert NE, O’Reilly JE, Chang CJ, Lin YC, Brueggemeier RW. Antiproliferative activity of gossypol and gossypolone on human breast cancer cells. Life Sci. 1995;57:61–7. doi: 10.1016/0024-3205(95)00243-y. [DOI] [PubMed] [Google Scholar]
- 23.Ligueros M, Jeoung D, Tang B, Hochhauser D, Reidenberg MM, Sonenberg M. Gossypol inhibition of mitosis, cyclin D1 and Rb protein in human mammary cancer cells and cyclin-D1 transfected human fibrosarcoma cells. Br J Cancer. 1997;76:21–8. doi: 10.1038/bjc.1997.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tuszynski GP, Cossu G. Differential cytotoxic effect of gossypol on human melanoma, colon carcinoma, and other tissue culture cell lines. Cancer Res. 1984;44:768–71. [PubMed] [Google Scholar]
- 25.Chen G, Wang R, Chen H, Wu L, Ge R-S, Wang Y. Gossypol ameliorates liver fibrosis in diabetic rats induced by high-fat diet and streptozocin. Life Sci. 2016;149:58–64. doi: 10.1016/j.lfs.2016.02.044. [DOI] [PubMed] [Google Scholar]
- 26.Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chemi. 1994;269:23757–63. [PubMed] [Google Scholar]
- 27.Gauter-Fleckenstein B, Fleckenstein K, Owzar K, Jiang C, Reboucas JS, Batinic-Haberle I, et al. Early and late administration of MnTE-2-PyP5+ in mitigation and treatment of radiation-induced lung damage. Free Radic Biol Med. 2010;48:1034–43. doi: 10.1016/j.freeradbiomed.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vancheri C, Failla M, Crimi N, Raghu G. Idiopathic pulmonary fibrosis: a disease with similarities and links to cancer biology. Eur Respir J. 2010;35:496–504. doi: 10.1183/09031936.00077309. [DOI] [PubMed] [Google Scholar]
- 29.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie N, Tan Z, Banerjee S, Cui H, Ge J, Liu R-M, et al. Glycolytic Reprogramming in Myofibroblast Differentiation and Lung Fibrosis. Am J Respir Crit Care Med. 2015;192:1462–74. doi: 10.1164/rccm.201504-0780OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Branton MH, Kopp JB. TGF-beta and fibrosis. Microbes Infect. 1999;1:1349–65. doi: 10.1016/s1286-4579(99)00250-6. [DOI] [PubMed] [Google Scholar]
- 32.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–24. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 33.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18:816–27. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 34.Anscher MS, Thrasher B, Rabbani Z, Teicher B, Vujaskovic Z. Antitransforming growth factor-beta antibody 1D11 ameliorates normal tissue damage caused by high-dose radiation. Int J Radiat Oncol Biol Phys. 2006;65:876–81. doi: 10.1016/j.ijrobp.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 35.Anscher MS, Thrasher B, Zgonjanin L, Rabbani ZN, Corbley MJ, Fu K, et al. Small molecular inhibitor of transforming growth factor-beta protects against development of radiation-induced lung injury. Int J Radiat Oncol Biol Phys. 2008;71:829–37. doi: 10.1016/j.ijrobp.2008.02.046. [DOI] [PubMed] [Google Scholar]
- 36.Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-beta signaling in fibrosis. Growth Factors. 2011;29:196–202. doi: 10.3109/08977194.2011.595714. [DOI] [PMC free article] [PubMed] [Google Scholar]


