Abstract
Background
Alzheimer’s disease (AD) results in progressive functional decline leading to loss of independence
Objective
To determine whether collaborative care plus two years of home-based occupational therapy delays functional decline
Design
Randomized controlled clinical trial
Setting
Urban public health system
Patients
180 community-dwelling subjects who were diagnosed with AD and their informal caregivers
Interventions
All subjects received collaborative care for dementia. Intervention patients also received in-home occupational therapy delivered in 24 sessions over 2 years.
Measurements
The primary outcome measures was the Alzheimer’s Disease Cooperative Studies Group Activities of Daily Living Scale (ADCS ADL); performance based measures included the Short Physical Performance Battery (SPPB) and Short Portable Sarcopenia Measure (SPSM)
Results
At baseline, there were no significant between group differences in clinical characteristics; the mean MMSE for both groups was 19 (SD=7). The intervention group received a median of 18 home visits from the study occupational therapists. Both groups declined in ADCS ADL scores over 24 months. At the primary endpoint of 24 months, there were no between group differences in ADCS ADL scores (mean difference 2.34, 95% CI −5.27, 9.96). We were also unable to definitively demonstrate between-group differences in the mean SPPB or SPSM.
Limitations
The results of this trial are indeterminate and do not rule out potentially clinically important effects of the intervention.
Conclusions
We were unable to definitively demonstrate whether the addition of two years of in-home occupational therapy to a collaborative care management model slows the rate of functional decline among persons with AD. This trial underscores the burden undertaken by family caregivers as they provide care for persons with AD and the difficulty in slowing functional decline.
Background
Alzheimer’s disease (AD) and related dementias lead to a high burden of suffering for patients, families, and society.(1) Over the typical disease course of 5–10 years, the condition results in progressive functional disability, frequent transitions in care, and excess health care costs.(2–5) There is no known cure for AD and no known disease modifying treatments.(6) In the context of AD, functional decline is believed to be the result of progressive deficits in cognitive, emotional, and physical function.
New models of care for AD focus on team-based care in support of the family caregiver and seek to improve patients’ quality of life.(7, 8) These new models of care emphasize coordination with community-based services, modifications to the patient’s home, and movement toward dementia-prepared communities.(9) Primary care practices often find these new models difficult to implement because they require practice redesign, a re-trained workforce, community outreach, and leadership in local advocacy. Ten years ago, we reported the results of a randomized controlled clinical trial testing the effectiveness of collaborative care among primary care patients with AD.(10) The intervention resulted in significant improvement in the quality of care and behavioral symptoms for patients and reduced stress for their family caregivers. Despite these improvements, the intervention did not slow the rate of patients’ functional decline.
Over the past decade, several studies focusing on functional decline among patients with AD have shown the potential to slow functional decline through home-based interventions.(11–16) The specific aim of this study was to conduct a two-year randomized, controlled clinical trial to delay functional decline among older adults with AD by comparing a control group receiving best practices primary care with an intervention group receiving best practice primary care plus a home-based occupational therapy intervention.
Methods
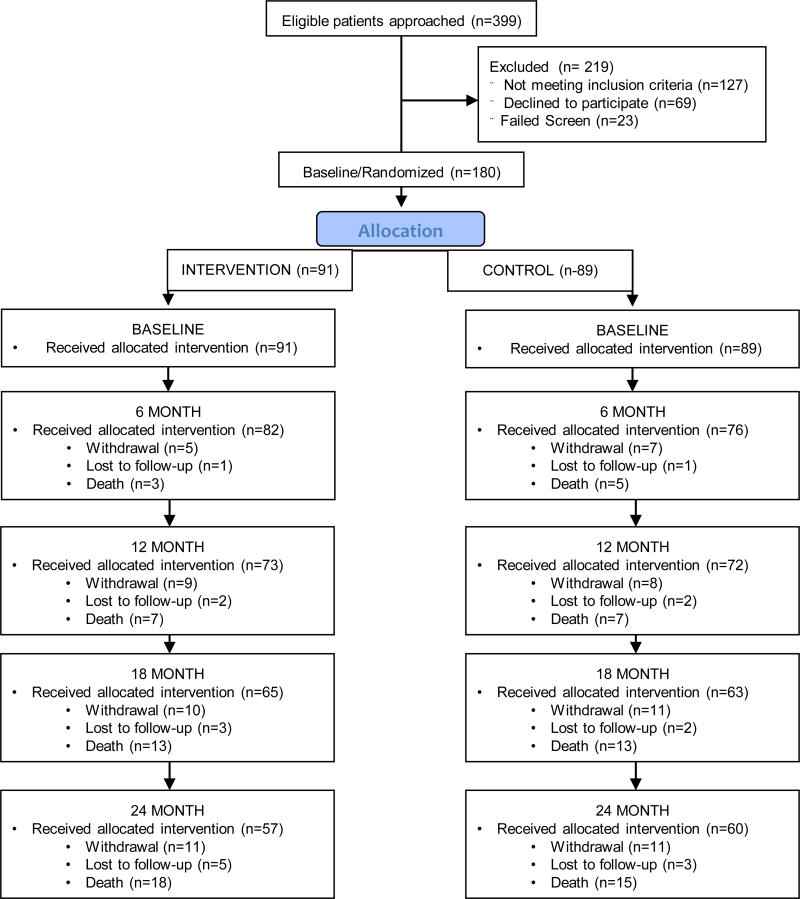
The study was approved by the Indiana University Purdue University-Indianapolis Institutional Review Board. A detailed description of the study design has been previously published.(17) This is a randomized single blind controlled clinical trial with a parallel design and a 1:1 allocation ratio. The trial was conducted at Eskenazi Health, an urban public health system serving Indianapolis, IN. Patients were enrolled from one of ten primary care practices or the one senior care practice affiliated with Eskenazi Health. Patients were eligible if they were aged 45 years and older and had a diagnosis of possible or probable AD as determined by physicians in a memory care practice affiliated with Eskenazi Health. Eligibility criteria also included: community-dwelling, English-speaking, a caregiver willing to participate in the study who had access to a telephone and was willing to receive home visits. For patients meeting eligibility criteria, research personnel assigned to each clinical site obtained written informed consent (or assent) from the patient and the participating family caregiver. The CONSORT diagram is shown in Figure 1
Figure 1.
CONSORT Diagram
Randomization was conducted at the patient l evel stratified by type of clinic (primary care clinic or senior care clinic). The statistical software SAS was used by the lead statistician (SG) to generate the randomization scheme in a block of 4. Sequentially numbered sealed envelopes containing the randomization assignment for patient for each of the two clinics were prepared by the study statistician. Actual randomization results were compared to the pre-planned randomization schedule and no deviation was found.
Description of the Control Condition
Both study groups received collaborative care for dementia facilitated through the Healthy Aging Brain Center, a memory care practice which provided co-management with the primary care practice.(18, 19) We considered this “best practices primary care” because it encompasses the collaborative care intervention tested in our prior clinical trial.(10) Based on the caregiver's reports of the patient’s current symptoms using the HABC Monitor,(20) individualized recommendations were made to manage a patient's behavioral symptoms.(21) Items reported by the caregiver dictated activation of specific behavioral intervention protocols by the care manager. Each of these protocols focused first on non-pharmacological interventions. If the non-pharmacological approach did not result in acceptable improvement, the care manager collaborated with the primary care physician or the memory care practice physician to consider protocol-based drug therapy.
Description of the Intervention
A description of the study intervention has been previously published.(22) Briefly, the intervention group receives all of the components of best practice primary care described above plus a home-based intervention designed to slow functional decline. Any study patient or caregiver, regardless of group, could receive any other concomitant care prescribed by their providers. The framework of the intervention is based on general occupational therapy principles,(22) as well as interventions described in prior published studies.(11–13, 23) The main goal was to support and augment the self-care functional capability of the patient as identified by goals established in negotiation with the patient and caregiver. The occupational therapist completed an initial in-home evaluation to develop a formal care plan that was tailored to the needs of the dyad. This evaluation was repeated at the beginning of each additional cycle by the occupational therapist. There were three cycles of the home-based intervention over two years. In the first cycle, there are eight 90-minute sessions delivered approximately every other week over 16 weeks. A new task is introduced with each visit based on a mutually agreed upon care plan. In the second cycle, the eight home visits were next spaced by four weeks and therefore completed over 32 weeks. In the third cycle, the eight home visits take place over one year. Phone calls were used to address problem solving or new concerns of the caregiver between home visits. Phone calls continued in the same progression throughout the three cycles but with more weeks between contacts. Over two years, each dyad could receive up to 24 90-minute homes visits by one or more of five occupational therapists or one occupational therapist assistant. Initial assessments and care plans were completed only by the occupational therapists and not the occupational therapy assistant.
Outcome Measures
Outcomes measures were completed in the patient’s home by a team of two research assistants who were blinded to the dyad’s randomization status. The primary outcome measure was the Alzheimer’s Disease Cooperative Study Group Activities of Daily Living Inventory (ADCS ADL). The instrument assesses the traditional basic activities of daily living as well as variations on instrumental activities of daily living and a number of more complex and explicit self-care tasks.(24) Scores vary from 0 to 75 with higher scores indicating better function. Because this scale is based on self-reports of the caregiver, we also completed two patient performance measures. The Short Physical Performance Battery (SPPB) is a standardized measure of lower extremity physical performance that includes walking, balance, and power tasks (25–27) Scores vary from 0 to 12 with higher scores indicating better function. The Short Portable Sarcopenia Measure (SPSM) was conceptualized as a measure of sarcopenia that combines muscle quantity and function.(28). The scale is based on timed chair rises, lean mass, and grip strength divided by height. Scores vary from 0 to 18 with higher scores indicating better function. We previously reported good correlation between these three scales and caregiver-reported function across a range of subjects’ cognitive function.(29) Those findings suggested that the patients were able to adequately understand and follow instructions for performing the SPPB and SPSM. To help compare our outcomes with the outcomes of our prior clinical trial of collaborative care alone, we also completed the ADCS Group Neuropsychiatric Inventory (NPI) at each assessment.(30–32) Scores vary from 0 to 144 with higher scores representing worse symptoms. We also collected a broad range of process of care data.
Analyses
The study was designed for 80% power based on a two-tailed test at 5% significance level to test the hypothesis that subjects in the intervention group will have improved function compared to the control group at 24 months with effect size of 0.23 standard deviation based on the ADCS ADL. Thus, the targeted sample size was 180 patients. All patients were randomized according to randomization scheme and were analyzed in the group to which they were randomized. Dementia-specific care processes were compared between the two groups using two sample t-test or Wilcoxon rank-sum tests for continuous variables and Fisher’s exact tests for categorical variables. For each outcome measure collected at baseline, 6, 12, 18 and 24 months, a mixed effects model was used with time and an interaction between group and time as independent variables while adjusting for randomization stratum and within patient correlation over time using an unstructured covariance matrix. Main effect for group was not included in the mixed effect models in order to enforce the equal group mean assumption at baseline given the randomized trial design.(33)
Two sensitivity analyses were conducted to assess the potential impact of missing data (see appendix). First, multiple imputation for subjects with missing follow-up data was completed for those subjects missing follow-up data not due to death. We used a regression imputation approach incorporating the patients’ baseline characteristics as well as observed outcomes with separate group means while adjusting for randomization stratum.(34) Second, we used a selection model approach in order to adjust for potentially non-ignorable missing data). All analyses were conducted using SAS 9.4.
The National Institute on Aging had no role in the design, conduct, and analysis of this study or the decision to submit the manuscript for publication.
Results
Table 1 compares the baseline characteristics of the participants. Consistent with a cohort of older adults with probable Alzheimer’s disease, the mean age of the study groups approaches 80 years, the majority of subjects are women, and subjects’ MMSE and Word List Learning scores demonstrate mild to moderate cognitive impairment. Study subjects also suffer from significant impairments in activities of daily living and from a high burden of behavioral problems. Caregivers showed mild to moderate levels of anxiety and depression. Subjects also suffered from a high burden of comorbid conditions including: diabetes (30% intervention, 30% control), depression (30% vs. 33%), congestive heart failure (12% vs. 16%), coronary artery disease (14% vs. 16%), and history of stroke (4% vs. 5%).
Table 1.
Baseline Comparison of Study Subject Characteristics
| Intervention (n=91)* |
Usual Care (n=89)† |
|
|---|---|---|
| Age, mean (SD), years | 79.6 (8.3) | 77.2 (9.4) |
| Male, (%) | 25 (27) | 28 (31) |
| Black, (%) | 53 (58) | 49 (55) |
| Not a high school graduate, (%) | 36 (40) | 44 (50) |
| Recruited from senior care clinic, (%) | 77 (85) | 77 (87) |
| Body Mass Index, mean (SD) | 27.2 (5.7) | 28.7 (6.4) |
| Mini-Mental State Examination, mean (SD) | 19.4 (6.9) | 19.0 (7.6) |
| Word List Learning, mean, (SD) | 9.5 (5.4) | 9.5 (5.9) |
| Activities of Daily Living Inventory, mean (SD) | 49.4 (17.6) | 47.8 (15.7) |
| Short Portable Sarcopenia Measure, mean (SD) | 3.3 (3.5) | 3.6 (3.7) |
| Short Portable Performance Battery, mean (SD) | 4.3 (2.7) | 4.2 (3.2) |
| Neuropsychiatric Inventory, mean (SD) | 15.6 (15.1) | 16.6 (18.9) |
| Caregiver is spouse, (%) | 20 (22%) | 28 (32%) |
| Age of caregiver, mean (SD), years | 56.0 (12.3) | 59.1 (12.5) |
| Caregiver’s GAD-7 anxiety scale, mean (SD) | 4.0 (4.5) | 3.6 (4.5) |
| Caregiver’s PHQ-9 depression scale, mean (SD) | 4.1 (4.1) | 3.7 (3.7) |
Sample size is smaller than 91 for the following individual variables in intervention group: education, n=90; body mass index, n=80; Mini-mental state examination, n=90, Word List Learning, n=90; Short Portable Sarcopenia Measure, n=88, Short Portable Performance Battery, n=87; PHQ-9, n=90
Sample size is smaller than 89 for the following individual variables in control group: education, n=88; body mass index, n=78; Word List Learning, n=88; Short Portable Sarcopenia Measure, n=86
Table 2 summarizes the level of dementia-specific care received by the two study groups. Both groups received best practices primary care for dementia through a locally adapted care management program provided in the Healthy Aging Brain Center.(18, 19) In both study groups, this dementia care approximates the collaborative care received in an earlier clinical trial.(10) All study subjects were diagnosed in a memory care practice and all were referred for care management to the home-based dementia care program. The majority of patients were treated with anti-dementia medications. There was no difference in the frequency of this dementia-specific care between groups.
Table 2.
Comparison of Concomitant Dementia-Specific Care Processes over 2 years
| Intervention (n=91) |
Usual Care (n=89) |
P value | |
|---|---|---|---|
| Physician visits in Healthy Aging Brain Center (HABC), median, (IQR) | 2 (0, 4) | 2 (0, 4) | 0.93 |
| HABC Care management visits face-to-face in clinic or home by nurse, social worker, or care coordinator assistant, median, (IQR) | 8 (4, 14) | 8 (4, 13) | 0.56 |
| HABC Care management telephone contacts by nurse, social worker, or care coordinator assistant, median, (IQR) | 8 (3, 17) | 8 (3, 13) | 0.174 |
| Receiving anti-dementia medication, % | 62 | 60 | 0.88 |
| Receiving anti-depressant medication, % | 48 | 54 | 0.46 |
| Non-study Occupational Therapy visits in hospital setting or in outpatient facility, median, (IQR) | 0 (0, 1) | 0 (0, 1) | 0.68 |
| Primary Care Practice visits, median, (IQR) | 6 (2, 10) | 7 (2, 12) | 0.56 |
| Specialty Care visits, median, (IQR) | 0 (0, 4) | 0 (0, 3) | 0.95 |
IQR: interquartile range.
Table 3 describes the frequency and content of the occupational therapy intervention. Over a period of two years, intervention patients received a median of 18 in-home evaluations and care visits that totaled 21 hours (median) of face-to-face time in the home with the occupational therapists. Because these visits are tailored to meet the expressed needs of the individual care recipient-caregiver dyads, and because these needs are expected to change over time, the content of the visits vary across subjects and within subjects over time. Table 3 shows the percentage of visits that focused on individual target areas as reported by the occupational therapist following each visit. The focus areas are ordered by frequency in the table and demonstrate the dominance of mobility interventions such as transfers, standing, household mobility, sitting, and home exercise, all of which were targeted in more the 50% of the home visits.
Table 3.
Frequency and Content of Occupational Therapy (OT) Intervention (n=91)
| Number of OT home visits over 2 years, median(IQR) | 18.0 (11, 21) |
| Total duration in hours of all OT home visits, median (IQR) | 20.7 (13.4, 24.8) |
| Average duration in minutes of each home OT visit, median, (IQR) | 68.5 (64.0, 73.2) |
| Number of OT telephone contacts between visits, median, (IQR) | 17 (11, 22) |
| Percent of visits by OTs targeting these priorities, mean (95% CI) | |
| Transfers | 66.5 (61.2 to 71.8) |
| Household Mobility | 64.5 (56.7 to 72.3) |
| Standing | 62.0 (56.7 to 72.3) |
| Home Exercise Program | 56.1 (50.5 to 61.6) |
| Sitting | 52.9 (46.1 to 59.8) |
| Patient or Caregiver Education | 41.8 (33.2 to 50.5) |
| Cognition | 39.6 (32.5 to 46.7) |
| Meaningful Activity | 36.0 (31.7 to 40.3) |
| Energy Conservation | 21.9 (16.7 to 27.0) |
| Safety | 21.0 (15.4 to 26.5) |
| Activities of Daily Living | 18.9 (13.9 to 23.9) |
| Toileting | 16.1 (12.0 to 20.2) |
| Dressing Lower | 13.1 (9.8 to 16.3) |
| Dressing Upper | 10.3 (7.4 to 13.3) |
| Gross Motor Coordination | 8.8 (6.0 to 11.7) |
| Feeding | 8.4 (6.0 to 10.9) |
| Fine Motor Coordination | 8.1 (5.6 to 10.6) |
| Grooming | 7.4 (4.8 to 10.1) |
| Light Housekeeping | 7.0 (4.6 to 9.5) |
| Cooking | 4.6 (2.5 to 6.8) |
| Bathing Lower | 3.3 (1.8 to 4.8) |
| Bathing Upper | 2.9 (1.5 to 4.3) |
IQR: interquartile range; CI: confidence interval
Table 4 compares the clinical outcomes between study groups. At 24 months, there was no statistically significant difference between groups in the ADCS ADL. Notably, the results are interpreted as indeterminate because the 95% confidence intervals (−5.27, 9.96) include clinically significant between-group differences (4.05 based on an effect size of 0.23). Both groups experienced progressive functional decline over time. Both groups also declined over time in the performance-based measures of the SPPB and the SPSM. In data not shown in the table, mean MMSE scores for both groups declined over time: the intervention group declined from 19.37 to 16.76; the control group declined from 19.02 to 17.26. There was no significant difference in mortality between the study groups over two years (20% vs. 17%) or in the average number of days participating in the study (577 vs. 575).
Table 4.
Mixed effects model results for outcome measures at 6, 12, 18 and 24 months*
| visit | Predicted Mean (95% CI) | Between-Group Difference |
95% CI of Difference |
P- value |
||
|---|---|---|---|---|---|---|
| Intervention | Usual care | |||||
| ADCS Group ADL Inventory | 6 Months | 45.49 (41.02 to 49.96) | 43.57 (38.97 to 48.18) | 1.92 | −3.49 to 7.32 | 0.49 |
| 12 Months | 43.25 (38.33 to 48.17) | 39.36 (34.33 to 44.39) | 3.89 | −2.24 to 10.01 | 0.21 | |
| 18 Months | 39.10 (33.96 to 44.24) | 36.32 (31.06 to 41.58) | 2.78 | −3.71 to 9.27 | 0.40 | |
| 24 Months | 34.47 (28.60 to 40.34) | 32.13 (26.17 to 38.08) | 2.34 | −5.27 to 9.96 | 0.54 | |
| SPPB Total | 6 Months | 3.88 (3.08 to 4.68) | 4.08 (3.25 to 4.91) | −0.20 | −1.19 to 0.78 | 0.68 |
| 12 Months | 3.88 (3.04 to 4.72) | 3.75 (2.88 to 4.61) | 0.14 | −0.91 to 1.18 | 0.80 | |
| 18 Months | 3.52 (2.65 to 4.38) | 3.16 (2.26 to 4.05) | 0.36 | −0.72 to 1.45 | 0.51 | |
| 24 Months | 2.45 (1.55 to 3.35) | 2.78 (1.87 to 3.69) | −0.33 | −1.46 to 0.80 | 0.57 | |
| SPSM Total | 6 Months | 1.85 (1.00 to 2.70) | 2.87 (1.98 to 3.76) | −1.02 | −2.05 to 0.02 | 0.05 |
| 12 Months | 2.00 (1.13 to 2.87) | 2.26 (1.35 to 3.16) | −0.25 | −1.32 to 0.82 | 0.64 | |
| 18 Months | 1.63 (0.72 to 2.53) | 2.06 (1.11 to 3.00) | −0.43 | −1.56 to 0.70 | 0.45 | |
| 24 Months | 1.48 (0.56 to 2.41) | 2.11 (1.15 to 3.07) | −0.62 | −1.78 to 0.53 | 0.29 | |
| NPI Frequency* Severity Score | 6 Months | 13.51 (9.44 to 17.57) | 17.80 (13.58 to 22.02) | −4.29 | −9.31 to 0.73 | 0.09 |
| 12 Months | 13.99 (9.66 to 18.31) | 18.29 (13.88 to 22.71) | −4.31 | −9.71 to 1.09 | 0.12 | |
| 18 Months | 14.96 (10.75 to 19.17) | 15.66 (11.30 to 20.02) | −0.71 | −5.96 to 4.55 | 0.79 | |
| 24 Months | 14.68 (9.97 to 19.38) | 19.13 (14.35 to 23.90) | −4.45 | −10.4 to 1.54 | 0.14 | |
| PHQ-9 Total | 6 Months | 3.48 (2.56 to 4.40) | 4.07 (3.11 to 5.03) | −0.59 | −1.73 to 0.56 | 0.31 |
| 12 Months | 3.65 (2.68 to 4.61) | 4.79 (3.80 to 5.78) | −1.14 | −2.34 to 0.06 | 0.06 | |
| 18 Months | 3.80 (2.81 to 4.79) | 3.83 (2.80 to 4.85) | −0.03 | −1.28 to 1.23 | 0.97 | |
| 24 Months | 3.72 (2.78 to 4.67) | 3.70 (2.73 to 4.67) | 0.02 | −1.15 to 1.20 | 0.97 | |
| GAD-7 Total | 6 Months | 3.22 (2.19 to 4.24) | 3.37 (2.30 to 4.43) | −0.15 | −1.42 to 1.12 | 0.82 |
| 12 Months | 3.21 (2.14 to 4.28) | 4.16 (3.07 to 5.26) | −0.95 | −2.29 to 0.39 | 0.16 | |
| 18 Months | 3.46 (2.48 to 4.44) | 2.75 (1.73 to 3.77) | 0.71 | −0.49 to 1.92 | 0.25 | |
| 24 Months | 2.86 (1.87 to 3.85) | 2.84 (1.83 to 3.86) | 0.01 | −1.20 to 1.23 | 0.98 | |
Results included predicted means and 95% confidence intervals from mixed effects models adjusting for randomization strata accounting for repeated assessments over time within the individual
ADCS: Alzheimer’s Disease Cooperative Studies Group; ADL: activities of daily living; SPPB: Short Portable Performance Battery; SPSM: Short Portable Sarcopenia Measure; NPI: Neuropsychiatric Inventory; PHQ-9: Patient Health Questionnaire 9 item depression scale; GAD-7: Generalized Anxiety Disorder 7 item scale.
We completed two additional analyses to explore the potential impact of missing data on the study outcomes. Data were primarily missing due to death of the subjects. First, we used multiple imputation to account for data missing not due to death. Second, we implemented the selection model approach by assuming the missing data are not ignorable and depend on unobserved outcomes. We also conducted a series of sensitivity analyses by varying the missing data assumption. Between group differences at 24 months remained non-significant in each of these analyses (see appendix).
Discussion
The goal of this study was to determine if a home-based occupational therapy intervention delivered over two years could slow the rate of functional decline among older adults with AD. Both study groups received best practices dementia care which has been previously demonstrated to improve behavioral outcomes and reduce caregiver stress but which did not slow functional decline.(10) In that prior trial, both groups declined by approximately 4 points per year on the 23-item scale ADCS ADL scale which has a range of scores from 0–75. Based on these findings and other studies in the literature, we estimated that between-group differences on this ADCS ADL in the range of 4 points over two years could be clinically significant. In the current study, we found between group differences of 2.34 points and we also report 95% confidence intervals that include the potential for clinically significant improvement (9.96) and clinically significant decline (−5.27) in the experimental group. For this reason, the results of this trial should be considered indeterminate.(35) Patients with AD and multiple chronic conditions suffer from a high mortality rate;(2) nearly 1 in 5 subjects died during the two year follow-up period of this study. However, the study findings remained consistent across a range of different analytic approaches to missing data.
Based on prior shorter-term studies of related interventions,(11–14) we had hypothesized that this intervention might slow the rate of functional decline through three potential mechanisms. First, the social, physical, and cognitive intervention encompassed by occupational therapy might actually slow the pathological processes of AD or stimulate compensatory cognitive mechanisms. Second, the intervention might have no impact on AD pathology, but instead might improve functional decline emanating from other diseases and conditions comorbid with the dementing illness, including frailty from consequential behaviors such as inactivity, boredom, or social withdrawal. Third, the occupational therapy might have no impact on any disease or the effects of sedentary behavior among the patients, but it might improve the caregiver’s perception of functional decline through caregiver training in areas such as transfers and toileting. Because caregiver-reported patient function and performance-based function measures as well as cognitive function measures continue to decline over time in both study groups, we were unable to provide support for these posited mechanisms of action for occupational therapy.
The trial has four important strengths beyond the randomized controlled study design. First, we were able to compare the impact of the intervention over and above the impact of best-practices dementia care. This aspect of the trial is fundamental to the examination of the unique contribution of longer term occupational therapy. Second, we included a broad range of outcome measures that included not only caregiver-reported patient function and performance-based measures of function, but also measures of cognition, mood, and behavioral symptoms. Third, we were able to document the process of care including the content and duration of the occupational therapy intervention, the content of concomitant dementia-specific care, and the content of the concomitant primary care and specialty care received by these study subjects. Fourth, this is the first trial of occupational therapy among persons with probable Alzheimer’s disease that followed subjects over a 2 year period.
Prior research exploring the capacity to slow functional decline in older adults with AD using different interventions has produced mixed results.(16, 36–39) In 2013, Pitkala et al. reported a home-based exercise study among persons with AD in Finland and compared this intervention with group-based exercise and with usual care.(40) Like the current study, the authors found that function among all three groups declined over time and that there was no significant differences in the SPPB between the groups at 12 months. However, unlike the current study, Pitkala et al. found that decline among the home-based exercise group was significantly less at 12 months as measured by the caregiver-reported Functional Independence Measure. In an accompanying editorial, the author questioned the limited clinical significance of this difference.(41) The present study enrolled older adults with similar ages and baseline MMSE scores as the Finnish study, but Pitkala et al. enrolled volunteer subjects who were able to walk independently at baseline (with or without a walking aid) and who had a spousal caregiver. Subjects in the current study were not excluded based on those two criteria and were enrolled from clinical populations. Patients in the current study had significantly lower SPPB scores at baseline, were less likely to have a spousal caregiver, had greater comorbidity, and were followed for two years. In a 2016 study, Toots et al. completed a randomized trial of intensive exercise among older adults with AD enrolled from residential care facilities in Sweden. The intervention included a functional exercise program that focused on lower limb strength and balance. This study also showed no evidence of a delayed decline in ADLs at 7 months.(42)
The present study has limitations. It is possible that a longer observation period, a more intensive occupational therapy intervention, enrollment of older adults earlier in the course of their dementing illness or older adults with lower levels of multi-morbidity might produce more encouraging results. It is possible that a larger sample size receiving the identical intervention might produce more encouraging results. Posited reasons why the intervention may have been less effective than anticipated include: (a) subjects may have been unable to learn the recommended tasks and activities promoted by the occupational therapy interventions; (b) caregivers enrolled in this study may have been less able or less motivated than other caregivers; (c) dyads may have had health priorities with regard to functional decline that are not captured by the ADCS ADL scale; and (d) occupational therapy may be necessary but not sufficient to slow the rate of functional decline and, had our intervention combined occupational therapy with other potential interventions, we may have produced different results. We stress that this trial is not a test of the potential benefits of occupational therapy for acute conditions among older adults with dementia nor is this trial a test of the benefits of collaborative care for older adults with dementia.
Long-term home-based occupational therapy is not currently the standard-of-care for older adults with Alzheimer’s disease, although many experts recommend strategies that promote continued physical, social, and cognitive activity. Medicare Part B and Medicaid do provide coverage for outpatient rehabilitation therapy when this therapy meets criteria as “medically necessary and reasonable”. For most Medicare beneficiaries, this benefit is capped at ~$2,000 per year and most beneficiaries would also be responsible for a 20% copayment although providers can request additional therapy based on medical necessity. In the present trial, subjects were not charged for the occupational therapy intervention but we estimated the cost of the occupational therapist effort for our intervention at ~$2100 per year. Our original hypothesis did presume that the benefit of occupational therapy in the targeted patient population was currently unproven. The definition of medically necessary and reasonable would be expected to vary across patients and health insurance plans but would often include provisions such that the therapy requires an occupational therapist’s expertise and that the service provided could reasonably be expected to provide benefit. For rehabilitation services, this benefit often requires that the patient show evidence of improvement or maintenance of function based on the provided therapy. From both a clinical and a policy perspective, the present study does not provide clear evidence that would support a change in current clinical practice or policy coverage.
Our findings suggest that persons with dementia face a steady decline in function that is not slowed by collaborative care and that may continue even with the provision of home-based occupational therapy. We report indeterminate results regarding the question of whether occupational therapy slows the rate of functional decline relative to collaborative care alone. Given the burden of caregiving for persons with dementia that is largely shouldered by family members, research must focus on identifying strategies that support caregivers in the home to provide care to persons with dementia. If the gradual functional decline attributable to AD is irreversible, a new generation of assistive devices, home modifications, community services, and technologies is needed to make longer term support in the home a practical reality for patients and families.
Acknowledgments
Supported by NIA grant R01 AG034946
Footnotes
ClinicalTrial.gov Identifier: NCT01314950
References
- 1.Alzheimer’s A. 2015 Alzheimer's disease facts and figures. Alzheimer's & dementia: the journal of the Alzheimer's Association. 2015;11(3):332. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Larson EB, Shadlen MF, Wang L, McCormick WC, Bowen JD, Teri L, et al. Survival after initial diagnosis of Alzheimer disease. Ann Intern Med. 2004;140(7):501–9. doi: 10.7326/0003-4819-140-7-200404060-00008. [DOI] [PubMed] [Google Scholar]
- 3.Callahan CM, Tu W, Unroe KT, LaMantia MA, Stump TE, Clark DO. Transitions in Care in a Nationally Representative Sample of Older Americans with Dementia. J Am Geriatr Soc. 2015;63(8):1495–502. doi: 10.1111/jgs.13540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arling G, Tu W, Stump TE, Rosenman MB, Counsell SR, Callahan CM. Impact of dementia on payments for long-term and acute care in an elderly cohort. Med Care. 2013;51(7):575–81. doi: 10.1097/MLR.0b013e31828d4d4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;368(14):1326–34. doi: 10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daviglus ML, Bell CC, Berrettini W, Bowen PE, Connolly ES, Jr, Cox NJ, et al. National Institutes of Health State-of-the-Science Conference Statement: Preventing Alzheimer Disease and Cognitive Decline. Ann Intern Med. 2010;153(3):176–81. doi: 10.7326/0003-4819-153-3-201008030-00260. [DOI] [PubMed] [Google Scholar]
- 7.Callahan CM, Sachs GA, Lamantia MA, Unroe KT, Arling G, Boustani MA. Redesigning systems of care for older adults with Alzheimer's disease. Health Aff (Millwood) 2014;33(4):626–32. doi: 10.1377/hlthaff.2013.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Waal H, Lyketsos C, Ames D, O'Brien J. Designing and Delivering Dementia Services. Chichester, West Sussex, UK: John Wiley & Sons, Ltd; 2013. [Google Scholar]
- 9.Lin SY, Lewis FM. Dementia friendly, dementia capable, and dementia positive: concepts to prepare for the future. Gerontologist. 2015;55(2):237–44. doi: 10.1093/geront/gnu122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callahan CM, Boustani MA, Unverzagt FW, Austrom MG, Damush TM, Perkins AJ, et al. Effectiveness of collaborative care for older adults with Alzheimer disease in primary care: a randomized controlled trial. JAMA. 2006;295(18):2148–57. doi: 10.1001/jama.295.18.2148. [DOI] [PubMed] [Google Scholar]
- 11.Teri L, Gibbons LE, McCurry SM, Logsdon RG, Buchner DM, Barlow WE, et al. Exercise plus behavioral management in patients with Alzheimer disease: a randomized controlled trial. JAMA. 2003;290(15):2015–22. doi: 10.1001/jama.290.15.2015. [DOI] [PubMed] [Google Scholar]
- 12.Graff MJ, Vernooij-Dassen MJ, Thijssen M, Dekker J, Hoefnagels WH, Rikkert MG. Community based occupational therapy for patients with dementia and their care givers: randomised controlled trial. Bmj. 2006;333(7580):1196. doi: 10.1136/bmj.39001.688843.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gitlin LN, Winter L, Burke J, Chernett N, Dennis MP, Hauck WW. Tailored activities to manage neuropsychiatric behaviors in persons with dementia and reduce caregiver burden: a randomized pilot study. Am J Geriatr Psychiatry. 2008;16(3):229–39. doi: 10.1097/JGP.0b013e318160da72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gitlin LN, Winter L, Dennis MP, Hodgson N, Hauck WW. Targeting and managing behavioral symptoms in individuals with dementia: a randomized trial of a nonpharmacological intervention. J Am Geriatr Soc. 2010;58(8):1465–74. doi: 10.1111/j.1532-5415.2010.02971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308(19):2020–9. doi: 10.1001/jama.2012.36918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McLaren AN, Lamantia MA, Callahan CM. Systematic review of non-pharmacologic interventions to delay functional decline in community-dwelling patients with dementia. Aging Ment Health. 2013;17(6):655–66. doi: 10.1080/13607863.2013.781121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Callahan CM, Boustani MA, Schmid AA, Austrom MG, Miller DK, Gao S, et al. Alzheimer's disease multiple intervention trial (ADMIT): study protocol for a randomized controlled clinical trial. Trials. 2012;13:92. doi: 10.1186/1745-6215-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boustani MA, Sachs GA, Alder CA, Munger S, Schubert CC, Austrom M, et al. Implementing innovative models of dementia care: The Healthy Aging Brain Center. Aging & Mental Health. 2011;15(1):13–22. doi: 10.1080/13607863.2010.496445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Callahan CM, Boustani MA, Weiner M, Beck RA, Livin LR, Kellams JJ, et al. Implementing dementia care models in primary care settings: The Aging Brain Care Medical Home. Aging Ment Health. 2011;15(1):5–12. doi: 10.1080/13607861003801052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monahan PO, Boustani MA, Alder C, Galvin JE, Perkins AJ, Healey P, et al. Practical clinical tool to monitor dementia symptoms: the HABC-Monitor. Clin Interv Aging. 2012;7:143–57. doi: 10.2147/CIA.S30663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Austrom M, Damush TM, Hartwell CW, Perkins T, Unverzagt F, Boustani M, et al. Development and implementation of nonpharmacologic protocols for the management of patients with Alzheimer's disease and their families in a multiracial primary care setting. Gerontologist. 2004;44(4):548–53. doi: 10.1093/geront/44.4.548. [DOI] [PubMed] [Google Scholar]
- 22.Schmid AA, Spangler-Morris C, Beuchamp RC, Wellington MC, Hayden WM, Porterfield HS, et al. The Home Based Occupational Therapy Intervention in the Alzheimer's Disease Multiple Intervention Trial (ADMIT) Occupational Therapy in Mental Health. 2015;31(1):19–34. doi: 10.1080/0164212X.2014.1002963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belle SH, Burgio L, Burns R, Coon D, Czaja SJ, Gallagher-Thompson D, et al. Enhancing the quality of life of dementia caregivers from different ethnic or racial groups: a randomized, controlled trial. Ann Intern Med. 2006;145(10):727–38. doi: 10.7326/0003-4819-145-10-200611210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galasko D, Bennett D, Sano M, Ernesto C, Thomas R, Grundman M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(Suppl 2):S33–9. [PubMed] [Google Scholar]
- 25.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 26.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–61. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDermott MM, Liu K, Guralnik JM, Mehta S, Criqui MH, Martin GJ, et al. The ankle brachial index independently predicts walking velocity and walking endurance in peripheral arterial disease. J Am Geriatr Soc. 1998;46(11):1355–62. doi: 10.1111/j.1532-5415.1998.tb06001.x. [DOI] [PubMed] [Google Scholar]
- 28.Miller DK, Malmstrom TK, Andresen EM, Miller JP, Herning MM, Schootman M, et al. Development and validation of a short portable sarcopenia measure in the African American health project. J Gerontol A Biol Sci Med Sci. 2009;64(3):388–94. doi: 10.1093/gerona/gln033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernard BL, Bracey LE, Lane KA, Ferguson DY, LaMantia MA, Gao S, et al. Correlation Between Caregiver Reports of Physical Function and Performance-based Measures in a Cohort of Older Adults With Alzheimer Disease. Alzheimer Dis Assoc Disord. 2015 doi: 10.1097/WAD.0000000000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaufer DI, Cummings JL, Christine D, Bray T, Castellon S, Masterman D, et al. Assessing the impact of neuropsychiatric symptoms in Alzheimer's disease: the Neuropsychiatric Inventory Caregiver Distress Scale. J Am Geriatr sSoc. 1998;46(2):210–5. doi: 10.1111/j.1532-5415.1998.tb02542.x. [DOI] [PubMed] [Google Scholar]
- 31.Weiner MF, Koss E, Wild KV, Folks DG, Tariot P, Luszczynska H, et al. Measures of psychiatric symptoms in Alzheimer patients: a review. Alzheimer Dis Assoc Disord. 1996;10(1):20–30. [PubMed] [Google Scholar]
- 32.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–14. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 33.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. 2. Hoboken, NJ: Wiley and Sons; 2011. pp. 124–138. [Google Scholar]
- 34.Tang L, Song J, Belin TR, Unutzer J. A comparison of imputation methods in a longitudinal randomized clinical trial. Stat Med. 2005;24(14):2111–28. doi: 10.1002/sim.2099. [DOI] [PubMed] [Google Scholar]
- 35.Alderson P. Absence of evidence is not evidence of absence. BMJ. 2004;328(7438):476–7. doi: 10.1136/bmj.328.7438.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Souto Barreto P, Demougeot L, Pillard F, Lapeyre-Mestre M, Rolland Y. Exercise training for managing behavioral and psychological symptoms in people with dementia: A systematic review and meta-analysis. Ageing Res sRev. 2015;24(Pt B):274–85. doi: 10.1016/j.arr.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 37.Ohman H, Savikko N, Strandberg TE, Pitkala KH. Effect of physical exercise on cognitive performance in older adults with mild cognitive impairment or dementia: a systematic review. Dement Geriatr Cogn Disord. 2014;38(5–6):347–65. doi: 10.1159/000365388. [DOI] [PubMed] [Google Scholar]
- 38.Pitkala K, Savikko N, Poysti M, Strandberg T, Laakkonen ML. Efficacy of physical exercise intervention on mobility and physical functioning in older people with dementia: a systematic review. Exp Gerontol. 2013;48(1):85–93. doi: 10.1016/j.exger.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 39.Forbes D, Forbes SC, Blake CM, Thiessen EJ, Forbes S. Exercise programs for people with dementia. Cochrane Database Syst Rev. 2015;4:CD006489. doi: 10.1002/14651858.CD006489.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pitkala KH, Poysti MM, Laakkonen ML, Tilvis RS, Savikko N, Kautiainen H, et al. Effects of the Finnish Alzheimer disease exercise trial (FINALEX): a randomized controlled trial. JAMA Intern Med. 2013;173(10):894–901. doi: 10.1001/jamainternmed.2013.359. [DOI] [PubMed] [Google Scholar]
- 41.Clarfield AM, Dwolatzky T. Exercise in Alzheimer disease: comment on" Effects of the Finnish Alzheimer disease exercise trial (FINALEX): a randomized controlled trial". JAMA Intern Med. 2013;173(10):901–2. doi: 10.1001/jamainternmed.2013.1215. [DOI] [PubMed] [Google Scholar]
- 42.Toots A, Littbrand H, Lindelof N, Wiklund R, Holmberg H, Nordstrom P, et al. Effects of a High-Intensity Functional Exercise Program on Dependence in Activities of Daily Living and Balance in Older Adults with Dementia. J Am Geriatr Soc. 2016;64(1):55–64. doi: 10.1111/jgs.13880. [DOI] [PMC free article] [PubMed] [Google Scholar]