Abstract
Global variations in the incidence of pediatric cancers have been described; however, the causes of such differences are not known. We investigated the relationship between the incidence of embryonal tumors and human development index on a global scale. Increasing incidence of neuroblastoma correlates significantly with an increasing index of human development, with greater incidence among countries with high socioeconomic development, in apparent contrast to the incidence of retinoblastoma. While more data are needed to corroborate this observation, our findings suggest new avenues for etiological research and serve as a call for support of population-based cancer registries in low–middle-income countries.
Keywords: cancer registries, neuroblastoma, pediatric oncology, socioeconomic status
1 INTRODUCTION
The interaction of environmental exposures and germline predisposition may play a significant role in the development of childhood cancer. Our understanding of cancer incidence and biology, however, derives largely from the study of populations from resource-rich countries, limiting the full capture of all socioeconomic landscapes. To understand how variations in exposure and germline susceptibilities may impact the development of childhood cancers, data from children in lower income settings must be considered in these analyses.1,2
Among high-income countries, neuroblastoma has been described as the most common extracranial solid tumor, with an incidence in the United States of 10.7 per million among children ages 0–14, approximately 7% of all childhood cancers.3 The incidence rates of retinoblastoma and Wilms tumor in the same population are lower at 4.0 and 8.3 per million, respectively.3 Among many low- and middle-income countries, in contrast, neuroblastoma has been noted to be relatively rare.4
Our institutional experiences with global oncology programs prompted us to explore these differences, and emerging data appear to corroborate these observations. In Guatemala, for example, the incidence of retinoblastoma appeared notably higher than in the United States based on the data from the main tertiary referral center for pediatric cancer.5 Between 2000 and 2007, neuroblastomas comprised only 1.5% of all cancer diagnoses, while retinoblastomas and renal tumors represented 10 and 4.0%, respectively.6
While ethnic and racial differences may play a role, these observations prompted us to consider whether a correlation between the incidence of neuroblastoma and human development existed on a global scale. We used data from the second volume of the International Incidence of Childhood Cancer7 and the human development index (HDI) to investigate the correlation between socioeconomic status and the worldwide incidence of extracranial embryonal tumors.
2 METHODS
Incidence (rates per million) of neuroblastoma, retinoblastoma, and Wilms tumor for ages 0–4 were abstracted from the International Incidence of Childhood Cancer, International Agency for Research on Cancer (IARC), Volume II7 into an electronic database. Hepatoblastoma was not included given small numbers. The HDI, a composite score incorporating life expectancy, education, and standard of living (www.hdr.undp.org), was used to represent each country’s level of development. In 2010, the methodology for calculating HDI was refined8 and values from http://hdr.undp.org/en/data-explorer (accessed July 14, 2016) were used in this study. The midpoint of each registry was approximated by the arithmetic mean, rounding upward to the nearest whole number year. (With the exception of Kuwait, each registry spanned continuous years). When more than one registry was available for a given country, the registry representing the largest population was selected (Supplementary Table S1). Registries without corresponding HDI information (n = 8) or incidence rates (n = 7) were excluded from this analysis. Correlations between incidence and HDI were performed using Spearman rank correlation coefficient.
To examine whether variations in common polymorphisms across populations may contribute to global differences in tumor incidence, we compared incidence with allele frequencies of 13 neuroblastoma and six Wilms tumor risk single-nucleotide polymorphisms (SNPs)9–15 within a subset of nine countries represented in both the IARC registries and the 1,000 Genomes (phase 3) dataset16 (Supplementary Tables S2 and S3).
3 RESULTS
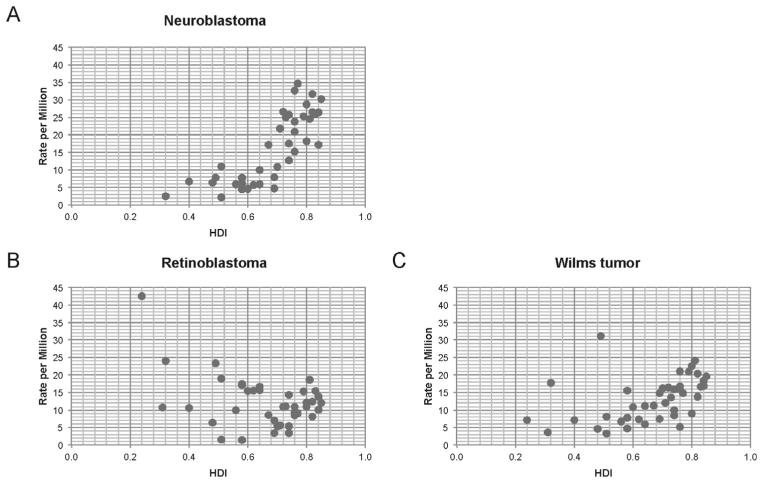
Incidence rates of neuroblastoma showed a direct relationship with HDI (r = 0.81; P < 0.001; n = 39) (Fig. 1A). In contrast, incidence of retinoblastoma was unrelated to HDI (r = −0.122; P = 0.45; n = 41) (Fig. 1B). The incidence of Wilms tumor (Fig. 1C) showed a correlation between HDI and incidence similar to that of neuroblastoma (r = 0.6; P < 0.001, n = 41). HDI and incidence rates are shown in Supplementary Table S1.
FIGURE 1.
Embryonal tumor incidence and HDI. (A) Neuroblastoma incidence is correlated with HDI, while (B) retinoblastoma incidence does not show this trend. (C) Wilms tumor incidence also correlates with HDI
Risk allele frequencies for neuroblastoma and Wilms tumor risk SNPs were not significantly associated with incidence after correcting for multiple testing, with or without controlling for HDI (Supplementary Table S2). Nonetheless, the positive association between incidence and HDI in the subset of nine countries remained significant for neuroblastoma (r = 0.82; P = 0.011), although the association was of only borderline significance for Wilms tumor (r = 0.65; P = 0.067).
4 DISCUSSION
Herein we demonstrate correlation between the incidence of neuroblastoma and HDI. Neuroblastoma is enriched in areas of high HDI, while retinoblastoma does not show this pattern. Underlying socioeconomic conditions are likely contributors, as HDI is a composite measure that includes life expectancy, anticipated education, and national standards of living. We hypothesize that the interplay of perinatal and early life environmental exposures combined with germline predisposition likely contributes to this observation.
Although no significant correlations between SNP allele frequency and incidence were observed, the analysis was limited by the availability of only nine matched registries with SNP data. Nonetheless, the positive association between incidence and HDI remained significant among this subset.
The trends observed could also be related to underdiagnosis of neuroblastoma given its frequent presentation as an intra-abdominal mass. Wilms tumor, with similar age at diagnosis and presenting signs and symptoms, was included in our analysis to address this. Given the similar trend observed with Wilms tumor, we are unable to rule out this possibility. Another potential limitation of such a study is the possibility of ecologic fallacy, that is, children in the registry may not have been exposed to the country’s HDI.
The IARC dataset used in this study was chosen as it represents the most comprehensive currently available database using standardized criteria for data inclusion. While the publication of Volume 3 is highly anticipated, at the writing of this report, only Volume 2 data were available for analysis; thus, HDI statistics were chosen to approximate the same time period. While we acknowledge that updated data are clearly needed, it will be informative to see how the incidence of these cancers evolve over time and to examine the impact of development. Similar associations and anticipated transitions have been described among adult-type cancers. Colorectal cancer, for example, has been shown to be overrepresented in areas of very high HDI, whereas infection-related cancers have been shown to be negatively associated with HDI, with projected changes in areas with economic transition.17 Other studies have examined the incidence of neuroblastoma by race/ethnicity. A Children’s Oncology Group study, for example, demonstrated that blacks and native Americans had a higher prevalence of high-risk disease compared with whites, suggesting the presence of biological factors affecting disease.18
We would be remiss not to acknowledge other limitations of the dataset used. Small numbers, for example, are expected to be less stable, and we were unable to capture regional differences in HDI. However, these data are beginning to emerge. Regional variations in cancer incidence have been described in Brazil, where cities with higher socioeconomic development had significantly higher neuroblastoma and lower retinoblastoma incidence, with a reversed phenomenon seen in cities with lower socioeconomic status.19 Similarly, fewer diagnoses of neuroblastoma and renal tumors have been described in higher poverty counties using Surveillance, Epidemiology, and End Results Program (SEER) data within regions of the United States.20
Examining the interaction of environmental and germline factors may provide critical insight into our understanding of cellular transformation and tumorigenesis. Pediatric tumors may be particularly informative, given the relatively short time to tumor formation. To fully understand the determinants of these cancers, a broader examination of the global landscape considering geography, ethnicity, and economic development are essential. Population-based cancer registries will undoubtedly be an important investment toward a fuller understanding of pediatric carcinogenesis.
Supplementary Material
Acknowledgments
The authors would like to thank P. Friedrich-Medina, MD, and A. Ricci, MD, for their input in manuscript preparation, as well as S. Bhatt Carreno and S. Block for their assistance with data management. M.A.O. is supported by NIH CA167833 and CA192662.
Abbreviations
- HDI
human development index
- IARC
International Agency for Research on Cancer
- SNP
single-nucleotide polymorphism
- SEER
Surveillance, Epidemiology, and End Results Program
Footnotes
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
Additional Supporting Information may be found online in the supporting information tab for this article.
References
- 1.Howard SC, Metzger ML, Wilimas JA, et al. Childhood cancer epidemiology in low-income countries. Cancer. 2008;112(3):461–472. doi: 10.1002/cncr.23205. [DOI] [PubMed] [Google Scholar]
- 2.Magrath I, Steliarova-Foucher E, Epelman S, et al. Paediatric cancer in low-income and middle-income countries. Lancet Oncol. 2013;14(3):e104–e116. doi: 10.1016/S1470-2045(13)70008-1. [DOI] [PubMed] [Google Scholar]
- 3.Siegel DA, King J, Tai E, Buchanan N, Ajani UA, Li J. Cancer incidence rates and trends among children and adolescents in the United States, 2001–2009. Pediatrics. 2014;134(4):e945–e955. doi: 10.1542/peds.2013-3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stiller CA, Parkin DM. International variations in the incidence of neuroblastoma. Int J Cancer. 1992;52(4):538–543. doi: 10.1002/ijc.2910520407. [DOI] [PubMed] [Google Scholar]
- 5.Dean M, Bendfeldt G, Lou H, et al. Increased incidence and disparity of diagnosis of retinoblastoma patients in Guatemala. Cancer Lett. 2014;351(1):59–63. doi: 10.1016/j.canlet.2014.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhojwani D, Antillon-Klussman F, Howard SC. Childhood cancer in the developing world. In: Carroll WL, Finlay JL, editors. Cancer in Children and Adolescents. Sudbury, MA: Jones and Bartlett Publishers; 2010. pp. 15–22. [Google Scholar]
- 7.Parkin DM, Kramarova E, Draper GJ, et al., editors. International Incidence of Childhood Cancer. 1998;II:391. [Google Scholar]
- 8.UNDP. Human Development Report 2010, The Real Wealth of Nations: Pathways to Human Development. 29. Vol. 20. New York: Palgrave Macmillan; 2010. Available from http://hdr.undp.org/sites/default/files/reports/270/hdr_2010_en_complete_reprint.pdf. [Google Scholar]
- 9.Capasso M, Devoto M, Hou C, et al. Common variations in BARD1 influence susceptibility to high-risk neuroblastoma. Nat Genet. 2009;41(6):718–723. doi: 10.1038/ng.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diskin SJ, Capasso M, Schnepp RW, et al. Common variation at 6q16 within HACE1 and LIN28B influences susceptibility to neuroblastoma. Nat Genet. 2012;44(10):1126–1130. doi: 10.1038/ng.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maris JM, Mosse YP, Bradfield JP, et al. Chromosome 6p22 locus associated with clinically aggressive neuroblastoma. N Engl J Med. 2008;358(24):2585–2593. doi: 10.1056/NEJMoa0708698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen le B, Diskin SJ, Capasso M, et al. Phenotype restricted genome-wide association study using a gene-centric approach identifies three low-risk neuroblastoma susceptibility Loci. PLoS Genet. 2011;7(3):e1002026. doi: 10.1371/journal.pgen.1002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burdett T, Hall PN, Hastings E, et al. [Accessed 2016 June 9, 2016];The NHGRI-EBI Catalog of published genome-wide association studies. 2016 Jun 9; http://www.ebi.ac.uk/gwas.
- 14.Turnbull C, Perdeaux ER, Pernet D, et al. A genome-wide association study identifies susceptibility loci for Wilms tumor. Nat Genet. 2012;44(6):681–684. doi: 10.1038/ng.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang K, Diskin SJ, Zhang H, et al. Integrative genomics identifies LMO1 as a neuroblastoma oncogene. Nature. 2011;469(7329):216–220. doi: 10.1038/nature09609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Genomes Project C. Auton A, Brooks LD, et al. A global reference for human genetic variation. Nature. 2015;526(7571):68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. Lancet Oncol. 2012;13(8):790–801. doi: 10.1016/S1470-2045(12)70211-5. [DOI] [PubMed] [Google Scholar]
- 18.Henderson TO, Bhatia S, Pinto N, et al. Racial and ethnic disparities in risk and survival in children with neuroblastoma: a Children’s Oncology Group study. J Clin Oncol. 2011;29(1):76–82. doi: 10.1200/JCO.2010.29.6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Camargo B, de Oliveira Ferreira JM, de Souza Reis R, Ferman S, de Oliveira Santos M, Pombo-de-Oliveira MS. Socioeconomic status and the incidence of non-central nervous system childhood embryonic tumours in Brazil. BMC Cancer. 2011;11:160. doi: 10.1186/1471-2407-11-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan IJ, Daniels JL, Zhu K. Poverty and childhood cancer incidence in the United States. Cancer Causes Control. 2010;21(7):1139–1145. doi: 10.1007/s10552-010-9528-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.