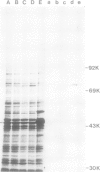
Abstract
A cDNA clone bank containing 30 000 clones was constructed from sucrose gradient-fractionated mRNA from human placenta. mRNA coding for transferrin receptor (TR) was enriched by polysome immuno-adsorbed chromatography with monospecific rabbit IgG and protein-A Sepharose. The library was screened for hybridisation to 32P-labelled cDNA synthesised from immunoselected TR mRNA and from poly(A)+ RNA of the polysome fraction that failed to bind to protein-A Sepharose. Plasmids isolated from colonies showing hybridisation only to the probe made from immunoselected mRNA were then subjected to hybrid selection. Two clones, pTR-48 and pTR-67, were able to hybridise the mRNA coding for the TR.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Delia D., Greaves M. F., Newman R. A., Sutherland D. R., Minowada J., Kung P., Goldstein G. Modulation of T leukaemic cell phenotype with phorbol ester. Int J Cancer. 1982 Jan 15;29(1):23–31. doi: 10.1002/ijc.2910290106. [DOI] [PubMed] [Google Scholar]
- Dobberstein B., Garoff H., Warren G., Robinson P. J. Cell-free synthesis and membrane insertion of mouse H-2Dd histocompatibility antigen and beta 2-microglobulin. Cell. 1979 Aug;17(4):759–769. doi: 10.1016/0092-8674(79)90316-7. [DOI] [PubMed] [Google Scholar]
- Goodfellow P. N., Banting G., Sutherland R., Greaves M., Solomon E., Povey S. Expression of human transferrin receptor is controlled by a gene on chromosome 3: assignment using species specificity of a monoclonal antibody. Somatic Cell Genet. 1982 Mar;8(2):197–206. doi: 10.1007/BF01538677. [DOI] [PubMed] [Google Scholar]
- Goubin G., Goldman D. S., Luce J., Neiman P. E., Cooper G. M. Molecular cloning and nucleotide sequence of a transforming gene detected by transfection of chicken B-cell lymphoma DNA. Nature. 1983 Mar 10;302(5904):114–119. doi: 10.1038/302114a0. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Helfman D. M., Feramisco J. R., Fiddes J. C., Thomas G. P., Hughes S. H. Identification of clones that encode chicken tropomyosin by direct immunological screening of a cDNA expression library. Proc Natl Acad Sci U S A. 1983 Jan;80(1):31–35. doi: 10.1073/pnas.80.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hutchings S. E., Sato G. H. Growth and maintenance of HeLa cells in serum-free medium supplemented with hormones. Proc Natl Acad Sci U S A. 1978 Feb;75(2):901–904. doi: 10.1073/pnas.75.2.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus J. P., Rosenberg L. E. Purification of low-abundance messenger RNAs from rat liver by polysome immunoadsorption. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4015–4019. doi: 10.1073/pnas.79.13.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurkinen M., Barlow D. P., Helfman D. M., Williams J. G., Hogan B. L. Isolation of cDNA clones for basal lamina components: type IV procollagen. Nucleic Acids Res. 1983 Sep 24;11(18):6199–6209. doi: 10.1093/nar/11.18.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrick J. W., Cresswell P. Modulation of cell surface iron transferrin receptors by cellular density and state of activation. J Supramol Struct. 1979;11(4):579–586. doi: 10.1002/jss.400110415. [DOI] [PubMed] [Google Scholar]
- Plowman G. D., Brown J. P., Enns C. A., Schröder J., Nikinmaa B., Sussman H. H., Hellström K. E., Hellström I. Assignment of the gene for human melanoma-associated antigen p97 to chromosome 3. Nature. 1983 May 5;303(5912):70–72. doi: 10.1038/303070a0. [DOI] [PubMed] [Google Scholar]
- Schneider C., Asser U., Sutherland D. R., Greaves M. F. In vitro biosynthesis of the human cell surface receptor for transferrin. FEBS Lett. 1983 Jul 25;158(2):259–264. doi: 10.1016/0014-5793(83)80591-2. [DOI] [PubMed] [Google Scholar]
- Schneider C., Sutherland R., Newman R., Greaves M. Structural features of the cell surface receptor for transferrin that is recognized by the monoclonal antibody OKT9. J Biol Chem. 1982 Jul 25;257(14):8516–8522. [PubMed] [Google Scholar]
- Sutherland R., Delia D., Schneider C., Newman R., Kemshead J., Greaves M. Ubiquitous cell-surface glycoprotein on tumor cells is proliferation-associated receptor for transferrin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4515–4519. doi: 10.1073/pnas.78.7.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodinelich L., Sutherland R., Schneider C., Newman R., Greaves M. Receptor for transferrin may be a "target" structure for natural killer cells. Proc Natl Acad Sci U S A. 1983 Feb;80(3):835–839. doi: 10.1073/pnas.80.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]