Abstract
Background
Hypertonic dextrose injection (prolotherapy) is reported to reduce pain including non-surgical chronic low back pain (CLBP), and subcutaneous injection of 5% dextrose is reported to reduce neurogenic pain, hyperalgesia and allodynia. The mechanism in both cases is unclear, though a direct effect of dextrose on neurogenic pain has been proposed. This study assessed the short-term analgesic effects of epidural 5% dextrose injection compared with saline for non-surgical CLBP.
Methods
Randomized double-blind (injector, participant) controlled trial. Adults with moderate-to-severe non-surgical low back pain with radiation to gluteal or leg areas for at least 6 months received a single epidurogram-confirmed epidural injection of 10 mL of 5% dextrose or 0.9% saline using a published vertical caudal injection technique. The primary outcome was change in a numerical rating scale (NRS, 0 - 10 points) pain score between baseline and 15 minutes; and 2, 4, and 48 hours and 2 weeks post-injection. The secondary outcome was percentage of participants achieving 50% or more pain improvement at 4 hours.
Results and Conclusions
No baseline differences existed between groups; 35 participants (54 ± 10.7 years old; 11 female) with moderate-to-severe CLBP (6.7 ± 1.3 points) for 10.6 ± 10.5 years. Dextrose participants reported greater NRS pain score change at 15 minutes (4.4 ± 1.7 vs 2.4 ± 2.8 points; P = 0.015), 2 hours (4.6 ± 1.9 vs 1.8 ± 2.8 points; P = 0.001), 4 hours (4.6 ± 2.0 vs 1.4 ± 2.3 points; P < 0.001), and 48 hours (3.0 ± 2.3 vs 1.0 ± 2.1 points; P = 0.012), but not at 2 weeks (2.1 ± 2.9 vs 1.2 ± 2.4 points; P = 0.217). Eighty four percent (16/19) of dextrose recipients and 19% (3/16) of saline recipients reported ≥ 50% pain reduction at 4 hours (P < 0.001). These findings suggest a neurogenic effect of 5% dextrose on pain at the dorsal root level; waning pain control at 2 weeks suggests the need to assess the effect of serial dextrose epidural injections in a long-term study with robust outcome assessment.
Keywords: Analgesia, Epidural, Anesthesia, Caudal, Dextrose
1. Background
Chronic low back pain (CLBP) is common, debilitating and consumes substantial health care resources (1, 2). Specific CLBP diagnoses include lumbar radiculopathy, spinal stenosis, non-specific low back pain and residual pain after low back surgery. Conventional therapies are often ineffective and some are known to have unacceptable adverse outcomes. For example, prescription opioids often used for CLBP have been identified as contributing to an “opioid epidemic (3).” Among the treatment options that have been assessed for CLBP are caudal epidural injection of anti-inflammatories, analgesics and anesthetics (4), and interventional procedures not involving injection (5). Effectiveness of each, however, is suboptimal (5, 6). The identification of safe and effective therapy for CLBP is a public health priority (7).
Dextrose injection (prolotherapy) is reported to safely reduce chronic musculoskeletal pain and improve function in a variety of conditions, though the mechanism is unclear (8, 9). Prolotherapy is identified as a regenerative treatment by some authors (10). A multi-factorial mechanism is proposed, including a direct analgesic effect. Dextrose has been injected into the epidural or intrathecal space to control the placement location of other epidural injectates due to its relatively high specific gravity (11-14). One rigorous trial reports outcomes favoring a dextrose-specific mechanism of prolotherapy for knee osteoarthritis (9) and a follow-up study suggested a direct sensorineural effect (15). Dextrose injections have also targeted superficial sensory nerves in uncontrolled and controlled studies (16-19). Anecdotal evidence from the authors’ clinics (LMS and HJR) suggested that epidural injection of 5% dextrose in patients with chronic low back and leg pain is associated with a rapid short-term analgesic response. However, this observation has not been empirically assessed.
2. Objectives
As part of a multi-method study, we conducted a randomized controlled trial (RCT) to test the hypothesis that participants with non-surgical CLBP with either buttock or leg pain who received injection of 5% dextrose in the caudal epidural space, compared with those who received normal saline injection, would report decreased pain within 15 minutes of injection lasting up to 2 weeks.
3. Methods
3.1. Study Design
This two-arm double-blind study was approved by the Western Institutional Review Board, with ClinicalTrials.gov identifier of NCT01547364. A non-blinded research assistant generated twenty randomly-permuted 2-person blocks via computer (randomization.com) for use in 1 to 1 group assignment. The research assistant used this password-protected list to consecutively allocate eligible participants to either dextrose or normal saline injection. The injector (LMS), participant, and outcome assessor were masked to treatment group. At the injection session, the research assistant consulted the allocation assignment list and prepared 10 mL of either dextrose or NS at the point of care in a separate room and presented the de-identified syringe to the blinded injector (LMS) for use on the blinded participant.
3.2. Inclusion and Exclusion Criteria
Inclusion criteria included 19 to 75 years old; simultaneous participation in a concurrent patient study of a vertical small needle caudal epidural injection technique (20); 6 months or more of self-reported moderate-to-severe CLBP including below the iliac crest as defined by a self-reported score of 5 or more on a 0 - 10 Numeric Rating Scale (NRS) in response to the question “What is the intensity of your back pain”; and failure of one or more non-injection therapies. NSAID use was not considered a modality of treatment. Exclusion criteria included progressive weakness; recent bowel or bladder dysfunction concerning for unstable or progressive surgical CLBP; increase in self-reported morphine equivalent dose of prescription pain medication in the past 3 months; local infection; unstable psychiatric disorder; other chronic pain; or current anticoagulation or medical condition rendering the potential participant unable to reliably participate in the study.
Single injection Intervention: Participants were treated in a prone position without an abdominal bolster and without reverse Trendelenburg. Sterile preparation of the injection site was with 2.3% chlorhexidine gluconate/24% isopropyl alcohol. A vertical short needle technique was utilized for injection in the caudal epidural space, using a 25 gauge 3.8 cm needle with needle entry at or below the sacral cornua (20). A positive epidurogram confirmed needle placement in the caudal epidural space. After confirmation, 10 mL of solution was injected over 1 minute as tolerated by the participant, with pressure sensation being the rate-limiting factor. Participants remained prone for at least 5 minutes. No post-injection analgesics were provided to participants. Participants were allowed to continue current medication usage during the 2-week period of the study.
3.3. Outcome Measures and Follow-Up
The primary outcome measure was a change in pain score on a 0 - 10 numerical rating scale (NRS) in response to the question “What is the intensity of your back pain?”, with anchors of 0 ("No pain") and 10 ("Most severe pain imaginable"). The NRS has been demonstrated to be reliable (21). In chronic musculoskeletal pain, a reduction of 15% or one point in the NRS represents a minimal clinically important difference (22). Participants pre-injection (baseline) and 15-minute follow-up post-injection pain scores were recorded in clinic and on a pre-printed pain-level-recording card. Participants were instructed to record their at-home pain levels at 2, 4 and 48 hours. They were called by the office manager/assessor at the conclusion of day 2 to report the pain levels indicated on their card through 48 hours. Their final in-person follow-up in this study was at two weeks. Participants were informed of their allocation assignment at two weeks; participants in both groups were offered enrollment in an open label study to assess the long-term effect of serial dextrose injections. Interested participants were enrolled; the study is ongoing and results will be reported separately.
3.4. Other Measures
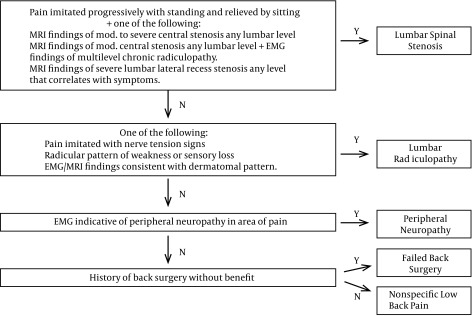
Age, sex, body mass index, pain severity, pain duration, prescription opioid use, medications for neuropathic pain, and diagnostic category were collected at baseline to characterize the sample and to evaluate as covariates for statistical analysis. Participants were assigned to one of five diagnostic categories based on magnetic resonance imaging, electromyographic and physical examination criteria: lumbar spinal stenosis, lumbar radiculopathy, peripheral neuropathy, failed back surgery or nonspecific low back pain. (Figure 1)
Figure 1. Diagnostic Category Assignment Method.
Pseudoclaudication plus moderate to severe radiographic findings were required for spinal stenosis assignment, hard neurologic examination or electromyographic findings for radiculopathy assignment, and electromyographic findings for peripheral neuropathy categorization.
Analysis: Using a projected between-group mean difference for improvement over time in NRS pain of 2.5 points on a 0 - 10 scale and a standard deviation of 2.0 points, an effect size of 1.25 was calculated. Assuming 10% loss to follow-up, 32 participants would provide 90% power to detect a difference in mean NRS scores between dextrose and normal saline at a significance level of 0.05. Analysis was per protocol. Data were analyzed using PASW 18 (Predictive Analytics Software 18.0.0, IBM). Descriptive statistics describe outcomes at each time point; mean value ± standard deviation (SD) was reported at baseline. A ANCOVA for pain scale was applied to compare the groups for magnitude of change in the 0 - 10 NRS pain score between baseline and follow-up for time points 15 minutes, 2, 4, 48 hours and 2 weeks. Preliminary observations suggested a maximal analgesic effect of epidural injection of dextrose at 4 hours. Efficacy studies on epidural injection for treatment of pain have defined a clinically relevant pain outcome of at least 50% improvement on the 0 - 10 NRS scale (23). Pearson chi square analysis was utilized to compare dextrose and saline groups for the percentage achieving ≥ 50% pain reduction at 4 hours, calculated by dividing the difference between baseline and follow-up scores by the initial score.
3.5. Presentation History
An abstract based on data from this clinical trial was presented as a poster at the annual meeting of American Congress of Rehabilitation Medicine, October 25-30, 2015, in Dallas, Texas.
4. Results
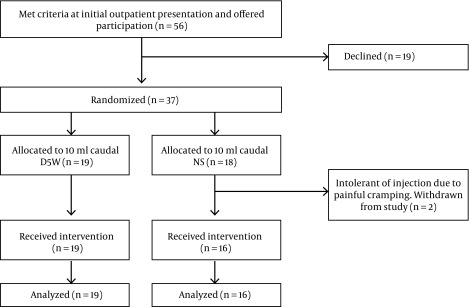
All participants were treated in Hilo, Hawaii, or Honolulu, Oahu, and were enrolled and treated between February 22, 2013, and November 12, 2013. Participation in the study was offered to the first 56 persons who met screening criteria (Figure 2). Nineteen declined participation. Thirty-seven were randomized. Nineteen received 5% dextrose and sixteen received normal saline injection into the caudal epidural space. Two participants complained of substantial cramping pain prior to receiving the full 10 mL injection volume. Both were exited from the study due to procedure intolerance, were unblinded at 15 minutes post injection, and determined to be in the saline group. There were no other adverse events. Data capture at 2-week follow-up was otherwise complete.
Figure 2. Consort Flow Diagram.
There were no significant baseline differences between groups (Table 1). The study sample was middle aged (54 ± 10.7 years) and 31% female; 77% were either pre-obese (BMI ≥ 25 kg/m2), or obese (BMI ≥ 30 kg/m2). Participants reported a mean of 10.6 ± 10.5 years of back pain. More than 50% were taking or had tried prescribed opioids. The most common primary diagnoses were lumbar spinal stenosis (34%), lumbar radiculopathy (26%) and nonspecific low back pain (26%). Pain severity was moderate to severe with a mean baseline pain level of 6.7 ± 1.3 points (range 5 to 9).
Table 1. Baseline Participant Characteristics by Treatment Groupa.
| Dextrose | Saline | P Valueb | |
|---|---|---|---|
| Non-Diagnostic Characteristics | |||
| No. | 19 | 16 | |
| Female | 6 (32) | 5 (31) | 0.983 |
| Age years | 54 ± 8.9 | 54 ± 12.8 | 0.960 |
| Pain duration years | 8.6 ± 6.6 | 12.9 ± 13.7 | 0.230 |
| NRS pain | 6.3 ± 1.3 | 7.1 ± 1.2 | 0.086 |
| ODI 2.0 | 43.2 ± 14.2 | 39.8 ± 13.4 | 0.477 |
| BMI | 30.8 ± 8.7 | 29.1 ± 5.2 | 0.509 |
| Narcotic Intake | 10 (53) | 8 (50) | 0.877 |
| SSRI/SNRI | 2 (11) | 1 (6) | 0.566 |
| Gabapentin/pregabalin | 3 (16) | 2 (13) | 0.585 |
| Epidural Steroid | 3 (16) | 4 (25) | 0.398 |
| Diagnostic Categories | |||
| Lumbar spinal stenosis | 8 (42) | 3 (19) | 0.195 |
| Lumbar radiculopathy | 6 (32) | 3 (19) | |
| Peripheral neuropathy | 0 | 2 (12) | |
| Post Laminectomy | 2 (10) | 2 (12) | |
| Nonspecific low back pain | 3 (16) | 6 (38) | |
aValues are expressed as No. (%) or mean ± SD.
bP values obtained from ANOVA for numeric variables. For non-numeric variables the Pearson chi square results were utilized except when cell counts were less than 5, in which case the Fisher’s exact test results were used.
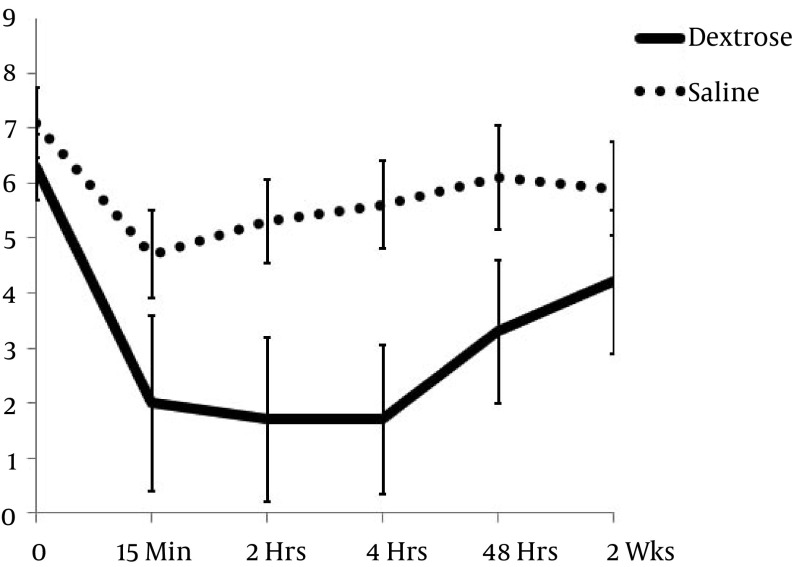
Between-group comparisons of NRS score change between baseline and follow-up time points favored the intervention group at all points except 2 weeks: 15 minutes (4.4 ± 1.7 vs 2.4 ± 2.8 points; P = 0.015), 2 hours (4.6 ± 1.9 vs 1.8 ± 2.8 points; P = 0.001), 4 hours (4.6 ± 2.0 vs 1.4 ± 2.3 points; P < 0.001), 48 hours (3.0 ± 2.3 vs 1.0 ± 2.1 points; P = 0.012), and 2 weeks (2.1 ± 2.9 vs 1.2 ± 2.4 points; P = 0.217), (Table 2, Figure 3). The number of participants achieving ≥ 50% improvement in pain at 4 hours was higher in dextrose recipients (16/19; 84%) than in saline recipients (3/16;19%; P < 0.001). Variables analyzed as covariates, including diagnostic category, were not predictive of point change in either group.
Table 2. Change in the NRS for Pain Severity From Baseline to 2 Weeks After Injection of 10 mL of Either 5% Dextrose or Normal Saline Into the Caudal Epidural Spacea.
| Raw score and Change Score | Dextrose (n = 19) | Saline (n = 16) | Significance: P Valueb |
|---|---|---|---|
| Baseline: Raw Score | 6.3 ± 1.3 | 7.1 ± 1.2 | NA |
| Change in Score | NA | NA | |
| 15 minutes: Raw score | 2.0 ± 1.6 | 4.7 ± 3.2 | 0.015 |
| Change in score | 4.4 ± 1.7 | 2.4 ± 2.8 | |
| 2 hours: Raw score | 1.7 ± 1.5 | 5.3 ± 3.0 | 0.001 |
| Change in score | 4.6 ± 1.9 | 1.8 ± 2.8 | |
| 4 hours: Raw score | 1.7 ± 1.6 | 5.6 ± 2.7 | < 0.001 |
| Change in score | 4.6 ± 2.0 | 1.5 ± 2.3 | |
| 48 hours: Raw score | 3.3 ± 1.9 | 6.1 ± 2.6 | 0.012 |
| Change in score | 3.0 ± 2.3 | 1.0 ± 2.1 | |
| 2 weeks: Raw score | 4.2 ± 1.7 | 5.9 ± 2.6 | 0.217 |
| Change in score | 2.1 ± 1.9 | 1.2 ± 2.4 |
aValues are expressed as mean ± SD.
bSignificance of the mean difference for change between groups.
Figure 3. Change in 0 - 10 NRS Pain Scores Over 2 Weeks (± Standard Error).
NRS is scored on a range of 0 to 10 points, with 10 anchored by “worst pain imaginable” and 0 by “no pain”. Non-overlapping confidence intervals indicate significance of change in dextrose scores compared with change in score of the saline (P < 0.05) group.
5. Discussion
This RCT found that, among participants with CLBP and either buttock or leg pain, 10 mL of dextrose injected in the caudal epidural space, compared with injection of 10 mL of normal saline, resulted in substantial, consistent, and significant analgesia within 15 minutes that lasted at least 48 hours. Pain improvement in the dextrose group at 15 minutes, 2 and 4 hours exceeded twice the minimal important change for pain improvement in low back pain as measured by NRS for pain (24). These results suggest a short-term analgesic effect of dextrose for CLBP with radiation to buttock or leg. Dextrose appears safe; 5% - 10% dextrose has been used to alter the spread of epidural anesthesia (11-14, 25-31) and has not been associated with complications. The current study was not powered to detect rare complications or adverse events. Previous studies including dextrose in the injectate did not assess for an analgesic effect attributable specifically to dextrose. These findings suggest for the first time that 5% dextrose injected in the caudal space may confer a pain-specific neurogenic effect at the dorsal root level. The selection of 10 mL volume as the dose of 5% dextrose was based on the authors’ clinical experience. It is unclear if this is optimal for all patients, as the dermatomal pain level for each patient is not the same. Given an analgesic effect in participants with pain at and above the iliac crest level, which is supplied by T12-L1, this suggests that the 10 mL volume introduced vertically at the sacral cornua level (20) was sufficient to allow cephalad flow of dextrose. Injection of larger volumes of 5% dextrose and radiographic confirmation of the extent of rostral movement of dye merit additional study.
This is the first study to assess the analgesic effect of dextrose injected in the caudal epidural space. Onset of analgesia compares well with reported onset with epidural morphine and fentanyl and may be of longer duration (32, 33). It did not alter sensation, although the analgesia was longer than that reported for single epidural injection of bupivacaine in one study (34).
These data are consistent with the effects of dextrose in two other contexts: First, hypertonic dextrose has been used for decades in prolotherapy, a technique that addresses pain receptors at entheses and intra-articular structures (8). Decreased pain and improved function after dextrose injection is reported in RCTs for several chronic conditions including rotator cuff tendinopathy (35), knee osteoarthritis (9, 36-38), Osgood Schlatter disease (39), hand osteoarthritis (40, 41), lateral epicondylosis (42, 43), and SI joint dysfunction (44). While dextrose injection has not been associated with changes in connective tissue assessed radiographically (35, 43), a recent open label study reports an association between intra-articular dextrose for knee osteoarthritis and histologically-assessed chondrogenesis, along with improved knee pain and function scores (45).
The current data are also consistent with three open-label studies and one randomized clinical trial of subcutaneous injection of dextrose over painful sensory nerves which suggest a potential therapeutic effect of dextrose injection on neuropathic pain (16-19). Taken as a whole, the clinical data suggest an independent effect of dextrose, though the precise mechanism is unclear, and other physiological effects of either 5% dextrose or 0.9% saline cannot be ruled out and may influence the results of the study.
Researchers have hypothesized that dextrose may reduce pain directly through a sensorineural mechanism. Afferent fibers expressing the transient receptor potential vanilloid receptor-1 (TRPV-1) cation channel, formerly known as the capsaicin-sensitive receptor, are widely accepted as the fibers on which much neuropathic pain depends (46, 47). Although long-term exposure to dextrose (in culture medium) may increase mRNA for TRPV-1 and predispose to neurogenic dysfunction (48), single dextrose injection may have a different effect on sensory nerves expressing the TRPV-1 cation channel. Mannitol, a molecule structurally chemically similar to dextrose, has been found to reduce capsaicin-induced burning pain upon application to the lip (49). Superficial dextrose injections targeting sensory nerves have been reported in a clinical trial to decrease trigger point-related pain more than lidocaine injections (50). Participants with Achilles tendinopathy in another study who received both exercise and dextrose injections targeting superficial sensory nerves report more improvement compared to exercise alone (19).
Limitations of this study include its short duration; however, the data support our hypothesis that dextrose reduces pain compared to control injection in the short term. In addition, this study cannot determine if the analgesic effect reported by dextrose participants is a one-time response, nor whether pain reduction can be repeated or endure with additional injections. We did not assess self-reported or objectively assessed function and so cannot comment on functional improvement, a key factor in treatment of CLBP. In addition, while participants did not report unexpected side effects nor adverse events, the study is not powered to detect rare events, nor events occurring in the long term. Results from a long-term study of the effects of serial dextrose injection on pain and functional abilities and monitoring for safety concerns will be separately reported. The small sample size and varied diagnostic criteria limit our ability to comment directly on the clinical effect of dextrose for any specific baseline CLBP diagnosis. However, the analgesic effect seen across various diagnostic categories suggests a potential common mechanism of neurogenic pain. The precise dosage of analgesic medication taken before and during the two week period of the study was not monitored, so we cannot comment on the short term effect of caudal epidural injection of D5W versus saline on analgesic intake. However, given that participants were stable regarding morphine equivalent dosing prior to the study, the immediate analgesic effect indicated herein appears to be independent of narcotic medication intake. Blinding was not assessed, possibly introducing bias; however, the randomization was effective and dextrose and saline are both colorless, transparent and of similar viscosity.
5.1. Conclusions
Compared with blinded saline, dextrose caudal epidural injection resulted in substantial analgesia within 15 minutes that persisted for 48 hours among chronic non-surgical LBP patients with buttock and/or leg pain, suggesting a neurogenic effect of dextrose in the caudal space. Basic science and clinical studies of longer duration and measuring both pain and functional outcomes are needed to elucidate the mechanism of action and potential clinical application of caudal epidural dextrose injection.
Acknowledgments
To colleagues of Liza Maniquis-Smigel including David Smigel, practice manager, Roquita Kaisen office manager, and Sasha Mooina, medical receptionist.
Footnotes
Financial Disclosure:Liza Maniquis-Smigel, M. D., Kenneth Dean Reeves, M.D., Howard Jeffrey Rosen, M.D. John Lyftogt, M.D., Cassie Coleman, R.N., An-Lin Cheng, Ph.D. (Statistics), and David Rabago M.D. have no financial interests related to the material in the manuscript.
References
- 1.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81(9):646–56. [PMC free article] [PubMed] [Google Scholar]
- 2.Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008;8(1):8–20. doi: 10.1016/j.spinee.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Wilkerson RG, Kim HK, Windsor TA, Mareiniss DP. The Opioid Epidemic in the United States. Emerg Med Clin North Am. 2016;34(2):e1–e23. doi: 10.1016/j.emc.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Rahimzadeh P, Sharma V, Imani F, Faiz HR, Ghodraty MR, Nikzad-Jamnani AR, et al. Adjuvant hyaluronidase to epidural steroid improves the quality of analgesia in failed back surgery syndrome: a prospective randomized clinical trial. Pain Physician. 2014;17(1):E75–82. [PubMed] [Google Scholar]
- 5.Patel VB, Wasserman R, Imani F. Interventional Therapies for Chronic Low Back Pain: A Focused Review (Efficacy and Outcomes). Anesth Pain Med. 2015;5(4):ee29716. doi: 10.5812/aapm.29716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veizi E, Hayek S. Interventional therapies for chronic low back pain. Neuromodulation. 2014;17 Suppl 2:31–45. doi: 10.1111/ner.12250. [DOI] [PubMed] [Google Scholar]
- 7.Hoy D, March L, Brooks P, Blyth F, Woolf A, Bain C, et al. The global burden of low back pain: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(6):968–74. doi: 10.1136/annrheumdis-2013-204428. [DOI] [PubMed] [Google Scholar]
- 8.Rabago D, Slattengren A, Zgierska A. Prolotherapy in primary care practice. Prim Care. 2010;37(1):65–80. doi: 10.1016/j.pop.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rabago D, Patterson JJ, Mundt M, Kijowski R, Grettie J, Segal NA, et al. Dextrose prolotherapy for knee osteoarthritis: a randomized controlled trial. Ann Fam Med. 2013;11(3):229–37. doi: 10.1370/afm.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeChellis DM, Cortazzo MH. Regenerative medicine in the field of pain medicine: Prolotherapy, platelet-rich plasma therapy, and stem cell therapy—Theory and evidence. Technique Region Anesth Pain Manag. 2011;15(2):74–80. doi: 10.1053/j.trap.2011.05.002. [DOI] [Google Scholar]
- 11.Mankowitz E, Brock-Utne JG, Cosnett JE, Green-Thompson R. Epidural ketamine. A preliminary report. S Afr Med J. 1982;61(12):441–2. [PubMed] [Google Scholar]
- 12.White JL, Stevens RA, Kao TC. Differential sensory block: spinal vs epidural with lidocaine. Can J Anaesth. 1998;45(11):1049–53. doi: 10.1007/BF03012390. [DOI] [PubMed] [Google Scholar]
- 13.Munishankar B, Fettes P, Moore C, McLeod GA. A double-blind randomised controlled trial of paracetamol, diclofenac or the combination for pain relief after caesarean section. Int J Obstet Anesth. 2008;17(1):9–14. doi: 10.1016/j.ijoa.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Park YR, Eastwood DW. Dextrose affects gravitational spread of epidural anesthesia. Anesthesiology. 1980;52(5):439–41. doi: 10.1097/00000542-198005000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Rabago D, Kijowski R, Woods M, Patterson JJ, Mundt M, Zgierska A, et al. Association between disease-specific quality of life and magnetic resonance imaging outcomes in a clinical trial of prolotherapy for knee osteoarthritis. Arch Phys Med Rehabil. 2013;94(11):2075–82. doi: 10.1016/j.apmr.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyftogt J. Subcutaneous prolotherapy for Achilles tendinopathy: The best solution? Aust Musculoskelet Med. 2007;12(2):107. [Google Scholar]
- 17.Lyftogt J. Subcutaneous prolotherapy treatment of refractory knee, shoulder, and lateral elbow pain. Aust Musculoskelet Med. 2007;12(2):110. [Google Scholar]
- 18.Lyftogt J. Prolotherapy for recalcitrant lumbago. Aust Musculoskelet Med. 2008;13(1):18. [Google Scholar]
- 19.Yelland MJ, Sweeting KR, Lyftogt JA, Ng SK, Scuffham PA, Evans KA. Prolotherapy injections and eccentric loading exercises for painful Achilles tendinosis: a randomised trial. Br J Sports Med. 2011;45:421–8. doi: 10.1136/bjsm.2009.057968. [DOI] [PubMed] [Google Scholar]
- 20.Maniquis Smigel L, Dean Reeves K, Jeffrey Rosen H, Patrick Rabago D. Vertical Small-Needle Caudal Epidural Injection Technique. Anesth Pain Med. 2016;6(3):ee35340. doi: 10.5812/aapm.35340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferraz MB, Quaresma MR, Aquino LR, Atra E, Tugwell P, Goldsmith CH. Reliability of pain scales in the assessment of literate and illiterate patients with rheumatoid arthritis. J Rheumatol. 1990;17(8):1022–4. [PubMed] [Google Scholar]
- 22.Salaffi F, Stancati A, Silvestri CA, Ciapetti A, Grassi W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain. 2004;8(4):283–91. doi: 10.1016/j.ejpain.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Manchikanti L, Knezevic NN, Boswell MV, Kaye AD, Hirsch JA. Epidural Injections for Lumbar Radiculopathy and Spinal Stenosis: A Comparative Systematic Review and Meta-Analysis. Pain Physician. 2016;19(3):E365–410. [PubMed] [Google Scholar]
- 24.Ostelo RW, Deyo RA, Stratford P, Waddell G, Croft P, Von Korff M, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine (Phila Pa 1976). 2008;33(1):90–4. doi: 10.1097/BRS.0b013e31815e3a10. [DOI] [PubMed] [Google Scholar]
- 25.Lee A, Ray D, Littlewood DG, Wildsmith JA. Effect of dextrose concentration on the intrathecal spread of amethocaine. Br J Anaesth. 1988;61(2):135–8. doi: 10.1093/bja/61.2.135. [DOI] [PubMed] [Google Scholar]
- 26.Hood DD, Mallak KA, Eisenach JC, Tong C. Interaction between intrathecal neostigmine and epidural clonidine in human volunteers. Anesthesiology. 1996;85(2):315–25. doi: 10.1097/00000542-199608000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Hasenbos MA, Gielen MJ. Anaesthesia for bullectomy. A technique with spontaneous ventilation and extradural blockade. Anaesthesia. 1985;40(10):977–80. doi: 10.1111/j.1365-2044.1985.tb10552.x. [DOI] [PubMed] [Google Scholar]
- 28.Connelly NR, Dunn SM, Ingold V, Villa EA. The use of fentanyl added to morphine-lidocaine-epinephrine spinal solution in patients undergoing cesarean section. Anesth Analg. 1994;78(5):918–20. doi: 10.1213/00000539-199405000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Liu S, Pollock JE, Mulroy MF, Allen HW, Neal JM, Carpenter RL. Comparison of 5% with dextrose, 1.5% with dextrose, and 1.5% dextrose-free lidocaine solutions for spinal anesthesia in human volunteers. Anesth Analg. 1995;81(4):697–702. doi: 10.1097/00000539-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 30.McLeod GA. Density of spinal anaesthetic solutions of bupivacaine, levobupivacaine, and ropivacaine with and without dextrose. Br J Anaesth. 2004;92(4):547–51. doi: 10.1093/bja/aeh094. [DOI] [PubMed] [Google Scholar]
- 31.Chauvin M, Samii K, Schermann JM, Sandouk P, Bourdon R, Viars P. Plasma concentration of morphine after i.m., extradural and intrathecal administration. Br J Anaesth. 1981;53(9):911–3. doi: 10.1093/bja/53.9.911. [DOI] [PubMed] [Google Scholar]
- 32.Torda TA, Pybus DA. Comparison of four narcotic analgesics for extradural analgesia. Br J Anaesth. 1982;54(3):291–5. doi: 10.1093/bja/54.3.291. [DOI] [PubMed] [Google Scholar]
- 33.Badner NH, Sandler AN, Koren G, Lawson SL, Klein J, Einarson TR. Lumbar epidural fentanyl infusions for post-thoracotomy patients: analgesic, respiratory, and pharmacokinetic effects. J Cardiothorac Anesth. 1990;4(5):543–51. doi: 10.1016/0888-6296(90)90402-2. [DOI] [PubMed] [Google Scholar]
- 34.Burm AG. Clinical pharmacokinetics of epidural and spinal anaesthesia. Clin Pharmacokinet. 1989;16(5):283–311. doi: 10.2165/00003088-198916050-00002. [DOI] [PubMed] [Google Scholar]
- 35.Bertrand H, Reeves KD, Bennett CJ, Bicknell S, Cheng AL. Dextrose Prolotherapy Versus Control Injections in Painful Rotator Cuff Tendinopathy. Arch Phys Med Rehabil. 2016;97(1):17–25. doi: 10.1016/j.apmr.2015.08.412. [DOI] [PubMed] [Google Scholar]
- 36.Dumais R, Benoit C, Dumais A, Babin L, Bordage R, de Arcos C, et al. Effect of regenerative injection therapy on function and pain in patients with knee osteoarthritis: a randomized crossover study. Pain Med. 2012;13(8):990–9. doi: 10.1111/j.1526-4637.2012.01422.x. [DOI] [PubMed] [Google Scholar]
- 37.Reeves KD, Hassanein K. Randomized prospective double-blind placebo-controlled study of dextrose prolotherapy for knee osteoarthritis with or without ACL laxity. Alternat Ther Health Med. 2000;6(2):68–80. [PubMed] [Google Scholar]
- 38.Sit RW, Chung V, Reeves KD, Rabago D, Chan KK, Chan DC, et al. Hypertonic dextrose injections (prolotherapy) in the treatment of symptomatic knee osteoarthritis: A systematic review and meta-analysis. Sci Rep. 2016;6:25247. doi: 10.1038/srep25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Topol GA, Podesta LA, Reeves KD, Raya MF, Fullerton BD, Yeh HW. Hyperosmolar dextrose injection for recalcitrant Osgood-Schlatter disease. Pediatrics. 2011;128(5):e1121–8. doi: 10.1542/peds.2010-1931. [DOI] [PubMed] [Google Scholar]
- 40.Jahangiri A, Moghaddam FR, Najafi S. Hypertonic dextrose versus corticosteroid local injection for the treatment of osteoarthritis in the first carpometacarpal joint: a double-blind randomized clinical trial. J Orthop Sci. 2014;19(5):737–43. doi: 10.1007/s00776-014-0587-2. [DOI] [PubMed] [Google Scholar]
- 41.Reeves KD, Hassanein K. Randomized, prospective, placebo-controlled double-blind study of dextrose prolotherapy for osteoarthritic thumb and finger (DIP, PIP, and trapeziometacarpal) joints: evidence of clinical efficacy. J Altern Complement Med. 2000;6(4):311–20. doi: 10.1089/10755530050120673. [DOI] [PubMed] [Google Scholar]
- 42.Scarpone M, Rabago DP, Zgierska A, Arbogast G, Snell E. The efficacy of prolotherapy for lateral epicondylosis: a pilot study. Clin J Sport Med. 2008;18(3):248–54. doi: 10.1097/JSM.0b013e318170fc87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rabago D, Lee KS, Ryan M, Chourasia AO, Sesto ME, Zgierska A, et al. Hypertonic dextrose and morrhuate sodium injections (prolotherapy) for lateral epicondylosis (tennis elbow): results of a single-blind, pilot-level, randomized controlled trial. Am J Phys Med Rehabil. 2013;92(7):587–96. doi: 10.1097/PHM.0b013e31827d695f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim WM, Lee HG, Jeong CW, Kim CM, Yoon MH. A randomized controlled trial of intra-articular prolotherapy versus steroid injection for sacroiliac joint pain. J Altern Complement Med. 2010;16(12):1285–90. doi: 10.1089/acm.2010.0031. [DOI] [PubMed] [Google Scholar]
- 45.Topol GA, Podesta LA, Reeves KD, Giraldo MM, Johnson LL, Grasso R, et al. Chondrogenic effect of intra-articular hypertonic-dextrose (prolotherapy) in severe knee osteoarthritis. PMR. 2016;8(11):1072–1082. doi: 10.1016/j.pmrj.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 46.Donaldson L. Neurogenic mechanisms in arthritis. In: Jancso G, editor. Neurogenic Inflammation in Health and Disease. Amsterdam: Elsevier; 2009. pp. 211–41. [Google Scholar]
- 47.Malek N, Pajak A, Kolosowska N, Kucharczyk M, Starowicz K. The importance of TRPV1-sensitisation factors for the development of neuropathic pain. Mol Cell Neurosci. 2015;65:1–10. doi: 10.1016/j.mcn.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 48.Mohammadi-Farani A, Ghazi-Khansari M, Sahebgharani M. Glucose concentration in culture medium affects mRNA expression of TRPV1 and CB1 receptors and changes capsaicin toxicity in PC12 cells. Iran J Basic Med Sci. 2014;17(9):673–378. [PMC free article] [PubMed] [Google Scholar]
- 49.Bertrand H, Kyriazis M, Reeves KD, Lyftogt J, Rabago D. Topical Mannitol Reduces Capsaicin-Induced Pain: Results of a Pilot-Level, Double-Blind, Randomized Controlled Trial. PM R. 2015;7(11):1111–7. doi: 10.1016/j.pmrj.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 50.Kim MY, Na YM, Moon JH. Comparison on treatment effects of dextrose water, saline, and lidocaine for trigger point injection. J Korean Acad Rehabil Med. 1997;21(5):967–73. [Google Scholar]