Abstract
Background
Adolescence is characterized by increasing prevalence of depressive symptomatology, along with significant structural brain development. While much research has examined focal abnormalities in gray matter structure underlying depression, we employed a structural coupling approach to examine whether longitudinal associations between amygdala and cortical development (referred to as maturational coupling) was related to concurrent changes in depressive symptomatology during adolescence.
Method
166 participants underwent up to three MRI scans (367 scans) between 11 and 20 years of age. Depressive symptoms were measured at three coinciding time points using the Center for Epidemiological Studies-Depression scale. Linear mixed models were employed to identify whether change in amygdala volume was related to development of cortical thickness, and if maturational coupling of these regions was related to changes in depressive symptomatology.
Results
Positive maturational coupling was identified between the right amygdala and (predominantly anterior) prefrontal cortex, as well as parts of the temporal cortices. Greater positive coupling of these regions was associated with reductions in depressive symptoms over time.
Conclusions
Findings highlight significant associations between cortico-amygdalar maturational coupling and the emergence of depressive symptoms during adolescence, suggesting that synchronous development of these regions might support more adaptive affect regulation and functioning.
Keywords: Structural coupling, depressive symptomatology, adolescence, brain development, affective functioning
1. Introduction
Adolescence is characterized by a dramatic rise in the incidence of depression – the leading cause of disability during the second decade of life in developed countries (World Health Organization, 2014). Future mental health trajectories are often shaped during this time, with the experience of a depressive symptoms during adolescence increasing the likelihood of future disorder onset (Costello and Maughan, 2015). Research has investigated the neurobiological underpinnings of depression, identifying structural abnormalities in the amygdala, subgenual anterior cingulate cortex (ACC), and various regions of the prefrontal cortex (Dohm et al., 2016; Kerestes et al., 2014; Miller et al., 2015; Singh and Gotlib, 2014). However, given documented maturation of both subcortical and prefrontal regions during adolescence (Dennis and Brotman, 2003; Goddings et al., 2014; Mutlu et al., 2013; Raznahan et al., 2011b; Tamnes et al., 2010; Vijayakumar et al., 2016), it can also be speculated that coordinated development of these regions may help support adaptive functioning, while aberrations to this pattern may result in poorer regulatory capacities that have enduring effects on mental health.
Indeed, it is widely theorized that depression emerges from the development of, and interaction between two broadly defined brain systems that work in concert – the subcortical system that supports emotion generation and reactivity, and the prefrontal cortex that is involved in the cognitive regulation of affective states (Badcock et al., 2017; Carver et al., 2008; Pfeifer and Allen, 2012; Siegle et al., 2007). Within the subcortical system, the amygdala is the most consistently implicated region in depression, with its extensive functional and anatomical connectivity highlighting the central role this region plays in emotional processing, learning and motivation (Mears and Pollard, 2016). As such, it is important to characterize coordinated development of the amygdala and PFC regions and understand how this may relate to the emergence of depressive symptomatology.
In comparison to traditional focal approaches, analyses investigating associations between brain regions have the potential to reveal more about cognitive and affective processes characterized by distributed neural activity (Evans, 2013). Within the structural neuroimaging field, this has resulted in a surge of studies on structural covariance or coupling of gray matter (i.e., how structural properties, such as volume or thickness, of different regions correlate with each other; (Alexander-Bloch et al., 2013b; Zielinski et al., 2010). This is frequently hypothesized to arise from coordinated neurobiological development through mutually trophic effects mediated by underlying axonal connections (i.e., Hebbian principals of “neurons that fire together wire together”; Hebb, 1949). Indeed, past investigations have also documented patterns of maturational coupling (i.e., patterns of correlated change) across the cortical mantle (Lerch et al., 2006; Raznahan et al., 2011a). However, coupling of subcortical and cortical structures may be particularly relevant to understanding associations between neurodevelopment and depression, as described above.
Only two studies have investigated normative structural coupling between subcortical and cortical structures. Albaugh and colleagues (2013) identified negative coupling between the amygdala and prefrontal structures. While they examined longitudinal data, no age-related effects were found (i.e., the relationship between amygdala volume and cortex thickness remained constant at any given time from 5-to-23 years of age). However, they did not investigate whether change in amygdala structure over time was related to change in cortical structures. To our knowledge, Walhovd and colleagues (2015) are the only authors to examine this question, showing that development of the hippocampus and basal ganglia were both positively related to cortical development, with greater subcortical reductions being related to greater cortical reductions. Each subcortical structure also exhibited unique coupling with largely non-overlapping cortical areas. Such an investigation is yet to be undertaken on cortico-amygdalar coupling.
In light of research supporting the importance of functional maturational coupling of the cortex and amygdala in affective processes (Gee et al., 2013), along with research implicating structural brain development in depression (Ducharme et al., 2014; Whittle et al., 2014), it can be hypothesized that similar associations may be present in relation to maturational coupling of structure. Initial research into the functional relevance of structural covariance networks focused on cognitive functions (Lee et al., 2013; Raznahan et al., 2014), but one recent study investigated associations with behavioral problems. Ameis and colleagues (2014) found amygdala-orbitofrontal cortex (OFC) coupling varied as a function of externalizing problems in adolescents, with a lack of coupling in adolescents being associated with higher levels of problems. However there has been no research examining how maturational coupling (i.e., change in structural coupling) may relate to internalizing symptoms and associated psychopathology.
Therefore, the current study characterized cortico-amygdalar maturational coupling during adolescence, and subsequently investigated whether this pattern of coupling was related to concurrent changes in depressive symptomatology. We focused on the amygdala given its prominent role in affective processing and prior research implicating functional and structural abnormalities in this region with depression (Kerestes et al., 2014; Singh and Gotlib, 2014). The research question was addressed in a community sample of adolescents examined longitudinally from 11-to-20 years of age, with up to three brain scans obtained per individual, as well as concurrent assessment of depressive symptoms. While structural correlates of depressive symptomatology have previously been examined in this sample (Whittle et al., 2014; 2011), this is the initial investigation into structural coupling. We first examined maturational cortico-amygdalar coupling, and given prior findings by Walhovd et al. (2015), hypothesized positive associations between change in amygdala volume and change in cortical thickness, particularly within regions implicated in modulating amygdala function (i.e., dorsolateral, dorsomedial and ventromedial PFC (dlPFC, dmPFC, vmPFC), OFC, and inferior parietal cortices; (Arnsten and Rubia, 2012; Burnett et al., 2011; Rempel-Clower, 2007). We subsequently examined whether these patterns of cortico-amygdalar maturational coupling were related to changes in depressive symptomatology. Although exploratory in nature, we hypothesized that adolescents with positive coupling (particularly greater correlated reductions in the size of the amygdala and associated cortical regions) would experience reductions in symptoms over time. In order to examine the specificity of findings to depression, particularly given the predominant role of the amygdala in anxiety (Blackford and Pine, 2012), we also investigated whether maturational coupling was related to changes in anxiety symptoms.
2. Methodology
2.1 Participants
The current sample was derived from a larger longitudinal cohort enrolled in the Orygen Adolescent Development Study (ADS), conducted in Melbourne, Australia. Students (N=2453) in the final year of primary school were recruited from schools to participate in an initial screening phase, which involved completion of the Early Adolescent Temperament Questionnaire-Revised (EATQR; Capaldi and Rothbart, 1992). Based on scores, a smaller sample of 415 students were selected by over-sampling adolescents at the extreme ends of the distribution for temperamental factors of Effortful Control and Negative Emotionality to maximize inter-individual differences in psychological well-being (an equal number of participants were invited to participate from the following standard deviation ranges above and below mean: i) 0-1 ii) 1-2 iii) 2-2.5 and iv) greater than 2.5, to emphasize distribution at the tails).
245 adolescents agreed to participate in the broader ADS. Of this sample, a number of adolescents declined participation in the Magnetic Resonance Imaging (MRI) assessments, resulting in 177 participants completing MRI scans at one to three time points when they were aged approximately 13 (time 1: T1), 17 (time 2: T2) and 19 (time 3: T3) years. Based on visual inspection of FreeSurfer processed MRI data (see below for details) by a researcher trained in neuroanatomy, nine participants were excluded due to poor image quality. Two additional participants with full scale IQ less than 70, as assessed by the Wechsler Intelligence Scale of Children – Version IV (Wechsler, 2003), were excluded from analyses. Following exclusions, 166 participants (n=86 males) aged 11-to-20 years were available for analyses. While this sample had greater variance on temperamental distributions compared to the school-screening sample (due to the sampling strategy, see Table S1), it also exhibited normal distribution on Effortful Control and Negative Emotionality based on the Kolmogorov-Smirnoff test (p > 0.05) and did not present with skewness or kurtosis (estimate/standard error < ±2).
Seventy-three participants had three scans, 55 had two scans and 38 had one scan. Table 1 provides a breakdown of the number of participants at each time point, and demographic and cognitive characteristics. Males and females did not differ on any of these variables (p>0.05). The final sample also did not differ from the initial school-screening sample (N=2453) on socioeconomic disadvantage [t(2439)=21.292; p=0.197] or sex (Pearson's χ2=2.245; p=0.691). Twenty-eight participants of the final sample met criteria for past or current psychiatric disorder at T1, and an additional 28 and 19 participants met criteria at T2 and T3, respectively, as assessed by the Schedule for Affective Disorder and Schizophrenia for School-Aged Children: Present and Lifetime Version (Kaufman and Schweder, 2004). Refer to supplementary material (Table S2) for further detail on administration and reliability of KSADS interviews. Table 2 provides further detail on psychiatric diagnoses. The prevalence of psychopathology in this sample is consistent with previous reports in large community samples (Merikangas et al., 2010). Informed consent was obtained from the child and at least one parent/guardian at each time point, consistent with the guidelines of the Human Research Ethics Committee at the University of Melbourne, Australia.
Table 1. Sample characteristics.
| Sex | Total | ||
|---|---|---|---|
|
| |||
| Male | Female | ||
| Number | |||
| Total | 85 | 81 | 166 |
| T1 | 69 | 63 | 132 |
| T2 | 65 | 67 | 132 |
| T3 | 50 | 53 | 103 |
| Age (years) | Mean; SD | Mean; SD | Mean; SD |
| T1 | 12.83; 0.452 | 12.77; 0.394 | 12.79; 0.425 |
| T2 | 16.70; 0.559 | 16.71; 0.480 | 16.70; 0.518 |
| T3 | 19.10; 0.507 | 19.05; 0.413 | 19.08; 0.460 |
| Delay time 1-2 (years) | 3.80; 0.158 | 3.87; 0.237 | 3.83; 0.204 |
| Delay time 2-3 (years) | 2.40; 0.177 | 2.35; 0.251 | 2.38; 0.219 |
| Estimate Full Scale IQ | 107.96; 15.51 | 107.75; 15.80 | 107.86; 15.60 |
| SES | 58.14; 20.42 | 58.01; 21.36 | 58.08; 20.80 |
NB: Values represent mean; standard deviation.
IQ was assessed using a short form of the Wechsler Intelligence Scale for Children, Fourth Version; SES (socioeconomic status) was assessed using the Australian National University Four Scale.
Table 2. Number of participants meeting criteria for psychiatric diagnoses.
| T1 | T1 - T2 | T2 - T3 | |
|---|---|---|---|
| Depressive | 3 | 18 | 19* |
| Anxiety | 16 | 11 | 12 |
| Attention | 6 | 0 | 0 |
| Oppositional/conduct | 6 | 10 | 1 |
| Substance | 0 | 9 | 14 |
| Eating | 0 | 1 | 1 |
| Adjustment | 0 | 4 | 5 |
NB: Values represent frequency
Includes one participant who developed bipolar disorder
2.2 MRI acquisition and analysis
2.2.1 Image Acquisition
At T1, MRI scans were performed on a 3Tesla GE scanner at the Brain Research Institute, Austin and Repatriation Medical Centre, Melbourne, Australia, with the following parameters: repetition time=36msec; echo time=9msec; flip angle=35°, field of view=20cm, 124 T1-weighted contiguous slices (voxel dimensions=0.4883×0.4883×1.5mm). At T2 and T3, all participants underwent MRI scans on a 3Tesla Siemens scanner at the Royal Children's Hospital, Melbourne, Australia, with the following parameters: repetition time=1900msec; echo time=2.24msec; flip angle=9°, field of view=23cm; 176 T1-weighted contiguous slices (voxel dimensions=0.9mm3).
2.2.2 Processing
Images were transferred to an SGI/Linux workstation for analysis at the Melbourne Neuropsychiatry Centre, Melbourne, Australia. Cortical reconstruction was performed using the FreeSurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu/), which provides tools for reconstructing topologically correct and geometrically accurate surface models of the inner and outer cortical boundaries, thus deriving anatomical measures such as cortical thickness and volume. To address issues arising from longitudinal and/or multisite studies (such as geometric distortion and voxel dimension drift), images were processed through the longitudinal stream of FreeSurfer 5.3 (Reuter et al., 2012), which creates a within-subject unbiased template space and average image from both time points using robust, inverse consistent registration. The template is used as an estimate to initialize subsequent segmentation processes in the longitudinal stream for each time point, providing common information regarding anatomical structures, and has been found to significantly increase reliability and statistical power (Reuter and Fischl, 2011; Reuter et al., 2010). The quality of the cortical reconstruction was visually inspected for all images and manual edits were made where necessary.
Given that different scanners were used at T1 vs. T2 and T3, a reliability analysis was undertaken to address concerns that changes in cortical thickness over time may be due to measurement bias from the different scanner platforms and acquisition parameters. This was relevant for all participants who completed T1 and one or more subsequent waves (i.e. T2 and/or T3). This analysis, involving an independent sample of adults who were scanned at both sites, indicated that changing scanners between T1 and T2 did not produce a systematic bias. Further detail has been outlined in previous papers with the cohort (Dennison et al., 2013; Vijayakumar et al., 2016; 2014) and is also presented in the supplementary section. In addition, some participants (N = ) only,
2.3 Internalizing symptoms
At all three time points, depressive and anxious symptoms were measured using the self-report Center for Epidemiological Studies-Depression Scale (CES-D; Radloff, 1977) and Beck Anxiety Inventory (BAI; Beck et al., 1988). The CES-D contains 20 items that relate to mood, somatic complaints, relations with others, and motor functioning during the past week, while the BAI contains 21 items relating to anxious symptoms in the past week. Both questionnaires have been used extensively on adolescents, and have been found to be valid and reliable measures for this population (Beck et al., 1988; Fusar Poli et al., 2013; Garrison et al., 1991).
2.5 Statistical analysis
Calculating change scores
Given the unbalanced dataset (i.e., different number of scans per individual, and scans from different waves of assessment), traditional change scores using two measurements (i.e., difference between follow-up and baseline) do not appropriately exploit all available data to improve statistical power and accuracy. Therefore, we calculated a change score for each individual in R as the random slope from linear mixed models (LMM). Random slopes combine information about the individual with that from the rest of the sample, thus allowing us to use all available data for the individual (i.e., one to three scans) to obtain a random slope or change variable. Change scores were calculated following the identification of best-fitting models, undertaken using a model selection procedure that compared the null model (Y = Intercept + di + d(age)i + eik) with the age-alone (Y = Intercept + di + d(age)i + β1(age) + eik), age-plus-sex Y = Intercept + di + d(age)i + β1(age) + β2(sex) + eik) and age-by-sex (Y = Intercept + di + d(age)i + β1(age) + β2(sex) + β3(age*sex) + eik ) models. The di and d(age)i terms represent the random effect of the intercept and age-slope for each ith subject, the eik represents the residual error term, and β represents the parameter estimates of fixed effects (sex, mean-centered age). A more complex model (i.e., has more fixed effects) was chosen if p<0.05 for the additional parameter and the AIC indicated better model fit (value smaller than two or more). Individual random slopes for age were then extracted from the best-fitting model. Positive and negative change scores represent more increase and decrease, respectively, relative to average group change. For information on correlations between random slopes and traditional difference scores, refer to supplementary material.
Analysis 1: Amygdala change
Development of the amygdala was outlined using the procedure outlined above (i.e., Y = amygdala volume), with separate models run for the right and left amygdala. Analyses were also re-run with the inclusion of intracranial volume (ICV) as a covariate to ensure findings were not driven by differences in whole brain size. Random slopes were extracted as a measure of amygdala change and subsequently used as a fixed effect measure in group-level analyses (described below: analysis 2). All 166 adolescents (367 observations) were used to model amygdala development.
Analysis 2: Cortico-amygdala maturational coupling
Vertex-wise cortical thickness analyses were conducted using SurfStat, a statistical toolbox for MATLAB (http://www.math.mcgill.ca/keith/surfstat/), with LMMs used to model cortical development. Change in amygdala volume was incorporated as a fixed effect, and the interaction between age and amygdala change examined maturational coupling. Specifically, for each amygdala (i.e., left and right hemispheres), the following equation was modeled at each jth vertex:
| Equation 1 |
Analyses were corrected for multiple comparisons using Random Field Theory, set at p < 0.025 (to account for left and right amygdala analyses) and a cluster-defining threshold of 0.001. To aid interpretation of significant age*amygdala change interactions, mean thickness estimates of cortical clusters were extracted for plotting and simple slope analyses. Using these estimates, change scores for each cortical ROI were also calculated as random slopes (as for the amygdala) and subsequently used in analysis 3 (outlined below). Separate models incorporating sex main effects and interactions (i.e., age*sex*amygdala change and all relevant lower-order effects) were also examined. Furthermore, analyses were re-run with the inclusion of ICV as a covariate.
To ensure that inclusion of individuals with a single time-point was not biasing random slope estimates, a subset of analyses 1 and 2 were re-analyzed using individuals with two or three time points alone. Analyses produced identical results to the full sample, suggesting there was no bias introduced by the inclusion of individuals with a single time point (refer to supplementary material).
Analysis 3: Internalizing symptoms and cortico-amygdala coupling
Best fitting developmental models for CES-D and BAI scores were investigated (as above). Subsequently, LMMs examined whether cortico-amygdala maturation was related to change in depressive/anxious symptoms using the following model:
| Equation 2 |
This was run for each ROI in combination with each internalizing scale (CES-D, BAI) separately. The three-way interaction specifically addressed whether an association between cortical and amygdala maturation was associated with CES-D/BAI development. Significant findings (following Bonferroni correction for the number of ROIs) were interpreted by plotting effects and testing the significance of simple slopes. Sex main effects and interactions (i.e., age*sex*Amygdala change*ROI change) were only incorporated to the model if sex-differences were identified in analysis 2 or CES-D/BAI development. Similarly, ICV correction was only undertaken if it was found to influence the results in analyses 1 or 2. Due to missing behavioral data, CES-D analysis was conducted on 161 adolescents with 351 observations, while BAI analysis was conducted on 162 adolescents with 358 observations.
For all analyses, we only examined linear developmental trajectories, as opposed to higher order (quadratic/cubic) functions, given the risk of over-fitting data to more complex trajectories when there is a maximum of three data points for each individual, along with limited age variance at each time point.
3. Results
Descriptive statistics for CES-D and BAI scores, and amygdala volumes, at each time point are reported in Table 3.
Table 3. Descriptive statistics for amygdala volume and internalizing symptoms.
| Right Amygdala | Left Amygdala | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| T1 | T2 | T3 | T1 | T2 | T3 | |
| Total | 1656; 219 | 1733; 222 | 1749; 219 | 1589; 231 | 1671; 203 | 1665; 206 |
| Males | 1714; 227 | 1824; 233 | 1850; 212 | 1639; 220 | 1753; 210 | 1766; 187 |
| Females | 1593; 191 | 1646; 170 | 1655; 181 | 1534; 231 | 1592; 162 | 1571; 177 |
| CES-Da | BAI | |||||
| T1 | T2 | T3 | T1 | T2 | T3 | |
| Total | 31.33; 9.61 | 29.94; 7.62 | 31.12; 10.01 | 8.36; 8.52 | 8.11; 7.78 | 7.68; 8.80 |
| Males | 32.27; 9.98 | 29.25; 7.73 | 29.77; 7.91 | 8.59; 8.61 | 6.83; 8.15 | 6.07; 7.46 |
| Females | 30.32; 9.17 | 30.56; 7.51 | 32.44; 11.6 | 8.12; 8.48 | 9.26; 7.28 | 9.27; 9.75 |
| Clinical-cut offs (%)b | 25 | 16 | 24 | 17 | 13 | 14 |
CES-D rating scale ranged from 1 to 4 in this study, as opposed to the traditional 0 to 3.
Refers to percentage of adolescents meeting recommended clinical cut-offs.
Analysis 1
Best fitting models for the left and right amygdala included a significant main effect of age, characterized by increases in volume with age. The best fitting model for the right amygdala additionally included a significant main effect of sex, with females having smaller volumes than males (but no sex-moderated age effect), while the best fitting model for the left amygdala included a significant age*sex interaction, with males exhibiting greater increases in amygdala volume with age compared to females (see Table 4). The inclusion of ICV as a covariate did not change these results.
Table 4. Best fitting models for amygdala volume.
| Intercept | Age | Sex | Age*sex | |
|---|---|---|---|---|
| Right Amygdala | 1781.13 (21.39)** | 12.85 (2.40)** | -147.40 (30.50)** | |
| Left Amygdala | 1708.63 (21.80)** | 17.22 (2.48)** | -137.75 (31.14)** | -7.68 (3.49)* |
p < 0.001;
p < 0.05
Analysis 2
Whole brain vertex-wise analyses identified a significant positive relationship between right amygdala volume change and cortical thickness development (i.e., age*amygdala change interaction predicting thickness) within multiple cortical clusters (model statistics reported in Table 5)1. As illustrated in Figure 1, these effects were present in the bilateral rostrolateral PFC extending into the OFC and ventromedial PFC (i.e., anterior PFC), anterior insula and parahippocampal gyri, as well as right dlPFC and left vlPFC, fusiform superior temporal and occipital cortices. Peaks vertices (RFT corrected) were also present in the anterior PFC, fusiform and superior temporal cortex. Graphical plotting of the anterior PFC cluster indicated that adolescents who exhibited less increases in right amygdala volume exhibited more reductions in cortical thickness. Simple slopes of most clusters revealed significant reductions in thickness across the sample, but comparatively greater thinning in those with more reductions in right amygdala volume (see Table S5).
Table 5. Maturational coupling between the right amygdala and cortex.
| MNI Coordinates | Broadmann's Area | Cluster size | RFT Corrected Significance | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| x | y | z | |||||
| 1 | Left superior temporal | -55.5 | -11.6 | -13.1 | 22 | 481 | <0.0001 |
| 2 | Left occipital | -7.4 | -92.9 | 28.4 | 19 | 347 | <0.0001 |
| 3 | Right anterior PFC | 11.3 | 62.0 | -13.2 | 10 | 274 | <0.0001 |
| 4 | Right PHG | 27.9 | -22.8 | -16.3 | 28 | 274 | <0.0001 |
| 5 | Left vlPFC | -39.1 | 21.2 | -15.3 | 47 | 227 | 0.0001 |
| 6 | Left fusiform | -33.5 | -29.9 | -28.8 | 36 | 282 | 0.0001 |
| 7 | Right dlPFC | 47.8 | 25.6 | 27.7 | 9 | 188 | 0.0002 |
| 8 | Left PHG | -25.8 | -22.0 | -17.7 | 35 | 163 | 0.0017 |
| 9 | Left anterior PFC | -7.4 | 63.1 | -19.0 | 10 | 154 | 0.0020 |
| 10 | Left anterior insula | -42.3 | 11.7 | 0.6 | 13 | 134 | 0.0026 |
| 11 | Right anterior insula | 31.1 | 21.3 | -7.0 | 47 | 99 | 0.02010 |
Figure 1.
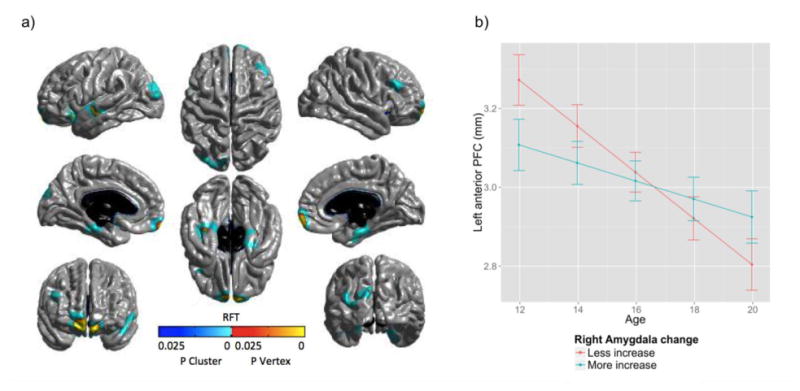
a) Significant interactions between age and right amygdala change predicting cortical thickness, following RFT correction for multiple comparisons (cluster (blue) / vertices (red): p < 0.05). Mean thickness estimates for the left anterior PFC cluster was extracted and used for plotting in R. As depicted in b), adolescents with less increase in amygdala volume exhibited more cortical thinning with age.
No significant associations between left amygdala change and cortical development were identified. No sex-moderated effects were present, and neither the inclusion of sex nor ICV as covariates changed the results. In order to examine the specificity of the cortical coupling map to the amygdala, we also examined maturational coupling of another subcortical structure: the thalamus. As depicted in Figure S3, the right and left thalamus exhibited significantly different coupling maps to that of the amygdala, supporting the specificity of the amygdala findings.
Analysis 3
Investigation of best-fitting developmental models for internalizing scales revealed that neither CES-D nor BAI scores changed with age for the sample as a whole2. Next, LMMs investigated the association between maturational cortico-amygdala coupling and CES-D/BAI development. Sex was not incorporated in these models given there were no sex differences in cortico-amygdala coupling or development of internalizing scales. Similarly, correction for whole brain size was not undertaken given ICV did not affect results of analyses 1 or 2.
While maturational cortico-amygdala coupling was not associated with BAI development, it was associated with CES-D development. Specifically, a significant three-way interaction between left anterior PFC development, right amygdala development and age was associated with CES-D scores (p = 0.002), surviving Bonferroni correction for multiple comparisons of 11 ROIs (see Table 6). This model also improved fit in comparison to the null-model (AIC value: 2464 vs. 2471). As illustrated in Figure 2, adolescents with similar developmental trajectories for the left anterior PFC and right amygdala (i.e., both structures exhibiting greater reductions in size or increases in size relative to the overall sample's trajectories) exhibited reductions in symptoms over time. In comparison, adolescents with opposing developmental patterns for these two structures exhibited increases in symptoms over time. Similar effects were identified in the right anterior PFC (p = 0.019), anterior insula (p = 0.010) and parahippocampal gyrus (p = 0.011), as well as left superior temporal (p = 0.020) and occipital (p = 0.012) cortices. These effects did not survive correction for multiple comparisons, but the inclusion of these terms improved model fit over the null model for all regions apart from the anterior insula (see Table S6 for model statistics and Table S7 for simple slopes analyses). Neither the addition of SES, IQ or baseline CES-D scores into these models changed the association between maturational coupling and CES-D development. Furthermore, 6% of this sample took antidepressant medication over the course of this study. Controlling for medication use also failed to change the results. Finally, the exclusion of participants with a diagnosis of MDD at any point over the course of the study produced similar results, suggesting that findings were not solely driven by participants with clinical diagnoses.
Table 6. LMMs predicting CES-D.
| Null model | Amygdala | Anterior PFC | Amygdala-PFC coupling | |
|---|---|---|---|---|
| Intercept | 30.678 (0.569)*** | 30.672 (0.570)*** | 30.674 (0.572)*** | 30.469 (0.600)*** |
| Age | -0.122 (0.174) | -0.134 (0.175) | 0.035 (0.176) | |
| Right Amygdala | 0.015 (0.042) | 0.004 (0.047) | ||
| ROI | 10.383 (33.956) | 2.082 (38.000) | ||
| Age*Right Amygdala | -0.017 (0.012) | -0.008 (0.013) | ||
| Age*Left anterior PFC | -12.750 (9.822) | -3.550 (10.531) | ||
| Left anterior PFC*Right Amygdala | 2.280 (1.661) | |||
| Age*Left anterior PFC*Right Amygdala | -1.447 (0.449)** |
p < 0.001,
p < 0.002
Figure 2.
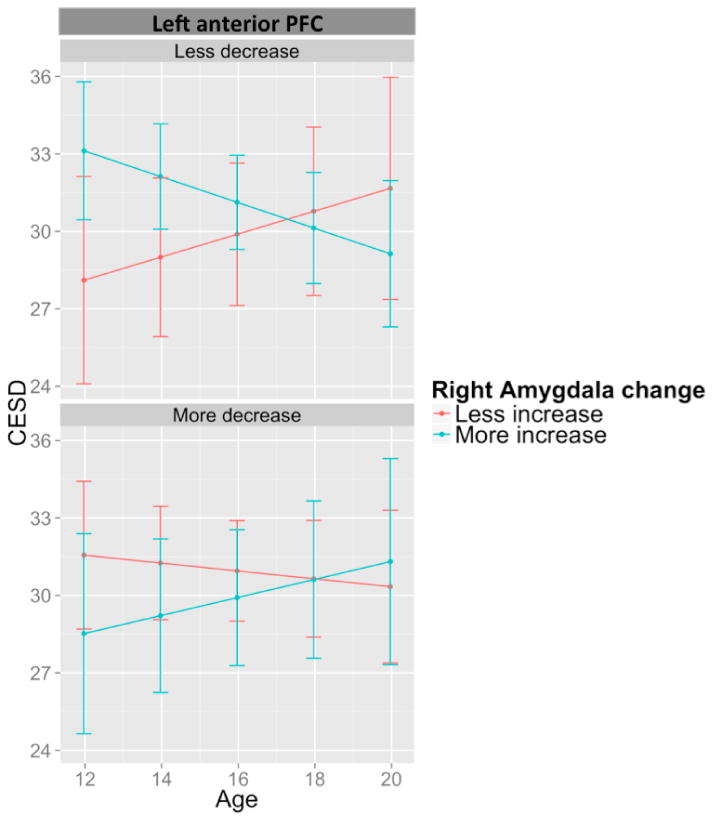
Positive maturational coupling of the right amygdala and left anterior PFC predicts reductions in CES-D with age.
Exploratory whole brain analyses revealed overlapping regions of significance, providing some support for the specificity of results to these ROIs (see Figure S5). In order to further address the question of specificity of these regions to CES-D development, we examined coupling between the amygdala and an un-related cortical region based on analysis 2 (specifically the left supplementary motor cortex, see Figure S6 for visualization of this region of interest). Consistent with the whole brain analyses, coupling between the left supplementary motor cortex and right amygdala was not associated with CES-D development (see Table S6).
Although not of primary relevance to the research question, exploratory analyses revealed that development of neither the right amygdala nor any of the ROIs identified above were associated with CES-D scores (i.e., models with main effect of brain development alone) or change in CES-D scores (i.e., models with age*brain development interaction and main effects; see Table 6 for anterior PFC results).
4. Discussion
This study identified positive maturational coupling between the right amygdala and various frontal and temporal cortices. Maturational coupling between the right amygdala and left anterior PFC was also associated with development of depressive symptomatology during adolescence. Specifically, greater positive coupling between these two regions was associated with reductions in depressive symptoms. This finding was specific to depressive symptomatology, with no such relationship identified with changes in anxiety symptoms. We hypothesize that this symptom specificity is reflective of the importance of maturational coupling during this time period for depression, which tends to have a later onset than anxiety problems (Kessler et al., 2007).
As hypothesized, adolescents exhibited positive coupling between changes in amygdala volume and changes in various frontal and temporal cortical structures. In order to interpret these findings, it is important to consider the pattern of change within each region. Amygdala volume increased with age in our sample of adolescents, along with predominant reductions in cortical thickness (documented previously in this sample by (Vijayakumar et al., 2016). As such, positive coupling likely represents greater reductions in cortical thickness combined with less increase in amygdala volume. This is in line with the only other study to examine the relationship between changes in cortical and subcortical structures, finding positive maturational coupling of the cortex with the hippocampus and striatum in healthy adolescents (Walhovd et al., 2015). Although Albaugh and colleagues (2013) previously identified negative cortico-amygdalar coupling in their healthy adolescent sample, their investigation did not examine changes in subcortical structures over time. Furthermore, they identified a negative coupling across 5-23 years of age. Our finding of positive maturational coupling actually supports this continued inverse association, as negative maturational coupling would likely result in a reversal of the cross-sectional relationship with age.
The exact mechanisms underlying structural covariance/coupling, and thus maturational coupling, remain unknown. However, it is commonly interpreted as reflecting anatomical and/or functional connectivity between brain regions (Alexander-Bloch et al., 2013a). Support for the “anatomical” hypothesis also comes from studies that have identified partial convergence between structural coupling networks and diffusion-based white matter networks (Gong et al., 2012), and interestingly, many of the cortical regions identified in this study have direct anatomical connections to the amygdala, such as the OFC, medial temporal cortices and anterior insula. However, there is also past support for similarities between functional connectivity and structural covariance networks (Raznahan et al., 2011a), and many of the identified cortical regions in this study are functionally linked to the amygdala through their involvement in affective processes. The OFC and ventromedial PFC are involved in the regulation of affect and motivation (Arnsten and Rubia, 2012), while the lateral prefrontal structures (i.e., dlPFC and vlPFC) are involved in voluntary forms of emotion regulation such as cognitive reappraisal and behavioral/impulse control (Kalisch, 2009; Ochsner and Gross, 2008). Past neuroimaging studies have revealed functional coupling between these regions when processing affective stimuli (Banks et al., 2007; Kanske et al., 2011; McRae et al., 2010; Ochsner and Gross, 2005; Ochsner et al., 2004) and functional connectivity during resting-state (Roy et al., 2009). Functional activity in the parahippocampal gyri, a region thought to play an important role for learning in the context of emotional arousal (Squire and Zola, 1996), has been found to be influenced by amygdala activation (Packard et al., 1994; Packard and Teather, 1998; Roesler et al., 2002). The anterior regions of the temporal lobe are also active during conditions of social and emotional salience, particularly when engaging in mentalizing processes (Blakemore, 2012), while the anterior insula is thought to play an important role in emotional awareness by integrating stimulus-driven interoceptive signals with top-down information (Gu et al., 2013). As such, it is also plausible that maturational coupling between the amygdala and these cortical structures may arise from synchronous activity during affective processing.
The functional relevance of this maturational coupling network was highlighted by the study's second aim, which found that greater positive maturational coupling between the right amygdala and left anterior PFC (encompassing the OFC and ventromedial PFC) was associated with concurrent reductions in depressive symptoms over adolescence. Thus it can be inferred that synchronous development of the amygdala and this prefrontal region might be associated with better affect regulation, and potentially top-down control of the amygdala. In support of this hypothesis, the OFC and ventromedial PFC are involved in the modulation of autonomic responses and generation of affect associated with emotional stimuli, and through their interactions with the dlPFC and vlPFC are also involved in executive functions that regulation affect. Furthermore, an association between this prefrontal region and the amygdala has consistently been implicated in neuroimaging studies of depression; for example, prior research has identified reduced functional coupling between the amygdala and OFC in individuals with depression when processing affective stimuli (Dannlowski et al., 2009; Matthews et al., 2008), as well as during resting conditions (Ramasubbu et al., 2014). Furthermore, DTI literature implicates abnormal white matter integrity in the uncinate fasiculus, a major tract connecting the amygdala and the OFC, in individuals with depression (Aghajani et al., 2013). Similar trending associations were identified with the right anterior PFC, as well as bilateral temporal cortices and anterior insula.
In comparison, development of neither the amygdala nor any of these cortical regions were individually associated with changes in depressive symptoms, thus providing further support for the functional relevance of the cortico-amygdalar maturational coupling network. This is also in line with the growing field of evidence that depression is characterized by impairments in functional and anatomical connections across distributed brain regions (Bracht et al., 2015; Mulders et al., 2015). While research on brain morphometry and activation has been important for advancing our understanding of depression thus far, there is increasing awareness of the interconnectivity across the brain and the distributed nature of activity that subserves affective and cognitive processes. Along with advances in image acquisition and analytic techniques, this has resulted in greater network-based investigations in depression (Hamilton et al., 2013).
This brain-behavior association begs the question as to whether maturational coupling arises from experience-dependent plasticity (i.e., engagement of these cortical and subcortical regions in affective processes) or mutually trophic influences on these brain regions. This issue of the microstructural basis of neuroanatomical covariance (extending on macrostructural “connectivity” outlined above) has been supported by both hypotheses. Reciprocal anatomical connections present early in development may have mutually trophic effects that lead to growth across these regions in a coordinated manner (Hebb, 1949), which might ultimately support more adaptive affective functioning. However, use-dependent synchronous firing can also induce synaptogenesis between neurons, thus potentially resulting in co-variance at a macro-anatomical level if it involves a large number such of connections (Alexander-Bloch et al., 2013a; Evans, 2013). We cannot comment on this question of causality given the observational nature of this study, but it remains an important area for future investigation as it would inform our understanding of either the neurotoxic effects of depressive symptoms on brain development trajectories, or of neurobiological processes that could be targeted by preventative interventions. A role for experience-dependent plasticity would particularly highlight this network as a potential indicator of intervention efficacy at a neurobiological level. Similarities of our structural coupling maps with functional and white matter networks implicated in affective processes also emphasize the value of examining gray matter properties using a similar network-based approach that may, in the future, help link information on structure, anatomical connections and function, thus ultimately improving our understanding of the neurobiological underpinnings of affective processes and associated psychopathology.
Limitations of the study include automated segmentation of the amygdala, which has been found to be less reliable than manual tracing (Morey et al., 2009). However, we chose to employ FreeSurfer given that reliability is also a concern for manual tracing of large samples. We also examined the amygdala as a single unit, as this region is comprised of distinct subnuclei with differing connectivity patterns (Alarcon et al., 2015). Future investigations that segment this region into its subcomponents may identify more detailed cortical coupling patterns. Furthermore, it is uncertain why maturational coupling maps were not identified with the left amygdala. Although a qualitative hemispheric difference (i.e., laterality was not statistically tested), similar differences have been identified in prior studies (Walhovd et al., 2015), while others have chosen to examine coupling of the left and right amygdala as a unitary structure (Albaugh et al., 2013). Therefore, future investigations examining quantitative evaluation of these hemispheric differences are needed to better understand these findings. As with other correlational analyses of “networks”, it is uncertain whether associations between brain regions represent unidirectional or bidirectional influences, or if development is linked to another common factor (i.e., gene expression). Our MRI scans were also acquired multisite, and there is a possibility of scanner/sequence bias affecting morphological estimates. However, post-acquisition procedures were adopted to minimize scanner effects on the acquired images. Our previous work has shown no interscanner bias (Dennison et al., 2013; Vijayakumar et al., 2016; 2014), and furthermore, it is unlikely that the measure of depressive symptomatology interacted with scanner type in a way that might bias the reported results. However, this remains an important limitation of the study that should be addressed in future investigations.
Despite these caveats, the current study has a number of important strengths. Our repeated assessment of both brain and psychopathological symptoms within participants allowed us to examine individual differences in the relationship between neurobiological maturation and affective functioning. Additionally, our risk-enriched community sample increased variance in affective symptomology, thus providing greater power to detect associations with the brain. This unique design enabled us to identify novel cortico-amygdalar maturational coupling associated with concurrent changes in depressive symptomatology. Our findings support the importance of examining associations between regions in structural neuroimaging given the distributed nature of neural function underlying affect.
Supplementary Material
Highlights.
Examined relationship of amygdala and cortical development during adolescence.
Positive maturational coupling between amygdala and anterior prefrontal cortex.
Greater positive coupling related to reductions in depressive symptoms.
Synchronous development of these brain regions supports mental well-being.
Acknowledgments
Neuroimaging analysis was facilitated by the Neuropsychiatry Imaging Laboratory at the Melbourne Neuropsychiatry Centre. The authors would like to thank the Brain Research Institute and Royal Children's Hospital for support in acquiring the neuroimaging data, and the families who participated in the study. This research was supported by grants from the Colonial Foundation, the National Health and Medical Research Council (NHMRC; Australia; Program Grant 350241) and the Australian Research Council (ARC; Discovery Grant DP0878136). Dr. Whittle is supported by a NHMRC Career Development Fellowship (ID: 1007716) and Dr Vijayakumar is supported by National Institute of Health R01 MH107418 (PI: Pfeifer)
Footnotes
Clinical status (i.e., healthy adolescents vs. those with lifetime case-level psychopathology) did not moderate this pattern of cortico-amygdalar coupling. For more information, refer to Supplementary Information.
Refer to figure S4 for a histogram of change in symptoms over time (using difference scores).
Financial Disclosures: The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aghajani M, Veer IM, van Lang NDJ, Meens PHF, van den Bulk BG, Rombouts SARB, Vermeiren RRJM, van der Wee NJ. Altered white-matter architecture in treatment-naive adolescents with clinical depression. Psychol Med. 2013:1–12. doi: 10.1017/S0033291713003000. [DOI] [PubMed] [Google Scholar]
- Alarcon G, Cservenka A, Rudolph MD, Fair DA, Nagel BJ. Developmental sex differences in resting state functional connectivity of amygdala sub-regions. NeuroImage. 2015;115:235–244. doi: 10.1016/j.neuroimage.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albaugh MD, Ducharme S, Collins DL, Botteron KN, Althoff RR, Evans AC, Karama S, Hudziak JJ, Brain Development Cooperative Group Evidence for a cerebral cortical thickness network anti-correlated with amygdalar volume in healthy youths: implications for the neural substrates of emotion regulation. NeuroImage. 2013;71:42–49. doi: 10.1016/j.neuroimage.2012.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander-Bloch A, Giedd JN, Bullmore E. Imaging structural co-variance between human brain regions. Nat Rev Neurosci. 2013a;14:322–336. doi: 10.1038/nrn3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander-Bloch A, Raznahan A, Bullmore E, Giedd J. The convergence of maturational change and structural covariance in human cortical networks. Journal of Neuroscience. 2013b;33:2889–2899. doi: 10.1523/JNEUROSCI.3554-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameis SH, Ducharme S, Albaugh MD, Hudziak JJ, Botteron KN, Lepage C, Zhao L, Khundrakpam B, Collins DL, Lerch JP, Wheeler A, Schachar R, Evans AC, Karama S. Cortical thickness, cortico-amygdalar networks, and externalizing behaviors in healthy children. Biological Psychiatry. 2014;75:65–72. doi: 10.1016/j.biopsych.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Rubia K. Neurobiological Circuits Regulating Attention, Cognitive Control, Motivation, and Emotion: Disruptions in Neurodevelopmental Psychiatric Disorders. JAAC. 2012;51:356–367. doi: 10.1016/j.jaac.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Badcock PB, Davey CG, Whittle S, Allen NB, Friston KJ. The Depressed Brain: An Evolutionary Systems Theory. Trends in Cognitive Sciences. 2017 doi: 10.1016/j.tics.2017.01.005. [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience. 2007;2:303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Blackford JU, PINE DS. Neural substrates of childhood anxiety disorders: a review of neuroimaging findings. Child and Adolescent Psychiatric Clinics of North America. 2012;21:501–525. doi: 10.1016/j.chc.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ. Imaging brain development: the adolescent brain. NeuroImage. 2012;61:397–406. doi: 10.1016/j.neuroimage.2011.11.080. [DOI] [PubMed] [Google Scholar]
- Bracht T, Linden D, Keedwell P. A review of white matter microstructure alterations of pathways of the reward circuit in depression. Journal of Affective Disorders. 2015;187:45–53. doi: 10.1016/j.jad.2015.06.041. [DOI] [PubMed] [Google Scholar]
- Burnett S, Sebastian C, Kadosh KC, Blakemore SJ. The social brain in adolescence: Evidence from functional magnetic resonance imaging and behavioural studies. Neuroscience and Biobehavioral Reviews. 2011;35:1654–1664. doi: 10.1016/j.neubiorev.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldi DM, Rothbart MK. Development and validation of an early adolescent temperament measure. The Journal of Early Adolescence. 1992;12:153–173. [Google Scholar]
- Carver CS, Johnson SL, Joormann J. Serotonergic function, two-mode models of self-regulation, and vulnerability to depression: what depression has in common with impulsive aggression. Psychological Bulletin. 2008;134:912–943. doi: 10.1037/a0013740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, Maughan B. Annual research review: Optimal outcomes of child and adolescent mental illness. Journal of Child Psychology and Psychiatry. 2015;56:324–341. doi: 10.1111/jcpp.12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Konrad C, Domschke K, Bauer J, Kugel H, Hohoff C, Schöning S, Kersting A, Baune BT, Mortensen LS, Arolt V, Zwitserlood P, Deckert J, Heindel W, Suslow T. Reduced amygdala-prefrontal coupling in major depression: association with MAOA genotype and illness severity. Int J Neuropsychopharmacol. 2009;12:11–22. doi: 10.1017/S1461145708008973. [DOI] [PubMed] [Google Scholar]
- Dennis TA, Brotman LM. Effortful control, attention, and aggressive behavior in preschoolers at risk for conduct problems. Annals of the New York Academy of Sciences. 2003;1008:252–255. doi: 10.1196/annals.1301.026. [DOI] [PubMed] [Google Scholar]
- Dennison M, Whittle S, Yücel M, Vijayakumar N, Kline A, Simmons J, Allen NB. Mapping subcortical brain maturation during adolescence: evidence of hemisphere-and sex-specific longitudinal changes. Developmental Science. 2013;16:772–791. doi: 10.1111/desc.12057. [DOI] [PubMed] [Google Scholar]
- Dohm K, Redlich R, Zwitserlood P, Dannlowski U. Trajectories of major depression disorders: A systematic review of longitudinal neuroimaging findings. Australian and New Zealand Journal of Psychiatry. 2016 doi: 10.1177/0004867416661426. [DOI] [PubMed] [Google Scholar]
- Ducharme S, Albaugh MD, Hudziak JJ, Botteron KN, Nguyen TV, Truong C, Evans AC, Karama S, for the Brain Development Cooperative Group Anxious/depressed symptoms are linked to right ventromedial prefrontal cortical thickness maturation in healthy children and young adults. Cereb Cortex. 2014;24:2941–2950. doi: 10.1093/cercor/bht151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AC. Networks of anatomical covariance. NeuroImage. 2013;80:489–504. doi: 10.1016/j.neuroimage.2013.05.054. [DOI] [PubMed] [Google Scholar]
- Fusar Poli P, Borgwardt S, Bechdolf A, Addington J, Riecher-Rössler A, Schultze-Lutter F, Keshavan M, Wood S, Ruhrmann S, Seidman LJ, Valmaggia L, Cannon T, Velthorst E, de Haan L, Cornblatt B, Bonoldi I, Birchwood M, McGlashan T, Carpenter W, McGorry P, Klosterkötter J, McGuire P, Yung A. The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry. 2013;70:107–120. doi: 10.1001/jamapsychiatry.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison CZ, Addy CL, Jackson KL, McKeown RE, Waller JL. The CES-D as a screen for depression and other psychiatric disorders in adolescents. Journal of the American Academy of Child & Adolescent Psychiatry. 1991;30:636–641. doi: 10.1097/00004583-199107000-00017. [DOI] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, Hare TA, Bookheimer SY, Tottenham N. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. Journal of Neuroscience. 2013;33:4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddings AL, Mills K, Clasen L, Giedd J, Viner R, Blakemore SJ. Longitudinal MRI to assess effect of puberty on subcortical brain development: an observational study. The Lancet. 2014;383:S52. doi: 10.1016/j.neuroimage.2013.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G, He Y, Chen ZJ, Evans AC. Convergence and divergence of thickness correlations with diffusion connections across the human cerebral cortex. NeuroImage. 2012;59:1239–1248. doi: 10.1016/j.neuroimage.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Gu X, Hof PR, Friston KJ, Fan J. Anterior insular cortex and emotional awareness. J Comp Neurol. 2013;521:3371–3388. doi: 10.1002/cne.23368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Chen MC, Gotlib IH. Neural systems approaches to understanding major depressive disorder: an intrinsic functional organization perspective. Neurobiology of Disease. 2013;52:4–11. doi: 10.1016/j.nbd.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb OD. The Organization of Behavior. Wiley and Sons; New York, NY: 1949. [Google Scholar]
- Heller AS. Cortical-Subcortical Interactions in Depression: From Animal Models to Human Psychopathology. Front Syst Neurosci. 2016;10:20. doi: 10.3389/fnsys.2016.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R. The functional neuroanatomy of reappraisal: Time matters. Neuroscience and Biobehavioral Reviews. 2009;33:1215–1226. doi: 10.1016/j.neubiorev.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Kanske P, Heissler J, Schonfelder S, Bongers A, Wessa M. How to Regulate Emotion? Neural Networks for Reappraisal and Distraction. Cerebral Cortex. 2011;21:1379–1388. doi: 10.1093/cercor/bhq216. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Schweder A. Schedule for Affective Disorders and Schizophrenia for School-Age Children- Present and Lifetime Version (K-SADS- PL), in: Comprehensive Handbook of Psychological Assessment, Personality Assessment. John Wiley and Sons; New Jersey: 2004. pp. 247–255. [Google Scholar]
- Kerestes R, Davey CG, Stephanou K, Whittle S, Harrison BJ. Functional brain imaging studies of youth depression: a systematic review. Neuroimage Clin. 2014;4:209–231. doi: 10.1016/j.nicl.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Angermeyer M, Anthony JC, DE Graaf R, Demyttenaere K, Gasquet I, DE Girolamo G, Gluzman S, Gureje O, Haro JM, Kawakami N, Karam A, Levinson D, Medina Mora ME, Oakley Browne MA, Posada-Villa J, Stein DJ, Adley Tsang CH, Aguilar-Gaxiola S, Alonso J, Lee S, Heeringa S, Pennell BE, Berglund P, Gruber MJ, Petukhova M, Chatterji S, Ustün TB. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization's World Mental Health Survey Initiative. World Psychiatry. 2007;6:168–176. [PMC free article] [PubMed] [Google Scholar]
- Lee NR, Raznahan A, Wallace GL, Alexander-Bloch A, Clasen LS, Lerch JP, Giedd JN. Anatomical coupling among distributed cortical regions in youth varies as a function of individual differences in vocabulary abilities. Hum Brain Mapp. 2013;35:1885–1895. doi: 10.1002/hbm.22299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch JP, Worsley K, Shaw WP, Greenstein DK, Lenroot RK, Giedd J, Evans AC. Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. NeuroImage. 2006;31:993–1003. doi: 10.1016/j.neuroimage.2006.01.042. [DOI] [PubMed] [Google Scholar]
- Matthews SC, Strigo IA, Simmons AN, Yang TT, Paulus MP. Decreased functional coupling of the amygdala and supragenual cingulate is related to increased depression in unmedicated individuals with current major depressive disorder. Journal of Affective Disorders. 2008;111:13–20. doi: 10.1016/j.jad.2008.05.022. [DOI] [PubMed] [Google Scholar]
- McRae K, Hughes B, Chopra S, Gabrieli JDE, Gross JJ, Ochsner KN. The neural bases of distraction and reappraisal. Journal of Cognitive Neuroscience. 2010;22:248–262. doi: 10.1162/jocn.2009.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mears D, Pollard HB. Network science and the human brain: Using graph theory to understand the brain and one of its hubs, the amygdala, in health and disease. J Neurosci Res. 2016;94:590–605. doi: 10.1002/jnr.23705. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, Benjet C, Georgiades K, Swendsen J. Lifetime Prevalence of Mental Disorders in U.S. Adolescents: Results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A) Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CH, Hamilton JP, Sacchet MD, Gotlib IH. Meta-analysis of Functional Neuroimaging of Major Depressive Disorder in Youth. JAMA Psychiatry. 2015;72:1045–1053. doi: 10.1001/jamapsychiatry.2015.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey Rajendra A, et al. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage. 2009;45(3):855–866. doi: 10.1016/j.neuroimage.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulders PC, van Eijndhoven PF, Schene AH, Beckmann CF, Tendolkar I. Resting-state functional connectivity in major depressive disorder: A review. Neuroscience and Biobehavioral Reviews. 2015;56:330–344. doi: 10.1016/j.neubiorev.2015.07.014. [DOI] [PubMed] [Google Scholar]
- Mutlu AK, Schneider M, Debbané M, Badoud D, Eliez S, Schaer M. Sex differences in thickness, and folding developments throughout the cortex. NeuroImage. 2013;82C:200–207. doi: 10.1016/j.neuroimage.2013.05.076. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. Cognitive emotion regulation: Insights from social cognitive and affective neuroscience. Current Directions in Psychological Science. 2008;17:153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: neural systems supporting the cognitive down-and up-regulation of negative emotion. NeuroImage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Packard MG, Cahill L, McGaugh JL. Amygdala modulation of hippocampal-dependent and caudate nucleus-dependent memory processes. Proceedings of the National Academy of Sciences. 1994;91:8477–8481. doi: 10.1073/pnas.91.18.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Amygdala modulation of multiple memory systems: hippocampus and caudate-putamen. Neurobiol Learn Mem. 1998;69:163–203. doi: 10.1006/nlme.1997.3815. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Allen NB. Arrested development? Reconsidering dual-systems models of brain function in adolescence and disorders. Trends in Cognitive Sciences. 2012;16:322–329. doi: 10.1016/j.tics.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Ramasubbu R, Konduru N, Cortese F, Bray S, Gaxiola-Valdez I, Goodyear B. Reduced intrinsic connectivity of amygdala in adults with major depressive disorder. Front Psychiatry. 2014;5:17. doi: 10.3389/fpsyt.2014.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Clasen LS, Giedd JN. Trail making test performance in youth varies as a function of anatomical coupling between the prefrontal cortex and distributed cortical regions. Developmental …. 2014 doi: 10.3389/fpsyg.2014.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Lerch JP, Lee N, Greenstein D, Wallace GL, Stockman M, Clasen L, Shaw PW, Giedd JN. Patterns of coordinated anatomical change in human cortical development: a longitudinal neuroimaging study of maturational coupling. Neuron. 2011a;72:873–884. doi: 10.1016/j.neuron.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Shaw P, Lalonde F, Stockman M, Wallace GL, Greenstein D, Clasen L, Gogtay N, Giedd JN. How does your cortex grow? Journal of Neuroscience. 2011b;31:7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempel-Clower NL. Role of orbitofrontal cortex connections in emotion. Annals of the New York Academy of Sciences. 2007;1121:72–86. doi: 10.1196/annals.1401.026. [DOI] [PubMed] [Google Scholar]
- Reuter M, Fischl B. Avoiding asymmetry-induced bias in longitudinal image processing. NeuroImage. 2011;57:19–21. doi: 10.1016/j.neuroimage.2011.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Rosas HD, Fischl B. Highly accurate inverse consistent registration: a robust approach. NeuroImage. 2010;53:1181–1196. doi: 10.1016/j.neuroimage.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. NeuroImage. 2012;61:1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesler R, Roozendaal B, McGaugh JL. Basolateral amygdala lesions block the memory-enhancing effect of 8-Br-cAMP infused into the entorhinal cortex of rats after training. Eur J Neurosci. 2002;15:905–910. doi: 10.1046/j.1460-9568.2002.01924.x. [DOI] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AMC, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of the human amygdala using resting state fMRI. NeuroImage. 2009;45:614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biological Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Singh MK, Gotlib IH. The neuroscience of depression: implications for assessment and intervention. Behaviour Research and Therapy. 2014;62:60–73. doi: 10.1016/j.brat.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Zola SM. Structure and function of declarative and nondeclarative memory systems. Proceedings of the National Academy of Sciences. 1996;93:13515–13522. doi: 10.1073/pnas.93.24.13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes CK, Østby Y, Fjell AM, Westlye LT, Due-Tønnessen P, Walhovd KB. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cerebral Cortex. 2010;20:534–548. doi: 10.1093/cercor/bhp118. [DOI] [PubMed] [Google Scholar]
- Vijayakumar N, Allen NB, Youssef G, Dennison M, Yücel M, Simmons JG, Whittle S. Brain development during adolescence: A mixed-longitudinal investigation of cortical thickness, surface area, and volume. Hum Brain Mapp. 2016 doi: 10.1002/hbm.23154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar N, Whittle S, Yücel M, Dennison M, Simmons J, Allen NB. Thinning of the lateral prefrontal cortex during adolescence predicts emotion regulation in females. Social Cognitive and Affective Neuroscience. 2014;9:1845–1854. doi: 10.1093/scan/nst183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Tamnes CK, Bjørnerud A, Due-Tønnessen P, Holland D, Dale AM, Fjell AM. Maturation of Cortico-Subcortical Structural Networks-Segregation and Overlap of Medial Temporal and Fronto-Striatal Systems in Development. Cereb Cortex. 2015;25:1835–1841. doi: 10.1093/cercor/bht424. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children-Fourth Edition. Harcourt Assessment, Inc; San Antonio TX: 2003. [Google Scholar]
- Whittle S, Lichter R, Dennison M, Vijayakumar N, Schwartz O, Byrne ML, Simmons JG, Yücel M, PANTELIS C, McGorry P, Allen NB. Structural brain development and depression onset during adolescence: a prospective longitudinal study. American Journal of Psychiatry. 2014;171:564–571. doi: 10.1176/appi.ajp.2013.13070920. [DOI] [PubMed] [Google Scholar]
- Whittle S, Yap MBH, Sheeber L, Dudgeon P, Yücel M, PANTELIS C, Simmons JG, Allen NB. Hippocampal volume and sensitivity to maternal aggressive behavior: a prospective study of adolescent depressive symptoms. Develop Psychopathol. 2011;23:115–129. doi: 10.1017/S0954579410000684. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Health for the World's Adolescents: A Second Chance in the Second Decade. World Health Organization; Geneva: 2014. [Google Scholar]
- Zielinski BA, Gennatas ED, Zhou J, Seeley WW. Network-level structural covariance in the developing brain. Proc Natl Acad Sci U S A. 2010;107:18191–18196. doi: 10.1073/pnas.1003109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


