Abstract
Context
An extensive body of literature indicates a relationship between insulin resistance and the up-regulation of the kynurenine pathway, i.e. the preferential conversion of tryptophan to kynurenine, with subsequent overproduction of diabetogenic downstream metabolites, such as kynurenic acid.
Case description
We have measured the concentration of kynurenine pathway metabolites (kynurenines) in the brain and pancreas of two young (27 and 28 yrs) insulin resistant, normoglycemic subjects (M-values 2 and 4 mg/kg/min, respectively) using quantitative C-11-alpha-methyl-tryptophan PET/CT imaging. Both subjects underwent a preventive 12-week metformin treatment regimen (500 mg daily) prior to the PET/CT study. Whereas treatment was successful in one of the subject (M-value increased from 2 to 12 mg/kg/min), response was poor in the other subjects (M-value changed from 4 to 5 mg/kg/min). Brain and pancreas concentrations of kynurenines observed in the responder were similar to that in a healthy control subject, whereas kynurenines determined in the non-responder were about 25% higher and similar to those found in a severely insulin resistant patient. Consistent with this outcome, M-values were negatively correlated with both kynurenic acid levels (R2 = 0.68, p = 0.09) as well as with the kynurenine to tryptophan ratio (R2 = 0.63, p = 0.11).
Conclusion
The data indicates that kynurenine pathway metabolites are increased in subjects with insulin resistance prior to overt manifestation of hyperglycemia. Moreover, successful metformin treatment leads to a normalization of tryptophan metabolism, most likely as a result of decreased contribution from the kynurenine metabolic pathway.
Keywords: Insulin resistance, Tryptophan metabolism, Kynurenine pathway, AMT PET imaging
1. Introduction
The insulin resistant (IR) state constitutes the major risk factor for the development of type 2 diabetes (T2D). There is evidence in literature that dysregulation of tryptophan (TRY) metabolism might be one of the mechanisms that contributes to IR [1]. TRY is an essential amino acid that is either incorporated into proteins or undergoes conversion to serotonin (5HT) or to kynurenin (KYN) via the KYN pathway. The KYN pathway is a fundamental mechanism of immunosuppression and peripheral tolerance [2], tightly controlled by the immune system. Extensive body of literature suggests that chronic stress and/or low-grade inflammation induce an up-regulation of the KYN pathway with subsequent overproduction of diabetogenic downstream metabolites [3]. Experimental studies clearly indicate a diabetogenic effect of KYN pathway metabolites, such as increased kynurenic acid (KYNA) levels in plasma of T2D patients [4]) and impairment of biosynthesis/activity [28] of insulin by KYN, KYNA and their derivatives [5]. In addition, as both TRY and KYN readily cross the blood-brain barrier [6], plasma fluctuations of peripheral KYN metabolites directly affect metabolism in the brain KYN pathway. In fact, about 60% of the metabolism along the KYN pathway in the CNS is initiated by KYN crossing the BBB [7]. Consequently, plasma fluctuations of KYN and its metabolites in the periphery directly affect metabolism in the brain KYN pathway, potentially affecting regulatory brain mechanisms that are in control of peripheral glucose levels.
Although metformin is one of the safest and most commonly prescribed drugs for treatment of IR, the variability of its therapeutic response is relatively high [8]. The exact mechanism for this differential response is currently unknown. It has been assumed that metformin acts primarily by enhancing the action of insulin in the liver by reducing the rate of hepatic glucose production via inhibition of glycogenolysis and gluconeogenesis. However, this assumption has been recently challenged, instead suggesting that the gut (and especially the distal small intestines) represents the main site of metformin action [9]. Moreover, available data suggests that KYNA is present in high concentration in the lumen of the small intestines (especially in the ileum) and is synthesized from TRY by the gut flora [10]. These new insights implying a possible connection between KYNA and metformin action are the motivation for our study investigating the relationship between regional TRY metabolism and the effect of metformin treatment among normoglycemic subjects with insulin resistance. This is accomplished using PET/CT imaging with [C-11]alpha-methyl-L-tryptophan (AMT) as tracer. AMT is an analogue of tryptophan and has been clinically used for the detection of epileptic tubers, which display increased AMT uptake even in the interictal state [11]. Tissue analysis of resected epileptic tubers demonstrated high concentration of KYN pathway metabolites (especially the concentration of the neurotoxin quinolinic acid [12]), indicating that increased conversion and trapping of AMT in these lesions is due to the up-regulation of the KYN pathway (which is activated in these lesions). More recently, high AMT uptake was demonstrated in gliomas and glioneuronal tumors with high indoleamine 2,3-dioxygenase 1 (IDO-1) expression [13]. Taken together, these results demonstrated that increased AMT uptake in tissue is related to the concentration of KYN pathway metabolites and can be used as a sensitive imaging tool for the quantification of regional levels of KYN pathway metabolites in vivo.
2. Subjects and methods
The study was approved by the Wayne State University Institutional Review Board. Written consent was obtained from the participants.
2.1. Clinical characteristics (Table 1)
Table 1.
Descriptive Statistics of subjects.
| Subject | IR | IS | ||
|---|---|---|---|---|
|
|
|
|||
| CJ | MZ | MK | DA | |
| M(mg/kg/min) | 2.5 | 5.0 | 12 | 13 |
| M(mg/kg/min, pre-treatment) | – | 4.0 | 1.5 | – |
| Age (yrs) | 20 | 27 | 28 | 25 |
| Height (cm) | 170 | 177 | 175 | 178 |
| Weight (kg) | 102 | 76 | 87 | 70 |
| Hb1Ac (%) | 4.7 | 5.6 | 5.1 | 4.9 |
| BMI (kg/m2) | 35 | 25 | 28 | 24 |
| Body Fat (%) | 40 | 20 | 24 | 18 |
| HR (beats/min) | 78 | 67 | 61 | 59 |
| BP (mm) | 106/61 | 112/66 | 118/71 | 119/50 |
| Tryptophan (uM) | 45.74 | 52.38 | 56.41 | 44.91 |
| Kynurenine (uM) | 2.47 | 1.58 | 1.32 | 1.28 |
| KT ratio (x 100) | 5.40 | 3.01 | 2.34 | 2.85 |
| Leptin (μg/L) | 8.07 | 0.56 | 0.36 | 0.23 |
| Adiponectin (μg/ml) | 2.71 | 3.05 | 1.01 | 10.32 |
| LA ratio | 2.98 | 0.18 | 0.36 | 0.02 |
| TNFα (pg/ml) | 0.01 | 0.06 | 0.06 | 0.22 |
LA: Leptin to Adiponectin.
Two healthy, non-diabetic volunteers were included in this study who underwent comprehensive screening tests such as vitals, Body Mass Index (BMI), urinalysis, ECG, body composition, medical/health history, international physical activity questionnaire, and complete blood chemistry, CBC, HbA1c, and lipid profile. After a 10 h fast, subjects underwent a hyperinsulinemic euglycemic clamp (HIEC) study which consisted of a 120min continuous infusion of human regular insulin (Humulin R; Eli Lilly, Indianapolis, IN) that started at a rate of 80 mU/m2/min. Euglycemia was targeted for 90 mg/dl by variable infusion of 20% D-glucose. Insulin-stimulated glucose disposal rates (M-value, infusion rate per kg per minute) were calculated as the average value during the final 30min of insulin infusion. After the clamp study, subjects underwent a 12-week preventive intervention with metformin (500 mg/day). After completing the 12 weeks of treatment, the HIEC study was repeated. One subject (MK) responded well to treatment (M-value changed from 1.5 to 12 mg/kg/min after treatment) whereas the other subject (MZ) responded poorly to treatment (M-value changed from 4 to 5 mg/kg/min during the same time period). In addition, two young treatment naïve subjects were included in this study for comparison, one with severe IR (M = 2.0 mg/kg/min) and the other with normal insulin sensitivity (M = 13.0 mg/kg/min).
2.2. Imaging procedures
All four subjects underwent 11C-alpha-methyl-tryptophan (AMT) PET/CT scans of the brain and the abdomen. In addition, structural T1w MR images of the brain were obtained and coregistered with the AMT PET images to guide definition of brain regions. Venous blood was obtained from all subjects yielding plasma values for TRY, KYN, leptin, adiponectin and TNFα. Based on the acquired abdominal CT images, regions were defined in the pancreas and the small intestines. Moreover, using the coregistered T1-w MR images, the following brain regions were defined: lower brainstem (pons and medulla), midbrain, thalamus, caudate and medial prefrontal cortex (mPFC). Regional TRY metabolism was quantified based on the standard uptake value (SUV), which represents AMT tracer concentration (uCi/cc) normalized to the injected activity (mCi) and patient weight (kg).
2.3. Laboratory methods
Venous blood was collected into pre-chilled K2EDTA vacutainer tubes, and placed on ice. Tubes were centrifuged at 3000 rpm for 10 min at 4 °C, plasma separated and stored at −80 °C until assay. TRY and KYN were determined by HPLC. Adiponectin, Leptin, and TNFα were determined by antibody based assay (AlphaLISA, PerkinElmer), average %CV for replicates were 0.52%, 9.83%, 2.77%, respectively.
3. Results
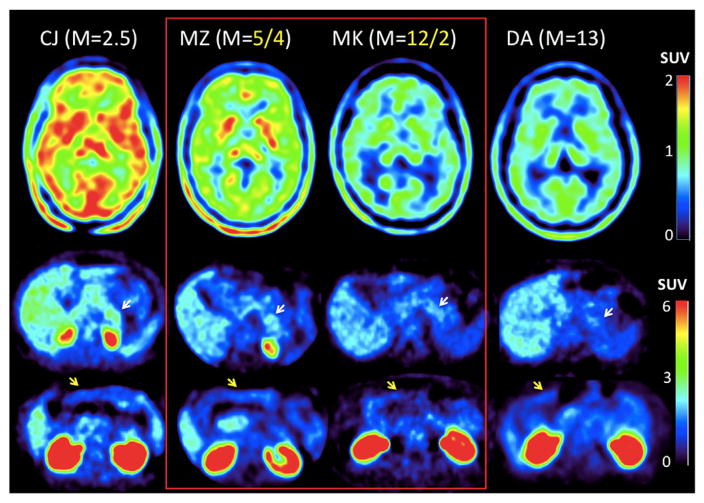
Visual assessment of SUV images (Fig. 1) showed increased concentration of KYN pathway metabolites in the non-responder (MZ) as compared to the responder (MK). This was true for both the brain (Fig. 1, top row) as well as for the pancreas (middle row). For comparison, SUVs determined in a subject with severe IR were found to be highest (CJ, far left column), whereas SUVs in a subject with normal insulin sensitivity (DA, far right column) were found to be lowest. The figure also indicates that metformin post-treatment SUVs in the brain of the responder (MK) are comparable to those in the control subject (see also Fig. 2A for regional SUVs), suggesting a normalization of brain TRY metabolism, most likely as a result of decreased contribution from the KYN metabolic pathway. In contrast, brain SUVs in the non-responder (MZ) were about 25% higher, conceivably a consequence of unchanged TRY conversion rate to KYN. The images also show increased SUVs in the pancreas (white arrows) in the two insulin resistant subjects (CJ and MZ, middle row) as compare to the two insulin sensitive subjects (MK and DA). In contrast, SUVs in the small intestines were similar in all subjects. This result implies greater vulnerability of the pancreas to increased levels of TRY metabolites via the KYN pathway.
Fig. 1.
AMT PET images in subjects with different levels of insulin sensitivity (M-value). Top row shows trans-axial SUV (activity in tissue normalized to injected activity and weight) images at the level of the thalamus, whereas the bottom two rows show SUV images at the level of the pancreas (white arrows) and at the level of the small intestines (yellow arrows). The two subjects who underwent metformin treatment are highlighted in the center (red line), with their post/pre-treatment M-values provided in yellow on top. SUV in the brain and pancreas is higher in subjects with low M-values, consistent with increased conversion of tryptophan via the kynurenine pathway in insulin resistant (CJ and MZ) as compared to insulin sensitive (MK and DA) subjects. The SUV in the small intestines is similar in all 4 subjects (bottom row). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
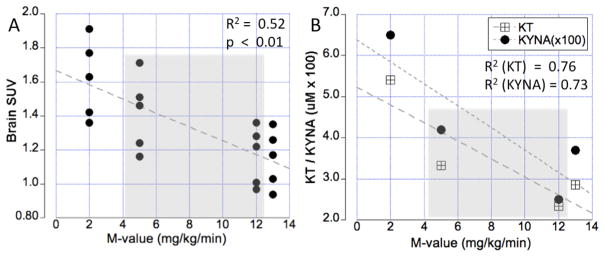
Fig. 2.
(A) Relationship between insulin sensitivity (M-value) and AMT tracer uptake in brain tissue (SUV), representing accumulation of kynurenine pathway metabolites. The gray area depicts two subjects who showed different responses to metformin treatment. The graph demonstrates that the concentration of kynurenine pathway metabolites in the subject who did not improve is similar to that found in an insulin resistant subjects, whereas kynurenine metabolite concentration in the subject who responded well to metformin are similar to an insulin sensitive control subject. (B) Corresponding kynurenic acid (KYNA) plasma concentration as well as the kynurenine to tryptophan ratio (KT). These values show a highly significant negative correlation with the M-value, indicating that brain kynurenine concentration is driven by peripheral kynurenine pathway metabolism.
The presented data suggests that insulin resistance is closely associated with the presence of KYN pathway metabolites in both the brain and peripheral tissues. Consistent with this interpretation, a highly significant (p < 0.01) inverse correlation between regional brain SUVs and M-values was established (Fig. 2A). Moreover, M-values were negatively correlated with both KYNA levels (R2 = 0.68, p = 0.09) as well as with the KT ratio (R2 = 0.63, p = 0.11, Fig. 2B), indicating that KYN pathway metabolites are increased in IR (low M-values). Finally, Table 2 reports regional SUVs for all 4 subjects, with a comparable rank order across the subjects (i.e. highest values for caudate and lowest for the brainstem).
Table 2.
Regional SUV in the CNS and periphery.
| Subject | IR | IS | ||
|---|---|---|---|---|
|
|
|
|||
| CJ | MZ | MK | DA | |
| Caudate | 1.91 | 1.71 | 1.36 | 1.35 |
| Thalamus | 1.77 | 1.51 | 1.28 | 1.26 |
| mPFC | 1.63 | 1.46 | 1.22 | 1.17 |
| Midbrain | 1.42 | 1.16 | 1.01 | 1.03 |
| Brainstem | 1.36 | 1.24 | 0.97 | 0.94 |
| Pancreas | 2.22 | 1.87 | 1.74 | 1.62 |
| Small Intestines | 1.85 | 1.88 | 1.79 | 1.82 |
4. Discussion
We show that TRY conversion via the KYN pathway is already up-regulated at the pre-diabetic stage, i.e. at a disease stage when insulin resistant subjects are still considered normoglycemic. Specifically, the severity of insulin resistance is related to the concentration of KYN pathway metabolites in the brain and the pancreas. Moreover, restoration of insulin sensitivity using metformin treatment is accompanied by a down-regulation of KYN pathway metabolism. These results support and augment recent findings that report increased levels of KYN pathway metabolites in plasma of diabetic patients [14]. Possible mechanisms mediating the contribution of KYN metabolites to the development of insulin resistance might be the formation of chelate complexes with insulin which are indistinguishable from insulin, but have ~50% lower activity than pure insulin [15]. In addition, KYNA is a competitive, broad-spectrum antagonist of glutamate receptors, inhibiting both NMDA and AMPA receptors in the brain. It was demonstrated that even modest increases in brain KYNA levels cause prompt reductions in extracellular glutamate and dopamine levels [16], affecting the firing rate of dopaminergic neurons in the midbrain and possibly interfering with the glucose regulatory network. The demonstration of a shift in TRY metabolism at a pre-diabetic stage might shed new light on the pathogenesis of diabetes.
Acknowledgments
The authors express their gratitude for the financial support received from the WSU Office of the Vice President (OVPR Grant 171904) as well as from the NIDDK (R01DK107666 and R01DK081750).
Footnotes
Disclosure summary
The authors declare no conflict of interest.
References
- 1.Oxenkrug G. Insulin resistance and dysregulation of tryptophan-kynurenine and kynurenine-nicotinamide adenine dinucleotide metabolic pathways. Mol Neurobiol. 2013;48(2):294–301. doi: 10.1007/s12035-013-8497-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones SP, Franco NF, Varney B, Sundaram G, Brown DA, de Bie J, Lim CK, Guillemin GJ, Brew BJ. Expression of the kynurenine pathway in human peripheral blood mononuclear cells: implications for inflammatory and neurodegenerative disease. PLoS One. 2015;10(6):e0131389. doi: 10.1371/journal.pone.0131389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heilbronn LK, Campbell LV. Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Curr Pharm Des. 2008;14:1225–1230. doi: 10.2174/138161208784246153. [DOI] [PubMed] [Google Scholar]
- 4.Oxenkrug GF. Increased plasma levels of xanthurenic and kynurenic acids in type 2 diabetes. Mol Neurobiol. 2015;52(2):805–810. doi: 10.1007/s12035-015-9232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okamoto H. Regulation of proinsulin synthesis in pancreatic islets and a new aspect to insulin-dependent diabetes. Mol Cell Biochem. 1981;37:43–61. doi: 10.1007/BF02355886. [DOI] [PubMed] [Google Scholar]
- 6.Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem. 1991;56:2007–2017. doi: 10.1111/j.1471-4159.1991.tb03460.x. [DOI] [PubMed] [Google Scholar]
- 7.Gál EM, Sherman AD. L-kynurenine: its synthesis and possible regulatory function in brain. Neurochem Res. 1980 Mar;5(3):223–239. doi: 10.1007/BF00964611. [DOI] [PubMed] [Google Scholar]
- 8.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) UK prospective diabetes study (UKPDS) group. Lancet. 1998;352(9131):854–865. [PubMed] [Google Scholar]
- 9.Buse JB, DeFronzo RA, Rosenstock J, Kim T, Burns C, Skare S, Baron A, Fineman M. The primary glucose-lowering effect of metformin resides in the gut, not the circulation: results from short-term pharmacokinetic and 12-week dose-ranging studies. Diabetes Care. 2016;39(2):198–205. doi: 10.2337/dc15-0488. [DOI] [PubMed] [Google Scholar]
- 10.Turski MP, Turska M, Paluszkiewicz P, Parada-Turska J, Oxenkrug GF. Kynurenic Acid in the digestive system-new facts, new challenges, Int. J Tryptophan Res. 2013 Sep 4;6:47–55. doi: 10.4137/IJTR.S12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chugani DC, Chugani HT, Muzik O, Shah JR, Shah AK, Canady A, Mangner TJ, Chakraborty PK. Imaging epileptogenic tubers in children with tuberous sclerosis complex using alpha-[11C]methyl-L-tryptophan positron emission tomography. Ann Neurol. 1998;44(6):858–866. doi: 10.1002/ana.410440603. [DOI] [PubMed] [Google Scholar]
- 12.Chugani DC, Muzik O. Alpha[C-11]methyl-L-tryptophan PET maps brain serotonin synthesis and kynurenine pathway metabolism. J Cereb Blood Flow Metab. 2000;20(1):2–9. doi: 10.1097/00004647-200001000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Batista CE, Juhász C, Muzik O, Kupsky WJ, Barger G, Chugani HT, Mittal S, Sood S, Chakraborty PK, Chugani DC. Imaging correlates of differential expression of indoleamine 2,3-dioxygenase in human brain tumors. Mol Imaging Biol. 2009 Nov-Dec;11(6):460–466. doi: 10.1007/s11307-009-0225-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munipally PK, Agraharm SG, Valavala VK, Gundae S, Turlapati NR. Evaluation of indoleamine 2,3-dioxygenase expression and kynurenine pathway metabolites levels in serum samples of diabetic retinopathy patients. Arch Physiol Biochem. 2011;117(5):254–258. doi: 10.3109/13813455.2011.623705. [DOI] [PubMed] [Google Scholar]
- 15.Kotaki Y, Ueda T, Mori T, Igaki S, Hattori M. Abnormal tryptophan metabolism and experimental diabetes by xanthurenic acid (XA) Acta Vitaminol Enzymol. 1975;29:236–239. [PubMed] [Google Scholar]
- 16.Rassoulpour A, Wu HQ, Ferré S, Schwarcz R. Nanomolar concentrations of kynurenic acid reduce extracellular dopamine levels in the striatum. J Neurochem. 2005;93:762–765. doi: 10.1111/j.1471-4159.2005.03134.x. [DOI] [PubMed] [Google Scholar]