Abstract
Purpose
To assess the visual outcomes of cataract surgery in eyes that received fluocinolone acetonide implant or systemic therapy with oral corticosteroids and immunosuppression during the Multicenter Uveitis Steroid Treatment (MUST) Trial.
Design
Nested prospective cohort study of patients enrolled in a randomized clinical trial.
Participants
Patients that underwent cataract surgery during the first 2 years of follow-up in the MUST Trial.
Methods
Visual outcomes of cataract surgery were evaluated 3, 6, and 9 months after surgery using logarithmic visual acuity charts. Change in visual acuity over time was assessed using a mixed-effects model.
Main Outcome Measures
Best-corrected visual acuity.
Results
After excluding eyes that underwent cataract surgery simultaneously with implant surgery, among the 479 eyes in the MUST Trial, 117 eyes (28 eyes in the systemic, 89 in the implant group) in 82 patients underwent cataract surgery during the first 2 years of follow-up. Overall, visual acuity increased by 23 letters from the preoperative visit to the 3-month visit (95% confidence interval [CI], 17–29 letters; P < 0.001) and was stable through 9 months of follow-up. Eyes presumed to have a more severe cataract, as measured by inability to grade vitreous haze, gained an additional 42 letters (95% CI, 34–56 letters; P < 0.001) beyond the 13-letter gain in eyes that had gradable vitreous haze before surgery (95% CI, 9–18 letters; P < 0.001) 3 months after surgery, making up for an initial difference of –45 letters at the preoperative visit (95% CI, –56 to –34 letters; P < 0.001). Black race, longer time from uveitis onset, and hypotony were associated with worse preoperative visual acuity (P < 0.05), but did not affect postsurgical recovery (P > 0.05, test of interaction). After adjusting for other risk factors, there was no significant difference in the improvement in visual acuity between the 2 treatment groups (implant vs. systemic therapy, 2 letters; 95% CI, –10 to 15 letters; P = 0.70).
Conclusions
Cataract surgery resulted in substantial, sustained, and similar visual acuity improvement in the eyes of patients with uveitis treated with the fluocinolone acetonide implant or standard systemic therapy.
Cataract is a common complication of uveitis. It can result from inflammation as part of the disease process or from the corticosteroids used to treat the inflammation. Cataract surgery in the setting of uveitis differs from senile cataract and can pose additional challenges and risks.1,2 Although the outcomes of cataract surgery in uveitic eyes are not as well studied as those in nonuveitic eyes, most studies suggest poorer visual outcomes and more postoperative complications among patients with uveitis. Furthermore, many of these studies are weighted toward the results of cataract surgery in patients with anterior uveitides.2–6
Preoperative control of inflammation is crucial for cataract surgery in uveitic eyes. A systematic review of the literature showed that eyes with controlled inflammation at the time of cataract surgery had better visual outcomes.3 A prospective study showed that eyes with active inflammation within 3 months before cataract surgery were more likely to have postoperative macular edema and that a short perioperative course of oral corticosteroids, started 2 days before surgery, significantly reduced the incidence of postoperative macular edema.6 Hence, preoperative addition or increase in systemic therapy, mainly corticosteroids, has become a standard approach in cataract surgery for uveitic eyes.
An alternative approach to systemic therapy for treating intermediate uveitis, posterior uveitis, and panuveitis is the fluocinolone acetonide implant (Retisert; Bausch & Lomb, Rochester, NY), which is designed to provide sustained, steady-state levels of corticosteroids directly to the eye for up to 3 years. In the Multicenter Uveitis Steroid Treatment (MUST) Trial, a randomized, clinical effectiveness trial of implant therapy versus standard systemic therapy with oral corticosteroids and immunosuppression for patients with noninfectious intermediate uveitis, posterior uveitis, and panuveitis, both the implant and systemic therapy controlled the inflammation in the large majority of patients. Nevertheless, implant therapy resulted in inactive inflammation in a greater proportion of eyes than did systemic therapy.7 Because cataracts occurred in both treatment groups, the MUST Trial provides the opportunity to compare the outcomes of cataract surgery in uveitic eyes with fluocinolone acetonide implants with those of patients treated with systemic corticosteroids and immunosuppression.
Methods
Eligibility
Patients included in this study were participants in the MUST Trial, a randomized, comparative effectiveness clinical trial comparing the fluocinolone acetonide intraocular implant with standard systemic therapy (systemic corticosteroids and immunosuppressive medications) for the treatment of noninfectious, recently active intermediate uveitis, posterior uveitis, or panuveitis (clinicaltrials.gov identifier, NCT00132691). The study enrolled 255 patients at 23 clinical centers in the United States (21 centers), United Kingdom, and Australia. The protocol was approved by the institutional review board of each center, and all participants provided written informed consent. The study complied with the Health Insurance Portability and Accountability Act and adhered to the tenets of the Declaration of Helsinki.7,8 Data on best-corrected visual acuity and a detailed ocular and medical history were collected at 3-month intervals for 2 years.
Uveitic eyes that underwent cataract surgery during follow-up were eligible for inclusion in this study unless the surgery was performed before or concurrent with implant surgery (Fig 1). Eyes that underwent concurrent cataract and implant surgery were excluded because of possible interference of implant surgery and its complications with cataract surgery-related outcomes. Visit windows were structured for the purpose of this analysis into a preoperative visit that occurred within 6 months of the date of surgery (−6 to 0 months) and postsurgical visits that occurred at 3, 6, and 9 months after surgery with windows of 0 to 4 months, 4 to 7 months, and 7 to 10 months, respectively. In addition, eyes that did not have a preoperative visual acuity measurement or a postsurgical visual acuity measurement within 10 months after surgery were excluded.
Figure 1.
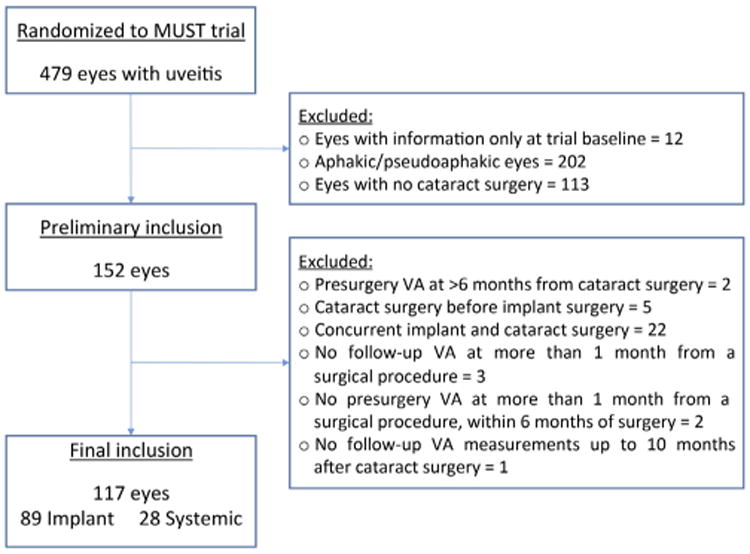
Flowchart showing the selection of uveitic eyes with cataract surgery during the Multicenter Steroid Treatment Trial for inclusion in analysis. VA = visual acuity.
Outcomes
The primary outcome of the current study was best-corrected visual acuity, measured using logarithmic visual acuity charts8,9 according to a standardized protocol. Secondary outcomes included binary categorizations of visual acuity patterns. The change from the preoperative to the first available postoperative visit was defined in 2 ways. For the purposes of this study, we considered a 5-letter improvement or more or a visual acuity outcome of 20/40 or better to be clinically meaningful. We first calculated the proportion of eyes with an improvement based on the number of letters (5 or 10 letters of improvement), and then the proportion of eyes that improved to 20/40 or better at the first postoperative visit was analyzed. The proportion of eyes with visual acuity of 20/40 or better at the first postoperative visit also was considered.
Statistical Analyses
Analyses were conducted based on the treatment being received at the time of the presurgical visit. The Kruskal-Wallis rank-sum test and the Fisher exact test were used to compare patient-level characteristics of the 2 therapies for eligible patients at the preoperative visit. A linear logistic (binary attribute) or multinomial (multicategory attribute) mixed-effects model with a patient-level random effect to adjust for between-eye correlation was used to compare preoperative eye-level characteristics between therapies. To model visual acuity over time, a means mixed-effects model that included terms for treatment, visit, and treatment-by-visit interaction was specified and estimated via maximum likelihood. A patient-level random intercept and a first-order autoregressive covariance structure for the within-eye repeated measurements were included to introduce between-eye correlation (via the random intercept) and longitudinal within-eye correlation (the first-order autoregressive component).
The association of other risk factors was assessed by adding them to the base model structure as follows: a main effect to assess the impact on baseline visual acuity and an interaction term with time to assess the impact on change in visual acuity after surgery. Secondary outcomes were modeled by logistic regression estimated via generalized estimating equations that account for the between-eye correlation for individuals with bilateral cataract surgery. Inability to grade vitreous haze was used as a surrogate for cataract severity. The risk factors considered included both individual characteristics (age, race, gender, uveitis location, presence of systemic disease) and preoperative eye-specific characteristics (disease duration, intraocular pressure, inability to grade vitreous haze). Robust standard errors were computed for all models. Statistical analyses were performed using SAS software version 9.3 (SAS Inc, Cary, NC), Stata 12 software (StataCorp LP, College Station, TX), and R software version 3.0.1 (The R project for Statistical Computing; available at: http://www.r-project.org/).
Results
From the 479 eyes with uveitis of the 255 individuals enrolled in the MUST Trial, a total of 117 eyes from 82 patients met the criteria for inclusion in the cataract study during the first 2 years of follow-up in the MUST Trial (Fig 1). A total of 28 eyes (24%) received systemic therapy and 89 eyes (76%) received implant therapy. Two eyes receiving systemic therapy and 15 of those receiving implant therapy originally were randomized to receive the other treatment. The most common reasons for exclusion included aphakia or pseudophakia at enrollment (n = 202 eyes), no surgery during follow-up (n = 113), or concurrent cataract and implant surgery (n = 22).
Demographic characteristics at the preoperative visit were similar between treatment groups, with 33% of patients being men, 18% of patients being black, and a median age of 46 years overall (Table 1). Fifty-two patients (63%) had posterior uveitis or panuveitis, with a nonsignificantly higher proportion in the implant group (67%) compared with the systemic group (55%). Preoperative visit visual acuities for eyes in the implant group were better than those in the systemic therapy group (median, 60 vs. 43 letters, respectively; P = 0.090), but this difference did not achieve conventional statistical significance. However, the proportion of eyes with visual acuity worse than 20/100 was significantly higher in the systemic group than in the implant group (64% vs. 36%, respectively; P = 0.031). Time since uveitis onset (or duration of uveitis) was similar between the 2 treatment groups, with an overall median of 2 years (interquartile range, 1–5 years). The proportion of eyes with ungradable vitreous haze at the preoperative visit was significantly higher in the systemic treatment group (43% vs. 16%; P = 0.003; Table 1). Preoperative intraocular pressure was within the normal range in both groups, but the median was 2-mmHg higher for eyes in the implant group, a magnitude that was statistically significant (P = 0.037), but not clinically meaningful.
Table 1. Characteristics of Patients and Eyes Undergoing Cataract Surgery in the Multicenter Uveitis Steroid Treatment Trial.
| Overall | Treatment Group | P Value* | ||
|---|---|---|---|---|
|
| ||||
| Systemic | Implant | |||
| Patient characteristics | ||||
| Participants, no. (%) | 82 | 22 (27) | 60 (73) | |
| Demographics | ||||
| Age at preoperative visit (yrs), median (IQR) | 46 (31–53) | 53 (38–58) | 44 (31–51) | 0.20 |
| Male gender, no. (%) | 27 (33) | 5 (23) | 22 (37) | 0.29 |
| Black race, no. (%) | 15 (18) | 3 (14) | 12 (20) | 0.75 |
| Clinical characteristics at the time of randomization in the MUST Trial | ||||
| Posterior uveitis or panuveitis, no. (%) | 52 (63) | 12 (55) | 40 (67) | 0.44 |
| Systemic disease, no. (%) | 22 (27) | 4 (18) | 18 (30) | 0.40 |
| Eye characteristics | ||||
| Eligible eyes, no. (%) | 117 | 28 (24) | 89 (76) | |
| Time since uveitis onset at the preoperative visit (yrs),‡ median (IQR)† | 2 (1–5) | 1 (0–3) | 2 (1–5) | 0.18‡ |
| Preoperative visual acuity (standard letters), no. (%) | ||||
| Median (IQR) | 56 (25–69) | 43 (5–59) | 60 (38–72) | 0.09 |
| 20/40 or better | 29 (25) | 5 (18) | 24 (27) | 0.33 |
| Worse than 20/100 | 50 (43) | 18 (64) | 32 (36) | 0.031 |
| 20/200 or worse | 33 (28) | 12 (43) | 21 (24) | 0.09 |
| Preoperative vitreous haze ungradable, no. (%) | 26 (22) | 12 (43) | 14 (16) | 0.003 |
| Preoperative intraocular pressure (mmHg), no. (%)† | ||||
| Median (IQR) | 18 (14–23) | 16 (13–20) | 18 (15–24) | 0.037 |
| ≤7 | 5 (4) | 3 (11) | 2 (2) | |
| 8–23 | 84 (72) | 21 (75) | 63 (72) | |
| 24–29 | 19 (16) | 2 (7) | 17 (19) | |
| ≥30 | 8 (7) | 2 (7) | 6 (7) | |
IQR = interquartile range (i.e. the 25th to 75th percentile); MUST = Multicenter Uveitis Steroid Treatment.
Unless otherwise specified, P values compare the distribution of all primary categories excluding missing values.
Missing values: 1 preoperative intraocular pressure observation missing (1%) from implant group, and 2 observations missing time since uveitis at the preoperative visit in the implant group (2%).
Based on a log transformation of time since uveitis onset at the preoperative visit.
Cataract surgery included intraocular lens placement in the overwhelming majority of eyes (113 eyes [97%]); the other 4 eyes (3%) were left aphakic. There was no difference by treatment group. In the implant group, 86 eyes (97%) had an intraocular lens placed, and in the systemic therapy group, 27 eyes (96%) had an intraocular lens placed (P = 0.91).
Overall, visual acuity improved by 23 letters (95% confidence interval [CI], 18–29 letters; P < 0.001) from the preoperative visit to the 3-month postoperative visit. The visual acuity was stable thereafter with an additional 2 letters at 6 months (95% CI, −0 to 4 letters; P = 0.05) that vanished by 9 months (P = 0.62). This pattern of improvement at 3 months that remained stable through 9 months was seen in both treatment groups (Fig 2). The point estimate of improvement was larger for eyes receiving systemic therapy (29 letters; 95% CI, 17–43 letters; P < 0.001) as compared with eyes receiving implant therapy (21 letters; 95% CI, 16–27 letters; P < 0.001). However, the difference between the groups in visual improvement was not statistically significant (mean, −9 letters for implant vs. systemic therapy; 95% CI, −23 to 6 letters; P = 0.24).
Figure 2.
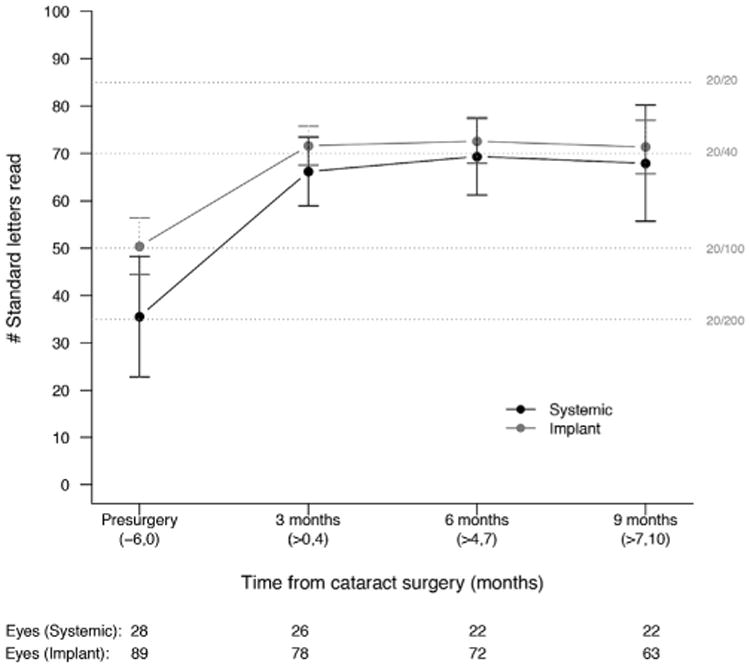
Mean visual acuity over time for eyes with cataract surgery during the Multicenter Steroid Treatment Trial stratified by treatment (implant [grey] vs. systemic [black] therapy). The 95% confidence intervals are included for each treatment group at each interval.
In the multivariate model, a number of factors were associated with worse visual acuity at the preoperative visit (Table 2). Eyes that had ungradable preoperative vitreous haze had a preoperative visual acuity that was 45 letters worse (−45 letters) than in eyes that were gradable (95% CI, −56 to −34 letters; P < 0.0001); eyes of black patients had a preoperative visual acuity that was 12 letters worse than those of nonblack patients (95% CI, −22 to −2 letters; P = 0.021), and preoperative hypotony (intraocular pressure, ≤7 mmHg) was associated with a visual acuity of 8 letters worse as compared with eyes with normal intraocular pressure (8–23 mmHg). For each decade since uveitis diagnosis, there was a 4.7-letter worse preoperative visual acuity (95% CI, −9 to −0.4 letters; P = 0.033). After adjusting for other risk factors, there was no significant difference in the preoperative visual acuity for the 2 treatment groups (implant vs. systemic therapy: median, 6 letters; 95% CI, −4 to 15 letters; P = 0.22).
Table 2. Adjusted* Risk Factors Affecting Visual Acuity at the Preoperative Visit and Affecting the Preoperative to Postoperative Visit Change in Visual Acuity in the Multicenter Uveitis Steroid Treatment Trial.
| Characteristics | Preoperative Visual Acuity | ||
|---|---|---|---|
|
| |||
| Estimated Mean Difference at Preoperative Visit, Letters (95% Confidence Interval) | P Value | ||
| Treatment | |||
| Systemic | Reference | ||
| Implant | 5.5 | −3.3 to 14.4 | 0.22 |
| Race | |||
| Nonblack | Reference | ||
| Black | −12.0 | −22.1 to −1.8 | 0.021 |
| Time from uveitis onset at preoperative visit (10 yrs), centered at 2 yrs | −4.7 | −9.0 to −0.4 | 0.033 |
| Preoperative intraocular pressure (mmHg) | |||
| ≤7 | −7.6 | −14.0 to −1.0 | 0.022 |
| 8–23 | Reference | ||
| 24–29 | −5.6 | −16.4 to 5.2 | 0.31 |
| ≤30 | 9.1 | −1.6 to 19.8 | 0.096 |
|
| |||
| Change from Preoperative to Postoperative Visual Acuity | |||
|
| |||
| Mean Difference from Preoperative to Postoperative Visits, Letters (95% Confidence Interval) | P Value | ||
|
| |||
| Eyes with gradable preoperative vitreous haze† | |||
| Preoperative visit | Reference | ||
| 3 mos | 13.4 | 9.7–17.1 | <0.001 |
| 6 mos | 15.9 | 12.6–19.2 | <0.001 |
| 9 mos | 15.4 | 11.7–19.0 | <0.001 |
| Eyes with ungradable preoperative vitreous haze | |||
| Preoperative visit | Reference | ||
| 3 mos | 55.4 | 42.9–67.9 | <0.001 |
| 6 mos | 58.0 | 45.4–70.6 | <0.001 |
| 9 mos | 57.4 | 44.7–70.1 | <0.001 |
Adjusted for all factors simultaneously.
The association of preoperative vitreous haze with preoperative visual acuity was significantly different from its association with postoperative visual acuity (P < 0.001).
Only the ability to grade preoperative vitreous haze was associated significantly with the amount of improvement in visual acuity after cataract surgery (Table 2). For eyes with gradable vitreous haze, visual acuity improved by a mean of 13 letters from the preoperative visit to the 3-month postoperative visit (95% CI, 9–18 letters; P < 0.001) and showed a significant additional gain at 6 months (mean, 3 letters; 95% CI, 1–4 letters; P = 0.001) that had vanished by 9 months (P = 0.52). Eyes that were not gradable for vitreous haze at the preoperative visit had an additional mean improvement of 42 letters (95% CI, 35 to −56 letters; P < 0.001) from the preoperative visit to the 3-month postoperative visit beyond that observed with gradable vitreous haze, for a mean total improvement of 55 letters. The difference in improvement was such that eyes with preoperative ungradable vitreous haze had postoperative visual acuities that were not statistically or clinically significantly different from those with gradable preoperative vitreous haze (mean, −3 letters; 95% CI, −12 to 7 letters; P = 0.55). After adjusting for other risk factors, there was no significant difference in the improvement in visual acuity between the 2 treatment groups (implant vs. systemic therapy: mean, 2 letters; 95% CI, −10 to 15 letters; P = 0.70).
Overall, most eyes improved by a clinically meaningful amount between the preoperative and first postoperative visit (Table 3). Ninety-two eyes (79%) had an improvement of 5 letters or more and 77 eyes (67%) had an improvement of 10 letters or more. In fact, 72 eyes (62%) had a postoperative visual acuity of 20/40 or better, including 45 eyes (63%) that improved from worse than 20/40 to 20/40 or better, all of whom had at least a 5-letter gain. Very few eyes experienced a 5-letter or more decline in visual acuity; 5 eyes (4%) had a decline of 5 letters or more and only 3 eyes (3%) had a decline of 10 letters or more.
Table 3. Risk Factors for Visual Acuity Improvement in Eyes Undergoing Cataract Surgery in the Multicenter Uveitis Steroid Treatment Trial.
| ≥5-Letter Improvement (n = 117) | ≥10-Letter Improvement (n = 115)* | Postoperative Visual Acuity 20/40 or Better (n = 117) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Odds Ratio | 95% Confidence Interval | P Value | Odds Ratio | 95% Confidence Interval | P Value | Odds Ratio | 95% Confidence Interval | P Value | |
| Patient characteristics | |||||||||
| Demographics | |||||||||
| Age at preoperative visit (yrs) | 1.0 | 0.9–1.1 | 0.47 | 1.0 | 0.9–1.1 | 0.19 | 1.0 | 0.9–1.1 | 0.13 |
| Gender | |||||||||
| Female | Ref | Ref | Ref | ||||||
| Male | 1.2 | 0.4–3.1 | 0.76 | 0.9 | 0.4–2.1 | 0.79 | 1.7 | 0.7–4.1 | 0.24 |
| Race | |||||||||
| Nonblack | Ref | Ref | Ref | ||||||
| Black | 0.9 | 0.3–2.8 | 0.82 | 1.3 | 0.4–4.0 | 0.59 | 0.7 | 0.3–2.1 | 0.57 |
| Clinical characteristics at the time of randomization in the MUST Trial | |||||||||
| Uveitis class | |||||||||
| Intermediate uveitis | Ref | Ref | Ref | ||||||
| Posterior uveitis or panuveitis | 0.3 | 0.1–1.1 | 0.06 | 0.4 | 0.2–1.1 | 0.07 | 1.0 | 0.4–2.4 | 0.98 |
| Systemic disease | |||||||||
| No | Ref | Ref | |||||||
| Yes | 1.0 | 0.3–2.8 | 0.96 | 1.0 | 0.4–2.6 | 0.97 | 1.6 | 0.6–4.1 | 0.34 |
| Eye characteristics | |||||||||
| Treatment | |||||||||
| Systemic | Ref | Ref | Ref | ||||||
| Implant | 1.4 | 0.5–4.0 | 0.49 | 1.0 | 0.5–2.5 | 0.99 | 1.36 | 0.6–3.3 | 0.49 |
| Time from uveitis onset at preoperative visit (yrs), centered at 2 yrs | 1.0 | 0.9–1.1 | 0.96 | 1.0 | 0.9–1.1 | 0.91 | 1.0 | 0.9–1.1 | 0.36 |
| Preoperative intraocular pressure (mmHg) | |||||||||
| ≤7 | 1.3 | 0.1–14.2 | 0.80 | 2.3 | 0.2–22.4 | 0.47 | 0.2 | 0.0–1.3 | 0.09 |
| 8–23 | Ref | Ref | Ref | ||||||
| 24–29 | 1.4 | 0.4–5.4 | 0.62 | 1.5 | 0.5–4.6 | 0.51 | 1.1 | 0.4–3.3 | 0.80 |
| ≥30 | 0.7 | 0.1–4.0 | 0.74 | 1.2 | 0.2–6.3 | 0.86 | 0.9 | 0.2–4.2 | 0.93 |
| Preoperative vitreous haze | |||||||||
| Gradable | Ref | Ref | Ref | ||||||
| Ungradable | 3.7 | 0.8–16.7 | 0.08 | 7.9 | 1.8–35.1 | 0.007 | 1.7 | 1.1–2.7 | 0.03 |
MUST = Multicenter Uveitis Steroid Treatment; Ref = reference.
Two eyes with a preoperative visual acuity of 89 letters, which precluded a 10-letter improvement, were excluded.
Similar to what was seen in the analysis of mean change in visual acuity, eyes with ungradable vitreous haze at the preoperative visit were more likely to experience postoperative visual acuity improvement by 10 letters or more (odds ratio [OR], 7.9 letters; 95% CI, 1.8–35.1 letters; P = 0.007) and a postoperative visual acuity of 20/40 or better regardless of preoperative visual acuity (OR, 1.7 letters; 95% CI, 1.1–2.7 letters; P = 0.03). There was no significant difference in the probability of postoperative improvement in vision by 5 letters or more, 10 letters or more, or having postoperative visual acuity of 20/40 or better for the 2 treatment groups (P > 0.49; Table 3). None of the other patient or eye characteristics were associated significantly with any of the postoperative improvement outcomes. However, there was a suggestion that having posterior uveitis or panuveitis at enrollment in the MUST Trial was associated with a lower probability of a 5- or 10-letter improvement (OR, 0.3; 95% CI, 0.1–1.1; P = 0.060; and OR, 0.4; 95% CI, 0.2–1.1; P = 0.07, respectively). Because of the small number of eyes with a decline, we were unable to evaluate risk factors associated with a loss of vision.
Discussion
Cataract is among the most common structural complications of uveitis and its treatment with corticosteroids, and cataract surgery is among the most common surgical procedures performed on patients with uveitis. Several studies have reported that substantial visual gain occurs in most uveitic eyes after cataract surgery.2–4,10–14 Although cataract is a well-recognized occurrence with the fluocinolone acetonide implant and cataract surgery is needed in more than 90% of eyes by 3 years after implantation,15 the MUST Trial provided the first opportunity to compare the outcomes of cataract surgery in eyes with the implant with those of patients treated with systemic therapy. The current study showed statistically significant, clinically meaningful, and sustained (for up to 9 months after surgery) improvement in visual acuity of similar magnitude after cataract surgery in both eyes treated with systemic therapy and those treated with the fluocinolone acetonide implant. Overall, 62% of eyes achieved a visual acuity of 20/40 or better after surgery. This success proportion is lower than that reported for uncomplicated cataract surgery in the general public (96%),16 but similar to that reported in other studies of uveitic cataract surgery.3
In a meta-analysis of the outcomes of cataract surgery, Mehta et al3 estimated that the overall percent of eyes with uveitis achieving 20/40 or better postoperative visual acuity was 68% and that anterior approaches, intraocular lens placement, nonsilicone lenses, and operating on eyes with inactive uveitis were associated with better outcomes. Uveitis class was not evaluated systematically, but Fuchs heterochromic uveitis seemed to respond better than other types of uveitis; intermediate uveitis seemed to have a similar success rate to the overall rate; and the one panuveitis evaluated, Behçet's disease, had worse outcomes. In this regard, there was a suggestion from our data that posterior uveitis and panuveitis may show a worse response than intermediate uveitis. Such a result would not be surprising because patients with posterior uveitis are more likely to encounter permanent structural damage that may limit visual improvement. Because the MUST Trial enrolled patients with only intermediate uveitis, posterior uveitis, or panuveitis, its results ay not be comparable directly with those of series including eyes with anterior uveitis. Nevertheless, the proportion of eyes with a postoperative visual acuity of 20/40 or better in the MUST Trial is on the same order as that in the meta-analysis.
The level of vision before cataract surgery differed between the 2 treatment groups, and it was likely that eyes in the systemic group had worse cataracts at the time of surgery. Eyes receiving systemic therapy were significantly more likely to have a preoperative visual acuity worse than 20/100 and, assuming that inability to grade the vitreous haze is a marker for denser cataract, worse cataracts. Reasons for this difference are unknown, but it is possible that ophthalmologists were likely to operate sooner on eyes treated with implant therapy because cataract surgery is an expected and nearly universal complication of implant therapy.7,15,17 It is also possible that eyes with more severe cataracts in the implant group had simultaneous cataract and implant surgery and were excluded from this study. Nevertheless, there was no significant difference in the visual improvement between the 2 treatment groups, even after adjusting for other risk factors.
Sheppard et al17 evaluated cataract outcomes in uveitic eyes that received the fluocinolone acetonide implant and compared them with those of fellow, nonimplanted eyes. They reported that eyes undergoing cataract surgery after the implantation had a better visual acuity gain and less postoperative inflammation than the fellow eyes, which were untreated or managed with regional corticosteroid injections.15 No details were given in this article about the duration of inactive disease or preoperative regimens in the nonimplant group, whereas given the duration of effect of the implant, these eyes were receiving preoperative corticosteroid therapy comparable with that resulting from systemic therapy. In this regard, the MUST results suggest that eyes with intermediate uveitis, posterior uveitis, or panuveitis will have similar postoperative visual improvement whether the eye has been treated with an implant or with systemic therapy.
Although black patients and eyes with hypotony were associated with poorer preoperative visual acuity, they were not associated with any worse likelihood of visual improvement after cataract surgery. The risk factors that were associated with lower preoperative visual acuity (black race, disease duration, hypotony) are known risk factors for poorer visual acuity in eyes with uveitis.18–20 Longer disease duration is known to be associated with poorer outcomes and more structural complications,18,21,22 and hypotony, which likely is reflective of the chronicity and severity of the underlying disease, has been associated with poorer visual acuity in patients with uveitis.19,21,22 Although these factors were associated with poorer overall vision, none of them had an impact on improvement in visual acuity after surgery (P > 0.05, test of interaction). The only characteristic associated with better gains in vision after surgery was ungradable vitreous haze before surgery. As noted above, it is likely that ungradable vitreous haze before surgery was a marker for denser and worse cataract, resulting in worse visual acuity before surgery and bigger gains in acuity at the postoperative visits.
The typical recommendation is to defer cataract surgery in uveitic eyes until the uveitis has been inactive for a minimum of 3 months before surgery and to pretreat patients with systemic corticosteroids briefly before cataract surgery and for a short time after surgery. A systematic review and meta-analysis of the literature found that active uveitis at the time of cataract surgery was associated with poorer outcomes. A prospective study of cataract surgery6 demonstrated that active uveitis within 3 months before cataract surgery and failure to use perioperative systemic corticosteroids each were associated with worse outcomes and evidenced by more macular edema. Unfortunately, the follow-up schedule and visit windows in this study (in which the preoperative visit could be as much as 6 months before surgery) precluded the evaluation of the effect of inactive uveitis in the 3 months before surgery. Similarly, the surgical data collection did not include information on the use of perioperative oral corticosteroids.
Our study is limited by the relatively small number of eyes in the systemic treatment group. We were unable to measure the severity of cataract directly and instead used a surrogate (inability to grade vitreous haze) to attempt to account for the impact of severity. The large majority of eyes showed an improvement in visual acuity, which limited our ability to perform multivariate analyses of risk factors related to visual acuity threshold. Nearly all eyes had placement of an intraocular lens, limiting any power to evaluate the effect of aphakia. Finally, the small number of eyes with a decline made it impossible to evaluate for risk factors related to decline. Nevertheless, our study suggested that patients with intermediate uveitis, posterior uveitis, or panuveitis show similar improvements in visual acuity after cataract surgery, regardless whether the uveitis was treated with the fluocinolone acetonide implant or with systemic therapy with oral corticosteroids and immunosuppression.
Supplementary Material
Acknowledgments
Supported by the National Eye Institute, National Institutes of Health, Bethesda, Maryland (Collaborative Agreement nos.: U10EY014655 [D.A.J.], U10EY014660 [J.T.H.], and U10EY014656 [M.M.A.]); and the National Eye Institute Intramural Research Program (H.N.S.) (Bethesda, MD). Bausch & Lomb (Rochester, NY) provided support to the study in the form of donation of fluocinolone implants for patients randomized to implant therapy who were uninsured or otherwise unable to pay for implants, or who were located at a site where implants could not be purchased (e.g., the United Kingdom). Additional support was provided by Research to Prevent Blindness, Inc., New York, NY; the Paul and Evanina Mackall Foundation (Chicago, IL); and the Lois Pope Life Foundation (Delray Beach, FL). A representative of the National Eye Institute participated in the conduct of the study, including the study design; collection, management, analysis, and interpretation of the data; and review and approval of this manuscript. The sponsor or funding organization had no role in the design or conduct of this research.
Abbreviations and Acronyms
- CI
confidence interval
- IQR
Interquartile range
- MUST
Multicenter Uveitis Steroid Treatment
- OR
odds ratio
Footnotes
Supplemental material is available at www.aaojournal.org.
Presented at: American Academy of Ophthalmology Annual Meeting, Chicago, IL, October 2014.
Financial Disclosure(s): The author(s) have made the following disclosure(s): D.A.J.: Consultant - Santen (Osaka, Japan); Data and Safety Monitoring Committee – Applied Genetic Technologies Corporation (Alachua, FL)
J.H.K: Consultant - AbbVie (North Chicago, IL); Alcon (Fort Worth, TX); Allergan (Irvine, CA) Can-Fite (Peta-Tikva, Israel); Clearside (Alpharetta, GA); Lux Biosciences (Jersey City, NJ); Roche (Basel, Switzerland) Xoma (Berkeley, CA)
Author Contributions: Conception and design: Kempen, Jabs, Abreu, Louis, Sugar, Sen
Analysis and interpretation: Kempen, Jabs, Abreu, Louis, Sugar, Sen
Data collection: Sen, Abreu, Louis, Sugar, Altaweel, Elner, Holbrook, Jabs, Kim, Kempen
Obtained funding: none
Overall responsibility: Kempen, Jabs, Sen
References
- 1.Jancevski M, Foster CS. Cataracts and uveitis. Curr Opin Ophthalmol. 2010;21:10–4. doi: 10.1097/ICU.0b013e328332f575. [DOI] [PubMed] [Google Scholar]
- 2.Van Gelder RN, Leveque TK. Cataract surgery in the setting of uveitis. Curr Opin Ophthalmol. 2009;20:42–5. doi: 10.1097/ICU.0b013e32831b9b22. [DOI] [PubMed] [Google Scholar]
- 3.Mehta S, Linton MM, Kempen JH. Outcomes of cataract surgery in patients with uveitis: a systematic review and meta-analysis. Am J Ophthalmol. 2014;158:676–92. doi: 10.1016/j.ajo.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Foster CS, Rashid S. Management of coincident cataract and uveitis. Curr Opin Ophthalmol. 2003;14:1–6. doi: 10.1097/00055735-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Meier FM, Tuft SJ, Pavesio CE. Cataract surgery in uveitis. Ophthalmol Clin North Am. 2002;15:365–73. doi: 10.1016/s0896-1549(02)00033-0. [DOI] [PubMed] [Google Scholar]
- 6.Bélair ML, Kim SJ, Thorne JE, et al. Incidence of cystoid macular edema after cataract surgery in patients with and without uveitis using optical coherence tomography. Am J Ophthalmol. 2009;148:128–35. doi: 10.1016/j.ajo.2009.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Multicenter Uveitis Steroid Treatment (MUST) Trial Research Group. Kempen JH, Altaweel MM, Holbrook JT, et al. Randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior, and panuveitis: the multicenter uveitis steroid treatment trial. Ophthalmology. 2011;118:1916–26. doi: 10.1016/j.ophtha.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Multicenter Uveitis Steroid Treatment Trial Research Group. Kempen JH, Altaweel MM, Holbrook JT, et al. The Multi-center Uveitis Steroid Treatment Trial: rationale, design, and baseline characteristics. Am J Ophthalmol. 2010;149:550–61. doi: 10.1016/j.ajo.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferris FL, III, Bailey I. Standardizing the measurement of visual acuity for clinical research studies: guidelines from the Eye Care Technology Forum. Ophthalmology. 1996;103:181–2. doi: 10.1016/s0161-6420(96)30742-2. [DOI] [PubMed] [Google Scholar]
- 10.Tomlins PJ, Sivaraj RR, Rauz S, et al. Long-term biocom-patibility and visual outcomes of a hydrophilic acrylic intraocular lens in patients with uveitis. J Cataract Refract Surg. 2014;40:618–25. doi: 10.1016/j.jcrs.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Ram J, Gupta A, Kumar S, et al. Phacoemulsification with intraocular lens implantation in patients with uveitis. J Cataract Refract Surg. 2010;36:1283–8. doi: 10.1016/j.jcrs.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 12.Okhravi N, Lightman SL, Towler HM. Assessment of visual outcome after cataract surgery in patients with uveitis. Ophthalmology. 1999;106:710–22. doi: 10.1016/S0161-6420(99)90155-0. [DOI] [PubMed] [Google Scholar]
- 13.Estafanous MF, Lowder CY, Meisler DM, Chauhan R. Phacoemulsification cataract extraction and posterior chamber lens implantation in patients with uveitis. Am J Ophthalmol. 2001;131:620–5. doi: 10.1016/s0002-9394(00)00909-0. [DOI] [PubMed] [Google Scholar]
- 14.Ganesh SK, Babu K, Biswas J. Phacoemulsification with intraocular lens implantation in cases of pars planitis. J Cataract Refract Surg. 2004;30:2072–6. doi: 10.1016/j.jcrs.2004.02.090. [DOI] [PubMed] [Google Scholar]
- 15.Callanan DG, Jaffe GJ, Martin DF, et al. Treatment of posterior uveitis with a fluocinolone acetonide implant: three-year clinical trial results. Arch Ophthalmol. 2008:1261191–201. doi: 10.1001/archopht.126.9.1191. [DOI] [PubMed] [Google Scholar]
- 16.Powe NR, Schein OD, Gieser SC, et al. Synthesis of the literature on visual acuity and complications following cataract extraction with intraocular lens implantation Cataract Patient Outcome Research Team. Arch Ophthalmol. 1994;112:239–52. doi: 10.1001/archopht.1994.01090140115033. [DOI] [PubMed] [Google Scholar]
- 17.Sheppard JD, Jr, Nguyen QD, Usner DW, Comstock TL. Post-cataract outcomes in patients with noninfectious posterior uveitis treated with the fluocinolone acetonide intravitreal implant. Clin Ophthalmol. 2012;6:79–85. doi: 10.2147/OPTH.S24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dana MR, Merayo-Lloves J, Schaumberg DA, Foster CS. Prognosticators for visual outcome in sarcoid uveitis. Ophthalmology. 1996;103:1846–53. doi: 10.1016/s0161-6420(96)30417-x. [DOI] [PubMed] [Google Scholar]
- 19.Sen HN, Vitale S, Gangaputra SS, et al. Periocular cortico-steroid injections in uveitis: effects and complications. Ophthalmology. 2014;121:2275–86. doi: 10.1016/j.ophtha.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maini R, O'Sullivan J, Reddy A, et al. The risk of complications of uveitis in a district hospital cohort. Br J Ophthalmol. 2004;88:512–7. doi: 10.1136/bjo.2002.013334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sen HN, Drye LT, Goldstein DA, et al. for the Multicenter Uveitis Steroid Treatment (MUST) Trial Research Group. Hypotony in patients with uveitis: the Multicenter Uveitis Steroid Treatment (MUST) trial. Ocul Immunol Inflamm. 2012;20:104–12. doi: 10.3109/09273948.2011.647228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daniel E, Pistilli M, Pujari SS, et al. Risk of hypotony in noninfectious uveitis. Ophthalmology. 2012;119:2377–85. doi: 10.1016/j.ophtha.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


