Abstract
Background
Although childhood cancer is a leading cause of childhood mortality in the US, evidence regarding the etiology is lacking. The goal of this study was to evaluate the association between benzene, a known carcinogen, and childhood acute leukemia.
Methods
We conducted a case-control study including cases diagnosed with acute leukemia between 1997 and 2012 (n=307) from the Oklahoma Central Cancer Registry and controls matched on week of birth from birth certificates (n=1,013). We used conditional logistic regression to evaluate the association between benzene, measured with the 2005 National-Scale Air Toxics Assessment (NATA) at census tract of the birth residence, and childhood acute leukemia.
Results
We observed no differences in benzene exposure overall between cases and controls. However, when stratified by year of birth, cases born from 2005-2010 had a three–fold increased unadjusted odds of elevated exposure compared to controls born in this same time period (4th Quartile OR: 3.53, 95% CI: 1.35, 9.27). Furthermore, the estimates for children with acute myeloid leukemia (AML) were stronger than those with acute lymphoid leukemia, though not statistically significant.
Conclusions
While we did not observe an association between benzene and childhood leukemia overall, our results suggest that acute leukemia is associated with increased benzene exposure among more recent births, and children with AML may have increased benzene exposure at birth. Using the NATA estimates allowed us to assess a specific pollutant at the census tract level, providing an advantage over monitor or point source data. Our study, however, cannot rule out the possibility that benzene may be a marker of other traffic-related exposures and temporal misclassification may explain the lack of an association among earlier births.
Keywords: benzene, pediatrics, leukemia, air pollution, cancer
1. Introduction
As a leading cause of childhood mortality, childhood cancer is an important health concern in the US (Heron, 2013). However, evidence regarding the etiology is lacking despite numerous studies (Belson et al., 2007; Ries et al., 1999). One environmental risk factor of recent interest is ambient air pollution. According to the International Agency for Research on Cancer (IARC), ambient air pollution has been classified as carcinogenic to humans (International Agency for Research on Cancer, 2013). Furthermore, IARC has classified diesel engine exhaust as carcinogenic and motor vehicle exhaust as possibly carcinogenic (Benbrahim-Tallaa et al., 2012). As the primary pollutant of concern in engine exhaust, benzene has been classified as a known carcinogen in adult acute myeloid leukemia (AML) (International Agency for Research on Cancer, 1982). The minimal risk level established by the Environmental Protection Agency (EPA) for benzene is 0.009 parts per million (ppm) (28.71 μg/m3) for acute exposure lasting less than 15 days and 0.003 ppm (9.57 μg/m3) for chronic exposure of ≥365 days (Department of Health and Human Services, 2007).
Important sources of exposure to benzene are occupational exposure, gasoline stations, auto-repair shops, traffic, and cigarette smoking (Department of Health and Human Services, 2007). In occupational settings, benzene exposure was higher than that of the general population, with health effects generally observed above 79.8 μg/m3 (Khalade et al., 2010; Rushton and Romaniuk, 1997). Benzene is also produced through drilling for oil and natural gas, by burning of oil and coal, and by oil and gas refineries (Department of Health and Human Services, 2007; Esswein et al., 2014; McKenzie et al., 2012; United States Environmental Protection Agency, 2015). While Oklahoma is a fairly rural state with an estimated 3.9 million people in 2016 and 34% of the population residing in rural areas, it is the third largest oil and gas producing state in the US (U.S. Energy Information Administration, 2016; United States Census Bureau, 2017a; United States Census Bureau, 2017b).
The biologic mechanism of benzene as a cause of leukemia, the most common form of childhood cancer, is not well understood. However, it is believed to occur through similar mechanisms as chemotherapy-induced AML, which is a result of chromosomal abnormalities and/or translocations secondary to previous chemotherapy treatments (Pedersen-Bjergaard et al., 2008). Benzene is metabolized primarily by the lungs and liver, but secondary metabolism occurs in the bone marrow, where blood cells are formed. Furthermore, benzene-induced chromosomal alterations are similar to those observed in AML cells (Department of Health and Human Services, 2007; McHale et al., 2012).
There have been few studies focusing specifically on benzene and childhood leukemia. However, studies to date have reported conflicting results for childhood leukemia overall (Crosignani et al., 2004; Garcia-Perez et al., 2015; Raaschou-Nielsen et al., 2001; Vinceti et al., 2012; Whitworth et al., 2008). Garcia-Perez et al. (2015) observed an association between residence ≤2.5 kilometers of an industrial facility releasing benzene and childhood leukemia in Spain (OR: 1.5, 95% CI: 1.1, 2.1). In a study using the EPA's National-Scale Air Toxics Assessment (NATA) estimates to measure benzene, Whitworth et al.(2008) reported an increased rate of leukemia among census tracts with benzene levels >2.36 μg/m3 compared to <1.28 μg/m3 in their ecologic study (Rate Ratio: 1.4, 95% Confidence Interval [CI]: 1.1, 1.8). Crosignani et al. (2004) reported a relative risk of 3.9 (95% CI: 1.4, 11.3) among those exposed to air concentrations of benzene >10 μg/m3 compared to <0.1 μg/m3. Among children less than 5 years of age, Vinceti et al. (2012) reported a 2.7 times higher income-adjusted odds of leukemia for each 10-fold increase in exposure to benzene when measured continuously in μg/m3 (95% CI: 1.1, 6.9). However, Raaschou-Nielsen et al. (2001) observed no association between benzene and leukemia in their case-control study, with rate ratio estimates near unity. In addition, previous studies suggest a stronger relationship between benzene and AML, with limited evidence of a relationship with acute lymphoid leukemia (ALL) (Heck et al., 2014; Houot et al., 2015; Symanski et al., 2016; Vinceti et al., 2012; Whitworth et al., 2008).
While dispersion models were used in previous studies of benzene and childhood acute leukemia, they primarily estimated benzene and other air pollutants along roadways (Crosignani et al., 2004; Raaschou-Nielsen et al., 2001; Vinceti et al., 2012). Our study aims to improve upon previous exposure assessments by using the NATA estimates, which take multiple sources of benzene exposure into account in addition to traffic, applying estimates to all census tracts. The NATA estimates also incorporated activity and microenvironment data to estimate exposures in addition to ambient concentration of benzene. The goal of this study was to determine if children in Oklahoma with acute leukemia had a higher odds of exposure to benzene than children without acute leukemia.
2. Materials and Methods
2.1 Study Design and Data Sources
We conducted a case-control study to compare children with acute leukemia from the Oklahoma Central Cancer Registry (OCCR) (n=360) with controls identified through birth certificate records matched on week of birth to cases (n=1,440). We linked the OCCR to birth certificates using Registry Plus™ Link Plus software v. 2.0 (CDC, Atlanta, GA), with 72% of leukemia cases linking to a birth certificate record. Details of the study design were published previously in an analysis of traffic-related air pollution and childhood leukemia and will be presented in brief (Janitz et al., 2016). Cases were diagnosed with leukemia prior to age 20 and during the years 1997-2012. Because we were unable to geocode all birth residences, our dataset available for analysis included 307 cases and 1,013 controls. We obtained data on cases related to their cancer diagnosis from OCCR and data on covariates related to birth, including residence, from birth certificates for all children included in the study. We used the 2014 TIGER/Line files (based on the 2010 U.S. Census) in ArcGIS (ESRI ®, Redlands, CA), using the North American Datum of 1983 as the geographic coordinate system, to geocode cases and controls. For those with rural routes or addresses unable to be geocoded in ArcGIS, we used the Melissa Data® service. We were unable to geocode children with Highway Contract (HC) Boxes or Post Office (PO) Boxes as no physical address was available. Institutional Review Board approval was obtained from both the University of Oklahoma Health Sciences Center and the Oklahoma State Department of Health.
We obtained data on benzene from the EPA's 2005 NATA, which estimated the average concentration of air toxics for the US at the census tract level to provide State/Local/Tribal agencies' directions for prioritization and research in order to better understand the health effects of pollution (United States Environmental Protection Agency, 2013). NATA models were based on data from various sources including state and local air toxics inventories, existing databases related to the EPA air toxics regulatory programs, and the TRI. Estimates from mobile sources, including motor vehicles, non-road engines, and equipment, were also incorporated into the models. Activity, fuel, and vehicle data were obtained from local, state, and federal agencies. Emissions estimates were obtained using emission factors of pollutants and sources of emissions included point (i.e., factory, ship, smokestack), and non-point (i.e., area pollution from small or ubiquitous sources such as dry cleaners) stationary sources, on-road (i.e., cars, trucks, buses) and non-road (i.e., airplanes, trains, lawnmowers) mobile sources, derived background from natural sources, and secondary formation and decay of air toxics from the Community Multiscale Air Quality Model from 2005 (ICF International, 2011).
More specifically, the 2005 NATA used the Assessment System for Population Exposure Nationwide (ASPEN) model and the Human Exposure Model-3 (HEM) American Meteorological Society – U.S. EPA Regulatory Model (AERMOD) Version to determine benzene estimates (ICF International, 2011; United States Environmental Protection Agency, 2011). ASPEN was used to model non-point sources at the census tract level using data from 2005 and census data from 2000. HEM was used to model point, on-road mobile, and non-road mobile emissions sources at the census block level along with dispersion and human exposure, using population data from both the 2000 and 2010 US Census.
To determine population exposure to benzene at the census tract level, the EPA used the Hazardous Air Pollutant Exposure Model (HAPEM). The estimates were calculated using ambient concentrations of air toxics, population data from the 2000 U.S. Census, population activity data, and microenvironmental data. Activity data were obtained from the Consolidated Human Activity Database (CHAD), where a sample of the population was surveyed in order to track activity levels in indoor and outdoor microenvironments.
These model-based assessments were conducted approximately every three to six years beginning in 1990. We used the assessment from 2005 as it incorporated the most advanced models available at the time this study was conducted. The EPA does not recommend combining assessments due to differing methodologies (United States Environmental Protection Agency, 2011).
2.2 Statistical Analysis
To evaluate the association between benzene and acute leukemia, we used conditional logistic regression to account for the matching variable of week of birth. We classified benzene into quartiles based on the distribution among controls since the logit was not linear for benzene. In a secondary analysis, we fitted a locally weighted scatterplot smoothing (LOESS) curve of the predicted log odds of a case by benzene resulting in additional exposure cutpoints at the 40th percentile (0.53 μg/m3), the 60th percentile (0.78 μg/m3), and the 95th percentile (1.33 μg/m3).
To evaluate confounding, we used a directed acyclic graph including the variables of race/ethnicity, age at diagnosis, gender, birth order, exposure to electromagnetic fields, urbanization, and maternal variables of education, age, and smoking during pregnancy, which was analyzed using DAGitty (Textor et al., 2011). The minimally sufficient set of confounding variables included urbanization, maternal education, and maternal tobacco use during pregnancy. We used backwards selection to quantitatively evaluate whether covariates changed the odds ratio (OR) >20% after removal from the model. Because tobacco use was only collected from 1991 forward, we evaluated confounding among children born from 1991-2010 and observed <10% change in the OR. Therefore, we analyzed the association between benzene and acute leukemia without tobacco as a confounder using all available data. Furthermore, because it was not clear whether urbanization may be a possible surrogate for socioeconomic status (SES) (Tselios, 2013) or benzene exposure, we evaluated models with and without urbanization as a potential confounder. Using a test for interaction, we evaluated effect modification of the association between benzene and leukemia by urbanization and other covariates.
To evaluate potential biases and limitations of the data, we conducted a series of sensitivity analyses. We explored whether the association between benzene exposure and leukemia differed by leukemia type. In addition, we stratified our analyses by age at diagnosis/index age and compared benzene exposure among cases who did and did not move between birth and cancer diagnosis to evaluate potential residential mobility. We also stratified by year of birth to assess temporal misclassification of benzene. To further evaluate the impact of potential misclassification related to using 2005 NATA data for children born in earlier years, we conducted a sensitivity analysis of children born from 1979 to 1996 and compared the results of our analysis using the 1996 NATA to the 2005 NATA. We also assessed for selection bias due to lack of geocoding for 316 children (n=53 [14.7%] cases and n=263 [18.3%] controls). We used the child's ZIP code (available for all but two children) to determine the census block with the highest population within the ZIP code and assigned benzene based on the appropriate census tract. To evaluate leukemia cases who were born in Oklahoma but did not link to a birth certificate, we compared cancer-related covariates with those of cases who linked using a Chi-Square Test, including type of cancer, year of birth, year of diagnosis, and age at diagnosis.
To identify observations that did not fit the conditional logistic regression model well, we calculated Pearson and Deviance residual measures and observed no poorly fit observations (all residual values <5). We used an alpha of 0.05 to define significance and SAS v. 9.4 (Cary, NC) for all statistical analyses.
3. Results
In Oklahoma, the average exposure concentration of benzene for all census tracts in the state (0.58 μg/m3) (average population size of 1530 people per census tract) was lower than that of the US (0.95 μg/m3). Oklahoma was ranked 39th lowest out of 53 states, territories, and Washington, DC, with Washington, DC (1.88 μg/m3), New York (1.85 μg/m3), and Colorado (1.49 μg/m3) having the highest total exposure concentration levels. Descriptively, several areas in central and northeastern Oklahoma had higher levels of benzene exposure (Figure 1). TRI sites, nine of which were oil refineries, were located in the Tulsa and Oklahoma City metropolitan areas and in both northern and southern Oklahoma (United States Environmental Protection Agency, 2015). While these sites were near the areas of highest benzene exposure, they did not correspond directly to the NATA estimates, especially in southern Oklahoma.
Fig 1.
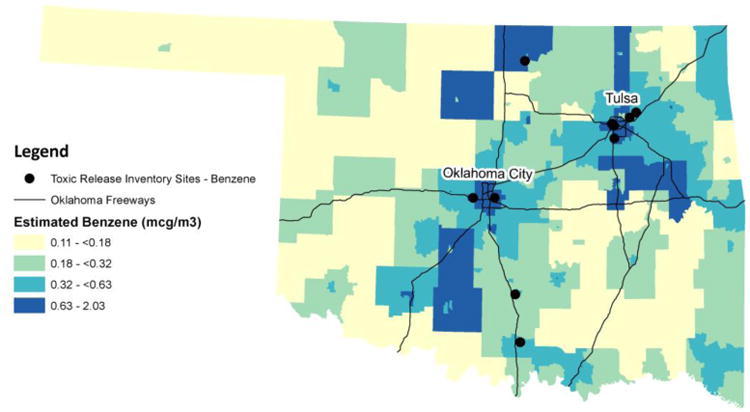
Distribution of benzene in Oklahoma using 2005 NATA estimates and locations of Toxic Release Inventory (TRI) sites. Source: Benzene levels were obtained from the 2005 National-level Air Toxics Assessment from the US EPA. Toxic Release Inventory sites were obtained from the US EPA in 2015. Oklahoma Freeways were obtained from the National Transportation Atlas Databases for the United States published by the Federal Highway Administration.
A higher percentage of cases were male and Hispanic compared to controls, but a lower percentage of cases were African American (Table 1). ALL was the most common type of acute leukemia among cases (74.3%), with precursor cell leukemias being the most common subtype of lymphoid leukemia (97.4%). Approximately half of all cases were diagnosed with leukemia prior to age five years (48.5%).
Table 1.
Distribution of birth characteristics of the child, mother, and residence by case/control status.
| Cases (n=307) | Controls (n=1,013) | p-valuea | |||
|---|---|---|---|---|---|
|
| |||||
| N | % | N | % | ||
| Female (v. Male) | 130 | 42.4 | 518 | 51.1 | 0.02 |
| Race/Ethnicityb | 0.001 | ||||
| Non-Hispanic (NH) White | 207 | 67.4 | 702 | 69.3 | |
| NH African American | 20 | 6.5 | 122 | 12.0 | |
| NH American Indian | 29 | 9.5 | 88 | 8.7 | |
| NH Other | 14 | 4.6 | 22 | 2.2 | |
| Hispanic | 37 | 12.1 | 79 | 7.8 | |
| Maternal Age at Child's Delivery | 0.34 | ||||
| <20 years | 40 | 13.0 | 162 | 16.0 | |
| 20-34 years | 240 | 78.2 | 778 | 76.8 | |
| ≥35 years | 27 | 8.8 | 73 | 7.2 | |
| Parity | 0.05 | ||||
| 0 previous deliveries | 112 | 36.5 | 430 | 42.5 | |
| 1 previous delivery | 91 | 29.6 | 320 | 31.6 | |
| 2 previous deliveries | 67 | 21.8 | 159 | 15.7 | |
| 3+ previous deliveries | 37 | 12.1 | 104 | 10.3 | |
| Tobacco Use During Pregnancy (1991-2010) | 0.87 | ||||
| Yes | 36 | 13.8 | 126 | 14.4 | |
| No | 198 | 75.9 | 689 | 78.7 | |
| Unknown | 27 | 10.3 | 60 | 6.9 | |
| Maternal Education | 0.33 | ||||
| >High School Education | 118 | 38.4 | 406 | 40.1 | |
| Completed High School | 103 | 33.6 | 361 | 35.6 | |
| <High School Education | 81 | 26.4 | 229 | 22.6 | |
| Unknown | 5 | 1.6 | 17 | 1.7 | |
| Urban Census Block at Birth Residence (v. Rural) | 249 | 81.1 | 830 | 81.9 | 0.63 |
| Complete Street Address (v. Rural Route) | 300 | 97.7 | 989 | 97.6 | 0.94 |
p-value based on univariate conditional logistic regression
Unknown Hispanic ethnicity classified as non-Hispanic, NH Other includes Asian, Pacific Islander, other unclassified ethnicity, and unknown ethnicity.
We observed no differences in benzene exposure between cases and controls in the bivariate analysis or after adjusting for the confounding factors of urbanization and maternal education (Table 2). In our evaluation of benzene exposure using cutpoints informed by the LOESS curve, benzene was again not associated with acute leukemia. While adjusting for urbanization and maternal education resulted in an OR further from the null, removal of these variables changed the OR <20%.
Table 2.
Odds ratios (OR) and 95% confidence intervals (CI) for the association between benzene and childhood acute leukemia.
| Cases | Controls | Unadjusted | Adjusted for Maternal Education | Adjusted for Urbanization and Maternal Education | |
|---|---|---|---|---|---|
|
| |||||
| N | N | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Quartiles of exposure | |||||
| 0.11-<0.39 μg/m3 | 73 | 251 | Reference | Reference | Reference |
| 0.39-<0.67 μg/m3 | 71 | 253 | 0.98 (0.68, 1.43) | 0.97 (0.67, 1.41) | 1.06 (0.71, 1.58) |
| 0.67-<0.91 μg/m3 | 77 | 253 | 1.05 (0.72, 1.51) | 1.07 (0.73, 1.55) | 1.21 (0.79, 1.87) |
| 0.91-2.03 μg/m3 | 86 | 256 | 1.17 (0.81, 1.68) | 1.13 (0.78, 1.63) | 1.28 (0.83, 1.97) |
| Benzene divided at 40th, 60th, and 95th percentiles | |||||
| 0.11-<0.53 μg/m3 | 117 | 405 | Reference | Reference | Reference |
| 0.53-<0.78 μg/m3 | 60 | 202 | 1.02 (0.71, 1.45) | 1.00 (0.69, 1.44) | 1.06 (0.72, 1.55) |
| 0.78-<1.33 μg/m3 | 116 | 355 | 1.13 (0.84, 1.53) | 1.13 (0.84, 1.53) | 1.23 (0.87, 1.72) |
| 1.33-2.03 μg/m3 | 14 | 51 | 0.94 (0.50, 1.77) | 0.81 (0.42, 1.56) | 0.88 (0.45, 1.73) |
In the analysis evaluating associations by leukemia type, the estimates for children with AML were stronger than those with ALL, though all of the confidence intervals contained the null value (Table 3). The point estimates suggest a potential dose-response relationship with AML, with the odds of exposure to the 4th quartile of benzene, relative to the lowest quartile, (OR: 2.42, 95% CI: 0.98, 5.96) approaching statistical significance among cases compared to controls after adjustment for urbanization and maternal education. In this model, removal of urbanization changed the OR >20%. Although we observed no significant differences when evaluating benzene and acute leukemia by age at diagnosis/index age, cases diagnosed between birth and 4 years of age had elevated ORs among those exposed to the 4th quartile compared to controls (Table 4). Among cases, 58.3% of children diagnosed between ages 0 and 4 years changed residence between birth and the date of cancer diagnosis, whereas 86.1% diagnosed from ages 15-19 years changed residence. We also observed a lower percentage of high benzene exposure based on quartiles among cases who did not change residence (21.7%) compared to those who moved between birth and cancer diagnosis (26.1%).
Table 3.
Odds ratios (OR) and 95% confidence intervals (CI) for the association between benzene and childhood acute leukemia, stratified by leukemia type.
| Acute Lymphoid Leukemia n=228 | Acute Myeloid Leukemia n=79 | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Unadjusted | Adjusted for Maternal Education | Adjusted for Urbanization and Maternal Education | Unadjusted | Adjusted for Maternal Education | Adjusted for Urbanization and Maternal Education | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Quartiles of benzene exposure | ||||||
| 0.11-<0.39 μg/m3 | Reference | Reference | Reference | Reference | Reference | Reference |
| 0.39-<0.67 μg/m3 | 0.93 (0.61, 1.42) | 0.89 (0.58, 1.37) | 0.91 (0.58, 1.44) | 1.20 (0.54, 2.66) | 1.24 (0.55, 2.79) | 1.60 (0.67, 3.81) |
| 0.67-<0.91 μg/m3 | 1.02 (0.67, 1.56) | 1.04 (0.68, 1.60) | 1.07 (0.66, 1.76) | 1.13 (0.54, 2.38) | 1.26 (0.58, 2.72) | 1.92 (0.77, 4.74) |
| 0.91-2.03 μg/m3 | 1.10 (0.72, 1.67) | 1.03 (0.67, 1.58) | 1.06 (0.65, 1.74) | 1.42 (0.69, 2.94) | 1.60 (0.75, 3.42) | 2.42 (0.98, 5.96) |
| Benzene divided at 40th, 60th, and 95th percentiles | ||||||
| 0.11-<0.53 μg/m3 | Reference | Reference | Reference | Reference | Reference | Reference |
| 0.53-<0.78 μg/m3 | 0.91 (0.61, 1.37) | 0.92 (0.61, 1.39) | 0.93 (0.60, 1.43) | 1.49 (0.69, 3.21) | 1.46 (0.66, 3.22) | 1.76 (0.79, 3.92) |
| 0.78-<1.33 μg/m3 | 1.08 (0.77, 1.53) | 1.07 (0.75, 1.51) | 1.08 (0.73, 1.59) | 1.33 (0.73, 2.40) | 1.50 (0.80, 2.78) | 1.74 (0.88, 3.46) |
| 1.33-2.03 μg/m3 | 0.81 (0.36, 1.84) | 0.66 (0.28, 1.57) | 0.67 (0.28, 1.62) | 1.24 (0.44, 3.48) | 1.34 (0.47, 3.86) | 1.58 (0.53, 4.69) |
Table 4.
Analysis of benzene classified into quartiles at the census tract of the birth residence and acute leukemia by age at leukemia diagnosis/index age for controls. Estimates are presented as odds ratios (OR) and 95% confidence intervals (CI).
| Age at Leukemia Diagnosis/Index Age | All Ages | 0-4 years | 5-9 years | 10-14 years | 15-19 years |
|---|---|---|---|---|---|
| Number of Cases | 307 | 149 | 76 | 37 | 45 |
| Number of Controls | 1013 | 514 | 250 | 110 | 139 |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Unadjusted | |||||
| 0.11-<0.39 μg/m3 | Referent | Referent | Referent | Referent | Referent |
| 0.39-<0.67 μg/m3 | 0.97 (0.67, 1.41) | 0.92 (0.54, 1.58) | 0.99 (0.46, 2.14) | 1.45 (0.54, 3.89) | 0.81 (0.28, 2.33) |
| 0.67-<0.91 μg/m3 | 1.07 (0.73, 1.55) | 1.22 (0.73, 2.05) | 0.69 (0.32, 1.50) | 1.22 (0.39, 3.88) | 1.00 (0.38, 2.62) |
| 0.91-2.03 μg/m3 | 1.13 (0.78, 1.63) | 1.28 (0.76, 2.17) | 1.26 (0.62, 2.56) | 0.79 (0.27, 2.29) | 0.95 (0.36, 2.51) |
| Adjusted for Urbanization and Maternal Education | |||||
| 0.11-<0.39 μg/m3 | Referent | Referent | Referent | Referent | Referent |
| 0.39-<0.67 μg/m3 | 1.06 (0.71, 1.58) | 1.01 (0.56, 1.81) | 1.07 (0.46, 2.44) | 1.66 (0.53, 5.17) | 0.71 (0.23, 2.19) |
| 0.67-<0.91 μg/m3 | 1.21 (0.79, 1.87) | 1.41 (0.77, 2.61) | 0.76 (0.31, 1.88) | 1.90 (0.44, 8.12) | 0.96 (0.33, 2.82) |
| 0.91-2.03 μg/m3 | 1.28 (0.83, 1.97) | 1.45 (0.77, 2.73) | 1.36 (0.59, 3.16) | 1.15 (0.31, 4.24) | 0.83 (0.27, 2.60) |
| Adjusted for Maternal Education | |||||
| 0.11-<0.39 μg/m3 | Referent | Referent | Referent | Referent | Referent |
| 0.39-<0.67 μg/m3 | 0.98 (0.68, 1.43) | 0.92 (0.54, 1.58) | 0.98 (0.45, 2.11) | 1.35 (0.48, 3.75) | 0.71 (0.23, 2.15) |
| 0.67-<0.91 μg/m3 | 1.05 (0.72, 1.51) | 1.25 (0.74, 2.10) | 0.67 (0.31, 1.47) | 1.37 (0.38, 4.95) | 0.96 (0.36, 2.56) |
| 0.91-2.03 μg/m3 | 1.17 (0.81, 1.68) | 1.28 (0.75, 2.17) | 1.21 (0.59, 2.48) | 0.86 (0.28, 2.66) | 0.83 (0.30, 2.33) |
When stratified by year of birth, cases born from 2005-2010 had a three–fold increased unadjusted odds of elevated exposure compared to controls born in this same time period (Table 5). After adjusting for both urbanization and maternal education, the odds of exposure increased for the third and fourth quartiles of benzene, but the confidence intervals were wider compared to the unadjusted estimates. In our sensitivity analysis comparing results of children born from 1979 to 1996 using both the 1996 and 2005 NATA estimates, we observed little change in the results (1996 NATA unadjusted 4th quartile OR: 0.81, 95% CI: 0.43, 1.51; 2005 NATA unadjusted 4th quartile OR: 0.78, 95% CI: 0.41, 1.49). Results were also similar after adjusting for urbanization and maternal education (data not shown).
Table 5.
Analysis of benzene classified into quartiles at the census tract of the birth residence and acute leukemia by year of birth. Estimates are presented as odds ratios (OR) and 95% confidence intervals (CI). Statistically significant estimates are indicated in bold.
| Year of Birth | All Years | 1979-1989 | 1990-1994 | 1995-1999 | 2000-2004 | 2005-2010 |
|---|---|---|---|---|---|---|
| Number of Cases | 307 | 34 | 45 | 67 | 102 | 59 |
| Number of Controls | 1013 | 102 | 142 | 216 | 355 | 198 |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Unadjusted | ||||||
| 0.11-<0.39 μg/m3 | Reference | Reference | Reference | Reference | Reference | Reference |
| 0.39-<0.67 μg/m3 | 0.97 (0.67, 1.41) | 0.70 (0.22, 2.24) | 1.03 (0.38, 2.81) | 0.80 (0.36, 1.76) | 1.16 (0.63, 2.15) | 1.37 (0.48, 3.91) |
| 0.67-<0.91 μg/m3 | 1.07 (0.73, 1.55) | 0.87 (0.29, 2.55) | 0.83 (0.28, 2.45) | 0.74 (0.36, 1.52) | 0.87 (0.45, 1.68) | 3.15 (1.22, 8.13) |
| 0.91-2.03 μg/m3 | 1.13 (0.78, 1.63) | 0.62 (0.20, 1.90) | 1.30 (0.51, 3.29) | 0.75 (0.35, 1.60) | 1.11 (0.59, 2.07) | 3.53 (1.35, 9.27) |
| Adjusted for Urbanization and Maternal Education | ||||||
| 0.11-<0.39 μg/m3 | Reference | Reference | Reference | Reference | Reference | Reference |
| 0.39-<0.67 μg/m3 | 1.06 (0.71, 1.58) | 0.53 (0.16, 1.79) | 1.70 (0.54, 5.28) | 0.80 (0.34, 1.86) | 1.15 (0.59, 2.25) | 2.22 (0.67, 7.39) |
| 0.67-<0.91 μg/m3 | 1.21 (0.79, 1.87) | 0.63 (0.17, 2.32) | 2.16 (0.58, 8.09) | 0.77 (0.34, 1.75) | 0.87 (0.40, 1.88) | 5.70 (1.69, 19.26) |
| 0.91-2.03 μg/m3 | 1.28 (0.83, 1.97) | 0.39 (0.10, 1.56) | 3.17 (0.96, 10.49) | 0.70 (0.30, 1.65) | 1.03 (0.49, 2.17) | 6.73 (1.86, 24.40) |
| Adjusted for Maternal Education | ||||||
| 0.11-<0.39 μg/m3 | Reference | Reference | Reference | Reference | Reference | Reference |
| 0.39-<0.67 μg/m3 | 0.98 (0.68, 1.43) | 0.59 (0.18, 1.91) | 1.12 (0.40, 3.17) | 0.78 (0.35, 1.75) | 1.18 (0.63, 2.19) | 1.40 (0.49, 4.01) |
| 0.67-<0.91 μg/m3 | 1.05 (0.72, 1.51) | 0.80 (0.26, 2.50) | 1.08 (0.34, 3.48) | 0.75 (0.36, 1.57) | 0.90 (0.46, 1.75) | 3.22 (1.24, 8.39) |
| 0.91-2.03 μg/m3 | 1.17 (0.81, 1.68) | 0.50 (0.15, 1.69) | 1.44 (0.54, 3.89) | 0.68 (0.31, 1.50) | 1.07 (0.56, 2.02) | 3.60 (1.35, 9.55) |
We observed no meaningful differences in the results in our sensitivity analysis including all cases (n=360) and all but two controls (n=1,438) compared to our primary analyses excluding those who did not geocode (n=1,320) (data not shown). Furthermore, in our analyses comparing cases who did and did not link to birth records, we observed no differences between the two groups among cancer-related covariates of type of leukemia (p=0.93), year of birth (p=0.18), year of diagnosis (p=0.24), and age at diagnosis (p=0.58).
4. Discussion
Although we observed no significant differences between cases and controls regarding benzene exposure, the ORs among children with AML were increased compared to controls. While our results suggested a potential dose-response relationship between benzene and AML based on the analysis of quartiles, this was not confirmed in our analysis using the cutpoints identified through LOESS curves. Although there were few observations in the higher levels of benzene exposure, we observed elevated ORs for both methods of classification. Previous studies have used varying methods to classify exposure to benzene (Crosignani et al., 2004; Raaschou-Nielsen et al., 2001; Vinceti et al., 2012; Whitworth et al., 2008). Thus, we conducted our analysis using a common method of classifying by quartiles and additionally determined cutpoints based on the LOESS curve.
Consistent with our findings, Whitworth et al. (2008) observed a potential dose-response relationship between benzene and AML in their ecologic study, which was only significantly elevated in the fourth quartile of benzene exposure (>2.36 μg/m3) (AML RR: 2.0, 95% CI: 1.0, 4.0) after adjusting for age, sex, race/ethnicity, and SES. Additionally, Vinceti et al. (2012) observed a stronger association between benzene and AML (OR: 5.5, 95% CI: 1.1, 26.5) compared to ALL (OR: 2.0, 95% CI: 0.6, 6.5) for every 10-fold increase in benzene, but only among children under five years of age after adjustment for coarse particulate matter (PM10). Houot et al. (2015) observed a positive association between benzene and AML among those exposed to benzene concentrations ≥1.3 μg/m3 and ≥309 meters of road within 150 meters of the child's residence (OR: 2.2, 95% CI: 1.1, 4.7) after adjustment for age. Furthermore when restricted to children under one year of age, Heck et al. (2014) observed an elevated association between one interquartile range increase in benzene and AML after adjustment for confounding factors including SES (OR: 2.6, 95% CI: 1.0, 7.0). The authors also observed significantly elevated associations with both AML (Adjusted OR: 1.8, 95% CI: 1.0, 2.9) and ALL (Adjusted OR: 1.5, 95% CI: 1.1, 2.1) during the third trimester of pregnancy (Heck et al., 2014).
In addition, two recent meta-analyses focused on air pollution and childhood leukemia observed an elevated summary OR for children with AML. Filippini et al. (2015) observed a summary OR of 2.3 for benzene and AML (95% CI: 1.1, 4.8), but no association with ALL (OR: 1.1, 95% CI: 0.7, 1.8). However, Carlos-Wallace et al. (2015) observed an elevated summary OR for both AML (OR: 2.1, 95% CI: 1.3, 3.2) and ALL (OR: 1.5, 95% CI: 1.1, 2.1) among studies that evaluated traffic density, traffic-related air pollution, or residential proximity to gas stations, excluding occupational or household product exposures. Filippini et al. (2015) reported summary results for the association between benzene and childhood leukemia whereas Carlos-Wallace et al. (2015) included multiple measures of traffic-related air pollution, which may partially explain the difference in the results for ALL. Furthermore, the meta-analysis by Carlos-Wallace et al. (2015) included studies with self-reported residential proximity to gas stations (Brosselin et al., 2009; Steffen et al., 2004), which may have biased the summary OR (Heinrich et al., 2005; Kuehni et al., 2006; Piro et al., 2008).
After adjustment for urbanization, the estimates for benzene and acute leukemia were larger than the unadjusted estimates. While there was little change in the OR after adjustment for urbanization in our sensitivity analysis with the entire dataset, we continued to observe an increased OR when restricting to children with AML. This increase was in contrast to other studies adjusting for urbanization as a confounder, which either observed no difference from the unadjusted model or a reduced OR after adjustment (Raaschou-Nielsen et al., 2001; Weng et al., 2009). We expected adjustment for urbanization to reduce the OR since it was positively associated with benzene and the levels of benzene were higher in urban than rural census tracts. However, an alternative explanation may be that urbanization functioned as a surrogate for traffic, indicating a stronger association among benzene sources other than traffic.
Because all of the cases and controls were not geocoded, we were concerned with potential selection bias in our study, with controls being less likely to geocode than cases (Janitz et al., 2016). Results from our sensitivity analysis including nearly all children available for the study (n=1,798) were similar to the results for the geocoded dataset (n=1,320), demonstrating that selection bias is unlikely to explain our results. However, we may have overestimated exposure to benzene by assigning the child to the census block with the highest population when they may have actually resided in a more rural census block. Depending on whether misclassification was differential, this may result in either an over or underestimate of the true OR.
In our study, we measured exposure at birth as a surrogate for maternal exposure during pregnancy. This assumption is only appropriate if the mother did not change residence during pregnancy and may not represent exposure during childhood. However, it is unclear when the relevant window of exposure occurs for childhood cancer. Chromosomal changes associated with childhood leukemia have been observed in blood spots collected at birth, indicating that at least one of the two or more genetic ‘hits,’ as described by Knudson (2001) occurred in utero (Wiemels et al., 1999; Wiemels et al., 2002). According to Smith (2010), environmental exposures, including benzene, could have occurred before birth and may be a result of parental exposure. While an estimated 13% to 30% of mothers change residence during pregnancy and up to two-thirds of children change residence between birth and cancer diagnosis (Chen et al., 2010; Lupo et al., 2010; Reynolds et al., 2001; Urayama et al., 2009), several studies have indicated minimal changes in exposure status, with kappa values of 0.69 to 0.99, when comparing exposure to ambient air pollution between the residence of the mother during the prenatal period and that listed on the birth certificate from case-control studies on congenital anomalies (Chen et al., 2010; Lupo et al., 2010). To estimate the potential impact of residential mobility in our study, we stratified by age at diagnosis/index age for controls. Younger children may have reduced residential mobility compared to older children, which we observed among cases and is supported by a stronger, though non-significant, association among children under 5 years of age. Although we were able to compare benzene exposure among cases who did and did not change residence between birth and cancer diagnosis, we had no information on controls with which to conduct a sensitivity analysis using the residence at diagnosis/index age. Studies with detailed data on residential mobility patterns are needed to explore the relevant windows of exposure and whether this occurs around the time of conception, in utero, or after birth.
Measurement of benzene biomarkers can be measured through breath, urine, and serum. While biomarkers are often measured in occupational studies, measurement is limited to recent exposures (Department of Health and Human Services, 2007). Although the NATA estimates were not a direct individual measure of benzene, they had several advantages over using monitor or TRI data, including adjustments for dispersion of the pollutants, meteorological variables, activity data and areas/sources of exposure. Collecting this type of information on individuals would not be feasible in a population-based study. In addition, NATA estimates incorporated many sources of benzene exposure, in addition to traffic. Because of the many factors that must be incorporated to accurately measure benzene exposure of the population, a comprehensive model, such as NATA, provided an improvement over models only estimating traffic-related benzene. However, NATA applied the same benzene levels to an entire census tract which may not reflect the actual exposure of all those residing in that census tract. Although the NATA estimates account for commuting between census tracts, the activity patterns of individual participants may vary. Using ecologic exposure data could result in misclassification, potentially biasing the results away or towards the null depending on the person's actual exposure.
By using a single year of benzene estimates, there was a risk for temporal misclassification. Births in this study ranged from 1979 to 2010 and benzene estimates from 2005 may not represent the exposure of those born in 1979. Ambient benzene levels have decreased approximately 56% from 1994-2002 in the US (Fortin et al., 2005). However, when comparing children born from 1979 to 1996 using both the 1996 and 2005 NATA estimates, we observed no meaningful difference in results. Among children born between 2005 and 2010, cases had a significantly higher odds of exposure to benzene than controls. While we expected a stronger association with benzene in children born in more distant years, children born during 2005-2010 may have less misclassification of exposure compared to children born prior to 2005. This suggests the potential association between benzene and leukemia may have been attenuated in our study by bias resulting from exposure misclassification, regardless of the NATA estimates used. However, it is unclear why there was no association among those born from 2000-2004.
Using birth certificate data as our source of covariates is a limitation of this study. While birth certificates may be a reliable source for some variables, the reliability for maternal tobacco use during pregnancy is reported to be poor and vary by education status (Vinikoor et al., 2010). However, Oklahoma updated the birth certificate in 2009 which improved measurement of smoking status. Prior to the update, smoking was classified as present or absent any time during pregnancy, whereas after the update smoking was assessed before pregnancy and by trimester. Although this improvement will be beneficial to future studies using birth certificate data, self-report of smoking status during pregnancy remains problematic due to the stigma of smoking during pregnancy.
5. Conclusions
Based on the reviewed literature, benzene has the strongest evidence of a relationship with acute leukemia compared to other components of motor vehicle exhaust. However, studies have not established whether one specific pollutant or a combination of pollutants is responsible for the association between air pollution and leukemia. Therefore, consideration of other markers of traffic, such as NO2 and road density, are important in understanding this relationship. In addition, improved measurement of benzene exposure at the individual level through adjustment for exposure misclassification from validation studies is necessary in future studies (Raaschou-Nielsen et al., 2001). While we did not observe statistically significant associations between benzene and leukemia, it is important to continue evaluating the effects in areas with higher exposure concentrations along with other potential health effects of benzene exposure.
Highlights.
Benzene is a suspected, but uncertain, risk factor for childhood acute leukemia.
Enhanced benzene estimates account for activity and multiple sources of exposure.
Potential dose-response relation revealed for benzene and acute myeloid leukemia.
Acknowledgments
We thank Dr. Derek Pate and Ryan Webb of the Oklahoma State Department of Health for their support in providing data for this study.
Funding Sources: This project was supported in part by Grant Number UB6HP27874 from the U.S. Department of Health and Human Services, Health Resources and Services Administration, Affordable Care Act (ACA) Public Health Training Centers Program and by Grant Number 1 U54GM104938 from the National Institutes of Health, National Institute of General Medical Sciences, an IDeA-CTR to the University of Oklahoma Health Sciences Center. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the Health Resources and Services Administration or of the U.S. Department of Health and Human Services.
Abbreviations
- ALL
Acute lymphoid leukemia
- AML
Acute myeloid leukemia
- PM10
Coarse particulate matter
- CI
Confidence Interval
- EPA
Environmental Protection Agency
- HC
Highway Contract Boxes
- IARC
International Agency for Research on Cancer
- LOESS
Locally weighted scatterplot smoothing
- NATA
National-Scale Air Toxics Assessment
- OR
Odds ratio
- OCCR
Oklahoma Central Cancer Registry
- PO
Post Office Boxes
- SES
Socioeconomic status
- TRI
Toxics Release Inventory
Footnotes
The authors declare no competing financial interests.
Ethics: We obtained IRB approval for this research from the University of Oklahoma Health Sciences Center and the Oklahoma State Department of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Belson M, et al. Risk factors for acute leukemia in children: a review. Environmental Health Perspectives. 2007;115:138–45. doi: 10.1289/ehp.9023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbrahim-Tallaa L, et al. Carcinogenicity of diesel-engine and gasoline-engine exhausts and some nitroarenes. Lancet Oncol. 2012;13:663–4. doi: 10.1016/s1470-2045(12)70280-2. [DOI] [PubMed] [Google Scholar]
- Brosselin P, et al. Acute childhood leukaemia and residence next to petrol stations and automotive repair garages: the ESCALE study (SFCE) Occup Environ Med. 2009;66:598–606. doi: 10.1136/oem.2008.042432. [DOI] [PubMed] [Google Scholar]
- Carlos-Wallace FM, et al. Parental, In Utero, and Early-Life Exposure to Benzene and the Risk of Childhood Leukemia: A Meta-Analysis. American Journal of Epidemiology. 2015 doi: 10.1093/aje/kwv120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, et al. Residential mobility during pregnancy and the potential for ambient air pollution exposure misclassification. Environmental Research. 2010;110:162–8. doi: 10.1016/j.envres.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Crosignani P, et al. Childhood leukemia and road traffic: A population-based case-control study. International Journal of Cancer. 2004;108:596–9. doi: 10.1002/ijc.11597. [DOI] [PubMed] [Google Scholar]
- Department of Health and Human Services, P. H. S Agency for Toxic Substances and Disease Registry. Toxicological Profile for Benzene. Agency for Toxic Substances and Disease Registry; Atlanta, GA: 2007. [PubMed] [Google Scholar]
- Esswein EJ, et al. Evaluation of some potential chemical exposure risks during flowback operations in unconventional oil and gas extraction: preliminary results. J Occup Environ Hyg. 2014;11:D174–84. doi: 10.1080/15459624.2014.933960. [DOI] [PubMed] [Google Scholar]
- Filippini T, et al. A review and meta-analysis of outdoor air pollution and risk of childhood leukemia. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2015;33:36–66. doi: 10.1080/10590501.2015.1002999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin TJ, et al. Temporal changes in U.S. benzene emissions inferred from atmospheric measurements. Environmental Science and Technology. 2005;39:1403–8. doi: 10.1021/es049316n. [DOI] [PubMed] [Google Scholar]
- Garcia-Perez J, et al. Childhood leukemia and residential proximity to industrial and urban sites. Environ Res. 2015;140:542–53. doi: 10.1016/j.envres.2015.05.014. [DOI] [PubMed] [Google Scholar]
- Heck JE, et al. Risk of leukemia in relation to exposure to ambient air toxics in pregnancy and early childhood. International Journal of Hygiene & Environmental Health. 2014;217:662–8. doi: 10.1016/j.ijheh.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich J, et al. Exposure to traffic related air pollutants: self reported traffic intensity versus GIS modelled exposure. Occup Environ Med. 2005;62:517–23. doi: 10.1136/oem.2004.016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron M. Deaths: Leading Causes for 2010. National Vital Statistics Reports. 2013;62 [PubMed] [Google Scholar]
- Houot J, et al. Residential Proximity to Heavy-Traffic Roads, Benzene Exposure, and Childhood Leukemia—The GEOCAP Study, 2002–2007. American Journal of Epidemiology. 2015;182:685–693. doi: 10.1093/aje/kwv111. [DOI] [PubMed] [Google Scholar]
- ICF International. An Overview of Methods for EPA's National-Scale Air Toxics Assessment. Durham, NC: 2011. [Google Scholar]
- International Agency for Research on Cancer. IARC Monographs. 4. 1 to 29. Lyon, France: 1982. IARC monographs on the evaluation of the carcinogenic risks to humans: Chemicals, industrial processes and industries associated with cancer in humans. [Google Scholar]
- International Agency for Research on Cancer. Air Pollution and Cancer. In: Straif K, et al., editors. IARC Scientific Publication No 161. Lyon, France: 2013. [Google Scholar]
- Janitz AE, et al. Traffic-related air pollution and childhood acute leukemia in Oklahoma. Environmental Research. 2016;148:102–111. doi: 10.1016/j.envres.2016.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalade A, et al. Exposure to benzene at work and the risk of leukemia: a systematic review and meta-analysis. Environmental Health. 2010;9:31. doi: 10.1186/1476-069X-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson AG. Two genetic hits (more or less) to cancer. Nature Reviews Cancer. 2001;1:157–62. doi: 10.1038/35101031. [DOI] [PubMed] [Google Scholar]
- Kuehni CE, et al. Association between reported exposure to road traffic and respiratory symptoms in children: evidence of bias. Int J Epidemiol. 2006;35:779–86. doi: 10.1093/ije/dyl022. [DOI] [PubMed] [Google Scholar]
- Lupo PJ, et al. Differences in exposure assignment between conception and delivery: the impact of maternal mobility. Paediatric and Perinatal Epidemiology. 2010;24:200–8. doi: 10.1111/j.1365-3016.2010.01096.x. [DOI] [PubMed] [Google Scholar]
- McHale CM, et al. Current understanding of the mechanism of benzene-induced leukemia in humans: implications for risk assessment. Carcinogenesis. 2012;33:240–52. doi: 10.1093/carcin/bgr297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie LM, et al. Human health risk assessment of air emissions from development of unconventional natural gas resources. Science of The Total Environment. 2012;424:79–87. doi: 10.1016/j.scitotenv.2012.02.018. [DOI] [PubMed] [Google Scholar]
- Pedersen-Bjergaard J, et al. Genetics of therapy-related myelodysplasia and acute myeloid leukemia. Leukemia. 2008;22:240–8. doi: 10.1038/sj.leu.2405078. [DOI] [PubMed] [Google Scholar]
- Piro FN, et al. A comparison of self reported air pollution problems and GIS-modeled levels of air pollution in people with and without chronic diseases. Environ Health. 2008;7:9. doi: 10.1186/1476-069X-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaschou-Nielsen O, et al. Air pollution from traffic at the residence of children with cancer. American Journal of Epidemiology. 2001;153:433–43. doi: 10.1093/aje/153.5.433. [DOI] [PubMed] [Google Scholar]
- Reynolds P, et al. A case-control pilot study of traffic exposures and early childhood leukemia using a geographic information system. Bioelectromagnetics. 2001;(5):S58–68. doi: 10.1002/1521-186x(2001)22:5+<::aid-bem1024>3.3.co;2-0. [DOI] [PubMed] [Google Scholar]
- Ries L, et al. National Cancer Institute. SEER Program; Bethesda, MD: 1999. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975-1995. [Google Scholar]
- Rushton L, Romaniuk H. A case-control study to investigate the risk of leukaemia associated with exposure to benzene in petroleum marketing and distribution workers in the United Kingdom. Occupational and Environmental Medicine. 1997;54:152–66. doi: 10.1136/oem.54.3.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MT. Advances in understanding benzene health effects and susceptibility. Annual Review of Public Health. 2010;31:133–48. doi: 10.1146/annurev.publhealth.012809.103646. 2 p following 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen C, et al. Acute childhood leukaemia and environmental exposure to potential sources of benzene and other hydrocarbons; a case-control study. Occup Environ Med. 2004;61:773–8. doi: 10.1136/oem.2003.010868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symanski E, et al. Air toxics and early childhood acute lymphocytic leukemia in Texas, a population based case control study. Environ Health. 2016;15:70. doi: 10.1186/s12940-016-0154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Textor J, et al. DAGitty: A Graphical Tool for Analyzing Causal Diagrams. Epidemiology. 2011;22:745. doi: 10.1097/EDE.0b013e318225c2be. [DOI] [PubMed] [Google Scholar]
- Tselios V. Urbanization and Socioeconomic Status in the European Regions: The Role of Population Ageing and Capital City Regions. European Planning Studies. 2013;22:1879–1901. [Google Scholar]
- U.S Energy Information Administration. Which States Consume and Produce the Most Natural Gas? 2016 [Google Scholar]
- United States Census Bureau. 2010 Census: Urban and Rural Universe: Total population 2010 Census Summary File 1 (Table P2) 2017a [Google Scholar]
- United States Census Bureau. QuickFacts. Oklahoma: 2017b. [Google Scholar]
- United States Environmental Protection Agency. Technology Transfer Network Air 2005. National-Scale Air Toxics Assessment: Assessment Methods. 2011 [Google Scholar]
- United States Environmental Protection Agency. National Air Toxics Assessments. 2013 [Google Scholar]
- United States Environmental Protection Agency. TRI Basic Data Files: Calendar Years 1987-2015. 2015 [Google Scholar]
- Urayama KY, et al. Factors associated with residential mobility in children with leukemia: implications for assigning exposures. Annals of Epidemiology. 2009;19:834–40. doi: 10.1016/j.annepidem.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinceti M, et al. Leukemia risk in children exposed to benzene and PM(10) from vehicular traffic: a case-control study in an Italian population. European Journal of Epidemiology. 2012;27:781–90. doi: 10.1007/s10654-012-9727-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinikoor LC, et al. Reliability of variables on the North Carolina birth certificate: a comparison with directly queried values from a cohort study. Paediatric and Perinatal Epidemiology. 2010;24:102–12. doi: 10.1111/j.1365-3016.2009.01087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng HH, et al. Childhood leukemia and traffic air pollution in Taiwan: petrol station density as an indicator. Journal of Toxicology and Environmental Health, Part A. 2009;72:83–7. doi: 10.1080/15287390802477338. [DOI] [PubMed] [Google Scholar]
- Whitworth KW, et al. Childhood lymphohematopoietic cancer incidence and hazardous air pollutants in southeast Texas, 1995-2004. Environmental Health Perspectives. 2008;116:1576–80. doi: 10.1289/ehp.11593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemels JL, et al. Prenatal origin of acute lymphoblastic leukaemia in children [see comment] Lancet. 1999;354:1499–503. doi: 10.1016/s0140-6736(99)09403-9. [DOI] [PubMed] [Google Scholar]
- Wiemels JL, et al. In utero origin of t(8;21) AML1-ETO translocations in childhood acute myeloid leukemia. Blood. 2002;99:3801–5. doi: 10.1182/blood.v99.10.3801. [DOI] [PubMed] [Google Scholar]


