Abstract
Cholesterol metabolism is vital for brain function. Previous work in cultured cells has shown that a number of psychotropic drugs inhibit the activity of 7-dehydrocholesterol reductase (DHCR7), an enzyme that catalyzes the final steps in cholesterol biosynthesis. This leads to the accumulation of 7-dehydrocholesterol (7DHC), a molecule that gives rise to oxysterols, vitamin D, and atypical neurosteroids. We examined levels of cholesterol and the cholesterol precursors desmosterol, lanosterol, 7DHC and its isomer 8-dehydrocholesterol (8DHC), in blood samples of 123 psychiatric patients on various antipsychotic and antidepressant drugs, and 85 healthy controls, to see if the observations in cell lines hold true for patients as well. Three drugs, aripiprazole, haloperidol and trazodone increased circulating 7DHC and 8DHC levels, while five other drugs, clozapine, escitalopram/citalopram, lamotrigine, olanzapine, and risperidone, did not. Studies in rat brain verified that haloperidol dose-dependently increased 7DHC and 8DHC levels, while clozapine had no effect. We conclude that further studies should investigate the role of 7DHC and 8DHC metabolites, such as oxysterols, vitamin D, and atypical neurosteroids, in the deleterious and therapeutic effects of psychotropic drugs. Finally, we recommend that drugs that increase 7DHC levels should not be prescribed during pregnancy, as children born with DHCR7 deficiency have multiple congenital malformations.
Keywords: cholesterol, haloperidol, clozapine, aripiprazole, trazodone, neurosteroids
Introduction
Cholesterol is an essential component of cellular membranes (Maxfield and Tabas, 2005). As much as 25% of cholesterol and cholesterol derivatives are contained in the human brain, even though the brain accounts for only 2% of total body weight (Dietschy and Turley, 2001, 2004). Although some of the body’s cholesterol is derived from nutritional sources, the brain depends predominantly on intrinsic de novo cholesterol biosynthesis as the blood-brain barrier limits the uptake of cholesterol from the circulation (Korade et al., 2009; Nicholas and Thomas, 1961).
Mitochondria play an important role in many aspects of cholesterol metabolism (Tatsuta et al., 2014). Endogenous cholesterol synthesis is essential for brain development, and intact cholesterol metabolism remains critical throughout life for normal brain function. In the elderly, high cholesterol is associated with better memory function, while low cholesterol is associated with an increased risk of depression (Huang and Chen, 2005; You et al., 2013).
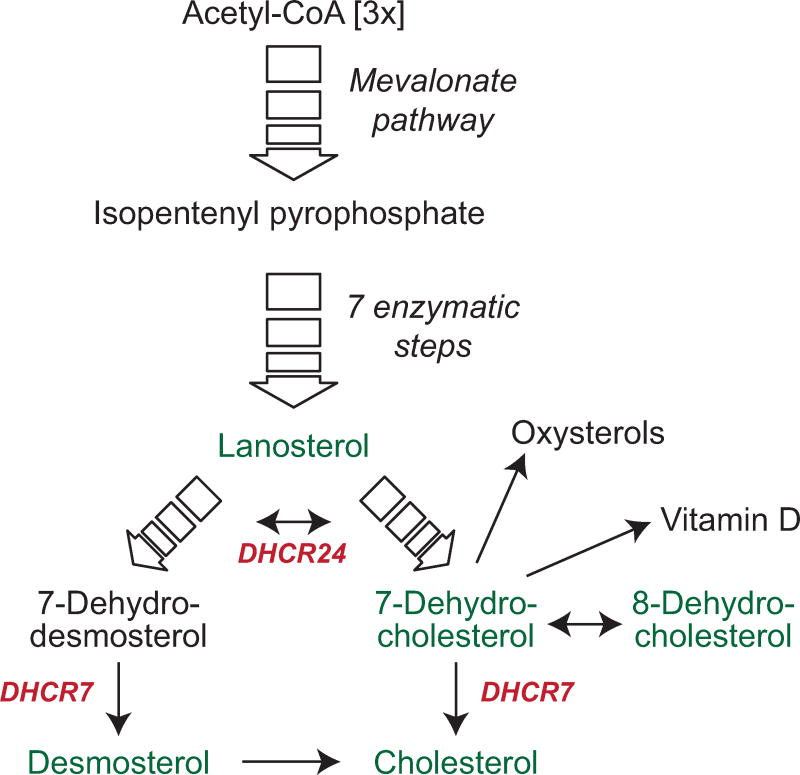
Acetyl-CoA, a central player in energy metabolism, is the seed molecule for cholesterol biosynthesis, which in over 20 enzymatic steps leads to cholesterol (fig. 1). Though the pathway branches at lanosterol, 7-dehydrocholesterol reductase (DHCR7) is crucial in both paths to complete the synthesis of cholesterol. In one branch DHCR7 catalyzes the conversion of 7-dehydrocholesterol (7DHC) into cholesterol, and in the other branch the conversion of 7-dehydrodesmosterol into desmosterol.
Figure 1. Synthesis of cholesterol, 7DHC and vitamin D.
Cholesterol synthesis commences with 3 molecules of acetyl-CoA, which in the mevalonate pathway are converted to isopentenyl pyrophosphate (IPP). IPP gives rise to lanosterol in a series of enzymatic reactions. Two separate enzymatic pathways generate cholesterol from lanosterol. Metabolites from both pathways are interconvertible via 24-dehydrocholesterol reductase (Dhcr24). For detailed metabolic pathway information see (Sharpe and Brown, 2013). Metabolites measured in the current study are shown in green, important enzymes are shown in red.
A number of psychotropic drugs have been shown to increase 7DHC levels in cell lines (Korade et al., 2016). A preliminary study suggested that patients on aripiprazole or trazodone might have elevated blood levels of 7DHC, indicating DHCR7 inhibition (Hall et al., 2013). However, a comparison and contrast of the effects of a larger group of psychotropic drugs on cholesterol metabolism, levels of 7DHC, and its isomer 8-dehydrocholesterol (8DHC), in patients’ blood has not been carried out yet.
Much of the current knowledge about DHCR7 comes from research of Smith-Lemli-Opitz syndrome (SLOS), caused by mutations in the DHCR7 gene. SLOS is characterized by altered CNS structure and function, manifested in developmental disabilities and autism (Bukelis et al., 2007; Nowaczyk and Irons, 2012). While cholesterol deficiency likely plays a central role in the disease, so might be the accumulation of 7DHC and 8DHC, the ensuing lipid peroxidation, and the formation of oxysterols (Liu et al., 2013; Xu et al., 2009). SLOS studies point to an important function of DHCR7 and cholesterol particularly during early brain development. Curiously, the disproportionate frequency of a small number of null alleles in SLOS has raised the possibility that decreased DHCR7 activity and increased levels of 7DHC confer an evolutionary advantage in heterozygous individuals, despite the devastating effects of homozygous mutations (Witsch-Baumgartner et al., 2000).
A competitive advantage of increased 7DHC levels could rest in its role as the sole source for endogenously synthesized vitamin D3 (cholecalciferol). Since polymorphisms in DHCR7 are associated with vitamin D levels, it has been suggested that higher concentrations of 7DHC might be protective against hypovitaminosis (Wang et al., 2010). Vitamin D deficiency is prevalent in Western populations and has been associated with memory function, depression, and psychosis (Anglin et al., 2013; Crews et al., 2013; Holick, 2009; McGrath et al., 2010). The presence of the vitamin D receptor in the brain indicates a role for vitamin D in brain function (Eyles et al., 2005). Moreover, the biologically active form of vitamin D, a metabolite of 7DHC and ligand for the vitamin D receptor, 1,25-dihydroxyvitamin D (dhVitD), mediates a diverse array of neuroprotective functions (Garcion et al., 2002).
Mitochondrial enzymes cleave 7DHC into novel, neurosteroid-like compounds (Acimovic et al., 2016; Marcos et al., 2004). Although neurosteroids were initially described as nuclear receptors that activate specific genetic programs, it is now accepted that they can also modulate neuronal excitability by rapid, non-genomic actions (Compagnone and Mellon, 2000; Omura, 2006; Slominski et al., 2015). Neurosteroids can have mood-stabilizing, anxiolytic, anticonvulsive and antidepressant effects, through modification of GABA and glutamate systems in the brain (Dubrovsky, 2005; Reddy et al., 2004; Zorumski et al., 2013). However, dependent on type, concentration and brain area in which they accumulate, they can also have the opposite effect (Dubrovsky, 2005). Thus, the effect of neurosteroids synthesized from elevated 7DHC on brain function is currently unknown.
Accumulation of 7DHC can also have negative consequences. As the clinical manifestations of SLOS demonstrate, disruption of DHCR7 activity, particularly during early development, has deleterious effects on brain function. While its role as a precursor for cholesterol, vitamin D and neurosteroids might be beneficial for brain function, 7DHC and 8DHC are a source of lipid peroxidation and oxysterols (Liu et al., 2013; Xu et al., 2009). Of relevance to treatment with psychotropic drugs, oxysterols have been associated with metabolic syndrome, a side effect of treatment with psychoactive drugs manifested in cardiovascular disease and obesity (Guillemot-Legris et al., 2016). Moreover, cellular stress caused by lipid peroxidation might contribute to the extrapyramidal side effects in the brain caused by treatment.
At this point, we are in need of more information about psychotropic drug effects on cholesterol metabolism and accumulation of 7DHC in patients. Understanding the individual profiles of psychotropic drugs is the first step toward determining if protection against lipid peroxidation and oxysterols in patients on these drugs can mitigate side effects, on one hand, and if an increase in vitamin D levels or formation of particular neurosteroids might contribute to the therapeutic profile, on the other. Furthermore, as the experience with SLOS shows, we need to identify which drugs inhibit DHCR7 activity and limit their prescription during pregnancy.
Materials and Methods
Materials
Unless otherwise noted, all chemicals were purchased from Sigma-Aldrich Co (St. Louis, MO). HPLC grade solvents were purchased from Thermo Fisher Scientific Inc (Waltham, MA). [25,26,26,26,27,27,27- d7] 7-DHC and 8-DHC were obtained by chemical synthesis as previously described (Anastasia et al., 1981; Xu et al., 2011b).
Study Participants
One hundred twenty-three patients with a psychiatric disorder (21 with major depressive disorder, MDD; 22 with bipolar disorder, BPD; and 80 with schizophrenia, SZ, schizoaffective disorder, SA, or schizophreniform disorder, grouped together into SZ+) were recruited through the inpatient unit and outpatient clinic at the Vanderbilt Psychiatric Hospital, and 85 healthy controls were recruited via advertisements within the community (table 1). The study was approved by the Vanderbilt University Institutional Review Board and all study subjects provided written informed consent. All participants were administered the Structured Clinical Interview for the DSM-IV-TR (SCID), which was reviewed by an experienced psychiatrist. Participants with any significant medical or neurological disease, head injury or a history of drug dependence, were excluded. The Vanderbilt Psychiatric Hospital has a detailed protocol to assess and verify diagnoses of study participants, and to capture clinical, psychological and demographic data (Sheffield et al., 2013; Woodward and Heckers, 2015).
Table 1. Demographics of study participants and their exposure to psychotropic drugs.
“Race” was self-declared. A number of study participants were either of other race (e.g. Asian, Pacific Islander) or declined to answer.
| Prescription | Total (n) | Sole psychoactive drug prescription (n) | Female (n, [%]) | Male (n, [%]) | White (n) | Black or African American (n) | Other/declined comment | Cholesterol μg/μl blood (mean ± STDEV) | MDD (n) | BPD (n) | SZ, SA, Schizophreni-form, (n) | Age (mean ± STDEV) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aripiprazole | 23 | 8 | 12 [52%] | 11 [48%] | 14 | 5 | 4 | 0.87 ± 0.16 | - | 5 | 18 | 32.8 ± 13.4 |
| Clozapine | 13 | 3 | 3 [23%] | 10 [77%] | 10 | 1 | 2 | 0.91 ± 0.14 | - | - | 13 | 38.1 ± 14.6 |
| Es/Citalopram | 15 | 10 | 11 [73%] | 4 [27%] | 13 | 2 | - | 0.87 ± 0.14 | 13 | - | 2 | 40.6 ± 9.1 |
| Haloperidol | 8 | 3 | 3 [38%] | 5 [63%] | 5 | 3 | - | 0.95 ± 0.20 | - | 1 | 7 | 32.5 ± 8.7 |
| Lamotrigine | 4 | - | 1 [25%] | 3 [75%] | 4 | - | - | 0.93 ± 0.16 | 1 | 2 | 1 | 27.5 ± 9.3 |
| Olanzapine | 13 | 13 | 3 [23%] | 10 [77%] | 9 | 4 | - | 0.91 ± 0.17 | - | 3 | 10 | 27.5 ± 12.3 |
| Risperidone | 28 | 15 | 6 [21%] | 22 [79%] | 17 | 7 | 4 | 0.94 ± 0.12 | 1 | 8 | 19 | 28.9 ± 12.8 |
| Trazodone | 14 | - | 7 [50%] | 7 [50%] | 12 | 2 | - | 0.87 ± 0.15 | 2 | 3 | 9 | 46.7 ± 10.2 |
| Patients not on medication | 5 | - | 2 [40%] | 3 [60%] | 4 | - | 1 | 0.97 ± 0.30 | 4 | - | 1 | 27.8 ± 8.3 |
| Control Participants | 85 | - | 30 [35%] | 55 [65%] | 60 | 20 | 5 | 0.97 ± 0.16 | - | - | - | 32.5 ± 12.7 |
|
| ||||||||||||
| Total | 208 | 52 | 78 [38%] | 130 [62%] | 148 | 44 | 16 | 21 | 22 | 80 | ||
Medication History
The medication history was based on self-report, and included current and prior medication. When available, the research group at the Vanderbilt Psychiatric Hospital cross-referenced the supplied information with current medical records. Despite best efforts, medication nonadherence, a well-known challenge in psychotic disorders, might have occurred in some instances (Chapman and Horne, 2013).
Collection of blood samples
A blood sample was drawn into PaxGene Blood RNA tubes (BD Biosciences, San Joes, CA), which contain 6.9 ml of RNA stabilization additive to which a maximum of 2.5 ml of blood can be added. Therefore, all analyses were corrected for a dilution factor of 3.8. Since the amount of blood collected per tube varied, we normalized sterol metabolites to free cholesterol levels measured in the same samples. Tubes were frozen and stored at −20°C.
A number of controls were carried out to ensure accuracy of measurements. First, a potential interference of the RNA stabilization additive with the measurements of sterol levels was examined. No effect on 7DHC measurements were observed, but desmosterol and lanosterol levels were reduced by approximately 50%. Second, storage effects were tested in 117 sample aliquots measured in 2014 and 2016, and no significant differences were observed (supplemental figure 1). Both measurements for each sample were averaged in the final analysis.
Animals
Twenty-four male Sprague-Dawley rats (Taconic Farms, Germantown, NY) weighing an average of 230 g before the first injection were housed three to a cage on a 12 hr light/dark cycle. The animals were allowed five days of habituation to the colony prior to the first drug administration. All rats received single, daily injections, of the respective drug or vehicle, administered intraperitoneally. Rats gained weight at the same rate, with an average weight of 350 g at the end of the experiment. Animal care and experimental procedures conformed to PHS Policy on Humane Care and Use of Laboratory Animals.
Drug treatment and tissue harvest of rats
Animals were assigned randomly to the following groups: controls (vehicle-treated; n=6), clozapine (8 mg/kg/day; n=6), haloperidol 0.2 (0.2 mg/kg/day; n=6) and haloperidol 0.05 (0.05 mg/kg/day; n=6). Haloperidol and clozapine were purchased from Sigma (St. Louis, MO) and Tocris Cookson, Inc. (Ellisville, MO), respectively, and dissolved in 0.2% lactic acid. The injections were administered i.p. once per day for twenty-four consecutive days. Vehicle injections consisted of 0.2% lactic acid. Animals were sacrificed by rapid decapitation twenty-four hours after the final injection and brains were immediately frozen. Tissue samples from prefrontal cortex and striatum were dissected from frozen brains on a freezing microtome and stored again at −80°C. Prefrontal cortex was defined as the cortical area rostral to bregma 2.2 mm, which included prelimbic, infralimbic, and cingulate cortex area 1 (Paxinos and Watson, 1998; Uylings et al., 2003). Striatum was obtained from a 3 mm round punch between bregma 1.20 mm to 0.20 mm (Paxinos and Watson, 1998).
Processing of tissue samples
Frozen tissue was homogenized in lysis buffer (125 mM NaCl, 50 mM HEPES, 1% Igepal) in the presence of 10 μg/ml butylated hydroxytoluene (BHT) and 25 μg/ml triphenylphosphine (TPP) using ultrasonic homogenization. After protein measurement using the Bio-Rad DC Protein Assay kit (Bradford, 1976), 50 μg protein were added to 800μl of Folch solution for lipid extraction.
Lipid extraction
Ten μl of human blood or 50 μg protein from rat samples were added to 800 μl of Folch solution containing 0.25 mg/ml TPP, 0.005% BHT, and the internal standards d7-7-DHC (13 ng), d7-8-DHC (30 ng), 13C3-Des (100 ng), 13C3-Lano (100 ng), d7-Chol (34 ng), followed by the addition of 400 μl of 0.9% NaCl. The resulting mixture was vortexed and centrifuged. The lower organic phase was recovered and dried under a stream of nitrogen. 200 μl of 1 mg/ml freshly prepared PTAD solution in MeOH was added to the residues of blood extracts, the solutions incubated for 30 minutes at room temperature with occasional shaking, and transferred into sample vials. The samples were stored at −80°C until analysis by LC-MS/MS.
LC-MS/MS conditions
LC separations were performed on a Waters Acquity UPLC system equipped with an autosampler (Waters, Milford, MA) using a Waters Acquity UPLC BEH C18 column (1.7 μm, 2.1 × 50 mm). A TSQ Quantum Ultra tandem mass spectrometer (ThermoFisher) was used for MS detections, and data were acquired with a Finnigan Xcalibur software package.
Analyses of 4-Phenyl-1,2,4-triazoline-3,5-dione (PTAD) derivatized samples were carried out with an isocratic solvent of methanol/0.1% acetic acid at a flow rate of 0.5 ml/min. MS/MS analysis of the PTAD derivatives was acquired in the positive ion mode using atmospheric pressure chemical ionization (APCI) and selected reaction monitoring (SRM). MS parameters were optimized for 7DHC-PTAD and were as follows: auxiliary nitrogen gas pressure at 55 psi and sheath gas pressure at 60 psi; discharge current at 22 μA and vaporizer temperature at 265°C. Collision induced dissociation (CID) was optimized at 12 eV under 1.0 mTorr of argon.
Statistical analyses
Boxplots, non-parametric Anovas, linear regression analyses and Dunnet’s two-tailed comparison to control with multiple comparison correction were carried out in SAS Studio, University Edition (Cary, NC).
Results
Haloperidol, but not clozapine, increased 7DHC and 8DHC levels in human blood
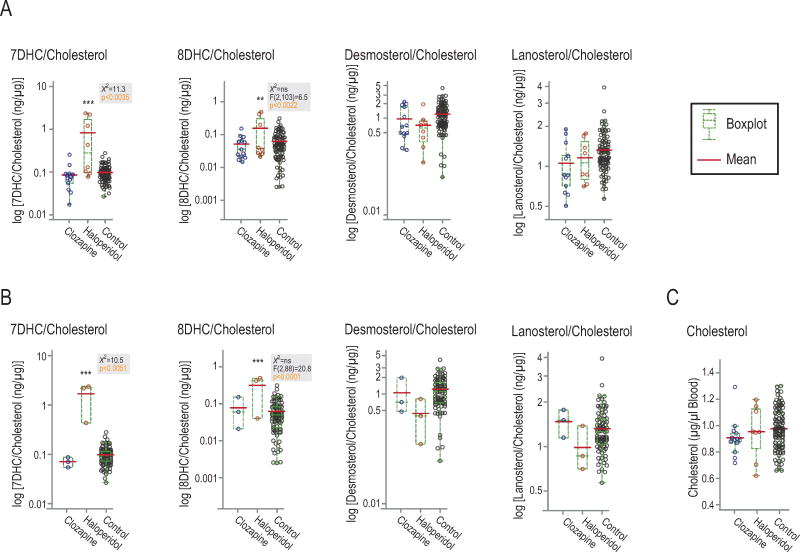
Haloperidol and clozapine are the prototypical conventional and atypical antipsychotics, respectively. Haloperidol treatment increased levels of 7DHC and 8DHC in whole blood of patients, while clozapine had no effect (fig. 2). In figure 2a, six patients were on a single antipsychotic drug, while 15 patients had additional prescriptions. Samples of patients on multiple psychotropic drugs were included only after it was established in preliminary tests that the secondary drug(s) did not affect sterol levels. In concordance with preliminary tests, a subgroup of patients on a single drug mirrored the pattern of the larger group (fig. 2b). The list of secondary drugs is provided in the supplemental information (supplemental table 1).
Figure 2. Haloperidol, but not clozapine, increased 7DHC and 8DHC levels in whole blood samples of patients.
A: Levels in all patients, including patients on more than one psychotropic drug. B: Subgroup of patients who were solely on haloperidol or clozapine. The bimodal distribution of haloperidol samples suggests that some patients might not have taken their medication (Griffiths et al., 2016). A, B, All sterols corrected for cholesterol levels; C: Cholesterol levels (μg/μl blood) were not affected by haloperidol or clozapine. Gray boxes show non-parametric Anova data, which were not significant for 8DHC, though parametric Anova analysis showed significance. Post-hoc analyses carried out using Dunnett’s comparison to control. ***p<0.0001, **p<0.01.
Desmosterol, lanosterol and cholesterol where not affected by either drug. However, as has been shown previously, desmosterol levels were lower in the female control group compared to male controls (supplemental fig. 2) (Matthan et al., 2013). Age had no significant effect on sterol levels in the control group (supplemental fig. 3).
Haloperidol, but not clozapine increased 7DHC and 8DHC levels in rat brain
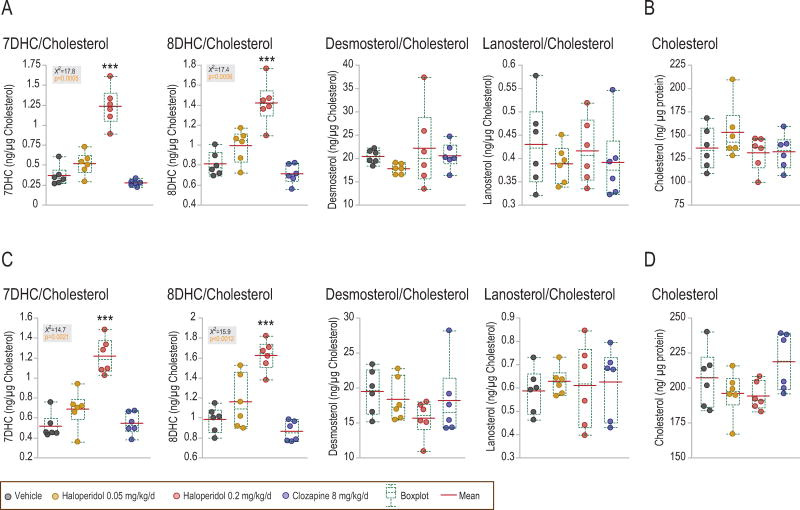
Rats treated chronically with haloperidol showed a dose-dependent increase of 7DHC and 8DHC levels in the striatum and the prefrontal cortex (fig. 3). Clozapine had no effect. Neither drug had an effect on desmosterol, lanosterol or cholesterol levels. Levels of 7DHC and 8DHC were correlated within each brain area, owing to the strong effect of haloperidol (supplemental fig. 4a).
Figure 3. Haloperidol, but not clozapine, increased 7DHC and 8DHC levels in the rat striatum and prefrontal cortex.
Chronic haloperidol treatment at 0.2 mg/kg/day, for 24 days, increased levels of 7DHC and 8DHC in the rat striatum, A, and prefrontal cortex, C. A trend toward increase was also observed at 0.05 mg/kg/day haloperidol, while chronic clozapine treatment had no effect. Patterns were similar if samples were normalized to protein (not shown), or to cholesterol levels. B, D, cholesterol levels (ng/μg protein) in striatum and prefrontal cortex, respectively. Gray boxes show non-parametric Anova data. ***p<0.0001 in post-hoc analysis using Dunnett’s comparison to control.
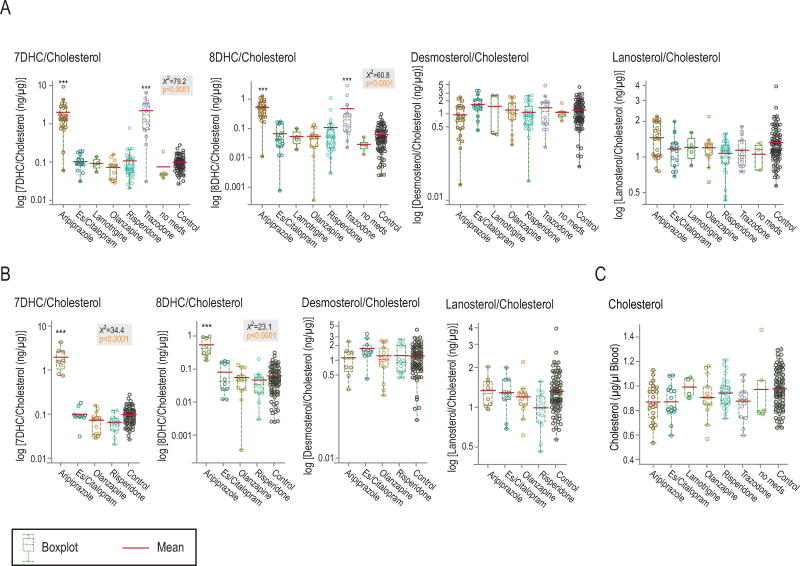
Aripiprazole and trazodone increased 7DHC and 8DHC levels in human blood
Aripiprazole, an atypical antipsychotic, and trazodone, an antidepressant and hypnotic, increased levels of 7DHC and 8DHC, but not of desmosterol, lanosterol or cholesterol (fig. 4a, 4c). Other drugs tested (es/citalopram, lamotrigine, olanzapine, and risperidone) did not affect circulating 7DHC levels. Levels of 7DHC and 8DHC were correlated (supplemental fig. 4b).
Figure 4. Aripiprazole and trazodone increased 7DHC and 8DHC levels in whole blood samples of patients.
A: Levels in all patients, including patients on more than one psychotropic drug. B: Patients who were on a single psychotropic drug. C: Treatment did not affect cholesterol levels. ‘Es/citalopram’: combined group of citalopram and its stereoisomer, escitalopram; ‘no meds’: five patients were not on psychotropic drugs. Gray boxes show non-parametric Anova data. ***p<0.0001 in post-hoc analysis using Dunnett’s comparison to control.
Although 51 patients were on multiple drugs, the subgroup of 46 patients on single drugs mirrored the pattern of the larger group (fig. 4b). Samples from patients on multiple psychotropic drugs were included only once and grouped with the drug of known effect on sterol levels (supplemental table 1). Trazodone, prescribed for its sleep-inducing effect, and lamotrigine, were always combined with other prescriptions.
Relationship of body-mass index (BMI), Positive and Negative Syndrome Scale (PANSS), Global Assessment of Functioning (GAF) scores and disease duration with sterol metabolites
For the analysis of BMI, PANSS, GAF and disease duration, sterol metabolite data were not normalized to cholesterol. BMI was available for 189 samples, and was not correlated with sterol metabolites (supplemental table 2). BMI was significantly increased with disease duration and with age, as well as in MDD patients who in our dataset had increased disease duration, and with es/citalopram treatment which was the main treatment for MDD.
In the rat experiments, all rats gained weight at the same rate and no correlations were observed between weight gain and sterol metabolites in either brain area examined.
PANSS scores (positive, negative, general, and total) were available for all patients diagnosed with SZ+, and 18 of the 22 patients with BPD. Pans scores were significantly higher in SZ+ (t[96]=3.7, p>=0.0004, for total score). No correlations were observed with sterol metabolites, drug treatment or disease duration.
GAF scores were available for 195 study participants, and showed a positive relationship with cholesterol, desmosterol in males, and lanosterol, in the whole dataset, but not within normal control samples only (supplemental table 3). GAF scores were decreased in all disorders when compared to control. Lowest levels were observed in SZ+, with increasing levels in BPD and MDD. Drug treatments followed the trend of the disorder they were treating.
Levels of sterols did not change with disease duration, with the qualifier that many of the patients were first-episode patients.
Discussion
Here we present the first comprehensive analysis of the effect of a number of psychotropic drugs on the levels of 7DHC, 8DHC, and other cholesterol metabolites in human blood. Our data support findings from a small previous study that retroactively associated increased plasma levels of 7DHC with prescriptions of aripiprazole and trazodone (Hall et al., 2013). We also show that metabolic adaptations in patients taking haloperidol, aripiprazole or trazodone mirror Neuro2a cells in vitro, though there were also differences (Korade et al., 2016). For example, risperidone, which increased 7DHC levels in cell lines, had no effect in patients (Canfran-Duque et al., 2013).
Lanosterol and desmosterol, two cholesterol precursors, were not altered in patients on haloperidol, aripiprazole or trazodone. Unlike in cell lines, the increases in 7DHC levels did not affect levels of desmosterol and cholesterol (fig. 1), (Kim et al., 2016; Korade et al., 2016). Because of the considerable differences of cholesterol regulation in cell culture systems and whole body, it is ultimately important to verify cell culture findings in the actual tissue of interest (Gordon et al., 2014; Kupferberg et al., 1991).
It is not known to what extent the effect of drugs on plasma sterol levels is reflected in the brain. However, we found that chronic administration of haloperidol in rats leads to a dose-dependent increase of 7DHC and 8DHC in the striatum and prefrontal cortex, while clozapine had no effect. These data parallel the data in human blood and support the assumption that the effects of psychotropic drugs on sterol metabolism in blood are likely to occur in brain as well.
An interesting remaining question concerns the common features of haloperidol, aripiprazole, and trazodone. Haloperidol, a conventional antipsychotic drug, and aripiprazole an atypical antipsychotic drug, are prescribed for SZ and BPD, while trazodone is prescribed for MDD and insomnia (de Oliveira et al., 2009; Docherty et al., 2010; Roth et al., 2011; Saletu-Zyhlarz et al., 2003). The piperazine ring in aripiprazole and trazodone has raised some interest because of similarities with known DHCR7 inhibitors (Hall et al., 2013). However, haloperidol increases 7DHC and 8DHC levels and does not contain a piperazine in its chemical structure, and other piperazines, such as clozapine or olanzapine, did not affect 7DHC or 8DHC levels in our study. Therefore, structural studies are needed to better understand if, and how, these drugs interfere with DHCR7 activity.
Many drugs that treat psychiatric disorders have unwanted side effects that limit their effectiveness and often lead to patient noncompliance. For example, extrapyramidal symptoms (EPS) are common with first generation antipsychotics. EPS are caused by oxidative stress and disruption of neuronal function in the basal ganglia (Casey, 1997; Lohr et al., 2003; Tsai et al., 1998). Though less common with subsequent generations of antipsychotics, EPS are still observed, such as with aripiprazole treatment (Bernagie et al., 2016; Etminan et al., 2016; Hall et al., 2009; Pena et al., 2011). The increased levels of the highly oxidizable 7DHC and 8DHC may accelerate oxidative stress and lipid peroxidation, and thus contribute to EPS (Korade et al., 2013; Xu et al., 2011a; Xu et al., 2013; Xu et al., 2011b). Therefore, measures to reduce oxidative stress, such as antioxidant supplementation, could be particularly important considerations for drugs that increase levels of 7DHC and 8DHC (Korade et al., 2014).
Another problem with psychotropic medications is their propensity to cause obesity and metabolic syndrome, manifested by hypertriglyceridemia, abnormal glucose tolerance, insulin resistance, and cardiovascular disease (Rojo et al., 2015; Rummel-Kluge et al., 2012). Metabolic syndrome has been associated with oxysterols (Guillemot-Legris et al., 2016). However, the incidence of metabolic syndrome under haloperidol exposure is comparable to, or even lower than with second generation antipsychotic drugs, and some second generation antipsychotic drugs that cause metabolic syndrome did not show elevated 7DHC levels in the blood samples we tested (Kahn et al., 2008).
7DHC could also have therapeutic effects. As a precursor of vitamin D, it can counteract vitamin D deficiency. Vitamin D deficiency is prevalent in Western populations and has been associated with depression and psychosis (Anglin et al., 2013; Crews et al., 2013; Holick, 2009; McGrath et al., 2010). The presence of the vitamin D receptor in the brain indicates a role for vitamin D in brain function (Eyles et al., 2005). Moreover, the biologically active form of vitamin D and ligand for the vitamin D receptor, dhVitD, mediates a diverse array of neuroprotective functions (Garcion et al., 2002). Similarly, neurosteroid-like compounds generated from 7DHC could have therapeutic potential and should be further investigated (Acimovic et al., 2016; Marcos et al., 2004). Since neurosteroids have been shown to protect against symptoms associated with stress, depression, and SZ, these novel neurosteroids could potentially have powerful therapeutic actions, or reveal further information on pathological processes in the brain (Wong et al., 2015; Zorumski et al., 2013). It is therefore possible that the increase in 7DHC and 8DHC by some psychotropic drugs contributes to their therapeutic benefits. This line of research should be further pursued.
While the adult brain can retrieve cholesterol from dietary sources, endogenous synthesis of cholesterol is important for early brain development and myelination (Marcos et al., 2007; Saher et al., 2005). Thus, a potential inhibition of DHCR7 by psychotropic medications in the developing brain could have adverse effects on brain function. This issue is particularly apparent in SLOS, where mutations of DHCR7 affect CNS structure and function, leading to developmental disabilities and autism (Bukelis et al., 2007; Nowaczyk and Irons, 2012). Experience with SLOS suggests that agents that inhibit DHCR7 function could have teratogenic consequences and should be avoided during pregnancy.
In conclusion, a number of psychotropic agents increase 7DHC and 8DHC levels in blood and brain. While the brain should be protected against 7DHC-mediated lipid peroxidation, the metabolic route of 7DHC to vitamin D should be supported. Furthermore, investigation of novel neurosteroid-like compounds could provide further insight in, and potential therapeutic approaches to, psychiatric disorders. Finally, the propensity of psychotropic drugs to increase 7DHC and 8DHC levels needs to be considered in prescription practices during pregnancy, as teratogenic effects of these drugs cannot be excluded.
Supplementary Material
7DHC and cholesterol were examined twice in a subset of patient and control samples (n=124), stored at −20°C and analyzed 2 years apart. No reduction in raw 7DHC or cholesterol measurements was observed, and 7DHC/cholesterol was highly correlated. Both measures were averaged in the final analysis.
Sex differences were observed in desmosterol levels (both raw data, not shown, and data normalized to cholesterol), but not in 7DHC, 8DHC or lanosterol levels. Data are normalized to cholesterol and are in line with previous studies (Matthan et al., 2013; Sato et al., 2015).
85 normal control samples, normalized to cholesterol levels were subjected to a linear regression analysis with ‘age’ as the X-variable. No significant trends were observed.
The isomers 7DHC and 8DHC are tightly correlated in the rat striatum and prefrontal cortex, A, as well as in human whole blood, B.
List of all psychotropic medications prescribed in addition to the medication the samples were grouped in. In cases where samples could be assigned to two or more groups, samples were grouped with the drug most likely to have an effect on 7DHC levels (‘primary medication’). For example, three patients on the primary medication aripiprazole had also prescriptions for es/citalopram, as had one patient on clozapine, one patient on haloperidol and two patients on trazodone. Such samples were only included with the primary medication and not with the secondary medication(s).
Effect of BMI on sterol metabolites (A), diagnosis (B), drug treatment (C) and disease duration (D). In our dataset BMI was not correlated with sterol levels (7DHC and 8DHC corrected for medication; desmosterol corrected for sex). MDD patients had longer disease durations and higher BMI. Es/citalopram, one of the main treatments in MDD, was also correlated with BMI. BMI data were available for 189 study participants; in this analysis, sterols were not normalized to cholesterol.
Interaction of GAF scores with sterol metabolites (A), diagnosis (B), drug treatment (C) and disease duration (D). A slightly positive correlation was observed between GAF scores and cholesterol levels, but 7DHC or 8DHC (corrected for treatment) were not affected by GAF. Desmosterol, corrected for sex, showed a positive correlation with GAF in males, but not females, and lanosterol showed a positive correlation with GAF scores. GAF scores were significantly lower in all diagnoses when compared to control, with lowest levels in SZ, followed by BPD and MDD. Drug treatments followed the trend of the disorder they were treating. GAF data were available for 195 study participants; in this analysis, sterols were not normalized to cholesterol.
Acknowledgments
This work was supported by The National Institutes of Health, NICHD HD064727 (NAP), NIEHS ES024133 (NAP and ZK) and NIMH MH074000 (CK). Recruitment and diagnosis of study participants, and collection of patient data and patient samples used in the study was carried out through the Psychiatric Genotyping-Phenotyping Project at the Vanderbilt Department of Psychiatry. The authors thank the members of the project as well as Dr. Stephan Heckers, Chair of the Vanderbilt Department of Psychiatry and project head.
Abbreviations
- DHCR7
7-dehydrocholesterol reductase
- 7DHC
7-dehydrocholesterol
- SLOS
Smith-Lemli-Opitz syndrome
- dhVitD
1,25-dihydroxyvitamin D
- 8DHC
8-dehydrocholesterol
- BHT
butylated hydroxytoluene
- TPP
triphenylphosphine
- PTAD
4-Phenyl-1,2,4-triazoline-3,5-dione
- APCI
atmospheric pressure chemical ionization
- SRM
selected reaction monitoring
- CID
Collision induced dissociation
- EPS
extrapyramidal symptoms
- IPP
isopentenyl pyrophosphate
- Dhcr24
24-dehydrocholesterol reductase
- MDD
Major depressive disorder
- BPD
bipolar disorder
- SZ
schizophrenia
- SA
schizoaffective disorder
- SZ+
SZ, SA and schizophreniform disorder
Footnotes
Contributions:
KA, collected patient data and samples
ZK, NAP, CK designed various parts of the study and wrote the protocol
ZK, WL, CK, processed tissue samples
CK, carried out animal experiments
WL, carried out LC-MS/MS analysis
CK, ZK, EBW, managed the literature searches and analyses
CK, undertook the statistical analysis
ZK, CK, wrote the first draft of the manuscript
All authors contributed to and have approved the final manuscript.
Conflict of interest disclosure:
The authors declare no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acimovic J, Goyal S, Kosir R, Golicnik M, Perse M, Belic A, Urlep Z, Guengerich FP, Rozman D. Cytochrome P450 metabolism of the post-lanosterol intermediates explains enigmas of cholesterol synthesis. Sci Rep. 2016;6:28462. doi: 10.1038/srep28462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasia M, Fiecchi A, Galli G. Synthesis of cholesta-5,8-dien-3-beta-ol. J Org Chem. 1981;46:3421–3422. [Google Scholar]
- Anglin RE, Samaan Z, Walter SD, McDonald SD. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013;202:100–107. doi: 10.1192/bjp.bp.111.106666. [DOI] [PubMed] [Google Scholar]
- Bernagie C, Danckaerts M, Wampers M, De Hert M. Aripiprazole and Acute Extrapyramidal Symptoms in Children and Adolescents: A Meta-Analysis. CNS Drugs. 2016;30(9):807–818. doi: 10.1007/s40263-016-0367-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bukelis I, Porter FD, Zimmerman AW, Tierney E. Smith-Lemli-Opitz syndrome and autism spectrum disorder. Am J Psychiatry. 2007;164(11):1655–1661. doi: 10.1176/appi.ajp.2007.07020315. [DOI] [PubMed] [Google Scholar]
- Canfran-Duque A, Casado ME, Pastor O, Sanchez-Wandelmer J, de la Pena G, Lerma M, Mariscal P, Bracher F, Lasuncion MA, Busto R. Atypical antipsychotics alter cholesterol and fatty acid metabolism in vitro. J Lipid Res. 2013;54(2):310–324. doi: 10.1194/jlr.M026948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey DE. The relationship of pharmacology to side effects. J Clin Psychiatry. 1997;58(Suppl 10):55–62. [PubMed] [Google Scholar]
- Chapman SC, Horne R. Medication nonadherence and psychiatry. Curr Opin Psychiatry. 2013;26(5):446–452. doi: 10.1097/YCO.0b013e3283642da4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21(1):1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- Crews M, Lally J, Gardner-Sood P, Howes O, Bonaccorso S, Smith S, Murray RM, Di Forti M, Gaughran F. Vitamin D deficiency in first episode psychosis: a case-control study. Schizophr Res. 2013;150(2–3):533–537. doi: 10.1016/j.schres.2013.08.036. [DOI] [PubMed] [Google Scholar]
- de Oliveira IR, Elkis H, Gattaz WF, Chaves A, de Sena EP, de Matos ESFG, Campos JA, Bueno JR, JA ES, Louza MR, de Abreu PB. Aripiprazole for patients with schizophrenia and schizoaffective disorder: an open-label, randomized, study versus haloperidol. CNS Spectr. 2009;14(2):93–102. doi: 10.1017/s1092852900000249. [DOI] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD. Cholesterol metabolism in the brain. Curr Opin Lipidol. 2001;12(2):105–112. doi: 10.1097/00041433-200104000-00003. [DOI] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD. Thematic review series: brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res. 2004;45(8):1375–1397. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- Docherty JP, Baker RA, Eudicone J, Mathew S, Marcus RN, McQuade RD, Mankoski R. Effect of aripiprazole versus haloperidol on PANSS Prosocial items in early-episode patients with schizophrenia. Schizophr Res. 2010;120(1–3):199–203. doi: 10.1016/j.schres.2010.03.040. [DOI] [PubMed] [Google Scholar]
- Dubrovsky BO. Steroids, neuroactive steroids and neurosteroids in psychopathology. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(2):169–192. doi: 10.1016/j.pnpbp.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Etminan M, Procyshyn RM, Samii A, Carleton BC. Risk of Extrapyramidal Adverse Events With Aripiprazole. J Clin Psychopharmacol. 2016;36(5):472–474. doi: 10.1097/JCP.0000000000000543. [DOI] [PubMed] [Google Scholar]
- Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13(3):100–105. doi: 10.1016/s1043-2760(01)00547-1. [DOI] [PubMed] [Google Scholar]
- Gordon K, Clouaire T, Bao XX, Kemp SE, Xenophontos M, de Las Heras JI, Stancheva I. Immortality, but not oncogenic transformation, of primary human cells leads to epigenetic reprogramming of DNA methylation and gene expression. Nucleic Acids Res. 2014;42(6):3529–3541. doi: 10.1093/nar/gkt1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths WJ, Abdel-Khalik J, Crick PJ, Ogundare M, Shackleton CH, Tuschl K, Kwok MK, Bigger BW, Morris AA, Honda A, Xu L, Porter NA, Bjorkhem I, Clayton PT, Wang Y. Sterols and oxysterols in plasma from Smith-Lemli-Opitz syndrome patients. J Steroid Biochem Mol Biol. 2016 doi: 10.1016/j.jsbmb.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot-Legris O, Mutemberezi V, Muccioli GG. Oxysterols in Metabolic Syndrome: From Bystander Molecules to Bioactive Lipids. Trends Mol Med. 2016 doi: 10.1016/j.molmed.2016.05.006. [DOI] [PubMed] [Google Scholar]
- Hall DA, Agarwal P, Griffith A, Segro V, Seeberger LC. Movement disorders associated with aripiprazole use: a case series. Int J Neurosci. 2009;119(12):2274–2279. doi: 10.3109/00207450903225553. [DOI] [PubMed] [Google Scholar]
- Hall P, Michels V, Gavrilov D, Matern D, Oglesbee D, Raymond K, Rinaldo P, Tortorelli S. Aripiprazole and trazodone cause elevations of 7-dehydrocholesterol in the absence of Smith-Lemli-Opitz Syndrome. Mol Genet Metab. 2013;110(1–2):176–178. doi: 10.1016/j.ymgme.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73–78. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TL, Chen JF. Serum lipid profiles and schizophrenia: effects of conventional or atypical antipsychotic drugs in Taiwan. Schizophr Res. 2005;80(1):55–59. doi: 10.1016/j.schres.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Kahn RS, Fleischhacker WW, Boter H, Davidson M, Vergouwe Y, Keet IP, Gheorghe MD, Rybakowski JK, Galderisi S, Libiger J, Hummer M, Dollfus S, Lopez-Ibor JJ, Hranov LG, Gaebel W, Peuskens J, Lindefors N, Riecher-Rossler A, Grobbee DE group E.s. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008;371(9618):1085–1097. doi: 10.1016/S0140-6736(08)60486-9. [DOI] [PubMed] [Google Scholar]
- Kim HY, Korade Z, Tallman KA, Liu W, Weaver CD, Mirnics K, Porter NA. Inhibitors of 7-Dehydrocholesterol Reductase: Screening of a Collection of Pharmacologically Active Compounds in Neuro2a Cells. Chem Res Toxicol. 2016;29(5):892–900. doi: 10.1021/acs.chemrestox.6b00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korade Z, Kenworthy AK, Mirnics K. Molecular consequences of altered neuronal cholesterol biosynthesis. J Neurosci Res. 2009;87(4):866–875. doi: 10.1002/jnr.21917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korade Z, Kim HY, Tallman KA, Liu W, Koczok K, Balogh I, Xu L, Mirnics K, Porter NA. The Effect of Small Molecules on Sterol Homeostasis: Measuring 7-Dehydrocholesterol in Dhcr7-Deficient Neuro2a Cells and Human Fibroblasts. J Med Chem. 2016;59(3):1102–1115. doi: 10.1021/acs.jmedchem.5b01696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korade Z, Xu L, Harrison FE, Ahsen R, Hart SE, Folkes OM, Mirnics K, Porter NA. Antioxidant supplementation ameliorates molecular deficits in Smith-Lemli-Opitz syndrome. Biol Psychiatry. 2014;75(3):215–222. doi: 10.1016/j.biopsych.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korade Z, Xu L, Mirnics K, Porter NA. Lipid biomarkers of oxidative stress in a genetic mouse model of Smith-Lemli-Opitz syndrome. J Inherit Metab Dis. 2013;36(1):113–122. doi: 10.1007/s10545-012-9504-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferberg A, Cremel G, Behr P, Van Dorsselaer A, Luu B, Mersel M. Differential sensitivity of astrocyte primary cultures and derived spontaneous transformed cell lines to 7 beta-hydroxycholesterol: effect on plasma membrane lipid composition and fluidity, and on cell surface protein expression. Mol Cell Biochem. 1991;101(1):11–22. doi: 10.1007/BF00238433. [DOI] [PubMed] [Google Scholar]
- Liu W, Xu L, Lamberson CR, Merkens LS, Steiner RD, Elias ER, Haas D, Porter NA. Assays of plasma dehydrocholesteryl esters and oxysterols from Smith-Lemli-Opitz syndrome patients. J Lipid Res. 2013;54(1):244–253. doi: 10.1194/jlr.M031732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr JB, Kuczenski R, Niculescu AB. Oxidative mechanisms and tardive dyskinesia. CNS Drugs. 2003;17(1):47–62. doi: 10.2165/00023210-200317010-00004. [DOI] [PubMed] [Google Scholar]
- Marcos J, Guo LW, Wilson WK, Porter FD, Shackleton C. The implications of 7-dehydrosterol-7-reductase deficiency (Smith-Lemli-Opitz syndrome) to neurosteroid production. Steroids. 2004;69(1):51–60. doi: 10.1016/j.steroids.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Marcos J, Shackleton CH, Buddhikot MM, Porter FD, Watson GL. Cholesterol biosynthesis from birth to adulthood in a mouse model for 7-dehydrosterol reductase deficiency (Smith-Lemli-Opitz syndrome) Steroids. 2007;72(11–12):802–808. doi: 10.1016/j.steroids.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthan NR, Zhu L, Pencina M, D’Agostino RB, Schaefer EJ, Lichtenstein AH. Sex-specific differences in the predictive value of cholesterol homeostasis markers and 10-year cardiovascular disease event rate in Framingham Offspring Study participants. J Am Heart Assoc. 2013;2(1):e005066. doi: 10.1161/JAHA.112.005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield FR, Tabas I. Role of cholesterol and lipid organization in disease. Nature. 2005;438(7068):612–621. doi: 10.1038/nature04399. [DOI] [PubMed] [Google Scholar]
- McGrath JJ, Eyles DW, Pedersen CB, Anderson C, Ko P, Burne TH, Norgaard-Pedersen B, Hougaard DM, Mortensen PB. Neonatal vitamin D status and risk of schizophrenia: a population-based case-control study. Arch Gen Psychiatry. 2010;67(9):889–894. doi: 10.1001/archgenpsychiatry.2010.110. [DOI] [PubMed] [Google Scholar]
- Nicholas HJ, Thomas BE. Cholesterol metabolism and the blood-brain barrier: an experimental study with 2-C14-sodium acetate. Brain. 1961;84:320–328. doi: 10.1093/brain/84.2.320. [DOI] [PubMed] [Google Scholar]
- Nowaczyk MJ, Irons MB. Smith-Lemli-Opitz syndrome: phenotype, natural history, and epidemiology. Am J Med Genet C Semin Med Genet. 2012;160C(4):250–262. doi: 10.1002/ajmg.c.31343. [DOI] [PubMed] [Google Scholar]
- Omura T. Mitochondrial P450s. Chem Biol Interact. 2006;163(1–2):86–93. doi: 10.1016/j.cbi.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. Academic Press; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- Pena MS, Yaltho TC, Jankovic J. Tardive dyskinesia and other movement disorders secondary to aripiprazole. Mov Disord. 2011;26(1):147–152. doi: 10.1002/mds.23402. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Castaneda DC, O’Malley BW, Rogawski MA. Anticonvulsant activity of progesterone and neurosteroids in progesterone receptor knockout mice. J Pharmacol Exp Ther. 2004;310(1):230–239. doi: 10.1124/jpet.104.065268. [DOI] [PubMed] [Google Scholar]
- Rojo LE, Gaspar PA, Silva H, Risco L, Arena P, Cubillos-Robles K, Jara B. Metabolic syndrome and obesity among users of second generation antipsychotics: A global challenge for modern psychopharmacology. Pharmacol Res. 2015;101:74–85. doi: 10.1016/j.phrs.2015.07.022. [DOI] [PubMed] [Google Scholar]
- Roth AJ, McCall WV, Liguori A. Cognitive, psychomotor and polysomnographic effects of trazodone in primary insomniacs. J Sleep Res. 2011;20(4):552–558. doi: 10.1111/j.1365-2869.2011.00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rummel-Kluge C, Komossa K, Schwarz S, Hunger H, Schmid F, Kissling W, Davis JM, Leucht S. Second-generation antipsychotic drugs and extrapyramidal side effects: a systematic review and meta-analysis of head-to-head comparisons. Schizophr Bull. 2012;38(1):167–177. doi: 10.1093/schbul/sbq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saher G, Brugger B, Lappe-Siefke C, Mobius W, Tozawa R, Wehr MC, Wieland F, Ishibashi S, Nave KA. High cholesterol level is essential for myelin membrane growth. Nat Neurosci. 2005;8(4):468–475. doi: 10.1038/nn1426. [DOI] [PubMed] [Google Scholar]
- Saletu-Zyhlarz GM, Anderer P, Arnold O, Saletu B. Confirmation of the neurophysiologically predicted therapeutic effects of trazodone on its target symptoms depression, anxiety and insomnia by postmarketing clinical studies with a controlled-release formulation in depressed outpatients. Neuropsychobiology. 2003;48(4):194–208. doi: 10.1159/000074638. [DOI] [PubMed] [Google Scholar]
- Sato Y, Bernier F, Yamanaka Y, Aoshima K, Oda Y, Ingelsson M, Lannfelt L, Miyashita A, Kuwano R, Ikeuchi T. Reduced plasma desmosterol-to-cholesterol ratio and longitudinal cognitive decline in Alzheimer’s disease. Alzheimers Dement (Amst) 2015;1(1):67–74. doi: 10.1016/j.dadm.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe LJ, Brown AJ. Controlling cholesterol synthesis beyond 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) J Biol Chem. 2013;288(26):18707–18715. doi: 10.1074/jbc.R113.479808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield JM, Williams LE, Blackford JU, Heckers S. Childhood sexual abuse increases risk of auditory hallucinations in psychotic disorders. Compr Psychiatry. 2013;54(7):1098–1104. doi: 10.1016/j.comppsych.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Li W, Kim TK, Semak I, Wang J, Zjawiony JK, Tuckey RC. Novel activities of CYP11A1 and their potential physiological significance. J Steroid Biochem Mol Biol. 2015;151:25–37. doi: 10.1016/j.jsbmb.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuta T, Scharwey M, Langer T. Mitochondrial lipid trafficking. Trends Cell Biol. 2014;24(1):44–52. doi: 10.1016/j.tcb.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Tsai G, Goff DC, Chang RW, Flood J, Baer L, Coyle JT. Markers of glutamatergic neurotransmission and oxidative stress associated with tardive dyskinesia. Am J Psychiatry. 1998;155(9):1207–1213. doi: 10.1176/ajp.155.9.1207. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behavioural Brain Research. 2003;146(1–2):3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL, Peltonen L, Cooper JD, O’Reilly PF, Houston DK, Glazer NL, Vandenput L, Peacock M, Shi J, Rivadeneira F, McCarthy MI, Anneli P, de Boer IH, Mangino M, Kato B, Smyth DJ, Booth SL, Jacques PF, Burke GL, Goodarzi M, Cheung CL, Wolf M, Rice K, Goltzman D, Hidiroglou N, Ladouceur M, Wareham NJ, Hocking LJ, Hart D, Arden NK, Cooper C, Malik S, Fraser WD, Hartikainen AL, Zhai G, Macdonald HM, Forouhi NG, Loos RJ, Reid DM, Hakim A, Dennison E, Liu Y, Power C, Stevens HE, Jaana L, Vasan RS, Soranzo N, Bojunga J, Psaty BM, Lorentzon M, Foroud T, Harris TB, Hofman A, Jansson JO, Cauley JA, Uitterlinden AG, Gibson Q, Jarvelin MR, Karasik D, Siscovick DS, Econs MJ, Kritchevsky SB, Florez JC, Todd JA, Dupuis J, Hypponen E, Spector TD. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376(9736):180–188. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witsch-Baumgartner M, Fitzky BU, Ogorelkova M, Kraft HG, Moebius FF, Glossmann H, Seedorf U, Gillessen-Kaesbach G, Hoffmann GF, Clayton P, Kelley RI, Utermann G. Mutational spectrum in the Delta7-sterol reductase gene and genotype-phenotype correlation in 84 patients with Smith-Lemli-Opitz syndrome. Am J Hum Genet. 2000;66(2):402–412. doi: 10.1086/302760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P, Sze Y, Chang CC, Lee J, Zhang X. Pregnenolone sulfate normalizes schizophrenia-like behaviors in dopamine transporter knockout mice through the AKT/GSK3beta pathway. Transl Psychiatry. 2015;5:e528. doi: 10.1038/tp.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Heckers S. Brain Structure in Neuropsychologically Defined Subgroups of Schizophrenia and Psychotic Bipolar Disorder. Schizophr Bull. 2015;41(6):1349–1359. doi: 10.1093/schbul/sbv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Davis TA, Porter NA. Rate constants for peroxidation of polyunsaturated fatty acids and sterols in solution and in liposomes. J Am Chem Soc. 2009;131(36):13037–13044. doi: 10.1021/ja9029076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Korade Z, Rosado DA, Jr, Liu W, Lamberson CR, Porter NA. An oxysterol biomarker for 7-dehydrocholesterol oxidation in cell/mouse models for Smith-Lemli-Opitz syndrome. J Lipid Res. 2011a;52(6):1222–1233. doi: 10.1194/jlr.M014498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Korade Z, Rosado DA, Jr, Mirnics K, Porter NA. Metabolism of oxysterols derived from nonenzymatic oxidation of 7-dehydrocholesterol in cells. J Lipid Res. 2013;54(4):1135–1143. doi: 10.1194/jlr.M035733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Liu W, Sheflin LG, Fliesler SJ, Porter NA. Novel oxysterols observed in tissues and fluids of AY9944-treated rats: a model for Smith-Lemli-Opitz syndrome. J Lipid Res. 2011b;52(10):1810–1820. doi: 10.1194/jlr.M018366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You H, Lu W, Zhao S, Hu Z, Zhang J. The relationship between statins and depression: a review of the literature. Expert Opin Pharmacother. 2013;14(11):1467–1476. doi: 10.1517/14656566.2013.803067. [DOI] [PubMed] [Google Scholar]
- Zorumski CF, Paul SM, Izumi Y, Covey DF, Mennerick S. Neurosteroids, stress and depression: potential therapeutic opportunities. Neurosci Biobehav Rev. 2013;37(1):109–122. doi: 10.1016/j.neubiorev.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
7DHC and cholesterol were examined twice in a subset of patient and control samples (n=124), stored at −20°C and analyzed 2 years apart. No reduction in raw 7DHC or cholesterol measurements was observed, and 7DHC/cholesterol was highly correlated. Both measures were averaged in the final analysis.
Sex differences were observed in desmosterol levels (both raw data, not shown, and data normalized to cholesterol), but not in 7DHC, 8DHC or lanosterol levels. Data are normalized to cholesterol and are in line with previous studies (Matthan et al., 2013; Sato et al., 2015).
85 normal control samples, normalized to cholesterol levels were subjected to a linear regression analysis with ‘age’ as the X-variable. No significant trends were observed.
The isomers 7DHC and 8DHC are tightly correlated in the rat striatum and prefrontal cortex, A, as well as in human whole blood, B.
List of all psychotropic medications prescribed in addition to the medication the samples were grouped in. In cases where samples could be assigned to two or more groups, samples were grouped with the drug most likely to have an effect on 7DHC levels (‘primary medication’). For example, three patients on the primary medication aripiprazole had also prescriptions for es/citalopram, as had one patient on clozapine, one patient on haloperidol and two patients on trazodone. Such samples were only included with the primary medication and not with the secondary medication(s).
Effect of BMI on sterol metabolites (A), diagnosis (B), drug treatment (C) and disease duration (D). In our dataset BMI was not correlated with sterol levels (7DHC and 8DHC corrected for medication; desmosterol corrected for sex). MDD patients had longer disease durations and higher BMI. Es/citalopram, one of the main treatments in MDD, was also correlated with BMI. BMI data were available for 189 study participants; in this analysis, sterols were not normalized to cholesterol.
Interaction of GAF scores with sterol metabolites (A), diagnosis (B), drug treatment (C) and disease duration (D). A slightly positive correlation was observed between GAF scores and cholesterol levels, but 7DHC or 8DHC (corrected for treatment) were not affected by GAF. Desmosterol, corrected for sex, showed a positive correlation with GAF in males, but not females, and lanosterol showed a positive correlation with GAF scores. GAF scores were significantly lower in all diagnoses when compared to control, with lowest levels in SZ, followed by BPD and MDD. Drug treatments followed the trend of the disorder they were treating. GAF data were available for 195 study participants; in this analysis, sterols were not normalized to cholesterol.