Abstract
A fundamental function of the intestinal epithelium is to act as a barrier that regulates interactions between the luminal contents, such as the intestinal microbiota, the underlying immune system, and the remainder of the body, while supporting vectorial transport of nutrients, water, and waste products. Epithelial barrier function requires a contiguous layer of cells as well as the junctions that seal the paracellular space between epithelial cells. Compromised intestinal barrier function has been associated with a number of disease states, both intestinal and systemic. Unfortunately, most current clinical data are correlative, making it difficult to separate cause from effect in interpreting significance of barrier loss. Some data from experimental animal models suggest that compromised epithelial integrity may play a pathogenic role in specific gastrointestinal diseases, but no FDA-approved therapies for targeting the epithelial barrier are presently available. In order for this goal to be achieved, a deeper understanding of both disease pathogenesis and mechanisms of barrier regulation must be reached.
Keywords: Cell Polarity, Epithelial Cells/cytology, Epithelium/growth & development, Tight junctions, Mammals/growth & development, Morphogenesis
An essential function of the intestinal mucosa is to act as a barrier between luminal contents and the underlying immune system. The term “intestinal barrier” is increasingly used to refer to the mucus layer or the underlying mucosal immune system. While each of these mucosal components provides a type of barrier, the physical epithelial barrier confers the property of selective permeability to the intestinal mucosa. We will therefore use the term “intestinal barrier function” to refer to the ability of the intestinal epithelium to restrict free exchange of water, ions, and macromolecules between the intestinal lumen and the underlying tissues. Intestinal permeability is the inverse of intestinal barrier function, and because the intestinal mucosa must simultaneously promote nutrient and water transport while serving as a protective barrier, neither property is absolute. Instead, intestinal barrier function depends on a variety of mucosal structural components that are tightly regulated in homeostasis and during disease.
The luminal surface of the intestinal mucosa is lined by a hydrated gel, composed of mucins secreted by goblet cells. This layer prevents large particles and intact bacteria from coming into direct contact with the underlying epithelium. The importance of the mucus layer is emphasized by the observations that mucin structure is markedly altered in active enterocolitis and that knockout mice lacking the protein mucin-2 (Muc2), which is the major component of intestinal mucin, develop spontaneous colitis 1. However, the mucus layer does not establish a significant barrier to transmucosal water or solute flux; that falls to the epithelial monolayer, which is the primary determinant of mucosal barrier function. The apical surface of the epithelium forms a single, continuous, community border as a result of the precise alignment of abutting cells. In an intact epithelium, this surface restricts passage of most hydrophilic solutes; however, in order to limit transmucosal flux, the paracellular space must also be sealed. The task of regulating paracellular transport is achieved by a series of intercellular junctions.
Molecular composition of the apical junctional complex (AJC)
From apical-to-basal, the interecellular junctions are the tight junction (zonula occludens), the adherens junction (zonula adherens), and the desmosome (Fig. 1). Together these three types of intercellular junctions comprise the apical junctional complex 2. The AJC is associated with a dense network of actin and myosin that encircles the apical aspect of each cell and surrounds the cortical actin web. The latter supports the dense microvillus brush border, while, as discussed below, the perijunctional actomyosin ring regulates epithelial barrier function.
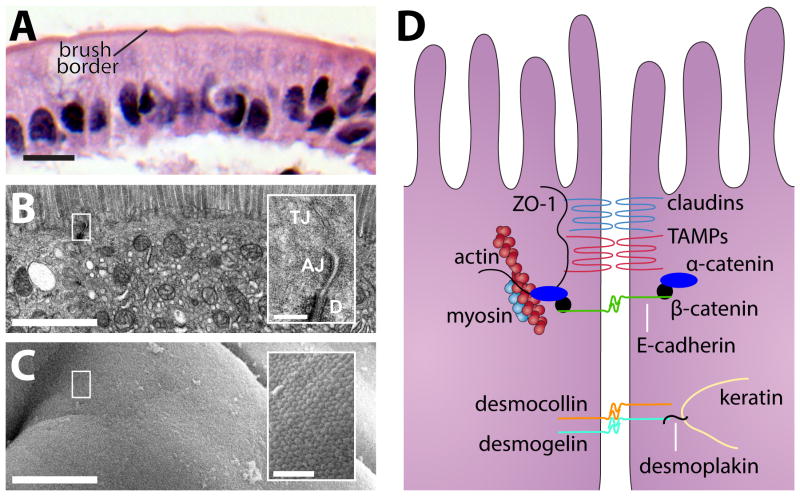
Figure 1. The apical junctional complex is necessary for epithelial barrier formation.
A) Intestinal epithelia consist of a single layer of epithelial cells separating the luminal contents (apical) from the underlying lamina propria (basal). This section of human jejunal epithelium stained with hematoxylin and eosin demonstrates that a series of individual cells form a community apical border which comprises the luminal surface. Bar = 10 μm. B) Transmission electron microscopy (TEM) of small intestinal epithelium demonstrates intercellular junctions and a dense microvillus brush border. Note the exclusion of membranous organelles from the dense band of cortical actin just beneath the brush border. Bar = 1 μm. Inset: Apical junctional complex, composed of the tight junction (TJ), adherens junction (AJ) and desmosome (D). Bar = 200 nm. C) Scanning electron microscopy (SEM) of small intestinal epithelium demonstrates the continuous brush border surface of the small intestine. Bar = 4 μm. Inset: Dense, tightly-packed microvillus array. Bar = 500 nm. D) Individual epithelial cells are held together and communicate with one another through a series of junctions within the apical junctional complex. The apical junctional complex is positioned near the apical surface along the lateral membrane and is comprised of the tight junction, adherens junction, and desmosomes. A simplified cartoon of the apical junctional complex is shown on the right.
Adherens junctions and desmosomes provide adhesive forces necessary for maintenance of cell-cell interactions. The most well-known component of the adherens junctions are the cadherins, single spanning transmembrane proteins that interact homotypically with the extracellular portion of cadherins on adjacent cells. On the cytoplasmic side, cadherins interact directly with p120-catenin and β-catenin, which in turn interact with α-catenin 3. Among other functions, α-catenin regulates perijunctional actin assembly, which provides further strength to these structures 4,5. In addition, the adherens junction is necessary for efficient tight junction assembly, a function that in vitro studies have attributed to both E-cadherin and α-catenin 6,7.
The tight junction is the primary determinant of paracellular permeability. When viewed by transmission electron microscopy, the tight junction appears to eliminate the intracellular space at so-called “kissing points,” and freeze-fracture electron microscopy clearly depicts that tight junctions consist of a series of anastomosing strands 2,8. Results from a study using direct rapid freezing methods suggest that tight junction strands may exist as pairs of inverted micelles formed by the fusion of the outer leaflets from plasma membranes from abutting cells 9,10, although this model was largely abandoned with the discovery of tight junction-associated structural and regulatory proteins. After the initial description of tight junctions, immuno-electron microscopy identified transmembrane tight junction proteins within tight junction strands 11. Multiple subsequent studies have shown that tight junction proteins reside in cholesterol-rich, detergent-insoluble lipid domains 12–14. These findings have led to speculation that dynamic fusion and fission of lipid-based tight junction strands may account for selective permeability, and a detailed review of the lipidic nature of tight junctions can be found elsewhere 10. It is probable that both specialized lipids and proteins are necessary components of the tight junction barrier; however, to date far more work has been done to identify structure and regulation of tight junction protein components.
Tight junction proteins can be broadly separated into transmembrane proteins, cytosolic plaque, or scaffolding, proteins, and regulatory proteins. Of the transmembrane tight junction proteins, the tetraspanning claudins are the most important. The extracellular domains of claudins on adjacent cells form pores to regulate tight junction ion selectivity 15. A seminal study determined that the expression level of a single claudin family member, claudin-2, is largely responsible for differences in trans-epithelial resistance (TER) between two clones of Madin Darby canine kidney (MDCK) cells 16. Subsequent analyses have shown that claudin-2 driven decreases in epithelial barrier function are due to increases in ion transport without accompanying alterations in flux of larger molecules. Recent data indicating that individual claudin-2-based channels are dynamically gated suggests that modulation of opening and closing of claudin-2 pores is a targetable process for barrier modulation 17. An alternative potential means of inhibiting claudin-2 function comes from the observation that prevention of casein kinase-2 (CK2) -mediated occludin phosphorylation promotes assembly of a tight junction complex that blocks claudin-2 pore function and can reverse IL-13-induced barrier loss in vitro 18. Such therapies must, however, be approached with caution, as trans-tight junction Na+-recycling, from the lamina propria into the lumen, is necessary to support critical transcellular vectorial transport processes, such as nutrient absorption, which are necessary for life 19–21.
The ZO family proteins (ZO-1, -2, and -3) are multi-domain scaffolding proteins that interact directly with transmembrane tight junction proteins such as claudins and the tight junction associated Marvel protein (TAMP) family, which includes occludin 11,22–24. ZO family proteins also interact with the actin cytoskeleton and a variety of actin regulatory elements 25. ZO-1, -2, and -3 have many similar structural domains, which has led to the hypothesis that they serve similar functions. These proteins must, however, also have unique functions, as either ZO-1 or ZO-2 knockout results in embryonic lethality 26,27. Nevertheless, studies of human patients have discovered two distinct pathogenic ZO-2 (TJP2) mutations 26–29. The first mutation impairs ZO-2 binding to with claudin-2 and results in an incompletely penetrant familial hypercholanemia, which presents with elevated serum bile acids, pruritis, and fat malabsorption 28. The more recently discovered mutation in ZO-2 encodes a truncated protein and is associated with severe cholestatic liver disease that presents early in life and frequently requires liver transplantation 29. Claudin-1, but not claudin-2, failed to localize to tight junctions within canalicular and cholangiocyte membranes. Interestingly, a study of mice lacking claudin-2, which forms a paracellular Na+ and water channel, found that these mice generated a more concentrated bile and were susceptible to gallstone disease 30. The ability of this truncated ZO-2 to support human life, while ZO-2 knockout is lethal in mice, suggests that by oligomerization with ZO-1 the shortened protein may be partially functional. Alternatively, the data may indicate species differences in redundancy of ZO-2. In either case, these data highlight the importance of tight junction proteins in homeostasis and the avoidance of gastrointestinal diseases. Although barrier function has not been measured in patients with either ZO-2 mutation, the differences in localization of claudin proteins to tight junctions implies that, like in the claudin-2 knockout mouse, altered epithelial barrier function can result in hepatobiliary disease 28,29.
Paracellular permeability pathways
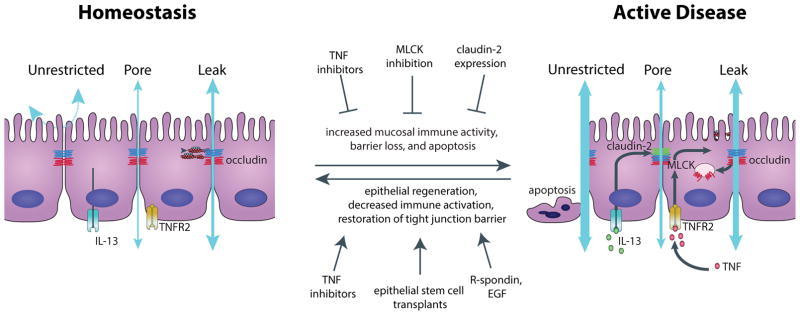
The tight junction barrier exhibits both size- and charge-selectivity. There are two distinct routes across tight junctions of an intact epithelial monolayer, termed the “pore” and “leak” pathways (Fig. 2). The pore pathway refers to a high-capacity, size- and charge-selective route, whereas the leak pathway is a low-capacity pathway that has limited selectivity. Pore pathway permeability appears to be determined primarily by the subset of claudins expressed, while leak pathway permeability can be regulated by ZO-1, occludin, and myosin light chain kinase (MLCK) 18,31,32. At sites of epithelial damage, e.g. erosions and ulcers, tight junctions are lost and, by definition, cannot contribute to local barrier function. Instead, luminal contents cross the intestinal barrier by a third pathway, termed the “unrestricted” pathway. As its name suggests, the unrestricted pathway is high capacity and permissive with respect to solute size and charge. Large proteins and even whole bacteria can cross the unrestricted pathway, which partially explains the severe disease initiated by epithelial damage. In the setting of extensive epithelial injury, such as that occurring in humans with necrotizing enterocolitis or rodents treated with DSS, the unrestricted pathway is often “unsealed” and is the predominant route of transmucosal flux. However, during homeostasis and less active inflammatory disease, the epithelium is generally intact and barrier function reflects flux across the paracellular pore and leak pathways.
Figure 2. Three distinct paracellular epithelial permeability pathways are disrupted during disease pathogenesis.
During homeostasis (left) there is little underlying mucosal immune activity, and the tight junction-regulated “leak” and “pore” pathways define intestinal permeability. In the presence of an intact epithelium, the tight junction-independent “unrestricted” pathway is sealed. During disease pathogenesis (right), increased mucosal immune activation leads to TNF and IL-13 production, which can lead to increased permeability across the leak and pore pathways, respectively. As disease progresses further, epithelial apoptosis occurs and permeability across the unrestricted pathway dominates. Multiple therapeutic approaches have been proposed to both inhibit disease progression or to restore epithelial barriers after disease onset.
Homeostasis and Physiologic Regulation of the Epithelial Barrier
At homeostasis, the intestinal epithelium is a highly dynamic structure and is estimated to completely self-renew every 4–7 days. Stem cells reside in the intestinal crypts where they proliferate, and daughter cells differentiate as they migrate up the crypt-villus axis and are eventually shed into the intestinal lumen. This constant turnover presents an opportunity for potential breaches in the epithelial barrier and development of an unrestricted pathway. However, both shedding events and oligocellular wounds are accompanied by formation and subsequent contraction of a multicellular actomyosin purse string, which drives tight junction expansion to the basal surface of the extruded cell to rapidly reestablish the contiguous epithelium and the tight junction barrier 33–35.
The most studied example of physiologic regulation of the tight junction barrier is the regulation that occurs upon activation of sodium-glucose cotransport. This co-transport leads to development of an osmotic gradient as well as activation of epithelial myosin light chain kinase (MLCK). MLCK activity increases paracellular permeability via the size-selective pore pathway, and in the setting of an osmotic gradient, this increased permeability allows for paracellular absorption of small nutrients, e.g. glucose, via solvent drag 36–41.
Pathophysiologic Regulation of Leak and Pore Pathways
The pore and leak pathways are also regulated in response to pathophysiologic stimuli. Perhaps the most well-studied is regulation of the pore pathway by interleukin (IL)-13 induction of claudin-2 expression. Notably, IL-13 is not the only immunologic regulator of claudin-2 expression and pore pathway permeability 42–44. It is however interesting that both claudin-2 expression and IL-13 production appear to be greater in ulcerative colitis relative to Crohn’s disease 45–47. While one study suggested that IL-13 causes barrier loss by inducing claudin-2 expression as well as by increasing apoptosis and inhibiting wound healing 47, both in vitro and in vivo studies using lower doses of IL-13 have shown claudin-2 upregulation and claudin-2-dependent pore-pathway activation in response to IL-13 exposure without associated increases in leak or unrestricted pathway flux 48. This is consistent with biophysical studies demonstrating that claudin proteins, e.g. claudin-2, form paracellular channels with exquisite size- and charge -selectivity and both closed and open states, similar to transmembrane ion channels.17,49–51 Interestingly, crypt, but not surface, epithelial express claudin-2, consistent with the greater paracellular permeability of the former.52–54
Tumor necrosis factor-α (TNF) has also been shown to regulate tight junction function, and the clinically relevant role of TNF to IBD pathogenesis is clearly demonstrated by the efficacy of anti-TNF antibodies in IBD, which reduce disease severity and restore intestinal barrier function 55. Restoration of epithelial barrier function by anti-TNF therapy may reflect mucosal healing in the setting of a dampened immune system; however, pre-clinical studies have shown that TNF signaling also modulates tight junction barrier function. This relationship was first recognized in vitro by the association between barrier loss and increased myosin light chain phosphorylation in response to TNF 56. Pharmacologic inhibition of MLCK activity rapidly reduced MLC phosphorylation and restored tight junction barrier function 56. Using both pharmacologic and genetic methods of MLCK inhibition, TNF-induced MLC phosphorylation and tight junction barrier dysfunction was shown to be required for diarrhea in vivo 57. It was subsequently shown that TNF could also upregulate claudin-2 expression, thereby enhancing pore pathway flux. This, however, only occurred after many hours of TNF treatment,43 in contrast to the rapid regulation of MLCK transcription,58 and is therefore best considered a secondary phase of TNF-induced barrier regulation. Notably, expression of constitutively-active MLCK within the intestinal epithelium also upregulated claudin-2 expression in vivo despite the absence of overt disease 48,59,60.
Further studies demonstrated the contribution of tight junction barrier loss to the pathogenesis of experimental colitis and are discussed in detail below 59,60. TNF diminishes epithelial barrier function in large part by inducing occludin internalization via a caveolin-1-dependent process 61. This was demonstrated in vivo as either pharmacologic or genetic inhibition of caveolin-1 function limited both occludin internalization and TNF-mediated diarrhea 61. Further, intestinal epithelial-specific occludin overexpression limited TNF-induced barrier loss and prevented TNF-induced diarrhea 61. Because in vivo occludin overexpression was associated with increased tight junction and lateral membrane occludin pools and relative preservation of tight junction occludin despite MLCK activation, these data indicate that it is removal of occludin, and not some other component, from tight junctions that leads to barrier loss. In vitro studies have corroborated this study by showing that occludin depletion results in decreased barrier function and that occludin-deficient monolayers are insensitive to further TNF-induced barrier loss 62,63. Given the greater paracellular permeability of crypt, relative to surface, epithelium, it is notable that crypt epithelia have significant intracellular occludin pools, while surface epithelia, do not.52–54
Subsequent domain analyses suggest that barrier regulation by occludin requires direct interactions between occludin and ZO-1 62. Unlike claudin channels that represent the structural pathway route of pore pathway conductance, the precise sites of paracellular leak pathway flux are not yet defined. The observation that overexpression of the occludin-related protein tricellulin reduces leak pathway conductance without affecting the pore pathway 64,65 suggests that tricellular junctions may be the sites of leak pathway flux. Interestingly, tricellulin is found at both tricellular to bicellular tight junctions, rather than only at the former, after occludin knockdown 62,66. This raises the possibility that redistribution of tricellulin following occludin endocytosis contributes to TNF-induced barrier loss 62,65–67. It is, however, worth noting that neither intestinal barrier defects nor intestinal disease have been reported in humans or mice with tricellulin mutations.68–70. Further, while occludin knockout mice do not have intestinal barrier defects,71,72 they are deaf and display tricellulin redistribution to bicellular tight junctions within the inner ear.73
While the leak and pore pathways represent distinct routes across the paracellular barrier, the two pathways are often impacted in parallel. For example, in the SAMP1/YitFc murine model of colitis, claudin-2 mRNA expression is increased and occludin expression is decreased, indicating that both leak and pore pathways are activated 74. Mechanistic interplay between the pathways was demonstrated using mice expressing a constitutively-active MLCK (CA-MLCK) within the intestinal epithelium. Colonic mucosae of these mice display increased cation selectivity that could not be explained by MLCK-dependent increases in leak pathway flux 48. Instead, in vivo responses to MLCK activation were shown to result in mucosal immune activation, enhanced IL-13 expression, and subsequent increases in claudin-2 expression that led to increased cation flux across the pore pathway 48.
Association of Intestinal Barrier Function with Intestinal disease
Intestinal barrier function has been associated with an increasing variety of diseases – both intestinal and systemic – and has led to the popularization of the catch-all diagnosis of “leaky gut syndrome.” The vast majority of these associations are merely correlative, but experimental evidence relating barrier dysfunction to disease pathogenesis exists in some cases, including inflammatory bowel disease and celiac disease 75. Some bacterial pathogens are also capable of regulating tight junction barrier function, e.g. secondary to MLCK activation in enteropathogenic E. coli infection,56,76 via direct interactions with specific claudins by C. perfringens enterotoxin,77,78 or by rho inhibition by C. difficile toxins.79,80
The association between intestinal barrier dysfunction and intestinal disease was first recognized by studies using an ex vivo approach that documented increased permeability in active IBD in both ulcerated and non-ulcerated epithelia 81–83. Subsequent studies revealed that tight junction function, ultrastructure, and protein composition are altered in active IBD 46,84. Expression and activity of the tight junction regulatory protein myosin light chain kinase as well as expression of the pore-forming protein claudin-2 are also increased in active disease, suggesting that tight junction dysregulation may play a pathogenic role in IBD, prior to epithelial ulceration 85,86.
Consistent with this idea, intestinal permeability has been reported as a fairly sensitive prognostic indicator of relapse to active disease in Crohn’s disease patients during clinical remission 87,88. These results were corroborated by a later study of 43 CD patients, which also reported increased levels of intestinal inflammation marker fecal calprotectin prior to relapse 89. This finding blurs the exact role of intestinal barrier dysfunction in relapse because, as implied by the in vitro and in vivo studies discussed above, subclinical levels of inflammation may be responsible for increased permeability. Consistent with this, inflammatory cytokine exposure is associated with increased epithelial cell turnover in vivo, and a recent clinical study using confocal laser endomicroscopy reported increased epithelial shedding and leakage of fluorescein dye across the intestinal epithelium in patients at risk of relapse within 1 year 34,90. Despite this, it is worth noting that the fluorescein dye flux is into the lumen, suggesting that any barrier defect results in local fluid efflux and, therefore, limited passive transport luminal materials into the mucosa. Further, many studies have shown relative maintenance of barrier function at sites of epithelial shedding 34,35,91.
The contribution of increased intestinal permeability to disease pathogenesis was first proposed with the realization that a subset of first-degree relatives of patients with Crohn’s disease, also display increased intestinal permeability 92. While first-degree relatives do have an increased risk of developing CD relative to the overall population, it remains to be determined if the subset with increased intestinal permeability are at greater risk than those without increased intestinal permeability. It is, however, interesting that the relatives with increased intestinal permeability tend to carry a specific disease-associated polymorphism of CARD15/NOD2 93. While interesting in the context of disease, it is also is important to note that these studies demonstrate that increased intestinal permeability alone is insufficient to cause overt clinical disease, as many healthy first-degree relatives harbor these deficits. Nevertheless, one case report has identified a first-degree relative with increased intestinal permeability prior to the clinical presentation of Crohn’s Disease, suggesting a pathogenic role for intestinal barrier function in IBD 94. This single case report must be interpreted with caution given the individual’s already increased risk of developing IBD and, as noted above, no studies have assessed long-term disease risk in first-degree relatives with increased intestinal permeability. However, a range of exciting experimental mouse models have provided evidence supporting the idea that intestinal barrier loss can be one component that contributes to a multi-hit mechanism of IBD pathogenesis.
Diminished intestinal barrier function has also been proposed to play a pathogenic role in celiac disease, as immune system exposure to gliadin is necessary for celiac disease to become clinically evident. However, the route by which gliadin is passed from the lumen to the lamina propria is controversial and may involve either the transcelluar or paracellular route. Support for this theory first came from the observations that intestinal permeability to non-metabolizable sugars is increased during active disease and decreases to normal ranges after consumption of a gluten-free diet 95. Additionally, a gluten challenge can increase intestinal permeability 95. Later studies found that intestinal permeability positively correlates with disease activity and is increased in both patients and their healthy relatives 96,97. Moreover, improvements in barrier function have been shown to precede histologic evidence of disease improvement after initiation of a gluten-free diet 98 and have even been reported in diarrhea-predominant irritable bowel syndrome (IBS-D) patients after challenge with gluten-free diet 99.
Animal models of celiac disease include Irish setter pups, a subset of whom are gluten sensitive. Like patients with celiac disease, gluten-sensitive Irish setter pups display gluten-dependent increases in intestinal permeability that precede histologic enteropathy 100. These observations are supported by multiple studies showing increased intestinal permeability upon gluten exposure in gluten-sensitive HLA-DQ8 transgenic mice 101,102. Each of these results can potentially be a result of immune signaling to epithelia that results in increased permeability. Consistent with this, removal of the immune stimulus (i.e. gliadin) restores intestinal barrier function. However, in vitro studies indicate that gliadin may have a direct effect on the epithelium, as exposure to gliadin and gliadin peptides produce a significant reduction in barrier function of confluent intestinal epithelial cell (IEC-6) monolayers 103. A similar result was reproduced using the human intestinal epithelial cell line Caco-2, and in the latter study, size-selectivity of gliadin-induced barrier defect was assessed by measuring flux of both 4 kDa and 70 kDa FITC-dextran across treated monolayers.104 This revealed that gliadin-exposed Caco-2 monolayers were significantly more permeable to small (4 kDa) but not large (70 kDa) dextrans, indicating an increase in leak pathway flux with preservation of the unrestricted pathway.104
The mechanism for gliadin-mediated reductions of epithelial barrier function has been proposed to involve up-regulation of zonulin, a putative regulator of tight junction permeability. Zonulin expression is increased in patients with active celiac disease and a zonulin antagonist, larazotide acetate (AT-1001), inhibits gliadin-induced reductions in epithelial permeability in vitro and in vivo 105,106. Unfortunately, clinical trials of larazotide have not produced similar reductions in intestinal permeability 107.
Other mechanism of barrier loss in celiac disease may reflect polymorphisms in myosin-IXb, which have been linked to celiac disease.108,109 Myosin-IXb is a Rho-GTPase activating protein (GAP) that plays a role in actin remodeling.110 The myosin-IXb polymorphisms linked to celiac disease are within the N-terminal portion of myosin-IXb, the region of the protein which confers Rho-GAP activity 110,111. However, studies of myosin-IXb variants in additional populations have yielded discrepant results with regard to association with celiac disease 112,113. These conflicting results may be due to population differences, unidentified environmental cofactors, or false positive or negative results. Nevertheless, some support for a pathogenic role of myosin-IXb polymorphisms comes from studies linking these to Crohn’s disease and ulcerative colitis 114–116. While it remains to be tested if the identified myosin-IXb variants are pathogenic, the association of polymorphisms in a single protein with multiple disease entities underscores the hypothesis that common cellular mechanisms may underlie multiple inflammatory diseases. In vitro studies of Caco-2 monolayers have shown an essential role of myosin-IXb in intestinal epithelial wound closure, tight junction protein localization, and epithelial barrier function at steady state 117. All of these data suggest that myosin-IXb may play an important role in maintaining the barrier by regulating both the tight junction and epithelial repair. Although intestinal permeability was not studied in subjects carrying myosin-IXb polymorphisms, it is interesting to speculate that these variants may increase disease susceptibility by enhancing flux across both tight junction leak and unrestricted pathways. Indeed, myosin IXb KO mice were recently shown to have diminished epithelial barrier function, characterized by increased 40kDa dextran flux 118. These observations are most likely explained by increased rates of epithelial apoptosis; however, intestinal epithelia from myosin IXb KO mice also display increased sub-apical phosphorylated MLC and reduced ZO-1 at tight junctions 118.
Other studies have identified changes in claudin protein expression that may also impact flux across the tight junction pore pathway 119,120. Thus, as in IBD, all three flux pathways likely contribute to permeability increases in celiac disease. One final factor that may affect transmucosal flux in celiac disease is the reduction in mucosal surface area as a result of villous blunting. This is often associated with reactive crypt hyperplasia. Together, this results in a skewing of the crypt:villus surface area ratio. Because the leak pathway of crypt tight junctions is far more permeable than in the villus 52–54, this likely increases leak pathway flux. However, pore pathway flux may be reduced as a result of the overall loss of surface area. These changes explain the increased lactulose permeability, as it is a leak pathway probe, decreased flux of the pore pathway probe mannitol, and increased lactulose:mannitol ratio in celiac disease 96,121,122.
Contribution of mouse models to understanding intestinal barrier function in disease
A variety of mouse models have led to a more sophisticated understanding of the contribution of intestinal barrier function to inflammatory diseases. Dysregulation of adherens junction proteins has been described in human inflammatory bowel disease, and the critical role of epithelial barrier function in homeostasis was demonstrated in a mouse line with chimeric expression of a dominant negative N-cadherin cytoplasmic tail 123,124. E-cadherin-mediated interactions were disrupted in intestinal epithelial lineages expressing the N-cadherin tail resulted aberrant epithelial differentiation, chronic active inflammation, and dysplasia 123. A histologically similar inflammatory process characterized by erosions and ulcerations was reported in mice lacking intestinal-epithelial p120-catenin, which display marked E-cadherin downregulation due to enhanced degradation in the absence of p120-catenin 125. More recently, mice with a targeted, conditional E-cadherin deletion within intestinal epithelium were generated.126 These also displayed altered differentiation patterns, enhanced epithelial apoptosis, and bloody diarrhea as well as impaired bacterial defense.126 Disease in each of these models likely reflects marked disruption of tight junctions secondary to adherens junction disassembly, and can therefore be considered a model of disease driven, at least partially, by unrestricted pathway defects. This may be a component of IBD pathogenesis, but is unlikely to reflect a primary mechanism in disease presenting after the neonatal period. Nevertheless, it is interesting that polymorphisms near the gene CDH1 that encodes E-cadherin have been linked to ulcerative colitis 127.
Similar to human patients, colitis development in IL-10 knockout mice is highly variable in penetrance, age of onset, and severity. Environmental stimuli and genetic factors, including both targeted changes and strain-specific differences, contribute to the observed variation 128,129. Moreover, enteric bacteria are necessary for colitis onset, as germ-free IL-10 knockout mice do not develop disease, and antibiotic treatment can attenuate colitis 128,130,131. While the primary defect on IL-10 knockout mice is, obviously, immune, it is notable that intestinal barrier defects are present prior to clinical evidence of disease onset and do not develop under germ-free conditions 130. From these data, it is unclear whether increased intestinal permeability is a key pathogenic component of colitis in IL-10 knockout mice or simply an early marker of mucosal injury. Several studies suggest that the former may be true. First, it is now well appreciated that the non-steroidal anti-inflammatory drug (NSAID) piroxicam can promote uniform disease development in IL-10 knockout mice 132. Given that NSAIDs are known to result in epithelial damage, it is likely that NSAID treatment provoked disease by increasing flux across the unrestricted pathway. Similarly, administration of a zonulin agonist enhanced intestinal permeability and modestly increased disease severity in IL-10 knockout mice 133. Conversely, a zonulin antagonist reduced intestinal permeability and disease severity in 1L-10 knockout mice 134. While the mechanism of action of these agents, including their specificity, is unclear, these data do suggest that modulating intestinal permeability can impact colitigenesis in IL-10 knockout mice.
Mouse models with more apical junctional complex defects may shed light onto the role of tight junction-mediated barrier in colitis development and progression. For example, mice lacking the junctional adhesion molecule-A (JAM-A), which facilitates tight junction assembly and leukocyte transmigration, display altered claudin protein expression, and increases in epithelial apoptosis, proliferation, and migration even in the absence of clinically-apparent disease. JAM-A deficient mice are also hypersensitive to DSS injury 135. This may indicate that JAM-A expression is either protective against intestinal epithelial damage or enhances regenerative capacity, but may also reflect the inability of knockout mice to mount an adequate response to DSS injury given the pre-existing chronic epithelial damage. Further, it is important to note that JAM-A is expressed in many tissues, and a specific role for intestinal epithelial JAM-A has not been assessed. Nevertheless, it is notable that JAM-A expression is downregulated in human disease 135.
A more targeted approach took advantage of the physiologically- and pathophysiologically-relevant tight junction regulator myosin light chain kinase to increase intestinal paracellular permeability. In this model, constitutively-active myosin light chain kinase (CA-MLCK) was expressed specifically within intestinal epithelia 59. Notably, this increased intestinal paracellular permeability without impacting epithelial maturation, proliferation, or turnover, much like the subset of healthy first-degree relatives of Crohn’s disease patients with increased intestinal permeability. CA-MLCK mice mature normally without developing spontaneous disease, but they do exhibit subclinical immune activation with TH1 polarization. Further, when challenged by adoptive transfer of effector T-lymphocytes, disease onset is accelerated, severity is worsened, and overall survival is reduced relative to non-transgenic littermates. These experimental data are consistent with clinical data indicating barrier dysfunction alone is insufficient to cause clinically detectable disease, and also provide direct evidence that isolated tight junction dysfunction can contributes to disease pathogenesis in susceptible hosts. As discussed above, it is also notable that the CA-MLCK-induced increase in leak pathway permeability resulted in claudin-2 upregulation and enhanced pore pathway flux 48.
A subsequent study investigated the interplay between immune activation, TNF signaling, intestinal epithelial MLCK expression, and intestinal barrier function using immune-mediated adoptive transfer colitis model 60. Similar to human disease 86, intestinal epithelial MLCK expression increased as colitis progressed 60. This was accompanied by increased intestinal epithelial transcription and expression of TNFR2, which had been shown to mediate TNF-induced increases in MLCK transcription in vitro 136. Consistent with this, TNFR2-deficient failed to upregulate MLCK expression or myosin light chain phosphorylation within intestinal epithelium. In contrast, deletion of TNFR1, which often regulates signaling on immune cells, had no effect on intestinal epithelial MLCK expression or activity. Further, mice lacking either TNFR2 or epithelial MLCK were significantly protected from increases in mucosal TNF production and permeability, and deletion of either gene markedly delayed onset of colitis 60. Interestingly, claudin-2 upregulation was also attenuated in MLCK-deficient mice. This was a specific effect of intestinal epithelial MLCK, as intestinal epithelial CA-MLCK expression was able to fully restore all features of disease, including claudin-2 expression, in MLCK-deficient mice 60. These data indicate that both TNFR2 and MLCK inhibition may be appropriate targets of future biologic therapies. They also raise the possibility that tNFR2 blockade may have advantages over TNF-targeted biologics in terms of reduced overall immunosuppression.
Intestinal barrier function and systemic disease
Increased intestinal permeability has been reported in patients with an array of autoimmune diseases, suggesting a link between exposure to microbial antigens and development of autoimmune disease. Most notable among these is the link between graft versus host disease, which develops in many patients following allogeneic stem cell (bone marrow) transplantation. For many years it was known that the magnitude of intestinal barrier defects, primarily representing increased flux across the unrestricted pathway, correlated with severity of experimental graft versus host disease 137,138. It was, however, not clear if this correlation merely represented the correlation between extent of epithelial damage and graft versus host disease severity or whether intestinal barrier loss played a specific causative role. One recent study has shown that intestinal barrier loss is not required for development of graft versus host disease in the context major antigen mismatch-driven bone marrow transplantation, which is the most commonly used experimental model 138–140. However, in the more clinically-relevant setting of minor antigen mismatch transplantation, intestinal epithelial damage, i.e. increased unrestricted pathway flux, was an essential cofactor in disease pathogenesis 139. Remarkably, this requirement could be overcome by intraperitoneal delivery of lipopolysaccharide, suggesting that transmucosal flux of bacterial products may be the key event triggered by intestinal epithelial damage 139. The specific role of tight junction-mediated barrier loss in graft versus host disease has not been defined.
Decreased intestinal barrier function has also been noted prior to clinical disease onset in patients with type 1 diabetes 141. The biobreeding diabetes-prone (BBDP) experimental rat model of type 1 diabetes displays increased intestinal permeability prior to the onset of disease 142. One study comparing the microbiome of BBDP rats to BB diabetes-resistant (BBDR) rats have shown more abundant Lactobacillus and Bifidobacterium in resistant rats; however, it remains unknown if alterations in microbiome composition are caused by diabetes or play a role in disease development. In another murine model of diabetes, the non-obese diabetic (NOD) mouse model, incidence of diabetes development can be influenced by exposure to and ability to sense luminal microbial stimuli 143,144. As the primary regulator of interaction between the immune system and luminal antigens, the epithelial barrier is therefore likely to be essential to preventing disease. Indeed, a pathogenic link between barrier dysfunction and diabetes has been proposed to work through the negative tight junction regulator zonulin, as zonulin expression is increased in BBDP rats, and administration of anti-zonulin antibodies decreases incidence of auto-antibody production and development of clinical type 1 diabetes in this model 145. While one study has reported that increased concentrations of serum zonulin correlate with intestinal permeability in patients with type I diabetes, a causative role in patients has not been demonstrated 146.
Targeting the epithelial barrier for therapy
Targeting and restoring the epithelial barrier is a tempting therapeutic goal. Unfortunately, no therapies currently exist to do so clinically, and one molecule shown to restore epithelial barrier function in pre-clinical studies did not replicate barrier-protective effects in clinical trials 106,107,147. Nevertheless, many promising approaches to target the epithelial barrier have been proposed and are discussed below.
Epithelial integrity restoration/regenerative strategies: the unrestricted pathway
Engraftment of intestinal stem cells has been proposed as a therapy for repairing damaged intestinal mucosa (i.e. the unrestricted pathway).148,149 Recent technological advances have made long-term culture and expansion of intestinal stem cells possible and have led many to believe that isolation, expansion, and transplantation of intestinal stem cells can aid in epithelial regeneration 150. This idea is supported by one study in which mice were subjected to DSS-induced epithelial damage and given either a mock enema or enema with cultured intestinal stem cells during recovery from DSS 151. Stem cells were able to engraft in areas of ulceration and serve as long-lived intestinal stem cells in vivo. However, in this study, engraftment efficiency was low, resulted in a minimal immediate improvement and no long-term improvement after removal of DSS, suggesting that the most effective way to restore the barrier is to remove the disease stimulus. Moreover, the Lgr5+ intestinal stem cells that are expanded and engrafted have been proposed to serve as cancer stem cells 152,153, and careful characterization of enteroid gene expression over many passages has not been performed, leaving open the possibility that engrafted enteroids may harbor malignant potential. While system characterization and improved culture and engraftment methods may make this method more promising, without removal of the underlying stimulus causing epithelial damage (DSS in this case), this approach is unlikely to provide significant benefit.
More targeted approaches have also been proposed and involve potentiating signaling pathways important for epithelial expansion. Two growth factors essential for growth and expansion of intestinal stem cells – EGF and R-spondin – are potential therapeutic agents for restoring damaged epithelia. Activation of EGFR protects against TNF-induced apoptosis 154, and R-spondin1 reduces disease severity in epithelial damage models of colitis 155. One may be cautious against this approach because both EGF and R-spondin are mitogens, and both EGF and the R-spondin-augmented Wnt pathway are dysregulated in colon cancer. For example, loss of negative regulator of EGF pathway (Lrig1) results in hyper-proliferation of intestinal cells in vivo 156,157. However, one study indicated that EGFR signaling actually decreased cancer incidence and altered colonic cytokine production in IL-10 knockout colitic mice, reigniting potential for this approach in a subset of colitis patients 158.
Tight junction regulation: pore and leak pathways
An alternative approach to barrier maintenance focuses on tight junction regulation and has potential roles in preventing initial IBD development in susceptible individuals and in promoting maintenance of remission. As discussed above, tight junction permeability is physiologically regulated to facilitate nutrient transport, raising concern of potential toxicity of this approach. While further studies are necessary to characterize and mitigate these potential undesired effects, two targets are particularly enticing. One promising target is MLCK, which has a well-defined mechanism of action with respect to barrier function in physiology and pathophysiology in vitro, in vivo, and in patients. Moreover, studies have shown beneficial effects of MLCK inhibition in mouse models of colitis when inhibition occurs specifically in the intestinal epithelium. However, MLCK inhibition harbors potentially detrimental off-target effects due to the fact that all MLCK isoforms share a common catalytic domain. For example, smooth muscle MLCK is essential for GI motility, blood pressure regulation, and airway contractility.159–161 While MLCK remains a promising target, more specific means of targeting long MLCK must be developed prior to considering MLCK a drug target for treating human disease. As discussed above, Claudin-2 also offers a potentially druggable target by either modulating claudin-2 mobility or directly targeting dynamic claudin-2 pore opening and closing events.17,18 Unfortunately, no drug for claudin-2 modulation currently exists.
Concluding thoughts
As suggested by the above discussion, currently, the best therapy for treating epithelial barrier loss is to treat the underlying disease, as increased permeability is as likely to be a consequence of the disease as it is to be a cause. For example, anti-TNF antibodies, which have been successful therapies for IBD, treat the underlying immune activation while also significantly reducing intestinal permeability to near normal levels 55. While there is promise in targeting the epithelial barrier, more research is needed to define mechanisms of epithelial homeostasis and fundamental disease pathogenesis prior to therapeutically targeting the epithelial barrier.
Table I.
Associations of representative diseases and disease models with intestinal barrier loss
| Inflammatory Bowel Disease | Celiac Disease | Graft vs. Host Disease | Type I Diabetes Mellitus | |
|---|---|---|---|---|
| Structural alterations | ||||
| Pore pathway | Increased claudin-2 expression45,47,60,85 | Increased claudin-2 expression119,120,162 | Increased claudin-2 expression139 | |
| Leak pathway | Reduced occludin expression47; increased MLCK expression and activity58,60,86, MLCK inactivation reduces severity60 | Reduced occludin expression104,119 | Reduced occludin expression163 | |
| Unrestricted pathway | Ulceration, epithelial apoptosis60,135,164 | Epithelial apoptosis165 | ||
| Functional alterations | ||||
| Pore and/or leak pathways | Increased lactulose:mannitol ratio and PEG-400 permeability in disease,82,83,166–168 impending relapse,87–89,169 and some healthy relatives92,93,170 | Increased lactulose:mannitol ratio in disease82,96,171 | Increased sucralose permeability137 | Increased lactulose:mannitol ratio141,142,172 |
| Leak and/or unrestricted pathways | Increased 4kD dextran and Evan’s blue flux in DSS-induced colitis135,173,174 | Pathogenic bacterial that increase intestinal permeability accelerate disease175 | Development of experimental minor mismatch disease requires intestinal damage 139, the extent of barrier loss induced by pre- transplant conditioning correlates with disease severity176 | |
Future Directions.
Establishing or refuting a pathogenic role for intestinal barrier dysfunction requires further investigation in both clinical studies and experimental models. It will also be important to determine if increased permeability in healthy first-degree relatives of Crohn’s disease patients is a risk factor for disease development.
Delineating the contributions of pore, leak, and unrestricted pathways to observations of increased intestinal permeability in disease will be necessary for mechanistic understanding of barrier function in disease and subsequent rational therapeutic design.
Claudin-2 and MLCK are potential therapeutic targets for modulation of tight junction pore and leak pathway permeability, respectively. It will, however, be challenging to develop means to inhibit intestinal epithelial MLCK (to limit leak pathway flux increases) without toxicities due to systemic MLCK inhibition. Likewise, modulation of claudin-2 pores (pore pathway) without negatively affecting overall epithelial water and ion transport may be complex.
Tight junction proteins have roles beyond barrier maintenance, including epithelial morphogenesis and differentiation. Definition of the underlying structure-function relationships and their contributions to diverse processes is a requisite precursor to targeting of barrier function without detrimental effects on other systems.
Key Points.
The intestinal epithelium is a dynamic cellular layer that serves as a barrier between luminal contents and the underlying immune system while simultaneously supporting water, nutrient, and ion transport.
Tight junctions are the primary determinants of barrier function in intact epithelia and are composed of a complex network of transmembrane and cytosolic proteins accompanied by cytoskeletal and regulatory proteins.
Two distinct pathways – termed pore and leak – regulate paracellular flux in intact epithelia while the unrestricted flux pathway is the dominant route across ulcerated or denuded epithelia.
Reduced intestinal epithelial barrier function is associated with a variety of gastrointestinal and systemic diseases, including inflammatory bowel disease and graft-versus-host disease, respectively.
Experimental evidence for barrier defects contributing to disease is in IBD, where mouse models demonstrate that increased paracellular permeability accelerates experimental colitis and that preservation of tight junction barrier function delays disease progression.
While no currently available therapeutics specifically modulate epithelial barrier function, promising approaches to target the pore, leak, and unrestricted pathways are being investigated.
Acknowledgments
Support
National Institute of Health grants F30DK103511 (M.A.O.), T32HD007009 (M.A.O.); R01DK61931 (J.R.T.), and R01DK68271 (J.R.T.).
Biographies
Matthew A. Odenwald, PhD, is an MD/PhD student at The University of Chicago Pritzker School of Medicine. He completed his PhD in the Department of Pathology under the mentorship of Dr. Jerrold Turner, MD, PhD, where he studied epithelial cell biology. His work is focused on the role of tight junctions in establishing epithelial barriers and has uncovered novel roles for tight junction proteins as integrators of epithelial morphogenesis.
Jerrold R. Turner, MD, PhD, recently moved to Brigham & Women’s Hospital and Harvard Medical School after service as Sara and Harold Lincoln Thompson Professor and Associate Chair of Pathology at The University of Chicago. He is an active gastrointestinal surgical pathologist, author of chapters in leading textbooks of pathology, gastroenterology, and mucosal immunology, and Editor of the new AGA journal Cellular and Molecular Gastroenterology and Hepatology. Dr. Turner’s laboratory focuses on epithelial cell biology and gastrointestinal pathophysiology using techniques from electrophysiology to intravital microscopy. Major discoveries from his group include the central roles and regulation of myosin light chain kinase (MLCK) in tight junction regulation, the dynamic nature and functional impact of tight junction protein interactions, and molecular underpinnings of distinct high capacity-high selectivity (pore) and low capacity-low selectivity (leak) tight junction flux pathways.
References
- 1.Van der Sluis M, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterol. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 2.Farquhar M, Palade G. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778:660–669. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maiden SL, Hardin J. The secret life of alpha-catenin: moonlighting in morphogenesis. J Cell Biol. 2011;195:543–552. doi: 10.1083/jcb.201103106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capaldo CT, Macara IG. Depletion of E-cadherin disrupts establishment but not maintenance of cell junctions in Madin-Darby canine kidney epithelial cells. Mol Biol Cell. 2007;18:189–200. doi: 10.1091/mbc.E06-05-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maiers JL, Peng X, Fanning AS, DeMali KA. ZO-1 recruitment to alpha-catenin--a novel mechanism for coupling the assembly of tight junctions to adherens junctions. J Cell Sci. 2013;126:3904–3915. doi: 10.1242/jcs.126565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodenough DA, Revel JP. A fine structural analysis of intercellular junctions in the mouse liver. J Cell Biol. 1970;45:272–290. doi: 10.1083/jcb.45.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kachar B, Reese TS. Evidence for the lipidic nature of tight junction strands. Nature. 1982;296:464–466. doi: 10.1038/296464a0. [DOI] [PubMed] [Google Scholar]
- 10.Lingaraju A, Long TM, Wang Y, Austin JR, 2nd, Turner JR. Conceptual barriers to understanding physical barriers. Semin Cell Dev Biol. 2015;42:13–21. doi: 10.1016/j.semcdb.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furuse M, et al. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stankewich MC, Francis SA, Vu QU, Schneeberger EE, Lynch RD. Alterations in cell cholesterol content modulate Ca(2+)-induced tight junction assembly by MDCK cells. Lipids. 1996;31:817–828. doi: 10.1007/BF02522977. [DOI] [PubMed] [Google Scholar]
- 13.Francis SA, et al. Rapid reduction of MDCK cell cholesterol by methyl-beta-cyclodextrin alters steady state transepithelial electrical resistance. Eur J Cell Biol. 1999;78:473–484. doi: 10.1016/s0171-9335(99)80074-0. [DOI] [PubMed] [Google Scholar]
- 14.Shen L, et al. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J Cell Sci. 2006;119:2095–2106. doi: 10.1242/jcs.02915. [DOI] [PubMed] [Google Scholar]
- 15.Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol. 2006;68:403–429. doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- 16.Furuse M, Furuse K, Sasaki H, Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J Cell Biol. 2001;153:263–272. doi: 10.1083/jcb.153.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weber CR, et al. Claudin-2-dependent paracellular channels are dynamically gated. eLife. 2015;4:e09906. doi: 10.7554/eLife.09906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raleigh DR, et al. Occludin S408 phosphorylation regulates tight junction protein interactions and barrier function. J Cell Biol. 2011;193:565–582. doi: 10.1083/jcb.201010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wada M, Tamura A, Takahashi N, Tsukita S. Loss of claudins 2 and 15 from mice causes defects in paracellular Na+ flow and nutrient transport in gut and leads to death from malnutrition. Gastroenterol. 2013;144:369–380. doi: 10.1053/j.gastro.2012.10.035. [DOI] [PubMed] [Google Scholar]
- 20.Tamura A, et al. Loss of claudin-15, but not claudin-2, causes Na+ deficiency and glucose malabsorption in mouse small intestine. Gastroenterol. 2011;140:913–923. doi: 10.1053/j.gastro.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Turner JR, Buschmann MM, Romero-Calvo I, Sailer A, Shen L. The role of molecular remodeling in differential regulation of tight junction permeability. Semin Cell Dev Biol. 2014;36:204–212. doi: 10.1016/j.semcdb.2014.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raleigh DR, et al. Tight junction-associated MARVEL proteins marveld3, tricellulin, and occludin have distinct but overlapping functions. Mol Biol Cell. 2010;21:1200–1213. doi: 10.1091/mbc.E09-08-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furuse M, et al. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol. 1994;127:1617–1626. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cording J, et al. In tight junctions, claudins regulate the interactions between occludin, tricellulin and marvelD3, which, inversely, modulate claudin oligomerization. J Cell Sci. 2013;126:554–564. doi: 10.1242/jcs.114306. [DOI] [PubMed] [Google Scholar]
- 25.Fanning AS, Anderson JM. Zonula occludens-1 and -2 are cytosolic scaffolds that regulate the assembly of cellular junctions. Ann N Y Acad Sci. 2009;1165:113–120. doi: 10.1111/j.1749-6632.2009.04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katsuno T, et al. Deficiency of zonula occludens-1 causes embryonic lethal phenotype associated with defected yolk sac angiogenesis and apoptosis of embryonic cells. Molecular biology of the cell. 2008;19:2465–2475. doi: 10.1091/mbc.E07-12-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu J, et al. Early embryonic lethality of mice lacking ZO-2, but not ZO-3, reveals critical and nonredundant roles for individual zonula occludens proteins in mammalian development. Mol Cell Biol. 2008;28:1669–1678. doi: 10.1128/MCB.00891-07. MCB.00891-07 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carlton VE, et al. Complex inheritance of familial hypercholanemia with associated mutations in TJP2 and BAAT. Nat Genet. 2003;34:91–96. doi: 10.1038/ng1147. [DOI] [PubMed] [Google Scholar]
- 29.Sambrotta M, et al. Mutations in TJP2 cause progressive cholestatic liver disease. Nat Genet. 2014;46:326–328. doi: 10.1038/ng.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsumoto K, et al. Claudin 2 deficiency reduces bile flow and increases susceptibility to cholesterol gallstone disease in mice. Gastroenterol. 2014;147:1134–1145 e1110. doi: 10.1053/j.gastro.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 31.Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol. 2011;73:283–309. doi: 10.1146/annurev-physiol-012110-142150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Itallie CM, Fanning AS, Bridges A, Anderson JM. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol Biol Cell. 2009;20:3930–3940. doi: 10.1091/mbc.E09-04-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russo JM, et al. Distinct temporal-spatial roles for rho kinase and myosin light chain kinase in epithelial purse-string wound closure. Gastroenterol. 2005;128:987–1001. doi: 10.1053/j.gastro.2005.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marchiando AM, et al. The epithelial barrier is maintained by in vivo tight junction expansion during pathologic intestinal epithelial shedding. Gastroenterol. 2011;140:1208–1218. e1201–1202. doi: 10.1053/j.gastro.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenblatt J, Raff MC, Cramer LP. An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr Biol. 2001;11:1847–1857. doi: 10.1016/s0960-9822(01)00587-5. [DOI] [PubMed] [Google Scholar]
- 36.Meddings JB, Westergaard H. Intestinal glucose transport using perfused rat jejunum in vivo: model analysis and derivation of corrected kinetic constants. Clin Sci (Lond) 1989;76:403–413. doi: 10.1042/cs0760403. [DOI] [PubMed] [Google Scholar]
- 37.Sadowski DC, Meddings JB. Luminal nutrients alter tight-junction permeability in the rat jejunum: an in vivo perfusion model. Can J Physiol Pharmacol. 1993;71:835–839. doi: 10.1139/y93-125. [DOI] [PubMed] [Google Scholar]
- 38.Madara JL, Pappenheimer JR. Structural basis for physiological regulation of paracellular pathways in intestinal epithelia. J Membr Biol. 1987;100:149–164. doi: 10.1007/BF02209147. [DOI] [PubMed] [Google Scholar]
- 39.Pappenheimer JR, Reiss KZ. Contribution of solvent drag through intercellular junctions to absorption of nutrients by the small intestine of the rat. The Journal of Membrane Biology. 1987;100:123–136. doi: 10.1007/bf02209145. [DOI] [PubMed] [Google Scholar]
- 40.Turner JR. Show me the pathway! Regulation of paracellular permeability by Na(+)-glucose cotransport. Adv Drug Deliv Rev. 2000;41:265–281. doi: 10.1016/s0169-409x(00)00046-6. [DOI] [PubMed] [Google Scholar]
- 41.Turner JR, Cohen DE, Mrsny RJ, Madara JL. Noninvasive in vivo analysis of human small intestinal paracellular absorption: regulation by Na+-glucose cotransport. Dig Dis Sci. 2000;45:2122–2126. doi: 10.1023/a:1026682900586. [DOI] [PubMed] [Google Scholar]
- 42.Wisner DM, Harris LR, 3rd, Green CL, Poritz LS. Opposing regulation of the tight junction protein claudin-2 by interferon-gamma and interleukin-4. J Surg Res. 2008;144:1–7. doi: 10.1016/j.jss.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 43.Mankertz J, et al. TNFalpha up-regulates claudin-2 expression in epithelial HT-29/B6 cells via phosphatidylinositol-3-kinase signaling. Cell Tissue Res. 2009;336:67–77. doi: 10.1007/s00441-009-0751-8. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki T, Yoshinaga N, Tanabe S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J Biol Chem. 2011;286:31263–31271. doi: 10.1074/jbc.M111.238147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeissig S, et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56:61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prasad S, et al. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab Invest. 2005;85:1139–1162. doi: 10.1038/labinvest.3700316. [DOI] [PubMed] [Google Scholar]
- 47.Heller F, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterol. 2005;129:550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 48.Weber CR, et al. Epithelial myosin light chain kinase activation induces mucosal interleukin-13 expression to alter tight junction ion selectivity. J Biol Chem. 2010;285:12037–12046. doi: 10.1074/jbc.M109.064808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu AS, et al. Molecular basis for cation selectivity in claudin-2-based paracellular pores: identification of an electrostatic interaction site. J Gen Physiol. 2009;133:111–127. doi: 10.1085/jgp.200810154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li J, Zhuo M, Pei L, Yu AS. Conserved aromatic residue confers cation selectivity in claudin-2 and claudin-10b. J Biol Chem. 2013;288:22790–22797. doi: 10.1074/jbc.M113.484238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li J, Zhuo M, Pei L, Rajagopal M, Yu AS. Comprehensive cysteine-scanning mutagenesis reveals Claudin-2 pore-lining residues with different intrapore locations. J Biol Chem. 2014;289:6475–6484. doi: 10.1074/jbc.M113.536888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marcial MA, Carlson SL, Madara JL. Partitioning of paracellular conductance along the ileal crypt-villus axis: a hypothesis based on structural analysis with detailed consideration of tight junction structure-function relationships. J Membr Biol. 1984;80:59–70. doi: 10.1007/BF01868690. [DOI] [PubMed] [Google Scholar]
- 53.Fihn BM, Sjoqvist A, Jodal M. Permeability of the rat small intestinal epithelium along the villus-crypt axis: effects of glucose transport. Gastroenterol. 2000;119:1029–1036. doi: 10.1053/gast.2000.18148. [DOI] [PubMed] [Google Scholar]
- 54.Mora-Galindo J. Maturation of tight junctions in guinea-pig cecal epithelium. Cell Tissue Res. 1986;246:169–175. doi: 10.1007/BF00219014. [DOI] [PubMed] [Google Scholar]
- 55.Suenaert P, et al. Anti-tumor necrosis factor treatment restores the gut barrier in Crohn’s disease. Am J Gastroenterol. 2002;97:2000–2004. doi: 10.1111/j.1572-0241.2002.05914.x. [DOI] [PubMed] [Google Scholar]
- 56.Zolotarevsky Y, et al. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterol. 2002;123:163–172. doi: 10.1053/gast.2002.34235. [DOI] [PubMed] [Google Scholar]
- 57.Clayburgh DR, et al. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J Clin Invest. 2005;115:2702–2715. doi: 10.1172/JCI24970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Graham WV, et al. Tumor necrosis factor-induced long myosin light chain kinase transcription is regulated by differentiation-dependent signaling events. Characterization of the human long myosin light chain kinase promoter. J Biol Chem. 2006;281:26205–26215. doi: 10.1074/jbc.M602164200. [DOI] [PubMed] [Google Scholar]
- 59.Su L, et al. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterol. 2009;136:551–563. doi: 10.1053/j.gastro.2008.10.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Su L, et al. TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterol. 2013;145:407–415. doi: 10.1053/j.gastro.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marchiando AM, et al. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol. 2010;189:111–126. doi: 10.1083/jcb.200902153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buschmann MM, et al. Occludin OCEL-domain interactions are required for maintenance and regulation of the tight junction barrier to macromolecular flux. Mol Biol Cell. 2013;24:3056–3068. doi: 10.1091/mbc.E12-09-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Itallie CM, Fanning AS, Holmes J, Anderson JM. Occludin is required for cytokine-induced regulation of tight junction barriers. J Cell Sci. 2010;123:2844–2852. doi: 10.1242/jcs.065581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Westphal JK, et al. Tricellulin forms homomeric and heteromeric tight junctional complexes. Cell Mol Life Sci. 2010;67:2057–2068. doi: 10.1007/s00018-010-0313-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krug SM, et al. Tricellulin forms a barrier to macromolecules in tricellular tight junctions without affecting ion permeability. Mol Biol Cell. 2009;20:3713–3724. doi: 10.1091/mbc.E09-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ikenouchi J, Sasaki H, Tsukita S, Furuse M, Tsukita S. Loss of occludin affects tricellular localization of tricellulin. Mol Biol Cell. 2008;19:4687–4693. doi: 10.1091/mbc.E08-05-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kojima T, et al. c-Jun N-terminal kinase is largely involved in the regulation of tricellular tight junctions via tricellulin in human pancreatic duct epithelial cells. J Cell Physiol. 2010;225:720–733. doi: 10.1002/jcp.22273. [DOI] [PubMed] [Google Scholar]
- 68.Nayak G, et al. Tricellulin deficiency affects tight junction architecture and cochlear hair cells. J Clin Invest. 2013;123:4036–4049. doi: 10.1172/JCI69031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Riazuddin S, et al. Tricellulin is a tight-junction protein necessary for hearing. Am J Hum Genet. 2006;79:1040–1051. doi: 10.1086/510022. S0002-9297(07)63466-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chishti MS, et al. Splice-site mutations in the TRIC gene underlie autosomal recessive nonsyndromic hearing impairment in Pakistani families. J Hum Genet. 2008;53:101–105. doi: 10.1007/s10038-007-0209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saitou M, et al. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schulzke JD, et al. Epithelial transport and barrier function in occludin-deficient mice. Biochim Biophys Acta. 2005;1669:34–42. doi: 10.1016/j.bbamem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 73.Kitajiri SI, et al. Deafness in occludin-deficient mice with dislocation of tricellulin and progressive apoptosis of the hair cells. Biology open. 2014 doi: 10.1242/bio.20147799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Olson TS, et al. The primary defect in experimental ileitis originates from a nonhematopoietic source. J Exp Med. 2006;203:541–552. doi: 10.1084/jem.20050407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Odenwald MA, Turner JR. Intestinal permeability defects: is it time to treat? Clin Gastroenterol Hepatol. 2013;11:1075–1083. doi: 10.1016/j.cgh.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yuhan R, Koutsouris A, Savkovic SD, Hecht G. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterol. 1997;113:1873–1882. doi: 10.1016/s0016-5085(97)70006-4. [DOI] [PubMed] [Google Scholar]
- 77.Sonoda N, et al. Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands: Evidence for direct involvement of claudins in tight junction barrier. J Cell Biol. 1999;147:195–204. doi: 10.1083/jcb.147.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saitoh Y, et al. Tight junctions. Structural insight into tight junction disassembly by Clostridium perfringens enterotoxin. Science. 2015;347:775–778. doi: 10.1126/science.1261833. [DOI] [PubMed] [Google Scholar]
- 79.Hecht G, Koutsouris A, Pothoulakis C, LaMont JT, Madara JL. Clostridium difficile toxin B disrupts the barrier function of T84 monolayers. Gastroenterol. 1992;102:416–423. doi: 10.1016/0016-5085(92)90085-d. S001650859200057X [pii] [DOI] [PubMed] [Google Scholar]
- 80.Just I, et al. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature. 1995;375:500–503. doi: 10.1038/375500a0. [DOI] [PubMed] [Google Scholar]
- 81.Hollander D. Crohn’s disease--a permeability disorder of the tight junction? Gut. 1988;29:1621–1624. doi: 10.1136/gut.29.12.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pearson AD, Eastham EJ, Laker MF, Craft AW, Nelson R. Intestinal permeability in children with Crohn’s disease and coeliac disease. Br Med J (Clin Res Ed) 1982;285:20–21. doi: 10.1136/bmj.285.6334.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ukabam SO, Clamp JR, Cooper BT. Abnormal small intestinal permeability to sugars in patients with Crohn’s disease of the terminal ileum and colon. Digestion. 1983;27:70–74. doi: 10.1159/000198932. [DOI] [PubMed] [Google Scholar]
- 84.Schmitz H, et al. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterol. 1999;116:301–309. doi: 10.1016/s0016-5085(99)70126-5. [DOI] [PubMed] [Google Scholar]
- 85.Weber CR, Nalle SC, Tretiakova M, Rubin DT, Turner JR. Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Lab Invest. 2008;88:1110–1120. doi: 10.1038/labinvest.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Blair SA, Kane SV, Clayburgh DR, Turner JR. Epithelial myosin light chain kinase expression and activity are upregulated in inflammatory bowel disease. Lab Invest. 2006;86:191–201. doi: 10.1038/labinvest.3700373. [DOI] [PubMed] [Google Scholar]
- 87.Wyatt J, Vogelsang H, Hubl W, Waldhoer T, Lochs H. Intestinal permeability and the prediction of relapse in Crohn’s disease. Lancet. 1993;341:1437–1439. doi: 10.1016/0140-6736(93)90882-h. [DOI] [PubMed] [Google Scholar]
- 88.D’Inca R, et al. Intestinal permeability test as a predictor of clinical course in Crohn’s disease. Am J Gastroenterol. 1999;94:2956–2960. doi: 10.1111/j.1572-0241.1999.01444.x. [DOI] [PubMed] [Google Scholar]
- 89.Tibble JA, Sigthorsson G, Bridger S, Fagerhol MK, Bjarnason I. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterol. 2000;119:15–22. doi: 10.1053/gast.2000.8523. S001650850064458X [pii] [DOI] [PubMed] [Google Scholar]
- 90.Kiesslich R, et al. Local barrier dysfunction identified by confocal laser endomicroscopy predicts relapse in inflammatory bowel disease. Gut. 2012;61:1146–1153. doi: 10.1136/gutjnl-2011-300695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Madara JL. Maintenance of the macromolecular barrier at cell extrusion sites in intestinal epithelium: physiological rearrangement of tight junctions. J Membr Biol. 1990;116:177–184. doi: 10.1007/BF01868675. [DOI] [PubMed] [Google Scholar]
- 92.Hollander D, et al. Increased intestinal permeability in patients with Crohn’s disease and their relatives. A possible etiologic factor. Ann Intern Med. 1986;105:883–885. doi: 10.7326/0003-4819-105-6-883. [DOI] [PubMed] [Google Scholar]
- 93.Buhner S, et al. Genetic basis for increased intestinal permeability in families with Crohn’s disease: role of CARD15 3020insC mutation? Gut. 2006;55:342–347. doi: 10.1136/gut.2005.065557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Irvine EJ, Marshall JK. Increased intestinal permeability precedes the onset of Crohn’s disease in a subject with familial risk. Gastroenterol. 2000;119:1740–1744. doi: 10.1053/gast.2000.20231. [DOI] [PubMed] [Google Scholar]
- 95.Hamilton I, Cobden I, Rothwell J, Axon AT. Intestinal permeability in coeliac disease: the response to gluten withdrawal and single-dose gluten challenge. Gut. 1982;23:202–210. doi: 10.1136/gut.23.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Smecuol E, et al. Gastrointestinal permeability in celiac disease. Gastroenterol. 1997;112:1129–1136. doi: 10.1016/s0016-5085(97)70123-9. S0016508597001595 [pii] [DOI] [PubMed] [Google Scholar]
- 97.van Elburg RM, Uil JJ, Mulder CJ, Heymans HS. Intestinal permeability in patients with coeliac disease and relatives of patients with coeliac disease. Gut. 1993;34:354–357. doi: 10.1136/gut.34.3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cummins AG, et al. Improvement in intestinal permeability precedes morphometric recovery of the small intestine in coeliac disease. Clin Sci (Lond) 2001;100:379–386. [PubMed] [Google Scholar]
- 99.Vazquez-Roque MI, et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterol. 2013;144:903–911 e903. doi: 10.1053/j.gastro.2013.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hall EJ, Batt RM. Abnormal permeability precedes the development of a gluten sensitive enteropathy in Irish setter dogs. Gut. 1991;32:749–753. doi: 10.1136/gut.32.7.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Verdu EF, et al. Gliadin-dependent neuromuscular and epithelial secretory responses in gluten-sensitive HLA-DQ8 transgenic mice. Am J Physiol - Gastrointest Liver Physiol. 2008;294:G217–225. doi: 10.1152/ajpgi.00225.2007. [DOI] [PubMed] [Google Scholar]
- 102.Natividad JM, et al. Host responses to intestinal microbial antigens in gluten-sensitive mice. PLoS One. 2009;4:e6472. doi: 10.1371/journal.pone.0006472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Clemente MG, et al. Early effects of gliadin on enterocyte intracellular signalling involved in intestinal barrier function. Gut. 2003;52:218–223. doi: 10.1136/gut.52.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sander GR, Cummins AG, Henshall T, Powell BC. Rapid disruption of intestinal barrier function by gliadin involves altered expression of apical junctional proteins. FEBS Lett. 2005;579:4851–4855. doi: 10.1016/j.febslet.2005.07.066. [DOI] [PubMed] [Google Scholar]
- 105.Fasano A, et al. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet. 2000;355:1518–1519. doi: 10.1016/S0140-6736(00)02169-3. [DOI] [PubMed] [Google Scholar]
- 106.Gopalakrishnan S, et al. Larazotide acetate regulates epithelial tight junctions in vitro and in vivo. Peptides. 2012;35:86–94. doi: 10.1016/j.peptides.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 107.Kelly CP, et al. Larazotide acetate in patients with coeliac disease undergoing a gluten challenge: a randomised placebo-controlled study. Aliment Pharm Ther. 2013;37:252–262. doi: 10.1111/Apt.12147. [DOI] [PubMed] [Google Scholar]
- 108.Monsuur AJ, et al. Myosin IXB variant increases the risk of celiac disease and points toward a primary intestinal barrier defect. Nat Genet. 2005;37:1341–1344. doi: 10.1038/ng1680. [DOI] [PubMed] [Google Scholar]
- 109.Van Belzen MJ, et al. A major non-HLA locus in celiac disease maps to chromosome 19. Gastroenterol. 2003;125:1032–1041. doi: 10.1016/s0016-5085(03)01205-8. [DOI] [PubMed] [Google Scholar]
- 110.Wirth JA, Jensen KA, Post PL, Bement WM, Mooseker MS. Human myosin-IXb, an unconventional myosin with a chimerin-like rho/rac GTPase-activating protein domain in its tail. J Cell Sci. 1996;109( Pt 3):653–661. doi: 10.1242/jcs.109.3.653. [DOI] [PubMed] [Google Scholar]
- 111.Post PL, Bokoch GM, Mooseker MS. Human myosin-IXb is a mechanochemically active motor and a GAP for rho. J Cell Sci. 1998;111( Pt 7):941–950. doi: 10.1242/jcs.111.7.941. [DOI] [PubMed] [Google Scholar]
- 112.Hunt KA, et al. Lack of association of MYO9B genetic variants with coeliac disease in a British cohort. Gut. 2006;55:969–972. doi: 10.1136/gut.2005.086769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Amundsen SS, et al. Association analysis of MYO9B gene polymorphisms with celiac disease in a Swedish/Norwegian cohort. Hum Immunol. 2006;67:341–345. doi: 10.1016/j.humimm.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 114.Wolters VM, et al. Replication of genetic variation in the MYO9B gene in Crohn’s disease. Hum Immunol. 2011;72:592–597. doi: 10.1016/j.humimm.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 115.van Bodegraven AA, et al. Genetic variation in myosin IXB is associated with ulcerative colitis. Gastroenterol. 2006;131:1768–1774. doi: 10.1053/j.gastro.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 116.Cooney R, et al. Association between genetic variants in myosin IXB and Crohn’s disease. Inflamm Bowel Dis. 2009;15:1014–1021. doi: 10.1002/ibd.20885. [DOI] [PubMed] [Google Scholar]
- 117.Chandhoke SK, Mooseker MS. A role for myosin IXb, a motor-RhoGAP chimera, in epithelial wound healing and tight junction regulation. Mol Biol Cell. 2012;23:2468–2480. doi: 10.1091/mbc.E11-09-0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hegan PS, et al. Mice lacking myosin IXb, an inflammatory bowel disease susceptibility gene, have impaired intestinal barrier function and superficial ulceration in the ileum. Cytoskeleton. 2016;73:163–179. doi: 10.1002/cm.21292. [DOI] [PubMed] [Google Scholar]
- 119.Schumann M, et al. Cell polarity-determining proteins Par-3 and PP-1 are involved in epithelial tight junction defects in coeliac disease. Gut. 2012;61:220–228. doi: 10.1136/gutjnl-2011-300123. [DOI] [PubMed] [Google Scholar]
- 120.Szakal DN, et al. Mucosal expression of claudins 2, 3 and 4 in proximal and distal part of duodenum in children with coeliac disease. Virchows Arch. 2010;456:245–250. doi: 10.1007/s00428-009-0879-7. [DOI] [PubMed] [Google Scholar]
- 121.Menzies IS, et al. Abnormal intestinal permeability to sugars in villous atrophy. Lancet. 1979;2:1107–1109. doi: 10.1016/s0140-6736(79)92507-8. [DOI] [PubMed] [Google Scholar]
- 122.Keating J, et al. Intestinal absorptive capacity, intestinal permeability and jejunal histology in HIV and their relation to diarrhoea. Gut. 1995;37:623–629. doi: 10.1136/gut.37.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hermiston ML, Gordon JI. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science. 1995;270:1203–1207. doi: 10.1126/science.270.5239.1203. [DOI] [PubMed] [Google Scholar]
- 124.Jankowski JA, et al. Alterations in classical cadherins associated with progression in ulcerative and Crohn’s colitis. Lab Invest. 1998;78:1155–1167. [PubMed] [Google Scholar]
- 125.Smalley-Freed WG, et al. p120-catenin is essential for maintenance of barrier function and intestinal homeostasis in mice. J Clin Invest. 2010;120:1824–1835. doi: 10.1172/JCI41414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schneider MR, et al. A key role for E-cadherin in intestinal homeostasis and Paneth cell maturation. PLoS One. 2010;5:e14325. doi: 10.1371/journal.pone.0014325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Barrett JC, et al. Genome-wide association study of ulcerative colitis identifies three new susceptibility loci, including the HNF4A region. Nat Genet. 2009;41:1330–1334. doi: 10.1038/ng.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. 0092-8674(93)80068-P [pii] [DOI] [PubMed] [Google Scholar]
- 129.Matharu KS, et al. Toll-like receptor 4-mediated regulation of spontaneous Helicobacter-dependent colitis in IL-10-deficient mice. Gastroenterol. 2009;137:1380–1390. e1381–1383. doi: 10.1053/j.gastro.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Madsen KL, et al. Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflamm Bowel Dis. 1999;5:262–270. doi: 10.1097/00054725-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 131.Madsen KL, et al. Antibiotic therapy attenuates colitis in interleukin 10 gene-deficient mice. Gastroenterol. 2000;118:1094–1105. doi: 10.1016/s0016-5085(00)70362-3. [DOI] [PubMed] [Google Scholar]
- 132.Berg DJ, et al. Rapid development of colitis in NSAID-treated IL-10-deficient mice. Gastroenterol. 2002;123:1527–1542. doi: 10.1053/gast.2002.1231527. [DOI] [PubMed] [Google Scholar]
- 133.Arrieta MC, Madsen KL, Field CJ, Meddings JB. Increasing small intestinal permeability worsens colitis in the IL-10−/− mouse and prevents the induction of oral tolerance to ovalbumin. Inflamm Bowel Dis. 2015;21:8–18. doi: 10.1097/MIB.0000000000000253. [DOI] [PubMed] [Google Scholar]
- 134.Arrieta MC, Madsen K, Doyle J, Meddings J. Reducing small intestinal permeability attenuates colitis in the IL10 gene-deficient mouse. Gut. 2009;58:41–48. doi: 10.1136/gut.2008.150888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vetrano S, et al. Unique role of junctional adhesion molecule-a in maintaining mucosal homeostasis in inflammatory bowel disease. Gastroenterol. 2008;135:173–184. doi: 10.1053/j.gastro.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 136.Wang F, et al. IFN-gamma-induced TNFR2 expression is required for TNF-dependent intestinal epithelial barrier dysfunction. Gastroenterol. 2006;131:1153–1163. doi: 10.1053/j.gastro.2006.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Brown GR, et al. Tumor necrosis factor inhibitor ameliorates murine intestinal graft-versus-host disease. Gastroenterol. 1999;116:593–601. doi: 10.1016/s0016-5085(99)70181-2. [DOI] [PubMed] [Google Scholar]
- 138.Cooke KR, et al. Tumor necrosis factor- alpha production to lipopolysaccharide stimulation by donor cells predicts the severity of experimental acute graft-versus-host disease. J Clin Invest. 1998;102:1882–1891. doi: 10.1172/JCI4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nalle SC, et al. Recipient NK cell inactivation and intestinal barrier loss are required for MHC-matched graft-versus-host disease. Sci Transl Med. 2014;6:243ra287. doi: 10.1126/scitranslmed.3008941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nalle SC, Turner JR. Intestinal barrier loss as a critical pathogenic link between inflammatory bowel disease and graft-versus-host disease. Mucosal Immunol. 2015;8:720–730. doi: 10.1038/mi.2015.40. [DOI] [PubMed] [Google Scholar]
- 141.Bosi E, et al. Increased intestinal permeability precedes clinical onset of type 1 diabetes. Diabetologia. 2006;49:2824–2827. doi: 10.1007/s00125-006-0465-3. [DOI] [PubMed] [Google Scholar]
- 142.Meddings JB, Jarand J, Urbanski SJ, Hardin J, Gall DG. Increased gastrointestinal permeability is an early lesion in the spontaneously diabetic BB rat. Am J Physiol. 1999;276:G951–957. doi: 10.1152/ajpgi.1999.276.4.G951. [DOI] [PubMed] [Google Scholar]
- 143.Wen L, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pozzilli P, Signore A, Williams AJ, Beales PE. NOD mouse colonies around the world--recent facts and figures. Immunol Today. 1993;14:193–196. doi: 10.1016/0167-5699(93)90160-M. [DOI] [PubMed] [Google Scholar]
- 145.Watts T, et al. Role of the intestinal tight junction modulator zonulin in the pathogenesis of type I diabetes in BB diabetic-prone rats. Proc Natl Acad Sci USA. 2005;102:2916–2921. doi: 10.1073/pnas.0500178102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sapone A, et al. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes. 2006;55:1443–1449. doi: 10.2337/db05-1593. [DOI] [PubMed] [Google Scholar]
- 147.Leffler DA, et al. A Randomized, Double-Blind Study of Larazotide Acetate to Prevent the Activation of Celiac Disease During Gluten Challenge. Am J Gastroenterol. 2012;107:1554–1562. doi: 10.1038/Ajg.2012.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Avansino JR, Chen DC, Woolman JD, Hoagland VD, Stelzner M. Engraftment of mucosal stem cells into murine jejunum is dependent on optimal dose of cells. J Surg Res. 2006;132:74–79. doi: 10.1016/j.jss.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 149.Tait IS, Evans GS, Flint N, Campbell FC. Colonic mucosal replacement by syngeneic small intestinal stem cell transplantation. Am J Surg. 1994;167:67–72. doi: 10.1016/0002-9610(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 150.Sato T, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. nature07935 [pii] [DOI] [PubMed] [Google Scholar]
- 151.Yui S, et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5(+) stem cell. Nat Med. 2012;18:618–623. doi: 10.1038/nm.2695. [DOI] [PubMed] [Google Scholar]
- 152.Schepers AG, et al. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science. 2012;337:730–735. doi: 10.1126/science.1224676. [DOI] [PubMed] [Google Scholar]
- 153.Barker N, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 154.Yamaoka T, et al. Transactivation of EGF receptor and ErbB2 protects intestinal epithelial cells from TNF-induced apoptosis. Proc Natl Acad Sci USA. 2008;105:11772–11777. doi: 10.1073/pnas.0801463105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhao J, et al. R-spondin1, a novel intestinotrophic mitogen, ameliorates experimental colitis in mice. Gastroenterol. 2007;132:1331–1343. doi: 10.1053/j.gastro.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 156.Powell AE, et al. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell. 2012;149:146–158. doi: 10.1016/j.cell.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wong VW, et al. Lrig1 controls intestinal stem-cell homeostasis by negative regulation of ErbB signalling. Nat Cell Biol. 2012;14:401–408. doi: 10.1038/ncb2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dube PE, et al. Epidermal growth factor receptor inhibits colitis-associated cancer in mice. J Clin Invest. 2012;122:2780–2792. doi: 10.1172/JCI62888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.He WQ, et al. Role of myosin light chain kinase in regulation of basal blood pressure and maintenance of salt-induced hypertension. Am J Physiol - Heart Circ Physiol. 2011;301:H584–591. doi: 10.1152/ajpheart.01212.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.He WQ, et al. Myosin light chain kinase is central to smooth muscle contraction and required for gastrointestinal motility in mice. Gastroenterol. 2008;135:610–620. doi: 10.1053/j.gastro.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang WC, et al. Myosin light chain kinase is necessary for tonic airway smooth muscle contraction. J Biol Chem. 2010;285:5522–5531. doi: 10.1074/jbc.M109.062836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Schumann M, et al. Defective tight junctions in refractory celiac disease. Ann N Y Acad Sci. 2012;1258:43–51. doi: 10.1111/j.1749-6632.2012.06565.x. [DOI] [PubMed] [Google Scholar]
- 163.Noth R, et al. Increased intestinal permeability and tight junction disruption by altered expression and localization of occludin in a murine graft versus host disease model. BMC Gastroenterol. 2011;11:109. doi: 10.1186/1471-230X-11-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Becker C, Watson AJ, Neurath MF. Complex roles of caspases in the pathogenesis of inflammatory bowel disease. Gastroenterol. 2013;144:283–293. doi: 10.1053/j.gastro.2012.11.035. [DOI] [PubMed] [Google Scholar]
- 165.Washington K, Jagasia M. Pathology of graft-versus-host disease in the gastrointestinal tract. Hum Pathol. 2009;40:909–917. doi: 10.1016/j.humpath.2009.04.001. S0046-8177(09)00118-X [pii] [DOI] [PubMed] [Google Scholar]
- 166.Andre F, et al. Assessment of the lactulose-mannitol test in Crohn’s disease. Gut. 1988;29:511–515. doi: 10.1136/gut.29.4.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Peeters M, et al. Increased permeability of macroscopically normal small bowel in Crohn’s disease. Dig Dis Sci. 1994;39:2170–2176. doi: 10.1007/BF02090367. [DOI] [PubMed] [Google Scholar]
- 168.Sundqvist T, Magnusson KE, Sjodahl R, Stjernstrom I, Tagesson C. Passage of molecules through the wall of the gastrointestinal tract. II Application of low-molecular weight polyethyleneglycol and a deterministic mathematical model for determining intestinal permeability in man. Gut. 1980;21:208–214. doi: 10.1136/gut.21.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Arnott ID, Kingstone K, Ghosh S. Abnormal intestinal permeability predicts relapse in inactive Crohn disease. Scand J Gastroenterol. 2000;35:1163–1169. doi: 10.1080/003655200750056637. [DOI] [PubMed] [Google Scholar]
- 170.May GR, Sutherland LM, Meddings JB. Lactulose/mannitol permeability is increased in relatives of patients with Crohn’s disease. Gastroenterol. 1992;102:A934. doi: 10.1016/0016-5085(93)90638-s. [DOI] [PubMed] [Google Scholar]
- 171.Smecuol E, et al. Sugar tests detect celiac disease among first-degree relatives. Am J Gastroenterol. 1999;94:3547–3552. doi: 10.1111/j.1572-0241.1999.01645.x. [DOI] [PubMed] [Google Scholar]
- 172.Secondulfo M, et al. Ultrastructural mucosal alterations and increased intestinal permeability in non-celiac, type I diabetic patients. Dig Liver Dis. 2004;36:35–45. doi: 10.1016/j.dld.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 173.Poritz LS, et al. Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis. J Surg Res. 2007;140:12–19. doi: 10.1016/j.jss.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 174.Yan Y, et al. Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulfate induced colitis. PLoS One. 2009;4:e6073. doi: 10.1371/journal.pone.0006073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lee AS, et al. Gut barrier disruption by an enteric bacterial pathogen accelerates insulitis in NOD mice. Diabetologia. 2010;53:741–748. doi: 10.1007/s00125-009-1626-y. [DOI] [PubMed] [Google Scholar]
- 176.Johansson JE, Ekman T. Gut toxicity during hemopoietic stem cell transplantation may predict acute graft-versus-host disease severity in patients. Dig Dis Sci. 2007;52:2340–2345. doi: 10.1007/s10620-006-9404-x. [DOI] [PubMed] [Google Scholar]