Abstract
Purpose
To evaluate the association between acute respiratory distress syndrome (ARDS) onset time and prognosis.
Methods
Patients with moderate to severe ARDS (N = 876) were randomly assigned into derivation (N = 520) and validation (N = 356) datasets. Both 28-day and 60-day survival times after ARDS onset were analyzed. A data-driven cutoff point between early- and late-onset ARDS was determined on the basis of mortality risk effects of onset times. We estimated the hazard ratio (HR) and odds ratio (OR) of late-onset ARDS using a multivariate Cox proportional hazards model of survival time and a multivariate logistic regression model of mortality rate, respectively.
Results
Late-onset ARDS, defined as onset over 48 h after intensive care unit (ICU) admission (N = 273, 31%), was associated with shorter 28-day survival time: HR = 2.24, 95% CI 1.48–3.39, P = 1.24 × 10−4 (derivation); HR = 2.16, 95% CI 1.33–3.51, P = 1.95 × 10−3 (validation); and HR = 2.00, 95% CI 1.47–2.72, P = 1.10 × 10−5 (combined dataset). Late-onset ARDS was also associated with shorter 60-day survival time: HR = 1.70, 95% CI 1.16–2.48, P = 6.62 × 10−3 (derivation); HR = 1.78, 95% CI 1.15–2.75, P = 9.80 × 10−3 (validation); and HR = 1.59, 95% CI 1.20–2.10, P = 1.22 × 10−3 (combined dataset). Meanwhile, late-onset ARDS was associated with higher 28-day mortality rate (OR = 1.46, 95% CI 1.04–2.06, P = 0.0305) and 60-day mortality rate (OR = 1.44, 95% CI 1.03–2.02, P = 0.0313).
Conclusions
Late-onset moderate to severe ARDS patients had both shorter survival time and higher mortality rate in 28-day and 60-day observations.
Keywords: ARDS, Early and late onset, Overall survival time, Mortality rate, Cox proportional hazards model, Logistic regression model
Introduction
Acute respiratory distress syndrome (ARDS) is life-threatening [1] and the major cause of morbidity and mortality in intensive care units (ICU) [2]. Few effective therapies exist for ARDS [3–7]. Even with protective ventilation [8], the mortality rate remains over 40% [9], resulting in a high public health impact [10].
ARDS patients with different predisposing conditions (e.g., sepsis or trauma) may have clinical and biological heterogeneity [11, 12]. Different ARDS onset times might also affect prognosis [13]. The association between ARDS onset time and prognosis (survival time or mortality rate) has been widely discussed for decades [14–18]. Late-onset ARDS patients have a slightly higher mortality rate than early-onset patients [15, 17, 18], though only one study reported a statistically significant mortality difference [15]. Further, late-onset patients may die significantly faster after onset of ARDS [14, 15], but the findings have not been consistent across studies [16]. Thus, the association between ARDS onset time and prognosis remains unclear.
We therefore conducted a two-stage study to determine the cutoff point between early- and late-onset ARDS and then test the association between ARDS onset time and prognosis.
Materials and methods
Study populations
ARDS patients were collected from the Molecular Epidemiology of ARDS (MEARDS) prospective cohort study (ClinicalTrials.gov Identifier: NCT00006496). All patients were recruited at the ICUs of Massachusetts General Hospital (MGH) and Beth Israel Deaconess Medical Center (BIDMC) between 1998 and 2014. Study population and procedures were described previously [19]. Briefly, eligible samples enrolled in MEARDS were critically ill patients with at least one predisposing condition for ARDS: bacteremia, sepsis, septic shock, pneumonia, multiple fractures, pulmonary contusion, aspiration, or massive blood transfusion, and without any of the exclusion criteria: age under 18 years, HIV infection, diffuse alveolar hemorrhage, chronic lung diseases other than chronic obstructive pulmonary disease or asthma, directive to withhold intubation, immunosuppression not secondary to corticosteroid, treatment with granulocyte colony-stimulating factor, cytotoxic therapy, and solid organ or bone marrow transplant. Two physicians performed independent X-ray readings. A third physician, who was blinded to former interpretations, reviewed any inconsistent decisions. We collected demographics, past history, vital signs, hematology, chemistry, and performed frequent arterial blood gas (ABG) analysis and chest X-ray (CXR) examinations within 24 h of admission. After 24 h, patients were followed daily for the development of ARDS. All ARDS patients met the Berlin definition [20]. ARDS is categorized into three grades. Optimal therapeutic approaches are based on their ARDS severity [21, 22]. We only enrolled moderate to severe ARDS patients in MEARDS study since they have higher mortality compared to mild ARDS patients [20]. Moderate to severe ARDS patients were retained in analyses only if they (1) had onset of ARDS after ICU admission; and (2) received initial ABG and CXR examinations within 24 h of ICU admission.
The study was reviewed and approved by institutional review boards of Harvard School of Public Health, MGH and BIDMC. All participants or their surrogate care providers gave written informed consent.
Clinical outcomes
Both 28-day and 60-day overall survival times (time to death) after ARDS onset were analyzed [15], which were calculated from ARDS onset to the 28th and 60th day of admission, respectively [23–25]. Survival time for patients alive or lost to follow-up at the end of study was a censored value.
ARDS onset time definition
ARDS onset time was defined as the interval from admission to the time all Berlin diagnosis criteria (including ABG and CXR criteria) were met. ARDS onset time within 24 h of ICU admission was defined as day 0, and 24 h to 48 h was day 1, etc. Since all ARDS patients had onset within 1 week, the onset time ranged from day 0 to day 6.
Statistical analysis
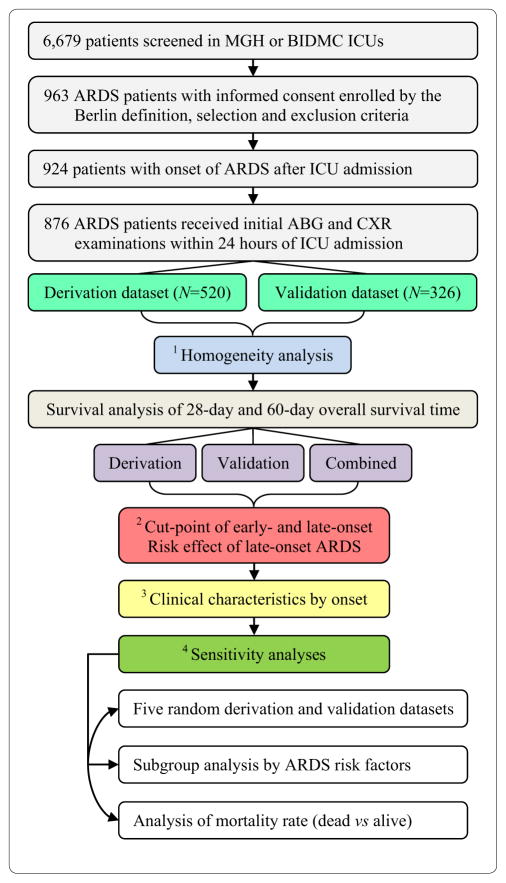
We applied a two-stage design (Fig. 1). ARDS patients were randomly assigned to derivation (N = 520, ≈60%) and validation (N = 356, ≈40%) datasets. First, we conducted a homogeneity analysis to compare patients in both datasets, including all collected demographic and clinical variables, as well as lung injury score [26], multiple organ dysfunction (MODS) score [27] and Acute Physiology and Chronic Health Evaluation (APACHE) III score [28]. Second, we used a multivariate Cox proportional hazards model of survival time to estimate the hazards ratio (HR) of onset time. In the derivation data-set, the data-driven cutoff point between early- and late-onset ARDS was determined on the basis of the HR of onset time. Then, association between late-onset ARDS and survival time was tested. We replicated these findings in the validation dataset. Third, we explored clinical characteristics associated with late-onset ARDS. Finally, we performed three types of sensitivity analyses to assess robustness of the association between ARDS onset time and prognosis: (1) survival analysis in five randomly generated derivation (60%) and validation (40%) datasets; (2) subgroup survival analysis by predisposing conditions of ARDS; and (3) analysis of death status (dead vs alive) using multivariate logistic regression models of mortality rate. The effect size of late-onset ARDS was measured by odds ratio (OR). Detailed statistical methods are provided in the online supplement.
Fig. 1.
Diagram of ARDS patient enrollment and statistical analyses
All P values were two-sided. The significance level was set to be 5%. STATA 14.0 (StataCorp, College Station, Texas, USA) was used to perform all statistical analyses.
Results
Homogeneity analysis for patients in derivation and validation datasets
We enrolled 876 qualified moderate to severe ARDS patients on the basis of predefined criteria (Fig. 1, Table S1). Patients were comparable for all variables across derivation and validation datasets (Table 1, Table S2). The main triggers of ARDS were sepsis, septic shock, or pneumonia. The distribution of ARDS onset time was positively skewed and comparable between datasets (Fig. S1).
Table 1.
Description of ARDS patients in the derivation dataset, the validation dataset, and the combined dataset
| Variable | Derivation dataset (N = 520) | Validation dataset (N = 356) | Combined dataset (N = 876) | P |
|---|---|---|---|---|
| Age | 56.71 ± 17.54 | 58.54 ± 18.10 | 57.45 ± 17.78 | 0.1362 |
| Gender | ||||
| Male | 329 (63.27%) | 221 (62.08%) | 550 (62.79%) | 0.7203 |
| Female | 191 (36.73%) | 135 (37.92%) | 326 (37.21%) | |
| Ethnicity | ||||
| White | 470 (90.38%) | 318 (89.33%) | 788 (89.95%) | 0.2999 |
| Black | 20 (3.85%) | 20 (5.62%) | 40 (4.57%) | |
| Hispanic | 22 (4.23%) | 13 (3.65%) | 35 (4.00%) | |
| Asian | 7 (1.35%) | 2 (0.56%) | 9 (1.03%) | |
| Other | 1 (0.19%) | 3 (0.84%) | 4 (0.46%) | |
| Body mass index | 28.82 ± 8.93 | 28.55 ± 9.19 | 28.71 ± 9.04 | 0.6750 |
| Smoking status | ||||
| Never | 143 (32.06%) | 104 (33.88%) | 247 (32.80%) | 0.2484 |
| Former | 129 (28.92%) | 101 (32.90%) | 230 (30.54%) | |
| Current | 174 (39.01%) | 102 (33.22%) | 276 (36.65%) | |
| Alcohol abuse within 12 months | 90 (17.31%) | 57 (16.01%) | 147 (16.78%) | 0.6140 |
| Prednisone prior ICU | 36 (6.92%) | 25 (7.02%) | 61 (6.96%) | 0.9547 |
| Vasopressors within 24 h | 391 (75.19%) | 258 (72.47%) | 649 (74.09%) | 0.3668 |
| Patient source | ||||
| Operating room | 69 (13.27%) | 46 (12.92%) | 115 (13.13%) | 0.6865 |
| Recovery room | 4 (0.77%) | 2 (0.56%) | 6 (0.68%) | |
| Emergency room | 182 (35.00%) | 126 (35.39%) | 308 (35.16%) | |
| Floor | 130 (25.00%) | 104 (29.21%) | 234 (26.71%) | |
| Other special care unit | 23 (4.42%) | 12 (3.37%) | 35 (4.00%) | |
| Other hospital floor | 112 (21.54%) | 66 (18.54%) | 178 (20.32%) | |
| ICU readmission | 52 (10.00%) | 38 (10.67%) | 90 (10.27%) | 0.7469 |
| ARDS risk factors | ||||
| Bacteremia | 90 (17.31%) | 50 (14.04%) | 140 (15.98%) | 0.1955 |
| Sepsis | 449 (86.35%) | 307 (86.24%) | 756 (86.30%) | 0.9628 |
| Septic shock | 360 (69.23%) | 235 (66.01%) | 595 (67.92%) | 0.3160 |
| Pneumonia | 393 (75.58%) | 258 (72.47%) | 651 (74.32%) | 0.3015 |
| Multiple fractures | 20 (3.85%) | 14 (3.93%) | 34 (3.88%) | 0.9481 |
| Pulmonary contusion | 23 (4.42%) | 20 (5.62%) | 43 (4.91%) | 0.4214 |
| Aspiration | 55 (10.58%) | 32 (8.99%) | 87 (9.93%) | 0.4402 |
| Multiple transfusion | 39 (7.50%) | 32 (8.99%) | 71 (8.11%) | 0.4278 |
| ARDS onset day | ||||
| 0 | 242 (46.54%) | 139 (39.04%) | 381 (43.49%) | 0.0966 |
| 1 | 135 (25.96%) | 87 (24.44%) | 222 (25.34%) | |
| 2 | 53 (10.19%) | 55 (15.45%) | 108 (12.33%) | |
| 3 | 37 (7.12%) | 30 (8.43%) | 67 (7.65%) | |
| 4 | 27 (5.19%) | 20 (5.62%) | 47 (5.37%) | |
| 5 | 13 (2.50%) | 16 (4.49%) | 29 (3.31%) | |
| 6 | 13 (2.50%) | 9 (2.53%) | 22 (2.51%) | |
| Lung injury score | 2.53 ± 0.76 | 2.44 ± 0.81 | 2.50 ± 0.78 | 0.0897 |
| APACHE III score | 76.63 ± 25.26 | 74.37 ± 22.42 | 75.71 ± 24.16 | 0.1650 |
| ICU duration days | 13.00 (7.00–21.00) | 14.00 (8.00–21.00) | 13.00 (8.00–21.00) | 0.2388 |
| ICU 28-day ventilator-free days | 2.00 (0.00–4.00) | 2.00 (0.00–4.00) | 2.00 (0.00–4.00) | 0.5795 |
Normal and non-normal distributed continuous variables were described using mean ± standard and median (25th quantile–75th quantile), respectively. ARDS onset within 24 h of ICU admission was defined as day 0, and 24–48 h was day 1, etc. ARDS patients between two phases are comparable if P > 0.05
APACHE Acute Physiology and Chronic Health Evaluation
Cutoff point between early- and late-onset moderate to severe ARDS
ARDS onset time was included as a dummy variable in the statistical model of 28-day survival time. Adjusted HRs of day 0 and day 1 ranged from 1 to 1.11, indicating weak risk effects. However, HRs of days 2–6 increased substantially, ranging from 1.46 to 2.86, indicating high risk effects (Table S3). Thus, early-onset was defined as day 0 to day 1 (within 48 h of admission), and late-onset was defined as day 2 to day 6 (over 48 h after admission). The onset time effect size pattern was similar in the derivation dataset, the validation dataset, and the combined dataset, indicating that this cutoff point was robust (Fig. S2a). This pattern was confirmed by using penalized smoothing splines in the statistical model (Fig. S3a). We also observed a similar pattern using 60-day survival time (Table S3, Fig. S2b, Fig. S3b). Our cutoff point was concordant with that adopted in several other studies [14–16, 18].
Risk effect of late-onset moderate to severe ARDS on survival time
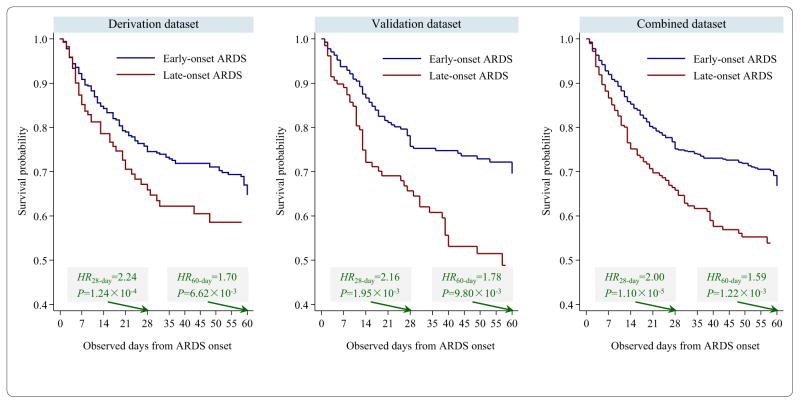
Late-onset ARDS was associated with shorter 28-day survival time: HR = 2.24, 95% CI 1.48–3.39, P = 1.24 × 10−4 (derivation); HR = 2.16, 95% CI 1.33–3.51, P = 1.95 × 10−3 (validation); and HR = 2.00, 95% CI 1.47–2.72, P = 1.10 × 10−5 (combined dataset) (Table 2). Late-onset ARDS was also associated with shorter 60-day survival time: HR = 1.70, 95% CI 1.16–2.48, P = 6.62 × 10−3 (derivation); HR = 1.78, 95% CI 1.15–2.75, P = 9.80 × 10−3 (validation); and HR = 1.20, 95% CI 1.22–2.10, P = 1.22 × 10−3 (combined dataset). Kaplan–Meier survival curve demonstrates that late-onset ARDS patients died more quickly than early-onset ARDS patients (Fig. 2).
Table 2.
Association between ARDS onset time and survival time in the derivation dataset, the validation dataset, and the combined dataset
| Dataset | Onset stage | N | % | 28-day survival time
|
60-day survival time
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||||||
| Derivation | Early | 377 | 72.50 | Ref | Ref | ||||||
| Late | 143 | 27.50 | 2.24 | 1.48 | 3.39 | 1.24 × 10−4 | 1.70 | 1.16 | 2.48 | 6.62 × 10−3 | |
|
| |||||||||||
| Validation | Early | 226 | 63.48 | Ref | |||||||
| Late | 130 | 36.52 | 2.16 | 1.33 | 3.51 | 1.95 × 10−3 | 1.78 | 1.15 | 2.75 | 9.80 × 10−3 | |
|
| |||||||||||
| Combined | Early | 603 | 68.84 | Ref | |||||||
| Late | 273 | 31.16 | 2.00 | 1.47 | 2.72 | 1.10 × 10−5 | 1.59 | 1.20 | 2.10 | 1.22 × 10−3 | |
HR hazard ratio, 95% CI 95% confidence interval, HR 95% CI and P values were adjusted for variables collected within 24 h of ICU admission (APACHE III score, septic shock, pneumonia, multiple fractures, transfusion within 7 days prior to admission, vasopressors prior to admission, intubation, ventilation, and highest potassium level), variables collected at time of ARDS onset (PaO2/FiO2, platelet count, and creatinine level), and ICU 28-day ventilation-free days in the Cox proportional hazards model; ARDS onset within 48 h after admission was defined as early-onset, and otherwise as late-onset stage
Fig. 2.
Kaplan–Meier curves of survival time after ARDS onset for early- and late-onset ARDS patients. ARDS onset within 48 h after admission was defined as early-onset, and otherwise as late-onset stage. HR and P value were derived from multivariate Cox proportional hazards model
However, for Cox proportional hazards model of 28-day and 60-day survival time, ARDS onset time only improved 1.3 and 1.7% of model prediction accuracy, having an average time-dependent area under receiver operating characteristic curve (AUC) value of 0.75 and 0.73 (Fig. S4), with other covariates (see Table 2) in the combined dataset, respectively.
Clinical characteristics by early- and late-onset moderate to severe ARDS
At time of admission, there was no significant difference between early- and late-onset patients for age, gender, ethnicity, smoking status, patient source, medical history, chronic disease, and MODS score. Most variables of vital signs, hematology, and chemistry were also comparable for early- and late-onset patients (Tables 3, S4, and S5). However, late-onset patients had less septic shock and pneumonia, and slightly more multiple fractures. Since late-onset patients had higher systolic blood pressure and mean arterial pressure, fewer of them received vasopressors before admission. Late-onset patients had higher PaO2/FiO2 ratios and lower lung injury scores, and fewer of them received intubation and ventilation at time of ICU admission. Generally, late-onset patients had lower APACHE III score.
Table 3.
Comparison of variables collected at time of ICU admission and ARDS onset for early- and late-onset ARDS patients
| Variable | Early-onset ARDS (N = 603) | Late-onset ARDS (N = 273) | P |
|---|---|---|---|
| At time of ICU admission | |||
| Age | 56.93 ± 17.99 | 58.62 ± 17.28 | 0.1922 |
| Gender (male) | 384 (63.68%) | 166 (60.81%) | 0.4148 |
| Ethnicity | |||
| White | 539 (89.39%) | 249 (91.21%) | 0.3507 |
| Black | 25 (4.15%) | 15 (5.49%) | |
| Hispanic | 29 (4.81%) | 6 (2.20%) | |
| Asian | 7 (1.16%) | 2 (0.73%) | |
| Smoking status | |||
| Never | 178 (34.17%) | 69 (29.74%) | 0.4581 |
| Former | 154 (29.56%) | 76 (32.76%) | |
| Current | 189 (36.28%) | 87 (37.50%) | |
| Other | 3 (0.50%) | 1 (0.37%) | |
| Year of sample enrollment | |||
| 1995–2000 | 33 (5.47%) | 20 (7.33%) | 0.2173 |
| 2001–2005 | 229 (37.98%) | 118 (43.22%) | |
| 2006–2010 | 254 (42.12%) | 97 (35.53%) | |
| 2011–2015 | 87 (14.43%) | 38 (13.92%) | |
| ARDS risk factors | |||
| Bacteremia | 98 (16.25%) | 42 (15.38%) | 0.7455 |
| Sepsis | 522 (86.57%) | 234 (85.71%) | 0.7338 |
| Septic shock | 434 (71.97%) | 161 (58.97%) | 0.0001 |
| Pneumonia | 464 (76.95%) | 187 (68.50%) | 0.0080 |
| Multiple fractures | 18 (2.99%) | 16 (5.86%) | 0.0412 |
| Pulmonary contusion | 28 (4.64%) | 15 (5.49%) | 0.5892 |
| Aspiration | 65 (10.78%) | 22 (8.06%) | 0.2124 |
| Multiple transfusion | 44 (7.30%) | 27 (9.89%) | 0.1927 |
| Prednisone prior admission | 46 (7.63%) | 15 (5.49%) | 0.2504 |
| Vasopressors prior admission | 468 (77.61%) | 181 (66.30%) | 0.0004 |
| Transfusion within 7 days prior admission | 305 (51.35%) | 158 (59.40%) | 0.0286 |
| Intubation within 24 h of admission | 473 (78.44%) | 170 (62.27%) | <0.0001 |
| Ventilation within 24 h of admission | 587 (97.35%) | 198 (72.53%) | <0.0001 |
| Ventilation days before ARDS onset | 1.00 (1.00–2.00) | 3.00 (0.00–4.00) | <0.0001 |
| Lung injury score | 2.63 ± 0.68 | 2.21 ± 0.91 | <0.0001 |
| APACHE III score | 77.18 ± 24.56 | 72.45 ± 22.94 | 0.0072 |
| Multiple organ dysfunction score | 6.27 ± 2.55 | 6.03 ± 2.83 | 0.2152 |
| At time of ARDS onset | |||
| Multiple organ dysfunction score | 5.98 ± 2.43 | 5.93 ± 2.60 | 0.7905 |
| Pulmonary: PaO2/FiO2 | 103.00 (72.00–137.00) | 118.00 (87.00–153.00) | <0.0001 |
| Hematologic: platelet (109/L) | 184.50 (114.00–288.50) | 151.50 (82.00–248.00) | 0.0002 |
| Thrombocytopenia | 224 (38.16%) | 132 (49.25%) | 0.0023 |
| Renal: creatinine (mg/dL) | 1.27 (0.81–2.10) | 1.10 (0.70–2.06) | 0.0203 |
| Hepatic: total bilirubin (mg/dL) | 0.80 (0.50–1.60) | 0.90 (0.50–2.40) | 0.1241 |
| Cardiovascular: vasopressors | 417 (73.81%) | 169 (69.60%) | 0.0691 |
| ARDS severity | |||
| Moderate | 318 (52.74%) | 182 (66.67%) | <0.0001 |
| Severe | 285 (47.26%) | 91 (33.33%) | |
Normal and non-normal distributed continuous variables were described using mean ± standard and median (25th quantile–75th quantile), respectively; ARDS onset within 48 h after admission was defined as early-onset, and otherwise as late-onset stage
Compared to early-onset patients, late-onset patients had greater decline of PaO2/FiO2 ratio from time of admission to time of ARDS onset (Table S5). Even though MODS scores were comparable between early- and late-onset patients, late-onset patients had higher PaO2/FiO2 ratios (more moderate ARDS), but lower platelet counts, more thrombocytopenia, and lower creatinine levels (Table 3, Table S6).
Sensitivity analyses
Association between ARDS onset time and 28-day survival time, as well as 60-day survival time, was significant in five randomly generated derivation and validation datasets (Table S7). In subgroup analysis by different ARDS risk factors, the association remained significant in ARDS patients with major triggers: sepsis, septic shock, and pneumonia (Table S8). Late-onset patients had both higher 28-day mortality (OR = 1.46, 95% CI 1.04–2.06, P = 0.0305) and 60-day mortality (OR = 1.44, 95% CI 1.03–2.02, P = 0.0313) (Table S9).
Discussion
Conflicting findings in previous studies of ARDS onset time are probably due to several factors. First, all studies had a small sample size (N < 400). Second, studies differed in ARDS definitions (American-European Consensus Conference [14–17] or Berlin [18]) and late-onset ARDS definition (over 48 h [14–16, 18] or 24 h [17] of admission). Third, outcome measures differed (in-hospital [18], out-of-hospital [16, 17] mortality or both [14, 15]). Finally, studies included ARDS patients with different major risk factors (trauma [14, 18], sepsis or pneumonia [15–17]).
We performed a two-stage association study of ARDS onset time and prognosis (survival time and mortality rate) using well-defined ARDS patients. We not only verified a cutoff point between early- and late-onset ARDS but also demonstrated that late-onset patients had both shorter survival time and higher mortality rate in 28-day and 60-day observations using large sample size.
The proportion of patients experiencing ARDS onset within 2 days in our study (69%) differs from that in the LUNG-SAFE study (93%) [29]. However, they might be incomparable because of the following factors. First, the LUNG-SAFE study defined onset day from the first day the acute hypoxemic respiratory failure criteria were satisfied, irrespective of ICU admission date, as used in our study. Thus, the onset set time point in the LUNG-SAFE study is later than ours, and some early-onset patients would likely be late-onset ones if their starting time point was earlier. Second, all our ARDS patients were diagnosed by clinical physicians. The LUNG-SAFE study used a computer algorithm to define ARDS, and 40% of ARDS cases were not diagnosed by clinicians. Finally, the LUNG-SAFE study enrolled all grades of ARDS patients, while we focused on moderate to severe ARDS. In the LUNG-SAFE study, there are 70% moderate to severe ARDS patients. Thus, there might be 65% (93% × 70%) moderate to severe ARDS patients within 2 days of onset, which is similar to our proportion.
Croce first reported that the early- and late-onset post-traumatic ARDS are two distinct clinical entities, and late-onset patients die significantly faster within 1 week of onset [14]. Our results were consistent with another study which focused on moderate to severe ARDS and an enrollment period of about 1.5 years [15]. In contrast, another study that enrolled all grades of ARDS patients over a 2-week period did not report a significant association [16].
We found that 31% of patients were likely to have late-onset ARDS. Late-onset patients had both shorter survival time and higher mortality rate in comparison with early-onset patients. Uncertainty remains regarding how diverse initial insults result in a sequence of events culminating in the clinical syndrome of ARDS. More research is needed to understand the mechanism of late-onset of ARDS and why late-onset patients have a worse prognosis.
Early- and late-onset ARDS patients may have different trajectories of recovery and dysfunction after ARDS onset [30]. In a “Big Hit” trajectory, ARDS patients have an acute loss of function during their critical illness, from which they may gradually recover. They may survive longer and have lower mortality. In a “Slow Burn” trajectory, patients have slight loss of function in the beginning, but keep more rapid function decline persistently later, resulting in worse prognosis.
At the time of both ICU admission and ARDS onset, early-onset patients had a lower PaO2/FiO2 ratio, indicating severe lung injury. Previous study also indicated that early-onset patients might be characterized by a higher degree of initial vascular injury and disrupted alveolar capillary barrier integrity [18]. On the other hand, early-onset patients had better prognosis. The available evidence thus suggests that early-onset patients may be on a Big Hit trajectory, while late-onset patients might be on a Slow Burn trajectory.
Our previous study indicated that low platelet count was associated with increased ARDS risk [31] and poor survival [32]. Here, we observed that late-onset patients, who had significantly lower platelet count at the time of ARDS onset, had worse prognosis. Platelets play a role in conjunction with fibrinogen to mediate endothelial damage through multiple signal transduction pathways [33]. If patients with ARDS develop thrombocytopenia or systemic coagulation disorders, such as platelet consumption or disseminated intravascular coagulation (DIC), they are at increased risk for a poor prognosis [34]. Our study suggests that late-onset patients with low platelet count might be an ideal population for anticoagulant treatment [35].
Our study has several advantages. First, to our knowledge, this is the first two-stage association study of ARDS onset time with the largest sample size to date. We observed consistent significant associations in two independent groups of samples. Thus, we had a lower probability of a false positive result. Second, the cutoff point between early- and late-onset ARDS remains consistent in all analyses, which is a robust finding. Third, we only recruited ARDS patients who met the Berlin definition. Fourth, we performed a comprehensive analysis using outcomes including both in-hospital and out-of-hospital survival time and mortality rate. Late-onset ARDS was significantly associated with all outcomes. Finally, we found significant associations in all scenarios of sensitivity analyses, which increased our confidence in the results. All results indicate that late-onset ARDS might be an independent risk factor of prognosis.
We also acknowledge limitations in this study. First, we cannot identify the molecular mechanisms of late-onset ARDS and why it is associated with worse prognosis in this clinical epidemiology study. Though we proposed possible explanations based on available evidence, further studies are warranted. Second, it is possible that some patients were already on the way to developing ARDS at the time of enrollment [17], or died before ARDS diagnosis. It is difficult to estimate the onset time from initial insult. Hence, all the previous studies calculated onset time from hospital [14, 15, 17] or ICU admission [16, 18, 36]. In our study, the onset was also defined from ICU admission to the date all Berlin definition criteria were met. Since ABG and CXR tests might delay ARDS diagnosis, only those patients who received their initial diagnosis within 24 h of admission were retained in the final analysis. Third, there is a possibility that patients developed ARDS as a result of a secondary insult, instead of initial risk factors, such as blood transfusions, amiodarone treatment, high tidal volume mechanical ventilation, and aspiration [37]. However, the real trigger of ARDS for each patient is difficult to distinguish. Fourth, ventilation before ARDS onset might affect survival and mortality. However, ventilation days for patients before ICU admission were not available. We could not evaluate severity of illness for each patient at time of ARDS onset based on the study design either. It would be more reasonable to adjust for ventilation days before ICU admission and APACHE III score at time of onset. Finally, we may miss some important but unmeasured variables (e.g., tidal volumes, plateau pressure, driving pressure, invasive ventilation, or prone positioning) that are correlated with mortality [6, 29], since our study was designed and initialized at pre-electronic medical record period. Additional data may help to improve statistical model prediction accuracy and explain mechanisms of late-onset ARDS and its worse prognosis. Further external studies exclusively designed for monitoring ARDS onset are warranted to confirm our findings.
Conclusion
This two-stage association study demonstrated that late-onset moderate to severe ARDS patients with onset time over 48 h after ICU admission had both shorter survival time and higher mortality rate during 28-day and 60-day observations.
Supplementary Material
Take-home message.
We conducted a two-stage study to determine the data-driven cutoff point between early- and late-onset moderate to severe ARDS and then tested the association between ARDS onset time and prognosis. This, the largest recent study, demonstrated that late-onset moderate to severe ARDS patients with onset time over 48 h after ICU admission had both shorter survival time and higher mortality rate.
Acknowledgments
We would like to thank Peggy S. Lai and Andrea Shafer (Pulmonary and Critical Care Division, Department of Medicine, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA) for comments on the original manuscript and study coordination, respectively. We appreciated Xihong Lin (Department of Biostatistics, Harvard School of Public Health, Boston, MA, USA) for reviewing overall statistical analysis and Elizabeth Anne Loehrer (Department of Environmental Health, Harvard School of Public Health, Boston, MA, USA) for grammar edits. We are indebted to all participants enrolled in this study. This study was supported by the National Heart, Lung, and Blood Institute (Grant R01HL060710 to D.C.C.) and National Institutes of Health (Grant P30 ES000002 to D.C.C), and partly supported by the National Science Foundation of China (Grant 81402763 to R.Z., 81402764 to Y.W. and 81530088 to F.C.).
Footnotes
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
The online version of this article (doi:10.1007/s00134-016-4638-3) contains supplementary material, which is available to authorized users.
References
- 1.Gotts JE, Matthay MA. Treating ARDS: new hope for a tough problem. Lancet Respir Med. 2014;2:84–85. doi: 10.1016/S2213-2600(13)70285-6. [DOI] [PubMed] [Google Scholar]
- 2.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 3.Spragg RG, Lewis JF, Walmrath HD, Johannigman J, Bellingan G, Laterre PF, Witte MC, Richards GA, Rippin G, Rathgeb F, Hafner D, Taut FJ, Seeger W. Effect of recombinant surfactant protein C-based surfactant on the acute respiratory distress syndrome. N Engl J Med. 2004;351:884–892. doi: 10.1056/NEJMoa033181. [DOI] [PubMed] [Google Scholar]
- 4.Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, Thompson BT, Ancukiewicz M. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354:1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 5.Papazian L, Forel J-M, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, Jaber S, Arnal J-M, Perez D, Seghboyan J-M, Constantin J-M, Courant P, Lefrant J-Y, Guérin C, Prat G, Morange S, Roch A. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 6.Guérin C, Reignier J, Richard J-C, Beuret P, Gacouin A, Boulain T, Mercier E, Badet M, Mercat A, Baudin O, Clavel M, Chatellier D, Jaber S, Rosselli S, Mancebo J, Sirodot M, Hilbert G, Bengler C, Richecoeur J, Gainnier M, Bayle F, Bourdin G, Leray V, Girard R, Baboi L, Ayzac L. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 7.Khandelwal N, Hough CL, Bansal A, Veenstra DL, Treggiari MM. Long-term survival in patients with severe acute respiratory distress syndrome and rescue therapies for refractory hypoxemia. Crit Care Med. 2014;42:1610–1618. doi: 10.1097/CCM.0000000000000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 9.Villar J, Blanco J, Anon JM, Santos-Bouza A, Blanch L, Ambros A, Gandia F, Carriedo D, Mosteiro F, Basaldua S, Fernandez RL, Kacmarek RM. The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med. 2011;37:1932–1941. doi: 10.1007/s00134-011-2380-4. [DOI] [PubMed] [Google Scholar]
- 10.Camporota L. The public health burden of acute lung injury. Thorax. 2006;61:56. [Google Scholar]
- 11.Gattinoni L, Pelosi P, Suter PM, Pedoto A, Vercesi P, Lissoni A. Acute respiratory distress syndrome caused by pulmonary and extrapulmonary disease. Different syndromes? Am J Respir Crit Care Med. 1998;158:3–11. doi: 10.1164/ajrccm.158.1.9708031. [DOI] [PubMed] [Google Scholar]
- 12.Calfee CS, Eisner MD, Ware LB, Thompson BT, Parsons PE, Wheeler AP, Korpak A, Matthay MA. Trauma-associated lung injury differs clinically and biologically from acute lung injury due to other clinical disorders. Crit Care Med. 2007;35:2243–2250. doi: 10.1097/01.ccm.0000280434.33451.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruyang Z, Zhaoxi W, Yongyue W, Paula TA, Zhaozhong Z, Li S, Ednan KB, Thompson BT, David CC. A two-stage association study identifies a high risk window of acute respiratory distress syndrome onset time correlated with poor 28 day overall survival. A53 Respiratory failure: risk factors and outcomes in ARDS. American Thoracic Society. 2016:A7794–A7794. [Google Scholar]
- 14.Croce MA, Fabian TC, Davis KA, Gavin TJ. Early and late acute respiratory distress syndrome: two distinct clinical entities. J Trauma. 1999;46:361–366. doi: 10.1097/00005373-199903000-00001. (discussion 366–368) [DOI] [PubMed] [Google Scholar]
- 15.Liao KM, Chen CW, Hsiue TR, Lin WC. Timing of acute respiratory distress syndrome onset is related to patient outcome. J Formos Med Assoc. 2009;108:694–703. doi: 10.1016/S0929-6646(09)60392-2. [DOI] [PubMed] [Google Scholar]
- 16.Vincent JL, Sakr Y, Groeneveld J, Zandstra DF, Hoste E, Malledant Y, Lei K, Sprung CL. ARDS of early or late onset: does it make a difference? Chest. 2010;137:81–87. doi: 10.1378/chest.09-0714. [DOI] [PubMed] [Google Scholar]
- 17.Shari G, Kojicic M, Li G, Cartin-Ceba R, Alvarez CT, Kashyap R, Dong Y, Poulose JT, Herasevich V, Garza JA, Gajic O. Timing of the onset of acute respiratory distress syndrome: a population-based study. Respir Care. 2011;56:576–582. doi: 10.4187/respcare.00901. [DOI] [PubMed] [Google Scholar]
- 18.Reilly JP, Bellamy S, Shashaty MG, Gallop R, Meyer NJ, Lanken PN, Kaplan S, Holena DN, May AK, Ware LB, Christie JD. Heterogeneous phenotypes of acute respiratory distress syndrome after major trauma. Ann Am Thorac Soc. 2014;11:728–736. doi: 10.1513/AnnalsATS.201308-280OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Su L, Zhai R, Sheu CC, Gallagher DC, Gong MN, Tejera P, Thompson BT, Christiani DC. Genetic variants in the angiopoietin-2 gene are associated with increased risk of ARDS. Intensive Care Med. 2009;35:1024–1030. doi: 10.1007/s00134-009-1413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 21.Rittayamai N, Brochard L. Recent advances in mechanical ventilation in patients with acute respiratory distress syndrome. Eur Respir Rev. 2015;24:132–140. doi: 10.1183/09059180.00012414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferguson ND, Fan E, Camporota L, Antonelli M, Anzueto A, Beale R, Brochard L, Brower R, Esteban A, Gattinoni L, Rhodes A, Slutsky AS, Vincent JL, Rubenfeld GD, Thompson BT, Ranieri VM. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38:1573–1582. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 23.Randomized, placebo-controlled trial of lisofylline for early treatment of acute lung injury and acute respiratory distress syndrome. Crit Care Med. 2002;30:1–6. doi: 10.1097/00003246-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Hernu R, Wallet F, Thiollière F, Martin O, Richard JC, Schmitt Z, Wallon G, Delannoy B, Rimmelé T, Démaret C, Magnin C, Vallin H, Lepape A, Baboi L, Argaud L, Piriou V, Allaouchiche B, Aubrun F, Bastien O, Lehot JJ, Ayzac L, Guérin C. An attempt to validate the modification of the American-European consensus definition of acute lung injury/acute respiratory distress syndrome by the Berlin definition in a university hospital. Intensive Care Med. 2013;39:2161–2170. doi: 10.1007/s00134-013-3122-6. [DOI] [PubMed] [Google Scholar]
- 25.Boissier F, Katsahian S, Razazi K, Thille AW, Roche-Campo F, Leon R, Vivier E, Brochard L, Vieillard-Baron A, Brun-Buisson C, Mekontso Dessap A. Prevalence and prognosis of cor pulmonale during protective ventilation for acute respiratory distress syndrome. Intensive Care Med. 2013;39:1725–1733. doi: 10.1007/s00134-013-2941-9. [DOI] [PubMed] [Google Scholar]
- 26.Atabai K, Matthay M. The pulmonary physician in critical care. 5: acute lung injury and the acute respiratory distress syndrome: definitions and epidemiology. Thorax. 2002;57:452–458. doi: 10.1136/thorax.57.5.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638–1652. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Zimmerman JE, Wagner DP, Draper EA, Wright L, Alzola C, Knaus WA. Evaluation of acute physiology and chronic health evaluation III predictions of hospital mortality in an independent database. Crit Care Med. 1998;26:1317–1326. doi: 10.1097/00003246-199808000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, Ranieri M, Rubenfeld G, Thompson BT, Wrigge H, Slutsky AS, Pesenti A. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 30.Iwashyna TJ. Trajectories of recovery and dysfunction after acute illness, with implications for clinical trial design. Am J Respir Crit Care Med. 2012;186:302–304. doi: 10.1164/rccm.201206-1138ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei Y, Wang Z, Su L, Chen F, Tejera P, Bajwa EK, Wurfel MM, Lin X, Christiani DC. Platelet count mediates the contribution of a genetic variant in LRRC16A to ARDS risk. Chest. 2015;147:607–617. doi: 10.1378/chest.14-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei Y, Tejera P, Wang Z, Zhang R, Chen F, Su L, Lin X, Bajwa EK, Thompson BT, Christiani DC. A missense genetic variant in LRRC16A/CARMIL1 improves ARDS survival by attenuating platelet count decline. Am J Resp Crit Care Med. 2016 doi: 10.1164/rccm.201605-0946OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dixon JT, Gozal E, Roberts AM. Platelet-mediated vascular dysfunction during acute lung injury. Arch Physiol Biochem. 2012;118:72–82. doi: 10.3109/13813455.2012.665463. [DOI] [PubMed] [Google Scholar]
- 34.Wu J, Sheng L, Wang S, Li Q, Zhang M, Xu S, Gan J. Analysis of clinical risk factors associated with the prognosis of severe multiple-trauma patients with acute lung injury. J Emerg Med. 2012;43:407–412. doi: 10.1016/j.jemermed.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 35.Frank AJ, Thompson BT. Pharmacological treatments for acute respiratory distress syndrome. Curr Opin Crit Care. 2010;16:62–68. doi: 10.1097/MCC.0b013e328334b151. [DOI] [PubMed] [Google Scholar]
- 36.Fuchs L, Talmor D. Outcomes of ventilated surgical and medical ICU patients: do patients die from ARDS or with ARDS? Crit Care. 2014;18:P29–P29. [Google Scholar]
- 37.Gajic O, Dabbagh O, Park PK, Adesanya A, Chang SY, Hou P, Anderson H, Hoth JJ, Mikkelsen ME, Gentile NT, Gong MN, Talmor D, Bajwa E, Watkins TR, Festic E, Yilmaz M, Iscimen R, Kaufman DA, Esper AM, Sadikot R, Douglas I, Sevransky J, Michael M on behalf of the USCI, Injury Trials Group: Lung Injury Prevention Study I. Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am J Resp Crit Care Med. 2011;183:462–470. doi: 10.1164/rccm.201004-0549OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.