Abstract
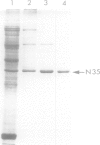
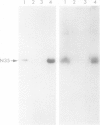
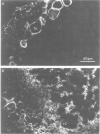
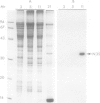
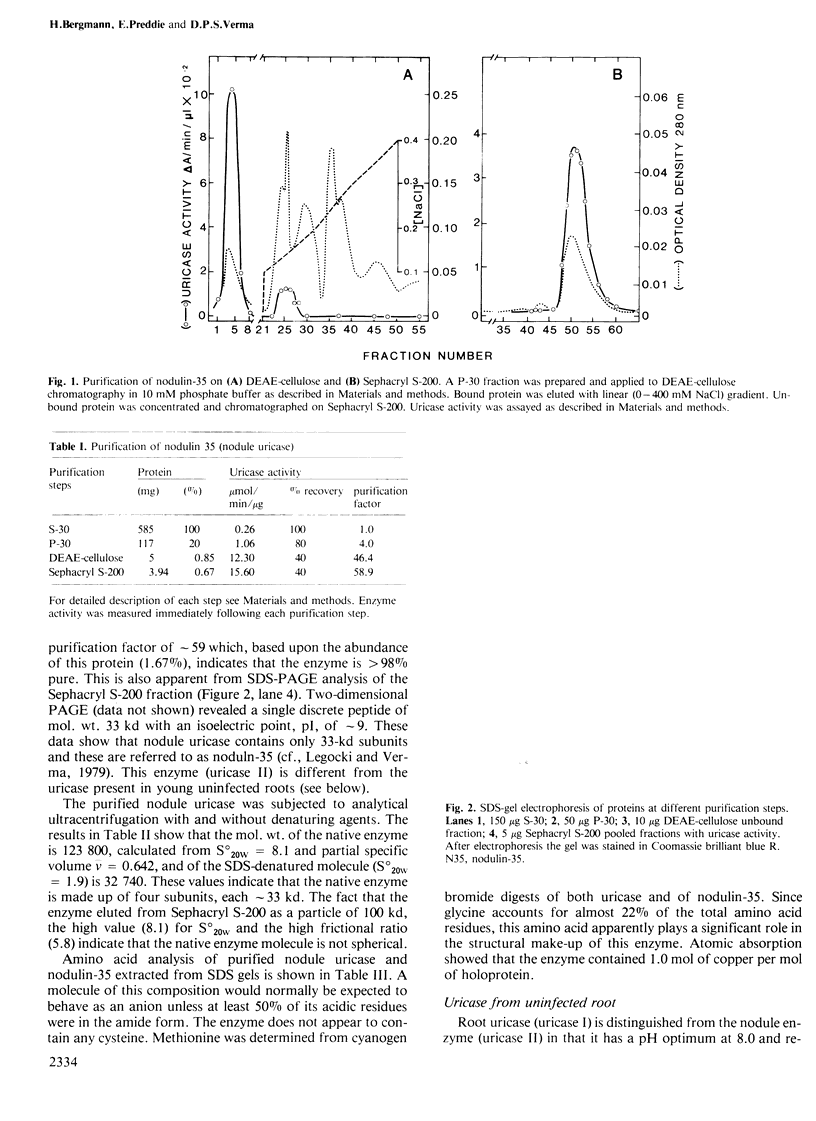
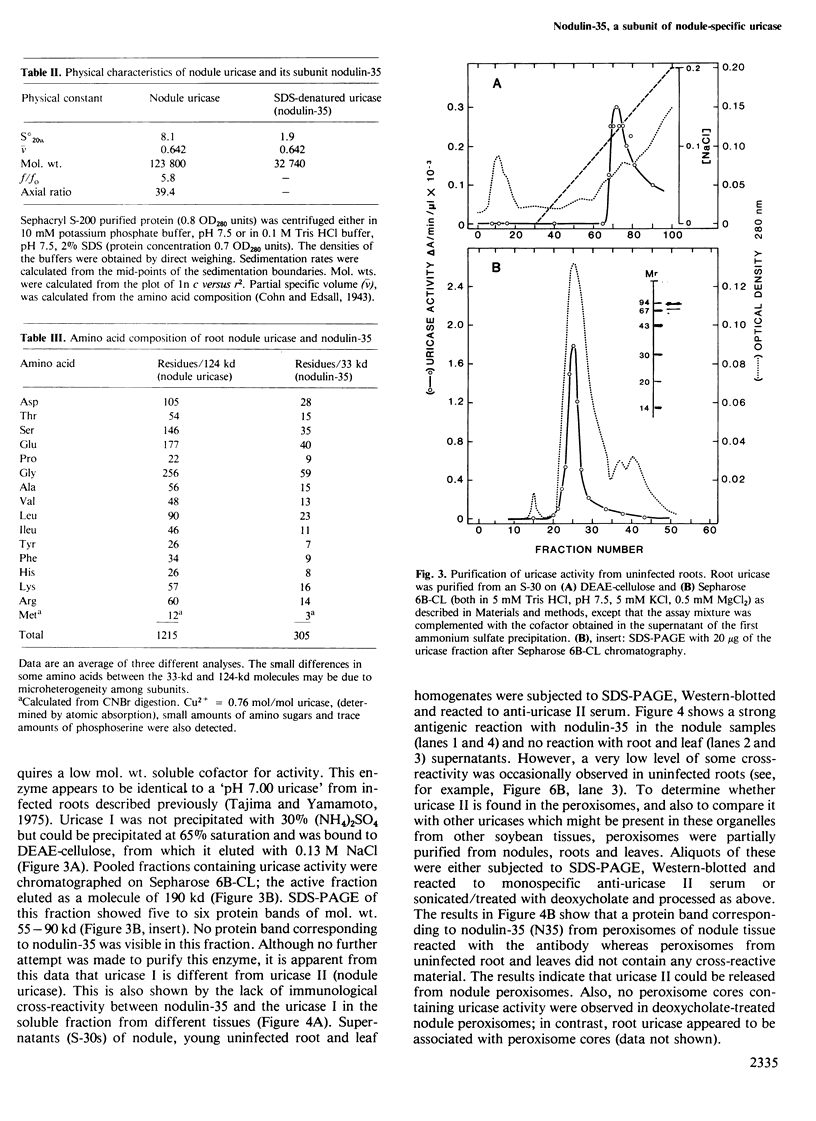
Nodulin-35, a protein specific to soybean root nodules, was purified under non-denaturing conditions (DEAE-cellulose followed by Sephacryl S-200 chromatography) to homogeneity. The holoprotein showed uricase (EC 1.7.3.3) activity. Analytical ultracentrifugation under non-denaturing conditions revealed a molecule of 124 kd, S°20W = 8.1; however, under denaturing conditions a value of 33 kd, S°20W = 1.9, was obtained. This indicated that nodulin-35 is the 33-kd subunit of a specific soybean root nodule uricase (uricase II) and that the enzyme contains four similar subunits. The native molecule contains ˜1.0 mol Cu2+ per mol, has an isoelectric point of ˜9.0 and a pH optimum for uricase activity at 9.5. Uricase activity found in young uninfected soybean roots is due to another form of enzyme (uricase I) which is of ˜190 kd, has maximum activity at pH 8.0 and does not contain any subunit corresponding in size to nodulin-35. Uricase I, also present in young infected roots, declines at a time when nodulin-35 appears. Monospecific antibodies prepared against uricase II (nodulin-35) showed no cross-reactivity. Uricase II was localized in the uninfected cells of the nodule tissue. These results are consistent with the concept that a nodule-specific ureide metabolism takes place in peroxisomes of uninfected cells, and suggest the participation of uricase II in this pathway.
Keywords: nodule-specific proteins, uricase, Glycine max, immunohistochemistry, N2-fixation
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bisseling T., Been C., Klugkist J., Kammen A., Nadler K. Nodule-specific host proteins in effective and ineffective root nodules of Pisum sativum. EMBO J. 1983;2(6):961–966. doi: 10.1002/j.1460-2075.1983.tb01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Fuller F., Künstner P. W., Nguyen T., Verma D. P. Soybean nodulin genes: Analysis of cDNA clones reveals several major tissue-specific sequences in nitrogen-fixing root nodules. Proc Natl Acad Sci U S A. 1983 May;80(9):2594–2598. doi: 10.1073/pnas.80.9.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks J. F., Schubert K., Tolbert N. E. Isolation and characterization of infected and uninfected cells from soybean nodules : role of uninfected cells in ureide synthesis. Plant Physiol. 1983 Apr;71(4):869–873. doi: 10.1104/pp.71.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks J. F., Tolbert N. E., Schubert K. R. Localization of enzymes of ureide biosynthesis in peroxisomes and microsomes of nodules. Plant Physiol. 1981 Jul;68(1):65–69. doi: 10.1104/pp.68.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. E., Oroszlan S., Konigsberg W. A micromethod for complete removal of dodecyl sulfate from proteins by ion-pair extraction. Anal Biochem. 1979 Feb;93(1):153–157. [PubMed] [Google Scholar]
- Herridge D. F., Atkins C. A., Pate J. S., Rainbird R. M. Allantoin and Allantoic Acid in the Nitrogen Economy of the Cowpea (Vigna unguiculata [L.] Walp.). Plant Physiol. 1978 Oct;62(4):495–498. doi: 10.1104/pp.62.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Legocki R. P., Verma D. P. A nodule-specific plant protein (nodulin-35) from soybean. Science. 1979 Jul 13;205(4402):190–193. doi: 10.1126/science.205.4402.190. [DOI] [PubMed] [Google Scholar]
- Legocki R. P., Verma D. P. Identification of "nodule-specific" host proteins (nodoulins) involved in the development of rhizobium-legume symbiosis. Cell. 1980 May;20(1):153–163. doi: 10.1016/0092-8674(80)90243-3. [DOI] [PubMed] [Google Scholar]
- Masters C., Holmes R. Peroxisomes: new aspects of cell physiology and biochemistry. Physiol Rev. 1977 Oct;57(4):816–882. doi: 10.1152/physrev.1977.57.4.816. [DOI] [PubMed] [Google Scholar]
- Newcomb E. H., Tandon S. R. Uninfected cells of soybean root nodules: ultrastructure suggests key role in ureide production. Science. 1981 Jun 19;212(4501):1394–1396. doi: 10.1126/science.212.4501.1394. [DOI] [PubMed] [Google Scholar]
- Rainbird R. M., Atkins C. A. Purification and some properties of urate oxidase from nitrogen-fixing nodules of cowpea. Biochim Biophys Acta. 1981 May 14;659(1):132–140. doi: 10.1016/0005-2744(81)90277-1. [DOI] [PubMed] [Google Scholar]
- Schubert K. R. Enzymes of Purine Biosynthesis and Catabolism in Glycine max: I. COMPARISON OF ACTIVITIES WITH N(2) FIXATION AND COMPOSITION OF XYLEM EXUDATE DURING NODULE DEVELOPMENT. Plant Physiol. 1981 Nov;68(5):1115–1122. doi: 10.1104/pp.68.5.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma D. P., Bal A. K. Intracellular site of synthesis and localization of leghemoglobin in root nodules. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3843–3847. doi: 10.1073/pnas.73.11.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma D. P., Ball S., Guérin C., Wanamaker L. Leghemoglobin biosynthesis in soybean root nodules. Characterization of the nascent and released peptides and the relative rate of synthesis of the major leghemoglobins. Biochemistry. 1979 Feb 6;18(3):476–483. doi: 10.1021/bi00570a016. [DOI] [PubMed] [Google Scholar]
- Verma D. P., Nash D. T., Schulman H. M. Isolation and in vitro translation of soybean leghaemoglobin mRNA. Nature. 1974 Sep 6;251(5470):74–77. doi: 10.1038/251074a0. [DOI] [PubMed] [Google Scholar]