Abstract
Smoking represents an important health risk for people living with HIV (PLHIV). Low adherence to smoking cessation pharmacotherapy may limit treatment effectiveness. In this study, 158 participants recruited from three HIV care centers in New York City were randomized to receive 12-weeks of varenicline (Chantix) either alone as standard care (SC) or in combination with text message (TM) support or TM plus cell phone-delivered adherence-focused motivational and behavioral therapy (ABT). Generalized linear mixed-effect models found a significant decline in varenicline adherence from week 1–12 across treatment groups. At 12-weeks, the probability of smoking abstinence was significantly higher in SC+TM+ABT than in SC. The study demonstrates the feasibility of delivering adherence-focused interventions to PLHIV who smoke. Findings suggest intensive behavioral support is an important component of an effective smoking cessation intervention for this population, and a focus on improving adherence self-efficacy may lead to more consistent adherence and higher smoking abstinence.
Keywords: HIV, Smoking cessation, Medication adherence, Text messaging, Telephone counseling
Introduction
People living with HIV (PLHIV) in the US are two to three times more likely to be current smokers (40–88 %) and significantly less likely to quit compared with the general population [1–3]. Due to treatment advances, PLHIV are living longer, making the issue of cigarette smoking in this population a major clinical concern. Tobacco-related illnesses, including cardiovascular disease and cancer, are now the leading causes of non HIV-related deaths among PLHIV [4, 5]. Cigarette smoking also places PLHIV at increased risk of unsuppressed viral load, low CD4 count, serious HIV-related co-morbidities and premature death [6, 7]. Despite the overwhelming burden of tobacco use, there is a lack of research demonstrating efficacious approaches to treating nicotine dependence in this population [8].
The few randomized controlled studies using varying combinations of medication and counseling have shown mixed results among PLHIV [8–17]. Five trials have found no difference between groups using different forms of behavioral therapy and nicotine-replacement therapy (NRT) [13–17]. In contrast, Vidrine et al. found that HIV- positive smokers randomized to 11 cell phone-delivered behavioral counseling sessions plus usual care (which included access to NRT) achieved significantly higher 3-month abstinence rates compared to standard care [11]. However, the effect was no longer significant at 6-months [12]. The current literature demonstrates the need to explore new approaches to increasing cessation rates among PLHIV
A key component of evidence-based smoking cessation treatment is pharmacotherapy. Higher rates of adherence are associated with a greater likelihood of smoking abstinence [8, 18–23]. However, similar to findings in the general population, adherence to smoking cessation pharmacotherapy among PLHIV is poor and declines over time [15, 16, 24–26]. Despite the significance of this problem, adherence to smoking cessation medications has received little attention in randomized clinical trials. Moreover, few studies have prospectively tested interventions to improve adherence to smoking cessation medication, and none have included PLHIV [27].
Innovative interventions that promote both adherence to cessation medications and provide intensive behavioral support (i.e., telephone counseling) are needed. Text messaging is particularly well suited to address behaviors like smoking. Text message interventions are able to interact with individuals in the context of the behavior [28, 29] and offer the opportunity to deliver medication reminders consistent with a patient’s dosing schedule. Two systematic reviews found increased cessation rates at 3 and/or 6 months for text message-based smoking cessation interventions that provided behavioral support [30, 31]. However, there are no smoking cessation studies in which text messages included both behavioral support and medication reminders and none of these studies included PLHIV. In addition, there are no studies that have tested interventions that combine telephone-delivered counseling with text messages to address barriers to adherence and cessation. The current literature suggests that more intensive support is needed to improve cessation outcomes in this population and therefore point to a potential advantage to combining these modes of behavioral support [11, 30].
In response to this research priority, we conducted a three-arm randomized controlled study that compared standard care with adherence-focused behavioral interventions through text messages alone or in combination with phone counseling. The goal of the study was to explore the feasibilty of each intervention component to facilitate adherence to varenicline (Chantix®) and 12-week smoking abstinence among smokers living with HIV.
Methods
Study Design
We conducted a three-arm randomized controlled study in which participants were randomized to receive 12 weeks of varenicline either alone as standard care (SC) or in combination with one of two adherence-focused support options: twice daily text message (TM) support, or TM plus seven telephone-delivered adherence-focused motivational and behavioral therapy sessions (ABT). The goal of the study was to test the feasibility and potential efficacy of text messaging alone and in combination with telephone counseling for varenicline adherence and was not designed to be a definitive test of the intervention although the sample size is larger than in a typical pilot study. As part of standard care, all participants also received a self-help information sheet, tailored to PLHIV who smoke with frequently asked questions about using varenicline, and a wallet card with the New York State Quitline number. Participants in all three arms could choose to use their own phone or a study provided cell phone during the 12 weeks of intervention period. The study phone provided unlimited text messaging and 250 min for telephone calls. Research staff monitored the study phone accounts and refilled them with additional minutes as needed. The study has been approved by New York University School of Medicine Institutional Review Board.
Setting and Participants
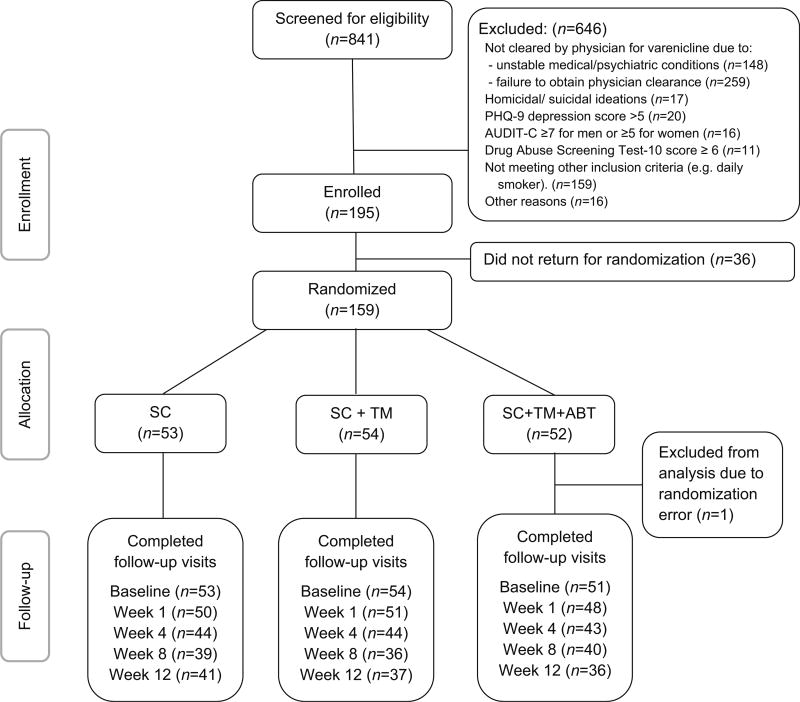
Between July 2013 and March 2014, we recruited and screened study participants for eligibility in the waiting area of three HIV care centers affiliated with St. Luke’s-Roosevelt Hospital Spencer Cox Centers for Health, located in New York City. Smokers were eligible if they were 18 years or older and diagnosed with HIV, smoked ≥5 cigarettes daily in the past week, were willing to quit within the next 2 weeks, and were cleared by their physician for varenicline use (i.e., did not have major depression, schizophrenia or bipolar disorder, unstable cardiovascular disease or renal impairment). Individuals were excluded if they did not speak or read English, were pregnant or nursing, using another FDA-approved smoking cessation medication, had suicidal/homicidal ideations or a PHQ 9 depression score >5, and either a substantial to severe drug use disorder defined as a score of ≥6 on the drug abuse screening test-10 and/or a hazardous or active alcohol use disorder defined as ≥7 for men and ≥5 for women on the alcohol use disorders identification test-consumption (AUDIT-C) [32, 33]. All eligible participants who gave informed consent were scheduled for a baseline visit at New York University School of Medicine. To reduce post-randomization attrition, participants were randomized to one of the three study groups at baseline instead of at enrollment. Randomization was stratified by number of cigarettes smoked per day at baseline (5–10 and >10 cigarettes/-day). A total of 841 patients were screened for eligibility and 158 were randomized (Fig. 1). Participants returned for follow-up visits at 1, 4, 8 and, 12 weeks postrandomization.
Fig. 1.
Consort diagram
Measures
Baseline Measures
Nicotine dependence was evaluated using the Heaviness of Smoking Index, which contains a four category-scoring scheme for “time to the first cigarette of the day” and “average daily consumption of cigarettes” (range 0–6) [34]. Alcohol and drug use were measured using the AUDIT-C [35], and the drug use disorders identification test (DUDIT) [36]. To measure beliefs and attitudes (motivation), we adapted Fucito’s 6-item beliefs and attitudes about bupropion measure which uses a 5-point Likert scale [25]. The 8-item varenicline information scale was adapted from The LifeWindows Information Motivation Behavioral Skills Adherence Assessment Questionnaire and was assessed on a 5-point Likert scale (1 = strongly disagree, 5 = strongly agree) [37]. We also measured varenicline adherence self-efficacy using a 17-item survey using a 4-point Likert scale (1 = not at all sure, 4 = extremely sure), with 12 items adapted from the Medication Adherence Self-Efficacy Scale (MASES) and 5 items from the Adherence Self-Efficacy Scale (ASES) [38, 39]. All negative questions were reverse coded before data analysis. To test internal consistency of the adapted scales, Cronbach’s alpha was calculated based on all participants who completed the baseline survey (n = 159, Crobach’s α = .86 for varenicline beliefs and attitudes measurement, Crobach’s α = .65 for varenicline information scale, Cronbach’s α = .92 for varenicline adherence self-efficacy measurement).
Adherence
Consistent with previous studies, adherence was defined as taking ≥80 % of prescribed varenicline since last visit, as determined by pill count and was assessed at 1, 4, 8 and 12 week follow up visits [22, 40–42]. Participants who did not bring their medication bottles for pill count or who did not come back for follow-up visits were considered non-adherent (intent-to-treat approach). Five participants became ineligible for varenicline during the course of intervention due to other medical reasons unrelated to varenicline use but were included in the analysis as per the intent to treat approach.
Abstinence
Self-reported 7-day point prevalence smoking abstinence was verified by a carbon monoxide (CO) <8 ppm and measured at 1, 4, 8, and 12 weeks. Participants with missing data due to loss-to-follow-up or withdrawal/discharge from the study were considered as non-abstinent (intent-to-treat approach).
Intervention Components
The text messaging and phone counseling interventions were guided by the information-motivation-behavioral skills model (IMB) of antiretroviral adherence [43]. This model incorporates factors from social cognitive theory and the theory of planned behavior that are associated with medication adherence and smoking abstinence [39, 44, 45]. It posits that adherence-related self-efficacy (behavioral skills), information/knowledge about the treatment, and positive attitudes and beliefs towards adherence (motivation) are critical determinants of medication adherence [46, 47].
The behavioral intervention components were designed to address factors hypothesized by the IMB model to influence the primary study outcomes of varenicline adherence and smoking cessation [48, 49]. Details about each intervention component are described below.
Varenicline
At baseline, participants were given a one-week supply of varenicline, and at each subsequent visit they were given enough to last until the next visit for a total of 12-weeks of treatment. We titrated the dosage of varenicline in the first week: .5 mg once daily for days 1–3, then .5 mg twice daily for days 4–7, followed by 1.0 mg twice daily from day 8 until week 12.
Text Messaging
The text messaging protocol was designed to address both medication adherence and tobacco cessation themes. Messages created by our study team were based on IMB constructs including motivation, social support, and expectancies and findings from previous studies testing the efficacy of text message interventions for tobacco cessation [50, 51]. We also drew from the National Cancer Institute’s QuitNowTXT library [52] and from a previous HIV-medication adherence study. [53] In addition, studies of adherence to HIV-related medications have found that “simply forgetting” is the most common self-reported reason for non-adherence [49]. Therefore, text messages that specifically prompted adherence were included daily. Based on findings from formative research, described in a previous publication, we developed a text library that included 168 text messages to ensure that each message was not repeated [54]. Each day participants in the two TM arms received one adherence-focused message and one IMB smoking cessation-themed message, at the time of their own choice.
ABT Phone Sessions
The seven-session standardized manual combined the principles of cognitive behavioral therapy with motivational interviewing techniques [18, 55–57]. The planning and quit date counseling sessions were approximately 30 min each, and the five follow-up sessions were scheduled 2 days, and then 2, 4, 6, and 10 weeks after the quit date, each lasting about 20 min. During the planning session, the counselor provided an overview of the program, discussed the effects of smoking on PLHIV, elicited the participant’s barriers and facilitators to quitting, and developed a quit plan. On the quit day, status of quit was assessed. The counselor discussed with the participant about withdrawal symptoms, external and internal triggers, high risk situations, and coping strategies. The five follow-up sessions were intended to maintain adherence to medication, to help prevent relapse, and to help those who relapse to make a quick recovery and resume quitting. All counselors had a master’s degree and were either a licensed clinical social worker or mental health counselor, or certified tobacco treatment specialist. Prior to the intervention, the counselors completed 4 days of training on the counseling manual with a member of the International Motivational Interviewing Network of Trainers organization. To prevent a decay of counseling skills, counselors also received ongoing coaching on their skills from the trainer.
Treatment Fidelity
Treatment fidelity was based on the expanded Lichtenstein treatment fidelity model developed by the Office of Behavioral Social Sciences Research Behavior Change Consortium [58]. Counselors completed a checklist indicating topics covered during the counseling sessions and recorded process notes after each session. All telephone sessions were audio-recorded and archived for systematic sampling of 10 % of the sessions and subsequently coded in order to assess adherence to the counseling manual using the Behavior Change Counseling Index [59]. Counselors were considered adherent if they scored an average score ≥3 on the index (range 1 [not at all]–4 [a great extent]). The average score across counselors throughout the 12-week period was three. Treatment fidelity measures also included number of completed counseling sessions and self-reported frequency of reading text messages.
Analysis
Baseline characteristics were described. Varenicline adherence and smoking abstinence outcomes at each time point were compared among three treatment groups using Chi square tests and Fisher’s exact tests. To investigate predictors of varenicline adherence and of smoking abstinence, two generalized linear mixed-effect models (GLMMs) were estimated, with repeated measurements nested under individual participants and randomly varying intercept coefficients. Variables that demonstrated group differences at baseline or known association with outcomes based on literature were chosen as covariates. In the model of varenicline adherence, treatment condition, time, baseline adherence self-efficacy, as well as the interaction term between time and treatment condition were included as predictors. Living in independent housing, on which there was a moderate imbalance across treatment arms despite randomization, was also included. In the model of smoking abstinence, treatment condition, time, varenicline adherence, baseline heaviness of smoking, living in independent housing, as well as the interaction term between time and treatment condition were included. In both models, the time variable was coded to contrast weeks 1 and 12. All analysis was conducted in version 14 of Stata [60], with xtmelogit used to fit GLMMs. Variance explained by the fixed and random effects in each GLMM was calculated [61]. Significance tests, including comparisons between specific treatment arms, were made without adjustments to p-values.
Power Analysis
Because this study was not meant to provide a definitive test of intervention efficacy, analyses relied on effect size calculation, confidence intervals and patterns of results in addition to null hypothesis significance testing. Nevertheless, a priori power calculations were undertaken to provide some idea of the magnitude of effects that could be reliably detected using conventional tests of significance for the proposed sample size. With a sample size of 50 participants per group that were deemed feasible to recruit, power is 80 % to detect an increase in the proportion with good varenicline adherence from 50 % (taking ≥80 % of prescribed varenicline) in the standard care condition to 77 % in one of the enhanced treatment conditions. This increase corresponds to an odds ratio of 3.29.
Results
Sample Demographics
Over 80 % of the sample was either Non Hispanic Black or Hispanic of any race, predominantly males in their mid 40 s and over 70 % were currently unemployed (Table 1). At baseline, over half of the participants smoked their first cigarette within 5 min after waking, and on average smoked 15 cigarettes per day.
Table 1.
Baseline characteristics
| Baseline variable | Mean ± SD, n (%) | |||
|---|---|---|---|---|
|
|
||||
| Total (n = 158) | SC (n = 53) | SC+TM (n = 54) | SC+TM+ABT (n = 51) | |
| Age in years | 46.79 ± 9.83 | 46.64 ± 10.77 | 46.00 ± 9.96 | 47.76 ± 8.74 |
| Gender | ||||
| Female | 29 (18.4 %) | 10 (18.9 %) | 10 (18.5 %) | 9 (17.6 %) |
| Male | 125 (79.1 %) | 39 (73.6 %) | 44 (81.5 %) | 42 (82.4 %) |
| Transgender | 4 (2.5 %) | 4 (7.5 %) | 0 (.0 %) | 0 (.0 %) |
| Race/ethnicity | ||||
| Non-hispanic black | 81 (51.3 %) | 21 (39.6 %) | 27 (50.0 %) | 33 (64.7 %) |
| Non-hispanic white | 21 (13.3 %) | 9 (17.0 %) | 7 (13.0 %) | 5 (9.8 %) |
| Other non-hispanic | 6 (3.8 %) | 2 (3.8 %) | 2 (3.7 %) | 2 (3.9 %) |
| Hispanic of any race | 50 (31.6 %) | 21 (39.6 %) | 18 (33.3 %) | 11 (21.6 %) |
| Education | ||||
| <HS | 35 (22.2 %) | 6 (11.3 %) | 15 (27.8 %) | 14 (27.5 %) |
| HS degree or GED | 46 (29.1 %) | 19 (35.8 %) | 10 (18.5 %) | 17 (33.3 %) |
| Some college | 52 (32.9 %) | 19 (35.8 %) | 21 (38.9 %) | 12 (23.5 %) |
| College or post-graduate degree | 25 (15.8 %) | 9 (17.0 %) | 8 (14.8 %) | 8 (15.7 %) |
| Housing | ||||
| Independent apartment/house | 132 (83.5 %) | 49 (92.5 %) | 38 (70.4 %) | 45 (88.2 %) |
| Other type of housing | 26 (16.5 %) | 4 (7.5 %) | 16 (29.6 %) | 6 (11.8 %) |
| Employment status | ||||
| Employed | 45 (28.5 %) | 14 (26.4 %) | 15 (27.8 %) | 16 (31.4 %) |
| Unemployed | 32 (20.3 %) | 15 (28.3 %) | 11 (20.4 %) | 6 (11.8 %) |
| Unable to work or disabled | 54 (34.2 %) | 14 (26.4 %) | 20 (37.0 %) | 20 (39.2 %) |
| Other | 27 (17.1 %) | 10 (18.9 %) | 8 (14.8 %) | 9 (17.6 %) |
| Used study cell phone | 101 (63.9 %) | 28 (52.8 %) | 36 (66.7 %) | 37 (72.5 %) |
| Baseline number of cigarettes per day | 14.8 ± 9.7 | 15.1 ± 10.1 | 14.2 ± 9.2 | 15.3 ± 9.9 |
| Time to first cigarette | ||||
| 5 min or less after waking | 85 (53.8 %) | 30 (56.6 %) | 28 (51.9 %) | 27 (52.9 %) |
| 6–30 min after waking | 55 (34.8 %) | 18 (34.0 %) | 17 (31.5 %) | 20 (39.2 %) |
| >30 min after waking | 18 (11.4 %) | 5 (9.4 %) | 9 (16.7 %) | 4 (7.8 %) |
| Heaviness of Smoking Index | 3.0 ± 1.2 | 3.2 ± 1.3 | 2.8 ± 1.2 | 3.1 ± 1.1 |
| DUDIT | 4.3 ± 6.5 | 4.4 ± 7.1 | 5.1 ± 7.2 | 3.2 ± 4.9 |
| AUDIT-C | 1.8 ± 1.8 | 1.9 ± 1.9 | 1.8 ± 1.9 | 1.7 ± 1.7 |
| Beliefs and attitudes about vareniclinea | 4.3 ± .6 | 4.4 ± .5 | 4.3 ± .7 | 4.2 ± .6 |
| Varenicline information scaleb | 34.9 ± 4.4 | 34.9 ± 4.1 | 34.9 ± 4.7 | 34.9 ± 4.4 |
| Adherence self-efficacyb | 55.6 ± 10.0 | 57.3 ± 8.6 | 54.9 ± 9.6 | 54.6 ± 11.5 |
Mean score was used for beliefs and attitudes about varenicline scale
Sum scores were used for adherence self-efficacy (MASES and ASES combined) and varenicline information scale
Intervention Feasibility
Recruitment and Retention
Figure 1 shows the CONSORT diagram. Among 841 individuals who were screened, 195 (23.2 %) were enrolled in the study and 158 (18.8 %) were randomized to one of three treatment arms. The overall retention rate was 72.2 % at 12 weeks. There was no difference in retention across treatment groups at any time point (data not shown; p = 1.00 at week 1, p = .93 at week 4, p = .40 at week 8, p = .57 at week 12; Chi square test).
Treatment Exposure
Among participants who completed week 12 surveys, 78.4 % (n = 29) in the SC+TM arm and 66.7 % (n = 24) in the SC+TM+ABT arm reported having always or usually read the intervention text messages (data not shown; p = .26; Chi square test). On average, participants in the SC+TM+ABT arm completed 4.25 of the seven counseling sessions (data not shown; n = 51, SD = 2.42).
Estimates of Efficacy for Adherence and Abstinence Outcomes
Varenicline Adherence and Smoking Abstinence at Weeks 1, 4, 8 and 12
Adherence and abstinence outcomes at each follow-up time point were compared across three arms, without adjusting for other factors (Table 2). No difference was found in varenicline adherence across treatment groups at any time point. At week 8, the abstinence rate among SC+TM+ABT was significantly higher (17.7 %) than the SC+TM (5.7 %) and SC groups (3.7 %) (p = .03). At week 12, the SC+TM+ABT group had a higher abstinence rate (15.7 %) compared with SC (5.7 %,) and SC+TM (3.7 %,) groups; however, the difference was only marginally significant (p = .07). Taking ≥80 % doses for at least two study visits in a row was associated with increased odds (data not shown, OR 11.33, p = .007, Fisher’s exact test) of smoking abstinence at week 12.
Table 2.
Varenicline adherence and smoking abstinence by treatment arms at each visit
| SC | SC+TM | SC+TM+ABT | p-valuea | |
|---|---|---|---|---|
| Week 1 | ||||
| Adherence rate | 66.0 % (n = 35) | 72.2 % (n = 39) | 78.4 % (n = 40) | .37 |
| Abstinence rate | 1.9 % (n = 1) | 7.4 % (n = 4) | .0 % (n = 0) | .13 |
| Week 4 | ||||
| Adherence rate | 54.7 % (n = 29) | 38.9 % (n = 21) | 43.1 % (n = 22) | .24 |
| Abstinence rate | 11.3 % (n = 6) | 18.5 % (n = 10) | 13.7 % (n = 7) | .59 |
| Week 8 | ||||
| Adherence rate | 35.9 % (n = 19) | 37.0 % (n = 20) | 35.3 % (n = 18) | .98 |
| Abstinence rate | 5.7 % (n = 3) | 3.7 % (n = 2) | 17.7 % (n = 9) | .03 |
| Week 12 | ||||
| Adherence rate | 34.0 % (n = 18) | 29.6 % (n = 16) | 29.4 % (n = 15) | .85 |
| Abstinence rate | 5.7 % (n = 3) | 3.7 % (n = 2) | 15.7 % (n = 8) | .07 |
Chi square tests were used for adherence rate and Fisher’s exact tests were used for abstinence rate Bold value is statistically significant (p < 0.05)
Longitudinal Modeling on Varenicline Adherence and Smoking Abstinence
Longitudinal modeling of varenicline adherence (Table 3) shows adherence decreased significantly from week 1 to week 12 (OR .09, p < .001). The trajectory of varenicline adherence over time did not differ across the three arms (data not shown, p = .50). Higher adherence self-efficacy at baseline was associated with significant increased odds of adherence (OR 2.20, p < .001). Fixed effects alone explained 19 % of the variance in adherence while the whole model (fixed and random effects) explained 64 % of the variance in adherence (data not shown).
Table 3.
Longitudinal models on varenicline adherence and smoking abstinence
| Adherence
|
Abstinence
|
|||||
|---|---|---|---|---|---|---|
| OR | p-value | 95 % CI | OR | p-value | 95 % CI | |
| Treatment | ||||||
| SC+TMa | 0.87 | .83 | 0.24, 3.09 | 0.62 | .64 | 0.08, 4.54 |
| SC+TM+ABTb | 0.70 | .59 | 0.20, 2.53 | 5.51 | .05 | 0.99, 30.58 |
| Timec | 0.09 | <.001 | 0.03, .25 | 1.53 | .62 | 0.28, 8.37 |
| Time*Treatment | ||||||
| SC+TM | 0.81 | .78 | 0.19, 3.51 | 0.17 | .14 | 0.02, 1.84 |
| SC+TM+ABT | 0.41 | .26 | 0.09, 1.93 | 8.84 | .07 | 0.84, 92.40 |
| Adherence to varenicline at the same visit | 2.01 | .13 | 0.82, 4.90 | |||
| Baseline heaviness of smoking Index | 0.61 | .09 | 0.35, 1.07 | |||
| Baseline adherence self-efficacyd | 2.20 | <.001 | 1.43, 3.39 | |||
| Independent housing | 1.02 | .97 | 0.34, 3.08 | 6.13 | .07 | 0.84, 44.96 |
Individuals’ intercepts vary with a standard deviation of 2.04 (95 % Cl 1.59–2.63) in the adherence model and 2.13 (95 % Cl 1.43–3.18) in the abstinence model
Bold values are statistically significant (p < 0.05)
The SC+TM treatment condition is compared with SC
The SC+TM+ABT treatment condition is compared with SC
Time variable is coded to contrast weeks 1 and 12
Standardized scores were used for baseline heaviness of smoking index and adherence self-efficacy
At 12-weeks, pairwise comparisons of the three intervention conditions suggest the probability of smoking abstinence was significantly higher in SC+TM+ABT than in SC (OR 5.51, p = .05). Adherence to varenicline at the same visit (OR 2.01, p = .13) and baseline smoking heaviness (OR .61, p = .09) were not associated with smoking abstinence. Fixed effects alone explained 14 % of the variance in abstinence while the whole model explained 64 % of the variance in abstinence (data not shown).
Discussion
The study demonstrated success in recruiting and retaining a racially diverse sample of cigarette smokers living with HIV in a smoking cessation trial. The medium to high level of treatment exposure reported by study participants confirmed the feasibility of implementing text messaging and phone counseling interventions with this vulnerable population. Although the objective of the study was not to draw definitive conclusions about intervention efficacy and the limited sample size did not allow precise estimates of intervention effects on outcomes, below we discuss interval estimates of intervention effects on varenicline adherence and smoking abstinence to identify strengths and weaknesses of the intervention design and to inform future studies.
Adherence
We found significant and similar declines in adherence over time across all three-study arms despite the addition of adherence-focused content in the text messaging and combined text and telephone counseling intervention arms. While confidence intervals for the effect of adding text messaging or text-messaging plus counseling include potential impacts on adherence with clinical and public health significance, even the upper limits of those intervals suggest modest effects of those enhancements to standard care (i.e., odds of good adherence multiplied by 3.5 at most). A 2015 Cochrane review also found limited evidence that interventions focused on improving adherence to smoking cessation medications enhanced adherence when added to behavioral support for smoking abstinence [62]. However, among the five studies reviewed, none included the use of text messaging. In contrast to our findings, text reminders have been shown to be effective in increasing adherence to antiretroviral therapy (ART) [27]. A recent meta-analysis of eight studies found text messaging yielded significantly higher rates of adherence to ART than control conditions. Larger effects were associated with interventions that used bidirectional communication, included personalized message content and were matched to the dosing schedule [27]. The current study used a unidirectional text message protocol that was not tailored to any individual characteristics, which may, in part, explain why effects on adherence were not stronger [63, 64]. Moreover, text reminders alone may not fully address the myriad social, economic and medical challenges of PLHIV, including an already complex medical regimen that may contribute to low rates of adherence to cessation medications [1, 65]. Notably, the addition of telephone counseling did not enhance adherence. However, our fidelity assessments indicated more than 75 % of the sessions did not focus significant time on this issue; rather, the counselor emphasized increasing motivation and behavioral support for cessation. Our findings do point to the potential for improving adherence self-efficacy as a strategy for increasing adherence to smoking cessation medications. Consistent with studies of ART adherence, adherence self-efficacy was a strong predictor of more consistent use of varenicline [46].
Together the literature, and findings from this study, suggests the need for further research to explore whether components of mobile-based phone interventions that are effective for increasing ART adherence can be applied to cessation pharmacotherapy. In addition, studies are needed that explore the impact of more consistent attention to adherence as part of counseling interventions with a specific focus on building adherence self-efficacy. Finally, to make progress in this area, studies should apply conceptual models that may help delineate how these interventions work to improve adherence [27].
Smoking Abstinence
In addition to the text message to remind participants to take varenicline, we sent daily messages that offered behavioral support for quitting informed by the IMB model. In contrast to previous studies of mobile phone interventions to increase cessation this component of the text intervention did not appear to improve cessation rates. A Cochrane review of five studies using mobile phone interventions reported increased quit rates at 6 months [30]. None of the studies included PLHIV. Again, differences in design elements may explain the discrepancy. Unlike these previous studies, the text delivered behavioral support was not customized, did not allow bidirectional interaction and did not vary in intensity. These factors appear to be important components of an effective text message cessation intervention.
Although the telephone counseling did not improve adherence rates, there was evidence of an effect on cessation. In the longitudinal model, the addition of cell phone delivered counseling was associated with significantly improved cessation rates even after controlling for varenicline adherence, suggesting an impact of the counseling’s emphasis on increasing motivation and behavioral support for cessation, other than improving adherence. The positive impact of telephone counseling is consistent with Vidrine et. al’s study in PLHIV, as are the relatively low quit rates [11, 66]. Based on the literature to date, more intensive counseling appears effective; however, rates of cessation are low and no studies to date have found evidence of long-term efficacy [12]. The longitudinal analysis did not demonstrate an association between adherence and abstinence. However, in combined analysis, participants who were more consistently adherent to the prescribed dose (≥80 % prescribed doses for at least two visits in a row) were significantly more likely to achieve 12 week smoking abstinence, pointing to a potential role for adherence focused interventions as a part of a comprehensive approach to smoking cessation in this population.
Our findings suggest that in a low SES patient population with significant comorbidities the multisession telephone-delivered behavioral support intervention component, which employed principles of motivational interviewing and cognitive behavioral therapy, was an important factor in achieving cessation. While evidence on association between adherence and smoking abstinence was weak, interventions that are able to lead to more sustained adherence may still lead to improved cessation outcomes. Adherence self-efficacy predicted medication adherence, suggesting a possible direction for interventions designed to enhance adherence.
There were several limitations. First, this study was not meant to provide a definitive test of intervention efficacy. Although our results demonstrated intervention feasibility, a detailed analysis on qualitative data collected with participants (not presented in this paper) will help further identify facilitators and barriers to adherence and abstinence. Second, this analysis presents data at end of treatment (12 weeks). A longer follow up assessment is needed to assess if the higher rates of cessation in telephone counseling intervention arm persist. Finally, as noted previously, the text message intervention lacked components that have been demonstrated to improve adherence rates in PLHIV.
In conclusion, the current study demonstrated the feasibility of delivering a smoking cessation intervention using text messaging in a HIV-positive group of smokers. Intensive behavioral support appears to be an important component of an effective smoking cessation intervention for PLHIV. Despite limitations in sample size and follow-up length, we observed trends in varenicline adherence and smoking abstinence worthy of further investigation. Future research needs to explore innovative approaches to delivering this support, including continuing to test the use of mobile-based interventions that address the multidimensional barriers to adherence and smoking abstinence and are tailored to the complex psychosocial needs of this vulnerable population.
Acknowledgments
Funding This research was sponsored by the National Institutes of Drug Abuse of the National Institutes of Health (R34 DA031636001A1, http:\\www.ClinicalTrials.gov ID# NCT01898195). The study medication was provided by Pfizer Inc, Mission, KS. This research was supported by the Center for Drug Use and HIV Research (CDUHR-P30 DA011041). Dr. Sherman is supported in part by a Grant from NIDA (#1K24DA038345).
Footnotes
Compliance with Ethical Standards
Conflict of Interest The authors declare that they have no conflict of interest.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Informed consent was obtained from all individual participants included in the study.
References
- 1.Burkhalter JE, Springer CM, Chhabra R, et al. Tobacco use and readiness to quit smoking in low-income HIV-infected persons. Nic Tob Res. 2005;7(4):511–22. doi: 10.1080/14622200500186064. [DOI] [PubMed] [Google Scholar]
- 2.Cooperman NA. Current research on cigarette smoking among people with HIV. Curr Addict Rep. 2016;3(1):19–26. [Google Scholar]
- 3.Mdodo R, Frazier EL, Dube SR, Mattson CL, Sutton MY, Brooks JT, Skarbinski J. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann Intern Med. 2015;162(5):335–44. doi: 10.7326/M14-0954. [DOI] [PubMed] [Google Scholar]
- 4.Sackoff JE, Hanna DB, Pfeiffer MR, et al. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med. 2006;145(6):397–406. doi: 10.7326/0003-4819-145-6-200609190-00003. [DOI] [PubMed] [Google Scholar]
- 5.Helleberg M, May MT, Ingle SM, et al. Smoking and life expectancy among HIV-infected individuals on antiretroviral therapy in Europe and North America. AIDS. 2015;29(2):221–9. doi: 10.1097/QAD.0000000000000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hile SJ, Feldman MB, Alexy ER, Irvine MK. Recent tobacco smoking is associated with poor HIV medical outcomes among HIV-infected individuals in New York. AIDS Behav. 2016;20(8):1722–9. doi: 10.1007/s10461-015-1273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reynolds NR. Cigarette smoking and HIV: more evidence for action. AIDS Educ Prev. 2009;21(suppl 3):106. doi: 10.1521/aeap.2009.21.3_supp.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niaura R, Chander G, Hutton H, Stanton C. Interventions to address chronic disease and HIV: strategies to promote smoking cessation among HIV-infected individuals. Curr HIV/AIDS Rep. 2012;9(4):375–84. doi: 10.1007/s11904-012-0138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cioe PA. Smoking cessation interventions in HIV-infected adults in North America: a literature review. J Addict Behav Ther Rehabil. 2013;2(3):1000112. doi: 10.4172/2324-9005.1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shuter J, Morales DA, Considine-Dunn SE, et al. Feasibility and preliminary efficacy of a web-based smoking cessation intervention for HIV-infected smokers: a randomized controlled trial. J Acquir Immune Defic Syndr. 2014;67(1):59–66. doi: 10.1097/QAI.0000000000000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vidrine DJ, Marks RM, Arduino RC, et al. Efficacy of cell phone-delivered smoking cessation counseling for persons living with HIV/AIDS: 3-month outcomes. Nicotine Tob Res. 2012;14(1):106–10. doi: 10.1093/ntr/ntr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gritz ER, Danysh HE, Fletcher FE, et al. Long-term outcomes of a cell phone-delivered intervention for smokers living with HIV/AIDS. Clin Infect Dis. 2013;57(4):608–15. doi: 10.1093/cid/cit349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moadel AB, Bernstein SL, Mermelstein RJ, et al. A randomized controlled trial of a tailored group smoking cessation intervention for HIV-infected smokers. J Acquir Immune Defic Syndr. 2012;61(2):208–15. doi: 10.1097/QAI.0b013e3182645679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lloyd-Richardson EE, Stanton CA, Papandonatos GD, et al. Motivation and patch treatment for HIV+ smokers: a randomized controlled trial. Addiction. 2009;104(11):1891–900. doi: 10.1111/j.1360-0443.2009.02623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanton CA, Papandonatos GD, Shuter J, Bicki A, Lloyd-Richardson EE, de Dios MA, Morrow KM, Makgoeng SB, Tashima KT, Niaura RS. Outcomes of a tailored intervention for cigarette smoking cessation among latinos living with HIV/AIDS. Nicotine Tob Res. 2015;17(8):975–82. doi: 10.1093/ntr/ntv014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ingersoll KS, Cropsey KL, Heckman CJ. A test of motivational plus nicotine replacement interventions for HIV positive smokers. AIDS Behav. 2009;13(3):545–54. doi: 10.1007/s10461-007-9334-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Humfleet GL, Hall SM, Delucchi KL, et al. A randomized clinical trial of smoking cessation treatments provided in HIV clinical care settings. Nicotine Tob Res. 2013;15(8):1436–45. doi: 10.1093/ntr/ntt005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiore MC, Jaen CR, Baker TB, et al., editors. Treating tobacco use and dependence: 2008 update. Clinical practice guideline. Rockville: U.S. DIANE Publishing; 2008. [Google Scholar]
- 19.Catz SL, Jack LM, McClure JB, et al. Adherence to varenicline in the COMPASS smoking cessation intervention trial. Nicotine Tob Res. 2011;5(5):361–8. doi: 10.1093/ntr/ntr003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liberman JN, Lichtenfeld MJ, Galaznik A, et al. Adherence to varenicline and associated smoking cessation in a community-based patient setting. J Manag Care Pharm. 2013;19(2):125–31. doi: 10.18553/jmcp.2013.19.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stanton CA, Lloyd-Richardson EE, Papandonatos GD, et al. Mediators of the relationship between nicotine replacement therapy and smoking abstinence among people living with HIV/AIDS. AIDS Educ Prev. 2009;21(suppl 3):65–80. doi: 10.1521/aeap.2009.21.3_supp.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hays JT, Leischow SJ, Lawrence D, et al. Adherence to treatment for tobacco dependence: association with smoking abstinence and predictors of adherence. Nicotine Tob Res. 2010;12(6):574–81. doi: 10.1093/ntr/ntq047. [DOI] [PubMed] [Google Scholar]
- 23.Raupach T, Brown J, Herbec A, et al. A systematic review of studies assessing the association between adherence to smoking cessation medication and treatment success. Addiction. 2014;109(1):35–43. doi: 10.1111/add.12319. [DOI] [PubMed] [Google Scholar]
- 24.Alterman AI, Gariti P, Cook TG, et al. Nicodermal patch adherence and its correlates. Drug Alcohol Depend. 1999;53(2):159–65. doi: 10.1016/s0376-8716(98)00124-0. [DOI] [PubMed] [Google Scholar]
- 25.Fucito LM, Toll BA, Salovey P, et al. Beliefs and attitudes about bupropion: Implications for medication adherence and smoking cessation treatment. Psychol Addict Behav. 2009;23(2):373–9. doi: 10.1037/a0015695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mooney ME, Sayre SL, Hokanson PS, et al. Adding MEMS feedback to behavioral smoking cessation therapy increases compliance with bupropion: a replication and extension study. Addict Behav. 2007;32(4):875–80. doi: 10.1016/j.addbeh.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 27.Finitsis DJ, Pellowski JA, Johnson BT. Text message intervention designs to promote adherence to antiretroviral therapy (ART): a meta-analysis of randomized controlled trials. PLoS One. 2014;9(2):e88166. doi: 10.1371/journal.pone.0088166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bock B, Heron K, Jennings E, et al. A text message delivered smoking cessation intervention: the initial trial of TXT-2-Quit: randomized controlled trial. JMIR MHealth UHealth. 2013;1(2):e17. doi: 10.2196/mhealth.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bock BC, Heron KE, Jennings EG, et al. User preferences for a text message-based smoking cessation intervention. Health Educ Behav. 2013;40(2):152–9. doi: 10.1177/1090198112463020. [DOI] [PubMed] [Google Scholar]
- 30.Whittaker R, McRobbie H, Bullen C, et al. Mobile phone-based interventions for smoking cessation. Cochrane Database Syst Rev. 2012;(11) doi: 10.1002/14651858.CD006611.pub3. [DOI] [PubMed] [Google Scholar]
- 31.Spohr SA, Nandy R, Gandhiraj D, et al. Efficacy of SMS text message interventions for smoking cessation: a meta-analysis. J Subst Abuse Treat. 2015;56:1–10. doi: 10.1016/j.jsat.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Skinner HA. The drug abuse screening test. Addict Behav. 1982;7(4):363–71. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- 33.Bush K, Kihlavan DR, McConell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Arch Int Med. 1998;15(16):1789–95. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 34.Heatherton TF, Kozlowski LT, Frecker RC, et al. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br J Addict. 1989;84(7):791–800. doi: 10.1111/j.1360-0443.1989.tb03059.x. [DOI] [PubMed] [Google Scholar]
- 35.Bradley KA, DeBenedetti AF, Volk RJ, et al. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res. 2007;31(7):1208–17. doi: 10.1111/j.1530-0277.2007.00403.x. [DOI] [PubMed] [Google Scholar]
- 36.Berman AH, Bergman H, Palmstierna T, et al. Evaluation of the drug use disorders identification test (DUDIT) in criminal justice and detoxification settings and in a Swedish population sample. Eur Addict Res. 2005;11(1):22–31. doi: 10.1159/000081413. [DOI] [PubMed] [Google Scholar]
- 37.The lifewindows project team [Internet] storrs (CT): the lifewindows information motivation behavioral skills ART adherence questionnaire (LW-IMBAAQ) [[cited 2015 Jul 28]];Center for health, intervention, and prevention, university of connecticut. 2006 Available from: http://www.chip.uconn.edu/chipweb/documents/Research/F_LWIMBARTQuestionnaire.pdf.
- 38.Fernandez S, Chaplin W, Schoenthaler AM, et al. Revision and validation of the medication adherence self-efficacy scale (MASES) in hypertensive African Americans. J Behav Med. 2008;31(6):453–62. doi: 10.1007/s10865-008-9170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson MO, Neilands TB, Dilworth SE, et al. The role of selfefficacy in HIV treatment adherence: validation of the HIV Treatment Adherence Self-Efficacy Scale (HIVASES) J Behav Med. 2007;30(5):359–70. doi: 10.1007/s10865-007-9118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nollen NL, Mayo MS, Ahluwalia JS, et al. Factors associated with discontinuation of bupropion and counseling among African American light smokers in a randomized clinical trial. Ann Behav Med. 2013;46(3):336–48. doi: 10.1007/s12160-013-9510-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buchanan TA, Carla BJ, Cox LS, et al. Adherence to varenicline among African American smokers: an exploratory analysis comparing plasma concentration, pill count, and self-report. Nicotine Tob Res. 2012;14(9):1083–91. doi: 10.1093/ntr/ntr333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nollen NL, Cox LS, Nazir N, et al. A pilot clinical trial of varenicline for smoking cessation in black smokers. Nicotine Tob Res. 2011;13(9):868–73. doi: 10.1093/ntr/ntr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fisher JD, Amico KR, Fisher WA, et al. The information-motivation-behavioral skills model of antiretroviral adherence and its applications. Curr HIV/AIDS Rep. 2008;5(4):193–203. doi: 10.1007/s11904-008-0028-y. [DOI] [PubMed] [Google Scholar]
- 44.Bandura A. Regulation of cognitive processes through perceived self-efficacy. Dev Psychol. 1989;25(5):729–35. [Google Scholar]
- 45.Fishbein M. A reasoned action approach to health promotion. Med Decis Making. 2008;28(6):834–44. doi: 10.1177/0272989X08326092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fisher JD, Fisher WA, Amico KR, et al. An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Health Psychol. 2006;25(4):462–73. doi: 10.1037/0278-6133.25.4.462. [DOI] [PubMed] [Google Scholar]
- 47.Starace F, Massa A, Amico KR, et al. Adherence to antiretroviral therapy: an empirical test of the information-motivation-behavioral skills model. Health Psychol. 2006;25:153–62. doi: 10.1037/0278-6133.25.2.153. [DOI] [PubMed] [Google Scholar]
- 48.Marlatt GA, Donovan DM, editors. Relapse prevention: maintenance strategies in the treatment of addictive behaviors. New York: Guilford Press; 2005. [Google Scholar]
- 49.Osterberg L, Blaschke T. Adherence to medication. New Engl J Med. 2005;353(5):487–97. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 50.Free C, Phillips G, Galli L, et al. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: a systematic review. PLoS Med. 2013;10(1):e1001362. doi: 10.1371/journal.pmed.1001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Free C, Whittaker R, Knight R, et al. Txt2stop: a pilot randomised controlled trial of mobile phone-based smoking cessation support. Tob Control. 2009;18(2):88–91. doi: 10.1136/tc.2008.026146. [DOI] [PubMed] [Google Scholar]
- 52.National Cancer Institute [Internet] [[cited 2015 Jul 27]];Bethesda (MD): Smoke-freeTXT. 2011 Available from: http://smokefree.gov/SmokefreeTXT/
- 53.Lewis MA, Uhrig JD, Bann CM, et al. Tailored text messaging intervention for HIV adherence: a proof-of-concept study. Health Psychol. 2013;32(3):248–53. doi: 10.1037/a0028109. [DOI] [PubMed] [Google Scholar]
- 54.Krebs P, Tseng TY, Pham H, et al. Formative evaluation of a text messaging intervention to promote varenicline adherence among tobacco-dependent persons with HIV. J Health Commun. 2015;9:1–5. doi: 10.1080/10810730.2015.1018595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Colby SM, Monti PM, Barnett NP, et al. Brief motivational interviewing in a hospital setting for adolescent smoking: a preliminary study. J Consult Clin Psychol. 1998;66(3):574–8. doi: 10.1037//0022-006x.66.3.574. [DOI] [PubMed] [Google Scholar]
- 56.Hall SM, Muñoz RF, Reus VI. Cognitive-behavioral intervention increases abstinence rates for depressive-history smokers. J Consult Clin Psychol. 1994;62(1):141–6. doi: 10.1037//0022-006x.62.1.141. [DOI] [PubMed] [Google Scholar]
- 57.Miller WR, Wilbourne PL. Mesa Grande: a methodological analysis of clinical trials of treatments for alcohol use disorders. Addiction. 2002;97(3):265–77. doi: 10.1046/j.1360-0443.2002.00019.x. [DOI] [PubMed] [Google Scholar]
- 58.Hollands GJ, McDermott MS, Lindson-Hawley N, et al. Interventions to increase adherence to medications for tobacco dependence. Cochrane Database Syst Rev. 2015;(2) doi: 10.1002/14651858.CD009164.pub2. [DOI] [PubMed] [Google Scholar]
- 59.Bellg AJ, Borrelli B, Resnick B, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH behavior change consortium. Health Psychol. 2004;23(5):443–51. doi: 10.1037/0278-6133.23.5.443. [DOI] [PubMed] [Google Scholar]
- 60.StataCorp, StataCorp. Stata statistical software: release 14. College Station: StataCorp LP; 2015. [Google Scholar]
- 61.Nakagawa S, Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol. 2013;4(2):133–42. [Google Scholar]
- 62.Lane C, Huws-Thomas M, Hood K, et al. Measuring adaptations of motivational interviewing: the development and validation of the behavior change counseling index (BECCI) Patient Educ Couns. 2005;56(2):166–73. doi: 10.1016/j.pec.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 63.Hardy H, Kumar V, Doros G, et al. Randomized controlled trial of a personalized cellular phone reminder system to enhance adherence to antiretroviral therapy. AIDS Patient Care STDs. 2011;25(3):153–61. doi: 10.1089/apc.2010.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baranoski AS, Meuser E, Hardy H, et al. Patient and provider perspectives on cellular phone-based technology to improve HIV treatment adherence. AIDS Care. 2014;26(1):26–32. doi: 10.1080/09540121.2013.802282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reynolds NR, Testa MA, Marc LG, et al. Factors influencing medication adherence beliefs and self-efficacy in persons naive to antiretroviral therapy: a multicenter, cross-sectional study. AIDS Behav. 2004;8(2):141–50. doi: 10.1023/B:AIBE.0000030245.52406.bb. [DOI] [PubMed] [Google Scholar]
- 66.Vidrine DJ, Arduino RC, Lazev AB, et al. A randomized trial of a proactive cellular telephone intervention for smokers living with HIV/AIDS. Aids. 2006;20(2):253–60. doi: 10.1097/01.aids.0000198094.23691.58. [DOI] [PubMed] [Google Scholar]