Abstract
Amphibole asbestos exposure is associated with production of mesothelial cell autoantibodies (MCAA). These MCAA have been linked with pleural fibrotic disease in the asbestos exposed community of Libby, Montana, and induce collagen deposition by cultured mesothelial cells. However, the exact intracellular mechanism by which these autoantibodies cause an increase in collagen deposition remains unknown. This study sought to gain insight into the transcription factors involved in the collagen production after human mesothelial cells are exposed to MCAA. In this study, transcription factor activation profiles were generated from human mesothelial cells (Met5A) treated with serum from Libby subjects, and were compared to cells treated with serum cleared of IgG, and therefore containing no MCAA. Analysis of those profiles indicated C/EBP-beta and hypoxia inducible factor 1 alpha (HIF-1α) are significantly increased in the nucleus, indicating activation, due to MCAA exposure compared to controls. Inhibition of either of these transcription factors significantly reduced collagen 1 deposition by these cells following exposure to MCAA. These data suggest autoantibodies are directly involved in type I collagen deposition and may elucidate potential therapeutic targets for autoantibody mediated fibrosis.
Keywords: Amphibole asbestos, Autoimmunity, Pleural Fibrosis
Introduction
A mine near Libby, Montana, USA, was the largest producer of vermiculite in the world from the early 1920s to 1990 (Meeker et al. 2003). Vermiculite is a mineral well known for its ability to resist changes in temperature and degradation, making this silicate mineral desirable for use in many products. Unfortunately, the vermiculite ore bed was contaminated with a unique type of amphibole asbestos now referred to as Libby Amphibole (LA) (Black et al. 2014). Many public structures in Libby were conditioned with this mineral, including homes, playgrounds, and the track and baseball field. Moreover, children would sometimes play in the piles of vermiculite, and families would use it in their gardens (Black et al. 2014). In 2002, the mine and the surrounding community were designated as a Superfund site by the U.S. Environmental Protection Agency (EPA) because of considerable health complications associated with LA exposure, now known to include reduced pulmonary function, enhanced autoimmune responses, pleural and interstitial fibrosis, and substantially increased mortality from lung cancer and malignant mesothelioma (Antao et al. 2012, Peipins et al. 2003, Pfau et al. 2005, Whitehouse et al. 2008). Vermiculite containing 0.3–7.0% LA was shipped to processing plants spread throughout the United States as well as other countries (Sullivan 2007), and millions of homes and other buildings still contain the material.
Asbestos-Related Diseases (ARD) noted in the Libby population include both malignant (mesothelioma) and non-malignant pleural disease, in addition to classical pulmonary fibrosis, termed asbestosis (Larson et al. 2010, Winters et al. 2012). Pleural disease, however, is the most prevalent pulmonary outcome in Libby, causing significant morbidity and mortality (Peipins et al. 2003), with an atypical clinical presentation including severe chest pain and rapid progression (Black et al. 2014). Moreover, an increase in the risk for systemic autoimmune diseases, specifically Systemic Lupus Erythematosus (SLE), Rheumatoid Arthritis (RA), and Systemic Sclerosis (SSc), has also been reported (Noonan et al. 2006, Pfau et al. 2014). An increased frequency of positive antinuclear autoantibody (ANA) tests occurs in this population and others exposed to amphibole asbestos (Marchand et al. 2012, Pfau et al. 2005, Pfau et al. 2014). Further examination of potential health effects of the Libby Amphibole revealed that it could induce autoantibodies in both mice and rats (Pfau et al. 2008, Salazar et al. 2012), while chrysotile asbestos did not (Ferro et al. 2013). Those data strongly support the ability of LA to induce autoimmune responses in exposed individuals, and there was some evidence that this immune dysfunction was associated with the incidence of pulmonary disease (Marchand et al. 2012, Pfau et al. 2005). Studies were undertaken to understand mechanisms whereby autoantibodies could play a role in the predominant non-malignant pulmonary diseases in Libby. This led to the discovery of tissue-specific autoantibodies in addition to the ANA, including anti-fibroblast antibodies (AFA) in LA-exposed mice (Pfau et al. 2011) and mesothelial cell autoantibodies (MCAA) in a LA-exposed cohort (Marchand et al. 2012). These autoantibodies subsequently were shown to induce collagen production in cultured fibroblasts and mesothelial cells, respectively (Pfau et al. 2011, Serve et al. 2013). We recently demonstrated that the LA-induced autoantibodies alone, in the absence of asbestos, could contribute to the fibrotic process in mice (Gilmer et al. 2016). The public health significance of this result is further highlighted by evidence that the LA non-malignant pleural disease is more severe and progressive than what is classically described for general occupational (chrysotile) asbestos exposures, and that the radiographic lesions were clearly associated with pulmonary function decline (Black et al. 2014, Larson et al. 2012, Whitehouse 2004).
The traditional mechanism of fibrogenesis has been well characterized, primarily focused on the pathways regulating production and remodeling of Type 1 collagen, one of the most prevalent proteins in the extracellular matrix, ECM (Bosman and Stamenkovic 2003). Growth factors such as platelet derived growth factor (PDGF), fibroblast growth factor 2 (FGF-2), and transforming growth factor beta 1(TGF-B1) are known to stimulate ECM deposition (Chaudhary et al. 2007). There are several transcription factors that play a role in the regulation of fibrosis including Smads, Sp1, and C/EBP-beta (Ghosh 2002).
However, the molecular mechanism of how mesothelial cell autoantibodies affect fibrosis has not been characterized, although we have shown that, in vitro, MCAA trigger collagen matrix deposition in the absence of epithelial-mesenchymal transition or TGF-beta signaling (Serve et al. 2013). Therefore, autoantibody-driven fibrosis may occur via a novel pathway, which could elucidate new potential targets for treatments of fibrotic diseases. We hypothesized that MCAA exposure leads to activation of a distinct set of transcription factors, and that this activation leads to collagen deposition in vitro. Based on transcription factor array data from mesothelial cells treated with MCAA, we predicted that transcription factors C/EBP-beta and hypoxia inducible factor (HIF-1α) would be activated by MCAA and that those activated transcription factors would result in an mRNA-expression profile unique to fibrosis. We further predicted that by inhibiting the transcription factors affected by MCAA exposure, collagen deposition stimulated by MCAA in vitro would return to normal levels, thereby establishing key steps in the MCAA-driven fibrotic pathway.
Materials and Methods
Human serum samples and IgG clearance
Serum samples were collected by the Center for Asbestos Related Disease (CARD) in Libby, Montana, USA in accordance with standard clinical protocols and the CARD clinic's IRB approval, then shipped to Idaho State University under IRB project approval #3292MOD and stored at −80°C until needed. Samples previously identified as MCAA positive were pooled (Serve et al. 2013) in order to provide enough serum for all experiments, and small aliquots were stored at −20°C. MCAA-positive serum cleared of IgG antibodies was used as a negative control treatment. Serum was cleared using Protein G Agarose Beads (Thermo Scientific, Rockford, IL) according to manufacturer’s instructions. Removal of detectable IgG was confirmed by SDS-PAGE, using a 12% Bis–Tris gel (Novex, Life Technologies, Carlsbad, CA), staining with GelCode Blue Stain Reagent (Thermo Scientific), and checking for lack of bands corresponding to the molecular weight of IgG heavy and light chains (50 and 25 kDa respectively). The IgG-cleared serum was subsequently shown to be MCAA-negative by cell binding ELISA, described below.
MCAA cell binding assay
A cell-based ELISA was performed as previously described (Serve et al. 2013) to confirm the presence of MCAA in serum samples. MeT-5A cells (ATCC, Manassas, VA) were seeded at confluency on 96 well plates, attached overnight and fixed in 1% paraformaldehyde. Following washes with phosphate-buffered saline (PBS)-Tween (0.05%), cells were blocked with 5% non-fat dried milk/PBS and then triplicate wells were exposed to either pooled MCAA-positive (MCAA+) serum samples or pooled MCAA+ serum samples cleared of IgG (Cleared), each diluted in 3% bovine serum albumin (BSA)/PBS (1:100). Following a 2-h incubation with serum as the primary antibody, cells were washed and blocked a second time. The secondary antibody HRP-conjugated goat anti-human IgG (Jackson ImmunoResearch, West Grove, PA) was applied at a dilution of 1:1000 in 3% BSA/PBS and incubated for 1 h. Excess antibody was removed and plates developed using TMB substrate reagent (Thermo Scientific), and stopped by 30 µL/ well of 1M HCl. Plates were analyzed at 450 nm on a microtiter plate reader (BioTek Instruments, Winooski, VT). Non-specific secondary antibody binding was corrected for on a plate-to-plate basis by subtracting the mean optical density (OD) for the secondary antibody-only control wells from the mean OD of each sample.
Transcription factor profile
Human mesothelial MeT-5A cells were seeded to confluency in two wells of a six well plate and allowed to adhere overnight. One well was treated with 10 µL of MCAA+ serum (1:200 dilution) and the other well was treated with 10 µL IgG-cleared MCAA+ serum. After 2h, a nuclear extraction (Signosis, Santa Clara, CA) was performed according to the manufacturer’s protocol and samples were stored at 4°C. To screen the nuclear extracts for 48 different transcription factors, TF Activation Profiling Plate Array I (Signosis) was utilized and performed according to the provided protocol. Briefly, nuclear extracts were mixed with biotin-labeled probes for the consensus sequences of specific transcription factor binding sites. Bound probes were then separated using a spin column. The bound probes were separated from the transcription factor complex and analyzed on the hybridization plate, which is coated with complementary sequences of the probes. The plate was then developed, with luminescence detected and quantified on the microtiter plate reader.
Transcription factor ELISA
To confirm activation (nuclear translocation) of C/EBP-beta and HIF-1α, a sandwich ELISA was performed. MeT-5A cells were seeded to confluency in six well plates and allowed to adhere overnight. Three wells were treated with 10 µL of pooled MCAA+ serum (1:200 dilution), three wells were treated with 10 µL of pooled MCAA+ serum cleared of IgG, three wells were treated with 1 µM hydrocortisone to induce C/EBP-beta as a positive control (Roos and Nord 2012), and three wells were treated with 26 µM N-acetyl-Leu-Leu-norleucinal (ALLN, Sigma Aldrich, St. Louis MO), a calpain protease inhibitor that has been reported to block C/EBP activation (Tang and Lane 2000), to act as a negative control. Three wells were treated with 50 µM cobalt chloride (Sigma) to induce HIF-1α as a positive control (Piret et al. 2002), and three wells were treated with 20 µM HIF-1α Inhibitor (Santa Cruz Biotechnology, Dallas TX) to act as a negative control. After 2h, a nuclear extraction was performed as before. Wells of a high binding 96 well plate were coated with 150 ng of C/EBP-beta rabbit polyclonal IgG (Santa Cruz) or HIF-1α rabbit polyclonal IgG (ABCAM, Cambridge, United Kingdom) per well for 2h. Wells were washed three times for four minutes with PBS/Tween-0.05%. Wells were blocked with 5% milk/PBS for 2h. Nuclear extracts from each of the wells of the 6-well plates (n=3 in each treatment group) were diluted 1:2, and 40µL of the extract was added to 3 replicate wells each, and incubated for 2h. Wells were washed and blocked as before. 150 ng of C/EBP-beta mouse monoclonal IgG (Santa Cruz) or HIF-1α mouse monoclonal IgG (ABCAM) in PBS with 3% BSA were added to each well and incubated for 2h. Wells were washed and blocked as before. HRP-conjugated goat anti-mouse IgG (Jackson ImmunoResearch) was applied at a dilution of 1:1000 in 3% BSA/PBS and incubated for 1 hour. Wells were washed as before. 100 µL of TMB substrate (Thermo Scientific) was added to each well and reaction was stopped with 50 µL of HCl per well. Plates were analyzed at 450 nm on a microtiter plate reader. Non-specific secondary antibody binding was corrected for on a plate-to-plate basis by subtracting the mean optical density (OD) for the secondary antibody-only control wells from the mean OD of each sample.
Collagen Assay with blocked HIF-1α and CEBP-beta
MeT-5A cells were seeded to confluency in a 96-well plate. For each of the following treatments, three wells were treated with either 2 µL of PBS to act as a negative control, 2 µL of pooled MCAA+ serum (1:50 dilution), 2 µL of pooled MCAA+ serum cleared of IgG (Cleared), 26 µM ALLN plus 2 µL of MCAA+ serum, 20 µM HIF-1α inhibitor plus 2 µL of MCAA+ serum. The plate was incubated for three days to allow for collagen deposition. Final concentration of diluents in the media were 1% ethanol for ALLN and 0.1% DMSO for HIF-1 α inhibitor. At these concentrations, neither of the diluents alone showed any toxicity to the cells, nor affected collagen production relative to either untreated cells (in the absence of MCAA+ serum, P=0.22 for ethanol, 0.89 for DMSO) or MCAA-treated cells (in the presence of MCAA+ serum, P=0.45 for ethanol, 0.16 for DMSO) (Data not shown). Cells were then blocked with 5% milk in PBS, and then primary antibody rabbit anti-collagen type 1 (ABCAM) was added at a 1:100 dilution with 3%BSA/PBS. After rinsing wells with PBS-Tween (0.05%), the wells were blocked as before. The secondary antibody used was HRP-conjugated goat anti-rabbit IgG (ABCAM) diluted 1:1000 with 3%BSA/PBS. Excess antibody was removed and plates developed using TMB reagent (Thermo Scientific), and stopped by 30 µL 1M HCl. Plates were read at 450 nm on a microtiter plate reader.
RT2 Profiler qPCR Array
Two T75 flasks were each seeded with 10 million MeT-5A cells, which were allowed to settle for 1 hour. One flask was treated with 20 µL of MCAA+ serum and the other flask was treated with 20 µL of cleared MCAA+ serum, and the flasks were incubated overnight. An RNA spin extraction was performed using RNeasy Mini Kit (SABiosciences, Frederick, MD) according to the manufacturer's protocol. RNA quantity and quality was determined by the ISU Molecular Research Core Facility using a ND-100 spectrophotometer (NanoDrop, Wilmington, DE) and a Fragment Analyzer (Advanced Analytical, Ankeny, IA). Relative mRNAs known to be related to fibrosis were quantified using a RT2 Profiler PCR Array for Human Fibrosis (Qiagen, Valencia, CA) according to the manufacturer’s instructions. This kit allowed for the screening of 83 genes (Table 1) associated with deposition, remodeling, and regulation of the extracellular matrix. According to the manufacturer, the assay has high (>85%) sensitivity with as little as 5 ng of mRNA from the extraction as long as there is no DNA contamination. In this experiment, NanoDrop analysis indicated highly pure RNA with negligible DNA.
Table 1.
All 83 fibrosis-associated genes analyzed in the RT qPCR gene expression experiment (RT2 Profiler PCR Array for Human Fibrosis, Qiagen, Valencia, CA).
| Gene Symbol | ||
|---|---|---|
| ACTA2 | IL4 | PLAU |
| AGT | IL5 | PLG |
| AKT1 | ILK | SERPINA1 |
| BCL2 | INHBE | SERPINE1 |
| BMP7 | ITGA1 | SERPINH1 |
| CAV1 | ITGA2 | SMAD2 |
| CCL11 | ITGA3 | SMAD3 |
| CCL2 | ITGAV | SMAD4 |
| CCL3 | ITGB1 | SMAD6 |
| CCR2 | ITGB3 | SMAD7 |
| CEBPB | ITGB5 | SNAI1 |
| COL1A2 | ITGB6 | SP1 |
| COL3A1 | ITGB8 | STAT1 |
| CTGF | JUN | STAT6 |
| CXCR4 | LOX | TGFB1 |
| DCN | LTBP1 | TGFB2 |
| EDN1 | MMP1 | TGFB3 |
| EGF | MMP13 | TGFBR1 |
| ENG | MMP14 | TGFBR2 |
| FASLG | MMP2 | TGIF1 |
| GREM1 | MMP3 | THBS1 |
| HGF | MMP8 | THBS2 |
| IFNG | MMP9 | TIMP1 |
| IL10 | MYC | TIMP2 |
| IL13 | NFKB1 | TIMP3 |
| IL13RA2 | PDGFA | TIMP4 |
| IL1A | PDGFB | TNF |
| IL1B | PLAT | |
Each well contained the appropriate primer for the individual gene of interest for that well. Real time quantitative PCR allowed for the simultaneous detection and amplification of mRNAs. By observing which wells crossed the threshold cycle earlier in the procedure, the relative amounts specific mRNAs was determined. Plates were analyzed using a Bio-Rad CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA). The threshold cycle was calculated and adjusted according to the manufacturer’s provided equation. Fluorescence data was exported to the Qiagen data analysis software where housekeeping genes and genomic DNA controls were analyzed to confirm validity of the protocol. The fluorescence data of the 83 wells were then analyzed using the same software to compare which treatment type contained relatively more mRNA for each appropriate gene. Kit instructions state that a difference in threshold cycles of 1 suggest a true up-or down-regulation to due the sensitivity of the assay, and are reportable.
Statistical analysis
Two-tailed, unpaired t-tests and one-way ANOVA (with Bonferroni post-hoc) tests were performed, using StatPlus (StatPlus Software, Walnut, CA). Statistical significance was defined as P < 0.05. Data are graphed with error bars indicating standard error of the mean (SEM). Graphs are representative of at least two experimental repetitions.
Results
Confirmation of the presence of MCAA in serum from LA-exposed subjects
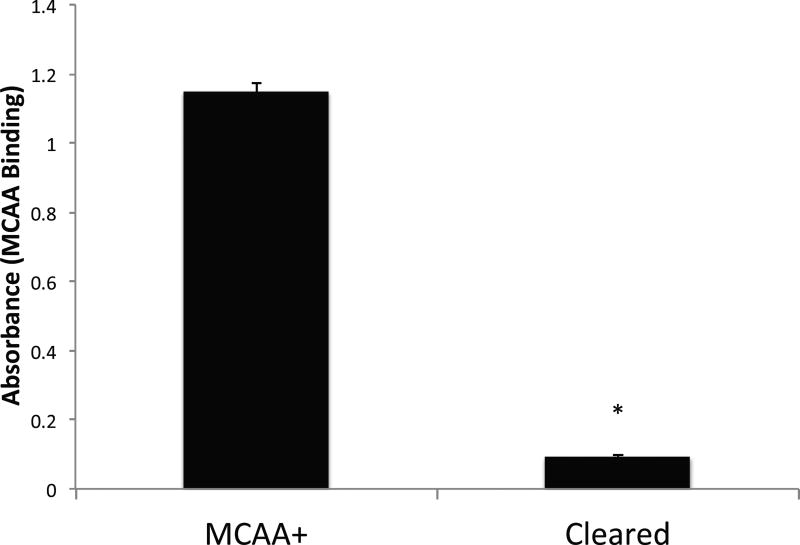
We hypothesized that exposing human cells to serum containing MCAA would cause increased activation of specific transcription factors responsible for regulation of fibrosis. In order to test the hypothesis, confirmation of the presence of MCAA in the human serum samples was required. The results of the MCAA cell binding assay (Figure 1) showed that clearing the IgG from the serum pooled from subjects already shown to be MCAA+ (Serve et al. 2013) reduced binding to a minimal level. This allowed us to use the pooled cleared serum as a MCAA-negative control in the subsequent experiments.
Figure 1.
Binding ELISA demonstrating the autoantibody binding to Met5A mesothelial cells due to the presence of MCAA in serum from human patients exposed to LA fibers. Sera were pooled from MCAA positive samples (MCAA+) and MCAA-positive samples cleared of all IgG (Cleared). Human mesothelial cells were plated in 96-well plates and then stained for MCAA binding using the pooled serum as the primary antibody and anti-human IgG - HRP for the secondary antibody. N= 3 samples per treatment group. Data are mean absorbance at 450 nm. *=p<0.001 by 2-tailed t-test.
Screening of transcription factors
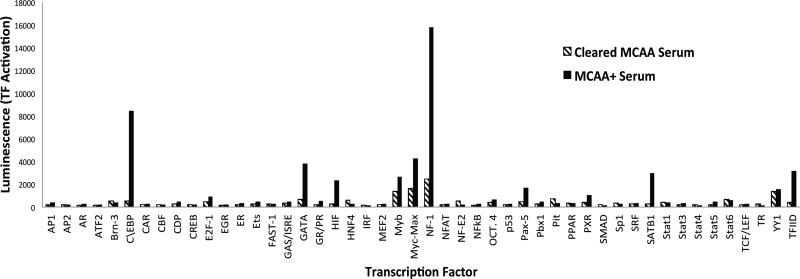
To determine which transcription factors were activated due to the presence of MCAA, we used a transcription factor profiling array that tested 48 different transcription factors. The nuclear extraction allowed us to observe only activated transcription factors that translocated to the nucleus. The kit specifies that readings between two samples must be over 2 fold (increase or decrease) to be significant. Treatment of the human mesothelial cells with serum containing MCAA resulted in differential activation greater than two-fold in 11 of the 48 examined transcription factors, compared to cells treated with serum cleared of MCAA. A representative set of the tested transcription factors are shown in Figure 2, where values represent the luminescence of hybridized sequences, indicating the relative amount of transcription factor in the nucleus. Of those 11 activated transcription factors, 9 have known binding sites to the COL1a1 promoter according to SABiosciences’ DECODE database (Table 2). Based on these results, we narrowed our investigation to C/EBP-beta and hypoxia inducible factor 1alpha, because their activation was increased more than three-fold, and the literature indicates that they have an established association with the extracellular matrix (Ghosh 2002).
Figure 2.
Transcription factor (TF) profile showing difference in amount of active transcription factor based on treatment. A nuclear extraction was performed and assessed for relative levels of activation by detection of presence of TF in the nucleus. Luminescence was detected when TF-bound sequences hybridized with complimentary sequences on the plate. Hatched bars indicate treatment of human mesothelial cells with pooled MCAA+ serum cleared of IgG. Black bars indicate treatment of human mesothelial cells with pooled MCAA+ serum.
Table 2.
Table showing results of the TF array, indicating the amount of increase in active transcription factor in cells that were exposed to MCAA serum relative to cells exposed to cleared serum. The 9 bolded pathways have a greater than two-fold increase and have bindings sites to the COL1A1 promoter.
| Pathway | Fold Change |
Pathway | Fold Change |
|---|---|---|---|
| AP1 | 1.664 | NF-1 | 6.346 |
| AP2 | 1.206 | NFAT | 1.318 |
| AR | 1.934 | NF-E2 | 0.361 |
| ATF2 | 1.883 | NFkB | 1.558 |
| Brn-3 | 0.810 | OCT. 4 | 1.715 |
| C\EBP | 16.166 | p53 | 1.372 |
| CAR | 1.500 | Pax-5 | 3.545 |
| CBF | 0.976 | Pbx1 | 1.714 |
| CDP | 1.763 | Pit | 0.471 |
| CREB | 0.839 | PPAR | 1.101 |
| E2F-1 | 1.981 | PXR | 2.695 |
| EGR | 1.463 | SMAD | 0.602 |
| ER | 1.610 | Sp1 | 0.880 |
| Ets | 1.642 | SRF | 1.356 |
| FAST-1 | 1.052 | SATB1 | 11.450 |
| GAS/ISRE | 1.509 | Stat1 | 1.029 |
| GATA | 5.866 | Stat3 | 1.443 |
| GR/PR | 2.424 | Stat4 | 0.746 |
| HIF | 9.016 | Stat5 | 2.231 |
| HNF4 | 0.404 | Stat6 | 0.864 |
| IRF | 0.943 | TCF/LEF | 1.190 |
| MEF2 | 1.125 | TR | 0.520 |
| Myb | 1.923 | YY1 | 1.148 |
| Myc-Max | 2.602 | TFIID | 7.440 |
Nuclear extract ELISA for activated transcription factors
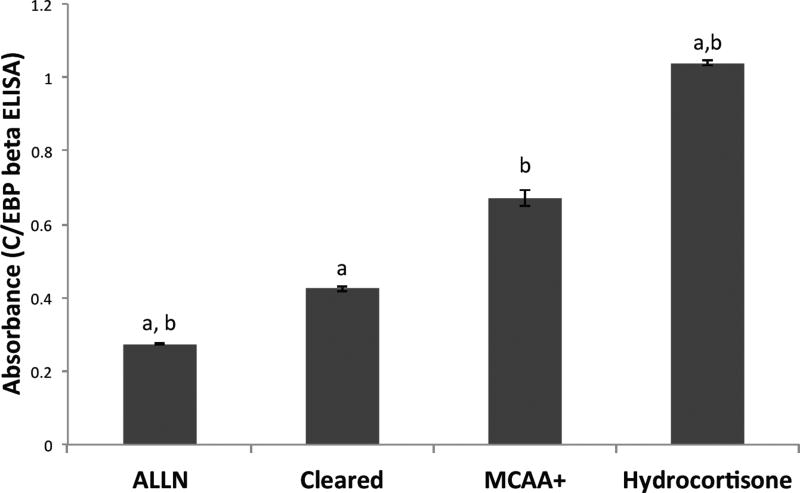
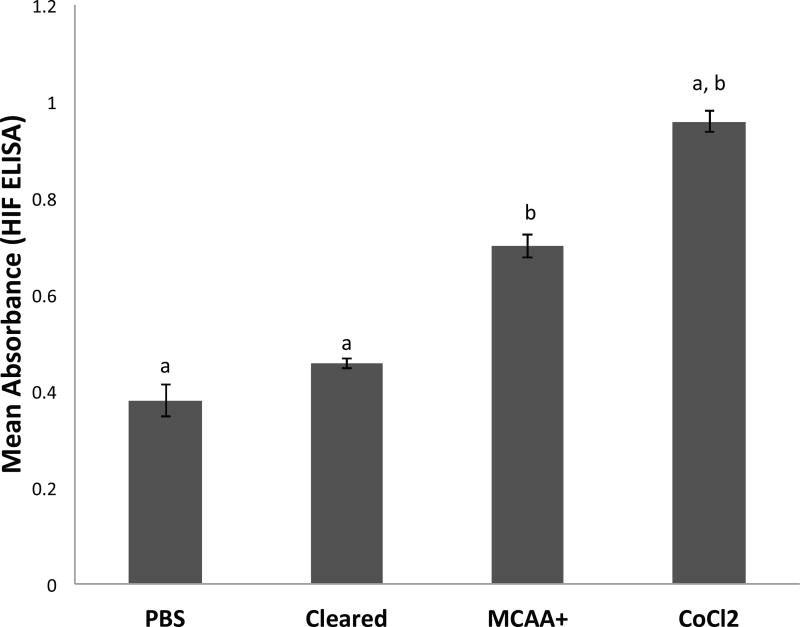
Following the screening assay, we used sandwich ELISA tests to confirm activation of C/EBP-beta and HIF-1α after exposure to MCAA serum. We were able to confirm the nuclear translocation of both C/EBP-beta and HIF-1α by performing the ELISA tests using nuclear extracts of human mesothelial cells to quantify the amount of active transcription factor. Human mesothelial cells treated with MCAA serum had significantly (N=3, p < 0.05) more active C/EBP-beta and HIF-1α than cells treated with cleared serum (Figure 3 and Figure 4). For C/EBP-beta (Figure 3), treatment with ALLN, a selective inhibitor of C/EBP (Tang and Lane 2000), significantly reduced C/EBP activation relative to MCAA+ serum, while treatment with hydrocortisone increased active C/EBP. For HIF-1α (Figure 4), treatment with PBS (negative control) or cleared serum showed similar levels, while both MCAA+ serum and cobalt chloride (HIF-1 inducer (Piret et al. 2002)) significantly increased activated HIF-1α compared to the controls.
Figure 3.
MCAA-induced increase in amount of active C/EBP-beta in nuclear extracts. Human mesothelial cells were plated in 6-well plates and then exposed to treatments (3 wells per treatment) for two hours before nuclear extraction. Negative control wells were treated with ALLN. Positive control wells were treated with hydrocortisone to induce C/EBP-beta. The nuclear extract of each well was then tested for the presence of C/EBP-beta by sandwich ELISA. N= 3 separate extracts per treatment group. a=p<0.05 compared to MCAA+; b=p<0.05 compared to Cleared, by both 2-tailed t-test and by 1-way ANOVA with Bonferroni post-hoc testing.
Figure 4.
MCAA-induced increase in amount of active hypoxia inducible factor in nuclear extracts, or positive control CoCl2. Human mesothelial cells were plated in 6-well plates and then exposed to treatments (3 wells per treatment) for two hours before nuclear extraction. The nuclear extract from each well was then tested for the presence of HIF-1α by sandwich ELISA. N= 3 separate extracts per treatment group. a=p<0.05 compared to MCAA+; b=p<0.05 compared to Cleared, by both 2-tailed t-test and by 1-way ANOVA with Bonferroni post-hoc testing.
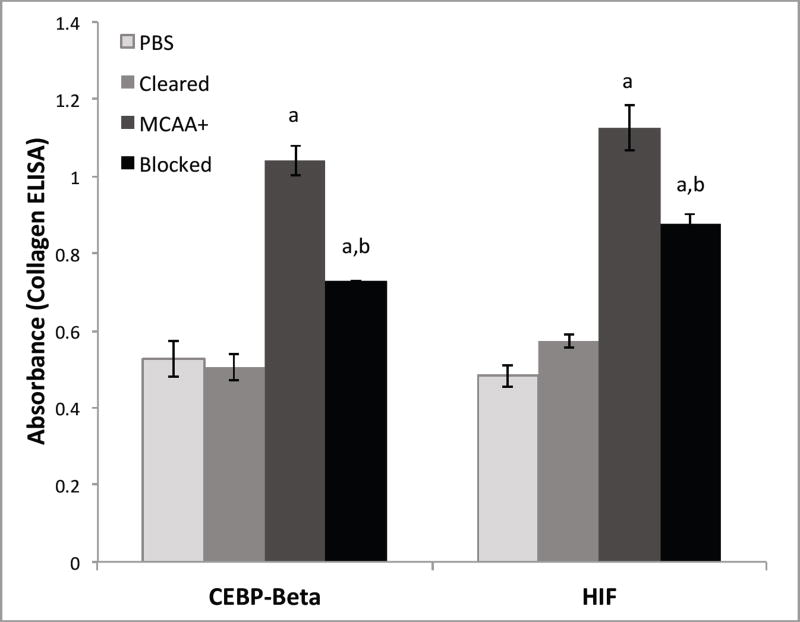
Blocking of collagen deposition
While C/EBP-beta and HIF-1α already have an established connection to the deposition, remodeling, and regulation of the extracellular matrix in the literature, it was not known whether they would be involved in autoantibody-induced collagen deposition. A firm connection between these transcription factors and the increase in collagen deposition induced by autoantibodies observed in vitro by Serve et al. (Serve et al. 2013) was needed. Blocking the activity of these transcription factors, and then quantifying the collagen deposited, tested the hypothesis that they play a key role in this novel mechanism for collagen deposition. As expected, the cells that received pooled MCAA+ serum produced significantly more collagen than those cells that received pooled serum cleared of IgG. Collagen levels were significantly reduced (p < 0.05) from the MCAA-induced levels when either C/EBP-beta or HIF activation was blocked with either ALLN (for C/EBP-beta) or HIF-1α Inhibitor (Figure 5). However, they did not return to the levels of the negative control nor cleared serum.
Figure 5.
| P-Value | HIF | C/EBP |
|---|---|---|
| MCAA vs Blocked | 0.029 | 0.041 |
| Cleared vs Blocked | 0.002 | 0.021 |
| Blocked vs Blocked | 0.027 | 0.027 |
Gene expression analysis
Analysis of mRNA induced by MCAA exposure was performed to further understand the molecular mechanism of this novel pathway of autoantibody-induced collagen deposition. Of the 83 genes associated with fibrosis that were analyzed, 56 were detected, 22 of which had differences in threshold cycles greater than 1 between treatment groups, and 8 of those had differences of 2 or greater (Table 3). Increases in transcription factor mRNA corresponded with the transcription factor profile that showed increases for MYC, SP1, and STAT1 and structural proteins (integrins), but these increases were quite small (less than 2 fold). More than 2-fold differences were seen for C/EBP-beta, LOX, PDGFA, TGFBR2, TIMP1, and STAT6 among the MCAA treated cells compared to the cells that received cleared serum (Table 3, bolded). The mRNAs for matrix metalloproteinases including MMP1, MMP14, MMP2, and MMP9 were all slightly decreased while mRNAs for metalloproteinase inhibitors such as TIMP1 and TIMP2 were increased, with MCAA treatment.
Table 3.
Table displaying relative differences in threshold cycles of genes studied in the RT2 Profiler Array for Human Fibrosis (Qiagen). The gene symbol corresponds to the abbreviation for the gene product. The difference was calculated by subtracting the threshold cycle values for MCAA+ serum treatment from those of the cleared serum treatment. Positive difference values indicate there was more starting mRNA in the MCAA+ samples than the cleared samples, while negative difference values indicate less starting mRNA in the MCAA+ samples than the cleared samples. Gene pathways of either treatment type that never crossed the threshold were omitted.
| Gene Symbol | Difference |
|---|---|
| CEBPB | 3.57 |
| CTGF | 0.58 |
| ENG | 0.85 |
| IL13 | −0.25 |
| IL13RA2 | −0.01 |
| IL1A | −0.40 |
| ILK | 1.93 |
| ITGA2 | 0.11 |
| ITGAV | 0.49 |
| ITGB1 | 0.62 |
| ITGB3 | 1.08 |
| ITGB5 | 1.60 |
| JUN | 0.76 |
| LOX | 2.37 |
| LTBP1 | −5.42 |
| MMP1 | −2.07 |
| MMP14 | −0.97 |
| MMP2 | −1.06 |
| MMP9 | −0.93 |
| MYC | 1.61 |
| NFKB1 | 1.40 |
| PDGFA | 2.40 |
| PDGFB | 1.11 |
| PLAU | −1.35 |
| SERPINE1 | 1.99 |
| SP1 | 0.68 |
| STAT1 | 1.03 |
| STAT6 | 2.23 |
| TGFB1 | 0.49 |
| TGFBR2 | 6.96 |
| TGIF1 | 1.82 |
| TIMP1 | 7.37 |
| TIMP2 | 1.01 |
Discussion
Our findings support the existence of a novel intracellular mechanism by which mesothelial cell autoantibodies induce collagen matrix deposition. Based on these data, it is clear that MCAA from LA-exposed subjects induce nuclear translocation of C/EBP-beta and HIF-1α. Furthermore, the data indicate those transcription factors play a key role in the increased collagen deposition in vitro, and therefore may be associated with LA-induced pleural fibrosis. Both C/EBP-beta and HIF-1α have known binding sites in promoters of genes responsible for regulation, remodeling, and deposition of collagen (Ghosh 2002). C/EBP-beta has been shown to act on the COL1A1 and COL1A2 promoters, for which the genes code for the fundamental precursor fibers of collagen (Ghosh et al. 2006). C/EBP-beta also acts on other transcription factors with known involvement to the regulation of the extracellular matrix including Sp1 (Lee et al. 1997). While the data suggest that C/EBP-beta plays a role in a significant proportion of the increased collagen deposition (Figure 5), there are other possible factors at play. The calpain-1 inhibitor, ALLN, used to block the activity of C/EBP-beta, widely inhibits cellular cysteine proteases, which may or may not affect the collagen output of these cells through other pathways. Hypoxia inducible factor is commonly induced by hypoxic conditions, such as those found in a mass of cancerous cells, where HIF is believed to play an important role in activating genes for angiogenesis and extracellular matrix deposition (Ghosh 2002, Wong et al. 2011). HIF triggers expression of the LOX gene, which codes for the lysyl oxidase protein. Lysyl oxidase is responsible for crosslinking between collagen and elastin in the extracellular matrix (Wong et al. 2011). Up-regulated expression of this specific remodeling gene might be expected during fibrosis; an increase in LOX mRNA was observed after treatment with MCAA (Table 3). These data show that HIF binding activity may be responsible for a portion of the overall increased collagen deposition (Figure 5). These data also suggest HIF can be activated by a different signaling pathway, unrelated to overt hypoxic conditions. Our findings reinforce the observation made by Serve et al (Serve et al. 2013) that cells exposed to MCAA in vitro produce more collagen than cells that receive only cleared serum, and that the collagen is produced in the absence of epithelial/mesenchymal transition (EMT). Moreover, we did not observe any significant changes in Smads, NF-kB, nor p53 activation (Table 2), which are traditionally associated with fibrosis (Serve et al. 2013). Taken together, these data suggest that autoantibody mediated fibrosis acts via a different intracellular pathway than cytokine mediated fibrosis.
The collagen assay specifically quantifies collagen matrix produced by the cells; however, fibrosis involves more than just the production of collagen. The gene expression data confirms that other genes for the structural subunits of the extracellular matrix such as integrins are also being transcribed. Integrins are important as they function to attach the extracellular matrix to the cytoskeleton of the cells. The gene expression data also show that the transcription factors that were activated correlated with an increase in respective mRNAs. While an increase in transcription factor mRNA does not confirm the presence of activated transcription factors, the correlation is worth pointing out because it sets the stage for continued responsiveness of these pathways. Beyond that, these data show a uniform decrease in mRNAs for all the matrix metalloproteinases studied. This decrease is noteworthy because the MMPs are responsible for breaking down the ECM (Visse and Nagase 2003), so if this reduced transcription correlates with low protein levels, this could contribute to reduced matrix degradation and thus the increase in the ECM observed. Contrary to what we expected, Serve et al observed an increase in presence and activity of MMP-8 and -9 when using a zymogen assay, which measures the actual proteins (Serve et al. 2013). The data also show an increase in the tissue specific metalloproteinase inhibitor (TIMP) mRNAs, which suggests another possible source of increased extracellular matrix as these proteins are responsible for inhibiting MMPs (Visse and Nagase 2003). Further studies need to be conducted to assess the amount and activity of these MMPs, TIMPs, and other ECM feedback proteins to better understand the dysregulation observed. Future studies also need to fully characterize the protein amounts and activity levels of the proteins in this pathway in order to understand MCAA-induced fibrosis.
Other autoimmune diseases have also been associated with fibrosis. Autoimmune hepatitis results in fibrosis of the liver (Bataller and Brenner 2005). While the exact mechanism behind this disease is not completely understood, the link between autoantibodies and fibrosis is strong (Bataller and Brenner 2005). Scleroderma is a systemic autoimmune disease involving fibrosis of connective tissue in various areas of the human body (Gabrielli et al. 2009). The severity of the disease correlates with presence of autoantibodies, but their specific role remains uncertain (Ihn et al. 2000). There is also a correlation between endothelial cell autoantibodies and pulmonary fibrosis (Ihn et al. 2000). Presence of autoantibodies is often used to aid in the diagnosis of a disease, but more consideration needs to be taken into the effect of these autoantibodies in the ultimate pathological outcome. This study connects the presence of autoantibodies to activation of specific transcription factors associated with fibrosis. Since LA induces these autoantibodies, this mechanism represents a critical candidate pathway contributing to the pleural fibrosis observed in the Libby patients.
Further studies need to be conducted to completely characterize this novel mechanism by which MCAA induce collagen deposition. This study only observed 48 transcription factors and 83 mRNAs. It is likely that the process of autoantibody-induced fibrosis involves more molecules than the ones investigated. Additional targets of these MCAA need to be identified in order to better understand the signal transduction pathway leading up to transcription factor activation. Determining whether MCAA induced collagen deposition is indeed connected with fibrosis in the pleural cavity will help to determine the connection to LA disease. The insight gained from this study reinforces the autoimmune component behind the disease, and helps illuminate new potential treatment approaches for this devastating disease.
Acknowledgments
This work was supported by grants from the National Institutes of Health: R15 ES21884-01 and INBRE P20 GM103408, and from the CDC/ATSDR TS000099-01 (Libby Epidemiology Research Program, LERP).
The Authors wish to acknowledge Dr. Kinta Serve, Mars Hill University, for her editorial and intellectual contributions to this work. We further dedicate this work to the memory of Dr. Steven Levin, who developed the LERP and whose insights inspired our continued research.
Footnotes
Disclosure of Interest
The authors report no conflicts of interest.
References
- Antao VC, Larson TC, Horton DK. Libby vermiculite exposure and risk of developing asbestos-related lung and pleural diseases. Current opinion in pulmonary medicine. 2012 Mar;18:161–167. doi: 10.1097/MCP.0b013e32834e897d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataller R, Brenner DA. Liver fibrosis. The Journal of clinical investigation. 2005 Feb;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black B, Szeinuk J, Whitehouse AC, Levin SM, Henschke CI, Yankelevitz DF, Flores RM. Rapid progression of pleural disease due to exposure to Libby amphibole: "Not your grandfather's asbestos related disease". American journal of industrial medicine. 2014 Nov;57:1197–1206. doi: 10.1002/ajim.22330. [DOI] [PubMed] [Google Scholar]
- Bosman FT, Stamenkovic I. Functional structure and composition of the extracellular matrix. The Journal of pathology. 2003 Jul;200:423–428. doi: 10.1002/path.1437. [DOI] [PubMed] [Google Scholar]
- Chaudhary NI, Roth GJ, Hilberg F, Muller-Quernheim J, Prasse A, Zissel G, Schnapp A, Park JE. Inhibition of PDGF, VEGF and FGF signalling attenuates fibrosis. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2007 May;29:976–985. doi: 10.1183/09031936.00152106. [DOI] [PubMed] [Google Scholar]
- Ferro A, Zebedeo CN, Davis C, Ng KW, Pfau JC. Amphibole, but not chrysotile, asbestos induces anti-nuclear autoantibodies and IL-17 in C57BL/6 mice. Journal of immunotoxicology. 2013 Oct 28; doi: 10.3109/1547691X.2013.847510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. The New England journal of medicine. 2009 May 7;360:1989–2003. doi: 10.1056/NEJMra0806188. [DOI] [PubMed] [Google Scholar]
- Ghosh AK. Factors involved in the regulation of type I collagen gene expression: implication in fibrosis. Experimental biology and medicine. 2002 May;227:301–314. doi: 10.1177/153537020222700502. [DOI] [PubMed] [Google Scholar]
- Ghosh AK, Bhattacharyya S, Mori Y, Varga J. Inhibition of collagen gene expression by interferon-gamma: novel role of the CCAAT/enhancer binding protein beta (C/EBPbeta) Journal of cellular physiology. 2006 Apr;207:251–260. doi: 10.1002/jcp.20559. [DOI] [PubMed] [Google Scholar]
- Gilmer J, Serve K, Davis C, Anthony M, Hanson R, Harding T, Pfau JC. Libby Amphibole Induced Mesothelial Cell Autoantibodies Promote Collagen Deposition in Mice. American journal of physiology Lung cellular and molecular physiology. 2016 Apr 22; doi: 10.1152/ajplung.00462.2015. ajplung 00462 02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihn H, Sato S, Fujimoto M, Igarashi A, Yazawa N, Kubo M, Kikuchi K, Takehara K, Tamaki K. Characterization of autoantibodies to endothelial cells in systemic sclerosis (SSc): association with pulmonary fibrosis. Clinical and experimental immunology. 2000 Jan;119:203–209. doi: 10.1046/j.1365-2249.2000.01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson TC, Antao VC, Bove FJ. Vermiculite worker mortality: estimated effects of occupational exposure to Libby amphibole. Journal of occupational and environmental medicine / American College of Occupational and Environmental Medicine. 2010 May;52:555–560. doi: 10.1097/JOM.0b013e3181dc6d45. [DOI] [PubMed] [Google Scholar]
- Larson TC, Lewin M, Gottschall EB, Antao VC, Kapil V, Rose CS. Associations between radiographic findings and spirometry in a community exposed to Libby amphibole. Occupational and environmental medicine. 2012 May;69:361–366. doi: 10.1136/oemed-2011-100316. [DOI] [PubMed] [Google Scholar]
- Lee YH, Williams SC, Baer M, Sterneck E, Gonzalez FJ, Johnson PF. The ability of C/EBP beta but not C/EBP alpha to synergize with an Sp1 protein is specified by the leucine zipper and activation domain. Molecular and cellular biology. 1997 Apr;17:2038–2047. doi: 10.1128/mcb.17.4.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand LS, St-Hilaire S, Putnam EA, Serve KM, Pfau JC. Mesothelial cell and anti-nuclear autoantibodies associated with pleural abnormalities in an asbestos exposed population of Libby MT. Toxicology letters. 2012 Jan 25;208:168–173. doi: 10.1016/j.toxlet.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker GP, Bern AM, Brownfield IK, Lowers HA, Sutley SJ, Hoefen TM, Vance JS. The composition and morphology of amphiboles from the Rainy Creek Complex, Near Libby, Montana. American Mineraologist. 2003;88:1955–1969. [Google Scholar]
- Miles LA, Castellino FJ, Gong Y. Critical role for conversion of glu-plasminogen to Lys-plasminogen for optimal stimulation of plasminogen activation on cell surfaces. Trends in cardiovascular medicine. 2003 Jan;13:21–30. doi: 10.1016/s1050-1738(02)00190-1. [DOI] [PubMed] [Google Scholar]
- Noonan CW, Pfau JC, Larson TC, Spence MR. Nested case-control study of autoimmune disease in an asbestos-exposed population. Environmental health perspectives. 2006 Aug;114:1243–1247. doi: 10.1289/ehp.9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peipins LA, Lewin M, Campolucci S, Lybarger JA, Miller A, Middleton D, Weis C, Spence M, Black B, Kapil V. Radiographic abnormalities and exposure to asbestos-contaminated vermiculite in the community of Libby, Montana, USA. Environmental health perspectives. 2003 Nov;111:1753–1759. doi: 10.1289/ehp.6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfau JC, Li S, Holland S, Sentissi JJ. Alteration of fibroblast phenotype by asbestos-induced autoantibodies. Journal of immunotoxicology. 2011 Jun;8:159–169. doi: 10.3109/1547691X.2011.562257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfau JC, Sentissi JJ, Li S, Calderon-Garciduenas L, Brown JM, Blake DJ. Asbestos-induced autoimmunity in C57BL/6 mice. Journal of immunotoxicology. 2008 Apr;5:129–137. doi: 10.1080/15476910802085756. [DOI] [PubMed] [Google Scholar]
- Pfau JC, Sentissi JJ, Weller G, Putnam EA. Assessment of autoimmune responses associated with asbestos exposure in Libby, Montana, USA. Environmental health perspectives. 2005 Jan;113:25–30. doi: 10.1289/ehp.7431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfau JC, Serve KM, Noonan CW. Autoimmunity and asbestos exposure. Autoimmune Dis. 2014;2014:1–11. doi: 10.1155/2014/782045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piret JP, Mottet D, Raes M, Michiels C. CoCl2, a chemical inducer of hypoxia-inducible factor-1, and hypoxia reduce apoptotic cell death in hepatoma cell line HepG2. Annals of the New York Academy of Sciences. 2002 Nov;973:443–447. doi: 10.1111/j.1749-6632.2002.tb04680.x. [DOI] [PubMed] [Google Scholar]
- Roos AB, Nord M. The emerging role of C/EBPs in glucocorticoid signaling: lessons from the lung. The Journal of endocrinology. 2012 Mar;212:291–305. doi: 10.1530/JOE-11-0369. [DOI] [PubMed] [Google Scholar]
- Salazar KD, Copeland CB, Wood CE, Schmid JE, Luebke RW. Evaluation of anti-nuclear antibodies and kidney pathology in Lewis rats following exposure to Libby amphibole asbestos. Journal of immunotoxicology. 2012 Dec 21;10:329–333. doi: 10.3109/1547691X.2012.747230. [DOI] [PubMed] [Google Scholar]
- Serve KM, Black B, Szeinuk J, Pfau JC. Asbestos-associated mesothelial cell autoantibodies promote collagen deposition in vitro. Inhalation toxicology. 2013 Dec;25:774–784. doi: 10.3109/08958378.2013.848249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PA. Vermiculite, respiratory disease, and asbestos exposure in Libby, Montana: update of a cohort mortality study. Environmental health perspectives. 2007 Apr;115:579–585. doi: 10.1289/ehp.9481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang QQ, Lane MD. Role of C/EBP homologous protein (CHOP-10) in the programmed activation of CCAAT/enhancer-binding protein-beta during adipogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2000 Nov 7;97:12446–12450. doi: 10.1073/pnas.220425597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse AC. Asbestos-related pleural disease due to tremolite associated with progressive loss of lung function: serial observations in 123 miners, family members, and residents of Libby, Montana. American journal of industrial medicine. 2004 Sep;46:219–225. doi: 10.1002/ajim.20053. [DOI] [PubMed] [Google Scholar]
- Whitehouse AC, Black CB, Heppe MS, Ruckdeschel J, Levin SM. Environmental exposure to Libby Asbestos and mesotheliomas. American journal of industrial medicine. 2008 Nov;51:877–880. doi: 10.1002/ajim.20620. [DOI] [PubMed] [Google Scholar]
- Winters CA, Hill WG, Rowse K, Black B, Kuntz SW, Weinert C. Descriptive analysis of the respiratory health status of persons exposed to Libby amphibole asbestos. BMJ open. 2012;2 doi: 10.1136/bmjopen-2012-001552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CC, Gilkes DM, Zhang H, Chen J, Wei H, Chaturvedi P, Fraley SI, Wong CM, Khoo US, Ng IO, et al. Hypoxia-inducible factor 1 is a master regulator of breast cancer metastatic niche formation. Proceedings of the National Academy of Sciences of the United States of America. 2011 Sep 27;108:16369–16374. doi: 10.1073/pnas.1113483108. [DOI] [PMC free article] [PubMed] [Google Scholar]