Abstract
Purpose
Tamoxifen therapy is integral in the treatment of patients with hormone receptor-positive breast cancer. However, there is an association between tamoxifen and thromboembolic events. Flap and systemic thromboembolic events have devastating consequences in microvascular breast reconstruction. Currently, there is conflicting data on the association between tamoxifen therapy and thromboembolic complications for patients undergoing microvascular breast reconstruction. The objective of this study is to determine if perioperative tamoxifen therapy modifies the risk of complications and thromboembolic events for patients with breast cancer undergoing microvascular breast reconstruction.
Methods
A comprehensive literature search was performed across 6 databases from January 2003 to February 2016. Pooled estimates and relative risk (RR) were calculated using a random-effects model, confounding was examined with meta-regression, and risk of bias was evaluated. Primary outcomes were thrombotic flap complications and total flap loss. Study quality was assessed using Downs and Black criteria.
Results
Of 95 studies reviewed, 4 studies comprising 1700 patients and 2245 procedures were included for analysis. Compared to non-recipients, patients on tamoxifen were at increased risk of developing thrombotic flap complications (pooled RR: 1.5; 95% CI: 1.14–1.98) and total flap loss (pooled RR: 3.35; 95% CI: 0.95–11.91). There was no significant heterogeneity present in either outcome and no evidence of publication bias.
Conclusions
Perioperative tamoxifen therapy may increase the risk of thrombotic flap complications and flap loss for patients with breast cancer undergoing microvascular reconstruction. These findings further the ability of providers to make evidence-based recommendations in the perioperative management of patients with breast cancer.
Keywords: Breast reconstruction, breast cancer, tamoxifen, selective estrogen receptor modulator, thromboembolism
INTRODUCTION
Breast cancer is the most common non-cutaneous malignancy among women in the United States.[1] For women diagnosed with breast cancer, distinct histological and molecular tumor subtypes contribute to different clinical presentations, treatment responses, and prognoses. A significant proportion of women with breast cancer have hormone receptor-positive tumors, subtypes that express estrogen receptors (ER-positive) or progesterone receptors (PR-positive).[2] Systemic hormone therapy reduces recurrence rates, prolongs survival, and lowers mortality for women with hormone receptor-positive breast tumors.[3,4] The most widely used therapy is tamoxifen, a selective estrogen receptor modulator (SERM). Treatment with tamoxifen for 5 years reduces recurrence rates in ER-positive breast cancer by half during treatment and by a third in the subsequent 5 years.[5] Furthermore, tamoxifen treatment reduces breast cancer mortality by nearly a third throughout the first 15 years and has efficacy in reducing the risk of contralateral breast cancer.[5,6]
Despite the efficacy of hormone therapy, there are significant potential adverse effects. Of particular concern, tamoxifen is an independent risk factor for thromboembolic events, a significant contributor to patient morbidity and mortality.[7,8] Breast cancer patients treated with tamoxifen have an estimated 1.5- to 7.1-fold increased risk of thromboembolic events compared to patients not treated with tamoxifen.[7,9,8] In patients undergoing surgical treatment for breast cancer, specifically mastectomy with microvascular breast reconstruction, this increased risk of thromboembolic events may be particularly problematic. There is increasing evidence that microvascular breast reconstruction is associated with improved quality of life and cosmetic outcomes for some patients, when compared to prosthetic reconstruction or mastectomy alone.[10–14] However, patients undergoing microvascular reconstruction have an increased susceptibility for systemic thromboembolic events given the prolonged duration of surgery, hospital length of stay, and period of non-ambulatory time following surgery.[13,15,16,14,17] In addition to systemic events, the hypercoagulable circulatory environment potentiated by tamoxifen may impact the microvascular anastomosis and contribute to thrombotic flap complications, including total flap loss. Therefore, there are concerns that patients on tamoxifen are at an increased risk for thromboembolic and flap complications following microvascular breast reconstruction.
Currently, there is a lack of convincing evidence on the association of tamoxifen therapy and thromboembolic complications for patients undergoing microvascular breast reconstruction as prior reports have produced conflicting results and conclusions.[18–21] Therefore, it is unclear whether tamoxifen cessation prior to reconstruction is warranted, representing a significant limitation in perioperative management. Thus, we undertook this study to determine if perioperative tamoxifen has an effect on complications and thromboembolic events for patients with breast cancer undergoing microvascular reconstruction. Our intention is to provide a qualitative and quantitative summation of current evidence that can lend clarity to this important area of research and guide evidence-based management decisions.
METHODS
The authors followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines throughout this investigation, as recommended by the EQUATOR Network.[22,23] The study research question, search strategy, inclusion and exclusion criteria, outcome measures, protocol for data extraction, and protocol for analyses were determined a priori by the authors.
Study Identification
The published literature was searched using strategies designed by a medical librarian for the concepts of breast cancer, flap or microvascular reconstruction, and hormone therapy including tamoxifen, selective estrogen receptor modulators, and aromatase inhibitors. These strategies were established using a combination of standardized terms and key words, and implemented in Ovid Medline, EMBASE, Scopus, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov databases. All searches were completed in February 2016. All results were exported to EndNote and the automatic duplicate finder was used to remove duplicate citations. References were then hand-searched and relevant articles retrieved.
Study Selection
All published observational studies and randomized-controlled trials comparing patients undergoing microvascular breast reconstruction on perioperative hormone therapy to patients not on perioperative hormone therapy were eligible for inclusion. Titles and abstracts were screened independently by two reviewers, with discrepancies resolved by discussion. A study was eligible if it included 1) patients with breast cancer undergoing microvascular reconstruction, 2) compared patients on perioperative tamoxifen therapy to those who were not, and 3) listed systemic and/or flap complications including, but not limited to, deep vein thrombosis, pulmonary embolism, partial flap loss, total flap loss, and arterial and/or venous thrombosis. We defined microvascular reconstruction as any procedure involving free flap tissue transfer to the breast that required an anastomosis. Articles in all languages were considered. A study was excluded if 1) subjects were not human, 2) there was no control group or comparison made, or 3) full article text could not be obtained.
Data Extraction and Quality Assessment
Articles that met inclusion criteria underwent independent data extraction by two reviewers using a standardized form. Data extracted included first author, publication year, publication country, journal, demographics, number of patients and flaps, tamoxifen timing and use, flap timing and type, flap and systemic complications, and potential confounders including medical comorbidities, radiation therapy, body-mass index (BMI), age, systemic or intraoperative anticoagulant use, and length of surgery. Primary outcomes were thrombotic flap complications and total flap loss. Secondary outcomes were systemic thromboembolic events, overall flap complications, partial flap loss, and arterial or venous anastomotic complications. Analyses were performed at the flap level as this data was available in each of the included studies. All patients receiving tamoxifen within 28 days of the procedure were considered to be on perioperative therapy. The Downs and Black tool was used by two reviewers to independently assess study quality, and modified to exclude four questions regarding randomized controlled trials.[24] Cutoffs were determined by dividing the possible 23 points into quartiles. A score of 19 or above was designated as high quality, 11–18 indicated moderate quality, 3–10 indicated fair quality, and 2 or below indicated low quality.
Statistical Analysis
Comparisons of the risk of thrombotic flap complication and total flap loss between patients who had perioperative tamoxifen therapy versus those who did not were treated as the primary endpoints of interest, while overall flap complications, partial flap loss, and arterial or venous anastomotic complications were treated as secondary endpoints of interest. As such, relative risks (RR) and 95% confidence intervals were selected as the primary summary measure of association and calculated for each study and endpoint, and weighted averages of the RRs were computed to obtain the pooled estimate as visualized on forest plots.
Statistical heterogeneity across studies was assessed using the chi-square (Cochran Q statistic) test and quantified using the I-square statistic, with P < 0.1 indicating significant between-study heterogeneity as opposed to the conventional 0.05 given our small number of studies.[25] Given inevitable heterogeneity between studies, particularly for secondary outcomes, we utilized the more conservative DerSimonian and Laird random-effects model.[26]
For secondary endpoints, there was a need to control for certain confounding variables such as age, BMI, smoking, exposure to radiation therapy, and cardiovascular comorbidities to minimize heterogeneity. These were performed using univariate meta-regressions, and the residual I-square were observed. Publication bias was assessed by visual appraisal of funnel plots. If any asymmetry was observed, the Begg and Harbord tests were performed to determine if “small study effect” was present using two-tailed P < 0.05 for significance.[27,28] Statistical Analyses were conducted in STATA 14.1 (StataCorp LP, College Station, TX).
RESULTS
Study selection
The results of the search strategy and selection process are outlined in the PRISMA flow diagram (Fig. 1). Initially, 154 citations were identified. After removal of duplicates, there were 95 unique studies. Abstracts and titles were screened, and 32 were preliminarily determined to meet criteria. Following full review, 4 studies met criteria for data extraction and analysis.[18–21]
Fig. 1.
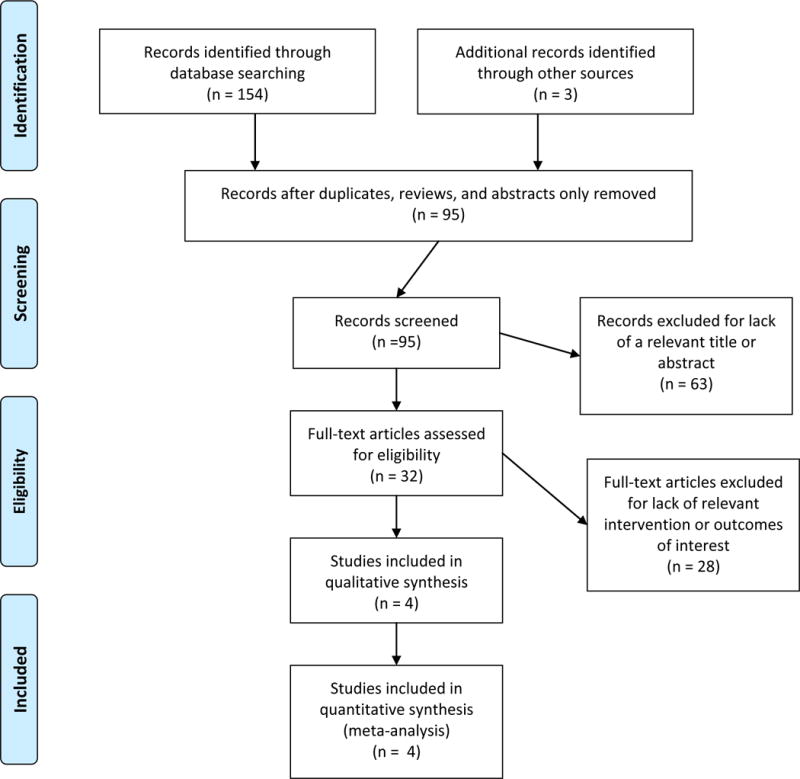
PRISMA flow diagram of articles screened and selected for meta-analysis.
Study Characteristics and Quality Assessment
Articles reported data collection for patients undergoing operations between 1993 and 2012, with all articles published after 2012. Characteristics of studies included are listed in Table 1. In total, studies evaluated 1,700 patients and 2,245 microvascular flaps. Of these, 320 patients undergoing 369 flaps were on tamoxifen and 1,380 patients undergoing 1876 flaps were not on tamoxifen. All studies reported the number of flaps experiencing a thrombotic complication, the number of flaps experiencing total flap loss, and the number of flaps with any complication; three of four studies reported the outcomes of partial flap loss, any arterial anastomotic complication, and any venous anastomotic complication. Additionally, all studies included data on relevant covariates of exposure to radiation therapy, BMI, age, tobacco use, and cardiovascular disease. However, data was not always complete: only two studies reported the incidence of systemic thromboembolic complications. Furthermore, three studies did not report data on systemic prophylactic anticoagulation or intraoperative anticoagulation and two studies did not include data on adjuvant chemotherapy. All studies were of moderate quality, with a median score of 14 and a range of 13–18, on modified Downs and Black Quality Assessment Scale. Inter-rater reliability was excellent and indicated substantial agreement (Cohen’s Kappa = 0.80).[29]
Table 1.
Characteristics of Studies Comparing Breast Reconstruction Outcomes for Patients on Tamoxifen versus Patients not on Tamoxifen
| Author | Location | Year | Study Design | N: Patients on Tamoxifen | N: Flaps performed in patients on tamoxifen | N: Patients not on tamoxifen | N: Flaps performed in patients not on tamoxifen | Quality Rating* |
|---|---|---|---|---|---|---|---|---|
| Kelley[19] | USA | 2012 | Cohort | 205 | 205 | 465 | 465 | Moderate |
| Jokuszies[18] | Germany | 2013 | Cohort | 5 | 5 | 24 | 24 | Moderate |
| Mirzabegi[20] | USA | 2015 | Cohort | 67 | 103 | 706 | 1120 | Moderate |
| Saliban[21] | USA | 2016 | Cohort | 43 | 56 | 185 | 267 | Moderate |
See Methods section, Data Extraction and Quality Assessment subsection, for details on determination of Quality Rating.
Clinical Outcomes
Patients undergoing microvascular breast reconstruction on perioperative tamoxifen, when compared to patients not on perioperative tamoxifen, were at an increased risk of developing thrombotic flap complications [pooled RR = 1.5 (95% CI: 1.14, 1.98)] and total flap loss [pooled RR = 3.35 (95% CI = 0.95–11.91)]. No significant heterogeneity was present in the data for the outcome thrombotic flap complications (χ2 = 0.98, I2 = 0.0%, P = 0.807) (Fig. 2). No evidence of publication bias was present for the outcome thrombotic flap complications on funnel plot visual appraisal and this was confirmed by Begg’s and Harbord tests (Fig. 3). For the outcome total flap loss, minor heterogeneity was present; however this did not achieve statistical significance (I2 = 33.9%, P = 0.209) (Fig. 4). No evidence of publication bias was present for the outcome total flap loss as assessed by visual appraisal of funnel plot and confirmed by Begg’s and Harbord tests. Sensitivity analyses were separately performed for the outcomes thrombotic flap complications and overall flap loss. Analyses of these outcomes demonstrated no significant change to the overall estimate with serial removal of studies.
Fig. 2.
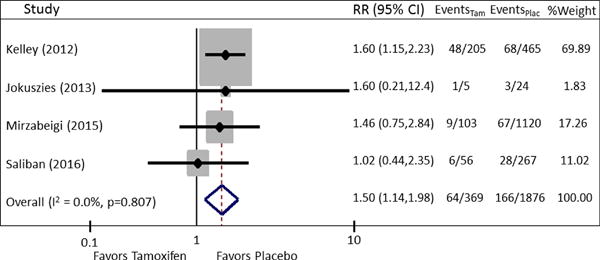
Forest Plot of pooled RR for the outcome thrombotic flap complications. Pooled RR is 1.50, 95% CI: 1.14 –1.98. No significant heterogeneity is present (χ2 = 0.98, I2 = 0.0%, P = 0.807).
Fig. 3.
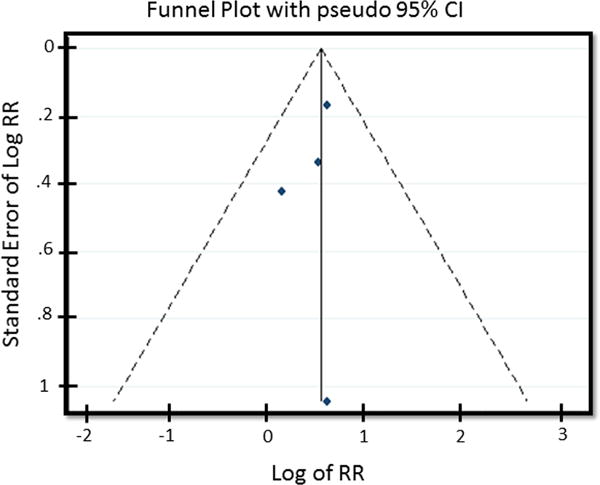
Funnel Plot assessing publication bias for outcome thrombotic flap complications.
Fig. 4.
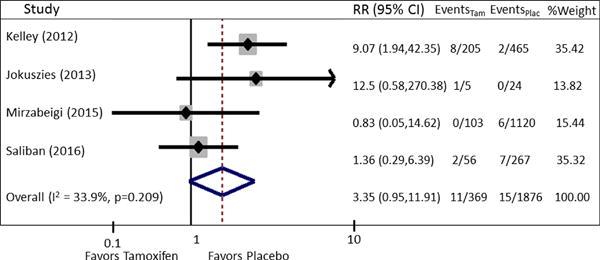
Forest plot of pooled RR for outcome of total flap loss. Pooled RR is 3.35, 95% CI: 0.95–11.91. Minor heterogeneity is present; however this did not achieve statistical significance (I2 = 33.9%, P = 0.209).
The risk of overall flap complications was slightly higher for patients on perioperative tamoxifen [RR = 1.11 (95% CI=0.72, 1.71)], however the data contained significant heterogeneity (I2 = 65.9%, P = 0.032) (Fig. 5). To explore sources of heterogeneity, a series of univariate regression analyses were performed including relevant clinical covariates of age, BMI, exposure to radiation therapy, current tobacco use, and presence of a cardiovascular comorbidity. After controlling for age, only 28% (P=0.27) residual heterogeneity remained (Fig. 6).
Fig. 5.
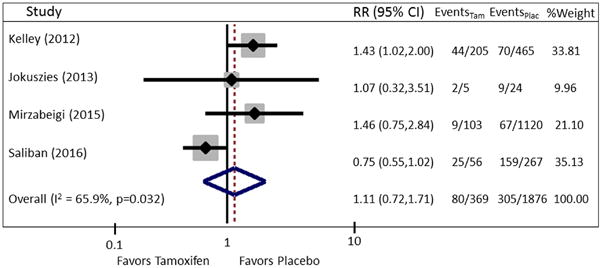
Forest plot of pooled RR for outcome of overall flap complications. Pooled RR is 1.11, 95% CI: 0.72–1.71. There is significant heterogeneity (I2 = 65.9%, P = 0.032) present.
Fig. 6.
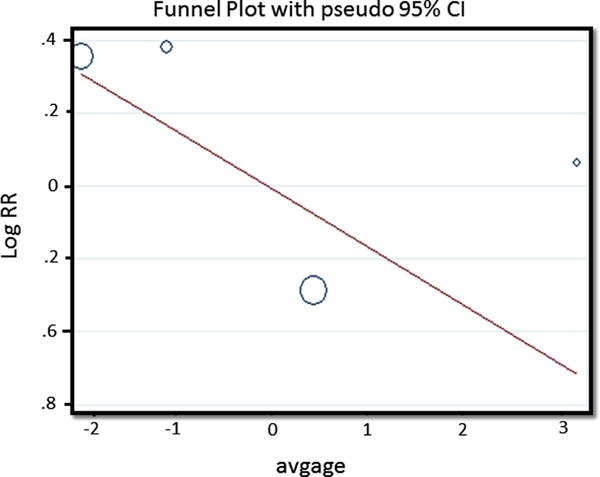
Regression analysis of the impact of age on overall flap complications. Residual I2 after adjusting for age = 28.1%
DISCUSSION
In this meta-analysis we demonstrated patients with breast cancer on perioperative tamoxifen hormone therapy, compared to patients not on tamoxifen, are at an increased risk for developing thrombotic flap complications and total flap loss when undergoing microvascular breast reconstruction. These findings add clarity to an important question that has previously lacked consensus: should tamoxifen therapy be held perioperatively for patients undergoing postmastectomy microvascular reconstruction? Pooled data from all available reports on the topic suggest temporarily discontinuing tamoxifen may be necessary to minimize the risk of complications.
This study has several implications for clinical practice. Tamoxifen is an integral part of adjuvant systemic treatment for a large proportion of women with breast cancer; therefore, it can be expected that a subset of women presenting for surgical evaluation will be on tamoxifen.[30,14] There is overwhelming evidence tamoxifen reduces mortality, the risk of contralateral cancer, and the risk of recurrence in patients with hormone positive breast cancer.[6,3,2] However, there is also substantial evidence supporting an association between tamoxifen and thromboembolic events.[31,7,9,8] In 1991, the Eastern Cooperative Oncology Group (ECOG) demonstrated patients with breast cancer receiving adjuvant tamoxifen were at an increased risk of venous and arterial thrombosis compared to patients not receiving adjuvant therapy.[32] These results have been corroborated in several landmark studies.[33–36] The National Surgical Adjuvant Breast and Bowel Project (NSABP) Breast Cancer Prevention Trial (P-1) and the first International Breast Cancer Intervention Study (IBIS-I) separately confirmed a significantly increased rate of thromboembolic events for patients on tamoxifen compared to placebo.[33,34] Furthermore, the risk is notably higher in the 3 months after initiation of tamoxifen, compared to the 3 months prior to therapy, and this risk may continue to be higher during the first 2 years after exposure.[9,37] This is important because women with tumors amenable to resection will likely undergo operative treatment within this timeframe. Patients undergoing surgical treatment for breast cancer are already considered high-risk for thromboembolic events, with an estimated 10-fold increased event rate; tamoxifen may compound this risk.[8]
In microvascular breast reconstruction, a concerning complication is thrombotic flap events, specifically small-vessel anastomotic thrombosis with clot propagation and embolization. This threatens flap survival by limiting the restoration of circulation, and maintenance of perfusion, in transferred tissue and directly contributes to flap loss. Despite advances in technique, thrombosis rates have not substantially improved and continue to compromise outcomes.[38–40] Flap loss resulting from thrombosis can contribute to additional operations and poor outcomes; therefore, efforts to minimize these complications are essential.[41] Based on overwhelming evidence tamoxifen increases the risk of systemic thromboembolic events in patients with breast cancer, there have been concerns a potentially prothrombotic environment would similarly increase the risk of flap thrombotic events. In 2012, Kelley, et al. lent this credibility by demonstrating an increased risk of flap loss and a lower rate of flap salvage for patients on tamoxifen undergoing microvascular reconstruction.[19] However, subsequent clinical reports presented conflicting data, leaving a lack of clarity regarding recommendations for perioperative tamoxifen use.[20,21] The perioperative management of women undergoing breast reconstruction requires a coordinated approach to ensure optimal oncological and reconstructive outcomes. Patients interact with multiple providers and a consistent approach based on the best available evidence facilitates comprehensive care.
The results of this meta-analysis support temporary discontinuation of tamoxifen at least 4 weeks, or 28 days, prior to surgical treatment. We considered patients who received tamoxifen within 28 days of surgery to be on tamoxifen to standardize the time to discontinuation of therapy as the existing literature used inconsistent definitions and time frames. The timing of discontinuing therapy is based on the studies included and supported by our understanding of pharmacokinetics and bioavailability of tamoxifen and its metabolites. Tamoxifen is biotransformed by cytochrome P450 enzymes into active metabolites, specifically 4-hydroxy tamoxifen and 4-hydroxy N-desmethyl tamoxifen (endoxifen), which have an approximately 30- to 100-fold greater antiestrogenic effect compared to tamoxifen.[42–45] The half-life of these metabolites is estimated at 14 days, compared to approximately 7 days for tamoxifen.[42,46,44,47] Furthermore, studies have confirmed tamoxifen and its metabolites may be retained in tissue and circulation up to 28 days after cessation at concentrations high enough to interfere with estrogen receptor activity.[48,46,49] Additionally, plasma concentrations of tamoxifen metabolites exhibit large interindividual variation in breast cancer patients, providing further support for holding tamoxifen treatment two half-lives to ensure adequate elimination.[50] It is important to acknowledge we were unable to determine optimal timing for restarting tamoxifen postoperatively as studies included did not report this. Our practice is to be conservative and restart tamoxifen at the initial 2-weeks postoperative visit. There are myriad studies supporting that a majority of flap thrombotic events occur within the first three days postoperatively; however, there are some reports of late venous thrombosis in microvascular reconstruction up to 12 days postoperatively.[38,51–54]
The mechanisms of tamoxifen increasing susceptibility to thrombosis are complex and represent a dynamic interplay of events yet to be fully elucidated. Thrombosis is a coordinated response to vascular injury consisting of a platelet plug, a fibrin-based clot, and activation of the inflammatory and repair processes.[55] In microvascular surgery, vessels are deliberately divided and subsequently reanastomosed, creating vessel wall injury, and exposing tissue factor on the subendothelial surface, resulting in the initiation of platelet activation, aggregation, and the coagulation cascade. Tissue factor is a principal initiator of the extrinsic pathway of coagulation, resulting in fibrin production which polymerizes to form a clot and strengthens the initial platelet plug.[41,56] This combination of fibrin production and platelet activation/aggregation are central events in arterial and venous thrombosis by contributing to intimal hyperplasia, smooth muscle cell degeneration, and formation of intraluminal thrombi at the anastomotic site.[41,57–59,40,60] The role of tamoxifen in increasing platelet activation and aggregation is considered the primary mechanism by which it induces a prothrombotic environment in patients with breast cancer. Recently, investigators demonstrated tamoxifen promotes calcium entry into platelets, which is critical to platelet activation and aggregation, and may lead to prothrombotic platelet phenotypes in breast cancer patients on hormone therapy.[61–65] Furthermore, tamoxifen increases the number of activated-platelet derived, tissue factor-bearing microparticles, which play a role in a variety of thromboembolic pathologies, including atherosclerosis, thrombocythemia, and thrombosis.[66–69] These mechanisms, combined with the existing thrombogenic environment present in patients with breast cancer, may explain the higher rate of systemic thromboembolic events observed in prior studies and thrombotic microvascular events observed in this meta-analysis.
There are certain limitations to this study that have implications for future research. This meta-analysis included a small sample size of studies. While there is a significant amount of literature on thrombotic complications following microvascular breast reconstruction, few studies report perioperative exposure to hormone therapy. Similar to other meta-analyses, we were limited by data available in the reports included for evaluation. By identifying these gaps in the reported literature, this meta-analysis can serve to improve the quality of future studies on the topic. We were unable to make conclusions regarding the risk of systemic thromboembolic events for patients on tamoxifen as these data were not consistently included in studies. Future studies reporting on systemic thromboembolic events are necessary to further delineate potential risks. Furthermore, there was substantial heterogeneity in regards to the reporting of relevant covariates, making it difficult to determine the impact of these potentially confounding variables. All future studies should report relevant covariates, including adjuvant chemotherapy, exposure to radiation therapy, and systemic prophylactic anticoagulation treatment, so these factors can be controlled for analytically.[70]
CONCLUSIONS
Perioperative tamoxifen therapy modifies the risk of thrombotic flap complications and total flap loss for patients undergoing microvascular breast reconstruction. Based on the results of this meta-analysis, temporarily discontinuing tamoxifen therapy at least 4 weeks prior to operative treatment may minimize the risk of complications. Further high quality studies that report and control for relevant covariates and report both systemic and flap thrombotic events are necessary to expand our understanding of the potential risks of tamoxifen therapy in microvascular breast reconstruction. Ultimately, the findings of this study further the ability of providers to make evidence-based recommendations in the perioperative management of patients with breast cancer.
Acknowledgments
Funding: RPP and EBO are supported by a National Institutes of Health (NIH) Ruth L. Kirschstein National Research Service Award Institutional Research Training Grant (T32CA190194, PI Colditz). No funding was provided directly for this study. The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
Footnotes
COMPLIANCE WITH ETHICAL STANDARDS:
Disclosure of potential conflicts of interest:
Conflict of Interest: The authors declare that they have no conflict of interest.
Research involving human participants and/or Animals:
Ethical Approval: This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent: Not applicable – this article does not contain any studies with human participants performed by any of the authors
References
- 1.American Cancer Society. Cancer Facts & Figures 2016. American Cancer Society; 2016. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. Accessed 05/02/2016. [Google Scholar]
- 2.Riggs BL, Hartmann LC. Selective estrogen-receptor modulators – mechanisms of action and application to clinical practice. N Engl J Med. 2003;348(7):618–629. doi: 10.1056/NEJMra022219. [DOI] [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. doi: 10.1016/s0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 4.Burstein HJ, Lacchetti C, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Solky AJ, Stearns V, Winer EP, Griggs JJ. Adjuvant Endocrine Therapy for Women With Hormone Receptor-Positive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update on Ovarian Suppression. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34(14):1689–1701. doi: 10.1200/jco.2015.65.9573. [DOI] [PubMed] [Google Scholar]
- 5.Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, Dowsett M, Ingle J, Peto R. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771–784. doi: 10.1016/s0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998;351(9114):1451–1467. [PubMed] [Google Scholar]
- 7.Deitcher SR, Gomes MP. The risk of venous thromboembolic disease associated with adjuvant hormone therapy for breast carcinoma: a systematic review. Cancer. 2004;101(3):439–449. doi: 10.1002/cncr.20347. [DOI] [PubMed] [Google Scholar]
- 8.Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS medicine. 2012;9(7):e1001275. doi: 10.1371/journal.pmed.1001275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernandez RK, Sorensen HT, Pedersen L, Jacobsen J, Lash TL. Tamoxifen treatment and risk of deep venous thrombosis and pulmonary embolism: a Danish population-based cohort study. Cancer. 2009;115(19):4442–4449. doi: 10.1002/cncr.24508. [DOI] [PubMed] [Google Scholar]
- 10.Eltahir Y, Werners LL, Dreise MM, van Emmichoven IA, Jansen L, Werker PM, de Bock GH. Quality-of-life outcomes between mastectomy alone and breast reconstruction: comparison of patient-reported BREAST-Q and other health-related quality-of-life measures. Plast Reconstr Surg. 2013;132(2):201e–209e. doi: 10.1097/PRS.0b013e31829586a7. [DOI] [PubMed] [Google Scholar]
- 11.Jagsi R, Jiang J, Momoh AO, Alderman A, Giordano SH, Buchholz TA, Pierce LJ, Kronowitz SJ, Smith BD. Complications After Mastectomy and Immediate Breast Reconstruction for Breast Cancer: A Claims-Based Analysis. Annals of surgery. 2016;263(2):219–227. doi: 10.1097/sla.0000000000001177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee KT, Mun GH. Prosthetic breast reconstruction in previously irradiated breasts: A meta-analysis. Journal of surgical oncology. 2015;112(5):468–475. doi: 10.1002/jso.24032. [DOI] [PubMed] [Google Scholar]
- 13.Matros E, Albornoz CR, Razdan SN, Mehrara BJ, Macadam SA, Ro T, McCarthy CM, Disa JJ, Cordeiro PG, Pusic AL. Cost-effectiveness analysis of implants versus autologous perforator flaps using the BREAST-Q. Plast Reconstr Surg. 2015;135(4):937–946. doi: 10.1097/prs.0000000000001134. [DOI] [PubMed] [Google Scholar]
- 14.Winters ZE, Benson JR, Pusic AL. A systematic review of the clinical evidence to guide treatment recommendations in breast reconstruction based on patient- reported outcome measures and health-related quality of life. Annals of surgery. 2010;252(6):929–942. doi: 10.1097/SLA.0b013e3181e623db. [DOI] [PubMed] [Google Scholar]
- 15.Pannucci CJ, Chang EY, Wilkins EG. Venous thromboembolic disease in autogenous breast reconstruction. Ann Plast Surg. 2009;63(1):34–38. doi: 10.1097/SAP.0b013e318188bedf. [DOI] [PubMed] [Google Scholar]
- 16.Pannucci CJ, MacDonald JK, Ariyan S, Gutowski KA, Kerrigan CL, Kim JY, Chung KC. Benefits and Risks of Prophylaxis for Deep Venous Thrombosis and Pulmonary Embolus in Plastic Surgery: A Systematic Review and Meta-Analysis of Controlled Trials and Consensus Conference. Plast Reconstr Surg. 2016;137(2):709–730. doi: 10.1097/01.prs.0000475790.54231.28. [DOI] [PubMed] [Google Scholar]
- 17.Butz DR, Lapin B, Yao K, Wang E, Song DH, Johnson D, Sisco M. Advanced age is a predictor of 30-day complications after autologous but not implant-based postmastectomy breast reconstruction. Plast Reconstr Surg. 2015;135(2):253e–261e. doi: 10.1097/prs.0000000000000988. [DOI] [PubMed] [Google Scholar]
- 18.Jokuszies A, R C, Betzler C, Branski L, Krämer R, Vogt PM. Is tamoxifen associated with an increased risk for thromboembolic complications in patients undergoing microvascular breast reconstruction? German medical science: GMS e-journal. 2013;11 doi: 10.3205/000173. Doc05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelley BP, Valero V, Yi M, Kronowitz SJ. Tamoxifen increases the risk of microvascular flap complications in patients undergoing microvascular breast reconstruction. Plast Reconstr Surg. 2012;129(2):305–314. doi: 10.1097/PRS.0b013e31823ae86c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirzabeigi MN, N JA, Fischer JP, Kovach SJ, Serletti JM, Wu LC, Kanchwala S. Tamoxifen (selective estrogen-receptor modulators) and aromatase inhibitors as potential perioperative thrombotic risk factors in free flap breast reconstruction. Plastic and reconstructive surgery. 2015;135(4):670e–679e. doi: 10.1097/PRS.0000000000001127. [DOI] [PubMed] [Google Scholar]
- 21.Salibian A, Gu J, Lee Y, Wirth GA, Paydar KZ, Kobayashi MR, Evans G. The Effects of Perioperative Tamoxifen Therapy on Microvascular Flap Complications in Transverse Rectus Abdominis Myocutaneous/Deep Inferior Epigastric Perforator Flap Breast Reconstruction. Annals of Plastic Surgery. 2016 doi: 10.1097/SAP.0000000000000707. [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 24.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. Journal of epidemiology and community health. 1998;52(6):377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in medicine. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 26.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 27.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 28.Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Statistics in medicine. 2006;25(20):3443–3457. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- 29.Hallgren KA. Computing Inter-Rater Reliability for Observational Data: An Overview and Tutorial. Tutorials in quantitative methods for psychology. 2012;8(1):23–34. doi: 10.20982/tqmp.08.1.p023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrow M, Li Y, Alderman AK, Jagsi R, Hamilton AS, Graff JJ, Hawley ST, Katz SJ. Access to breast reconstruction after mastectomy and patient perspectives on reconstruction decision making. JAMA Surg. 2014;149(10):1015–1021. doi: 10.1001/jamasurg.2014.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amir E, Seruga B, Niraula S, Carlsson L, Ocana A. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. Journal of the National Cancer Institute. 2011;103(17):1299–1309. doi: 10.1093/jnci/djr242. [DOI] [PubMed] [Google Scholar]
- 32.Saphner T, Tormey DC, Gray R. Venous and arterial thrombosis in patients who received adjuvant therapy for breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1991;9(2):286–294. doi: 10.1200/JCO.1991.9.2.286. [DOI] [PubMed] [Google Scholar]
- 33.Cuzick J, Forbes JF, Sestak I, Cawthorn S, Hamed H, Holli K, Howell A. Long-term results of tamoxifen prophylaxis for breast cancer–96-month follow-up of the randomized IBIS-I trial. Journal of the National Cancer Institute. 2007;99(4):272–282. doi: 10.1093/jnci/djk049. [DOI] [PubMed] [Google Scholar]
- 34.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. Journal of the National Cancer Institute. 1998;90(18):1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 35.Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, Hoctin-Boes G, Houghton J, Locker GY, Tobias JS. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365(9453):60–62. doi: 10.1016/s0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 36.Margolese RG, Cecchini RS, Julian TB, Ganz PA, Costantino JP, Vallow LA, Albain KS, Whitworth PW, Cianfrocca ME, Brufsky AM, Gross HM, Soori GS, Hopkins JO, Fehrenbacher L, Sturtz K, Wozniak TF, Seay TE, Mamounas EP, Wolmark N. Anastrozole versus tamoxifen in postmenopausal women with ductal carcinoma in situ undergoing lumpectomy plus radiotherapy (NSABP B-35): a randomised, double-blind, phase 3 clinical trial. Lancet. 2016;387(10021):849–856. doi: 10.1016/s0140-6736(15)01168-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walker AJ, West J, Card TR, Crooks C, Kirwan CC, Grainge MJ. When are breast cancer patients at highest risk of venous thromboembolism? A cohort study using English health care data. Blood. 2016;127(7):849–857. doi: 10.1182/blood-2015-01-625582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bui DT, Cordeiro PG, Hu QY, Disa JJ, Pusic A, Mehrara BJ. Free flap reexploration: indications, treatment, and outcomes in 1193 free flaps. Plast Reconstr Surg. 2007;119(7):2092–2100. doi: 10.1097/01.prs.0000260598.24376.e1. [DOI] [PubMed] [Google Scholar]
- 39.Khansa I, Chao AH, Taghizadeh M, Nagel T, Wang D, Tiwari P. A systematic approach to emergent breast free flap takeback: Clinical outcomes, algorithm, and review of the literature. Microsurgery. 2013;33(7):505–513. doi: 10.1002/micr.22151. [DOI] [PubMed] [Google Scholar]
- 40.Khouri RK, Cooley BC, Kunselman AR, Landis JR, Yeramian P, Ingram D, Natarajan N, Benes CO, Wallemark C. A prospective study of microvascular free-flap surgery and outcome. Plast Reconstr Surg. 1998;102(3):711–721. doi: 10.1097/00006534-199809030-00015. [DOI] [PubMed] [Google Scholar]
- 41.Hanasono MM, Butler CE. Prevention and treatment of thrombosis in microvascular surgery. Journal of reconstructive microsurgery. 2008;24(5):305–314. doi: 10.1055/s-2008-1080530. [DOI] [PubMed] [Google Scholar]
- 42.Adam HK, Patterson JS, Kemp JV. Studies on the metabolism and pharmacokinetics of tamoxifen in normal volunteers. Cancer treatment reports. 1980;64(6–7):761–764. [PubMed] [Google Scholar]
- 43.Hoskins JM, Carey LA, McLeod HL. CYP2D6 and tamoxifen: DNA matters in breast cancer. Nature reviews Cancer. 2009;9(8):576–586. doi: 10.1038/nrc2683. [DOI] [PubMed] [Google Scholar]
- 44.Johnson MD, Zuo H, Lee KH, Trebley JP, Rae JM, Weatherman RV, Desta Z, Flockhart DA, Skaar TC. Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast cancer research and treatment. 2004;85(2):151–159. doi: 10.1023/B:BREA.0000025406.31193.e8. [DOI] [PubMed] [Google Scholar]
- 45.Desta Z, Ward BA, Soukhova NV, Flockhart DA. Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. The Journal of pharmacology and experimental therapeutics. 2004;310(3):1062–1075. doi: 10.1124/jpet.104.065607. [DOI] [PubMed] [Google Scholar]
- 46.Gjerde J, Gandini S, Guerrieri-Gonzaga A, Haugan Moi LL, Aristarco V, Mellgren G, Decensi A, Lien EA. Tissue distribution of 4-hydroxy-N-desmethyltamoxifen and tamoxifen-N-oxide. Breast cancer research and treatment. 2012;134(2):693–700. doi: 10.1007/s10549-012-2074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lien EA, Solheim E, Ueland PM. Distribution of tamoxifen and its metabolites in rat and human tissues during steady-state treatment. Cancer research. 1991;51(18):4837–4844. [PubMed] [Google Scholar]
- 48.Kisanga ER, Gjerde J, Guerrieri-Gonzaga A, Pigatto F, Pesci-Feltri A, Robertson C, Serrano D, Pelosi G, Decensi A, Lien EA. Tamoxifen and metabolite concentrations in serum and breast cancer tissue during three dose regimens in a randomized preoperative trial. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10(7):2336–2343. doi: 10.1158/1078-0432.ccr-03-0538. [DOI] [PubMed] [Google Scholar]
- 49.Lien EA, Solheim E, Lea OA, Lundgren S, Kvinnsland S, Ueland PM. Distribution of 4-hydroxy-N-desmethyltamoxifen and other tamoxifen metabolites in human biological fluids during tamoxifen treatment. Cancer research. 1989;49(8):2175–2183. [PubMed] [Google Scholar]
- 50.Stearns V, Johnson MD, Rae JM, Morocho A, Novielli A, Bhargava P, Hayes DF, Desta Z, Flockhart DA. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. Journal of the National Cancer Institute. 2003;95(23):1758–1764. doi: 10.1093/jnci/djg108. [DOI] [PubMed] [Google Scholar]
- 51.Chen KT, Mardini S, Chuang DC, Lin CH, Cheng MH, Lin YT, Huang WC, Tsao CK, Wei FC. Timing of presentation of the first signs of vascular compromise dictates the salvage outcome of free flap transfers. Plast Reconstr Surg. 2007;120(1):187–195. doi: 10.1097/01.prs.0000264077.07779.50. [DOI] [PubMed] [Google Scholar]
- 52.Kroll SS, Schusterman MA, Reece GP, Miller MJ, Evans GR, Robb GL, Baldwin BJ. Timing of pedicle thrombosis and flap loss after free-tissue transfer. Plast Reconstr Surg. 1996;98(7):1230–1233. doi: 10.1097/00006534-199612000-00017. [DOI] [PubMed] [Google Scholar]
- 53.Nelson JA, Kim EM, Eftekhari K, Low DW, Kovach SJ, Wu LC, Serletti JM. Late venous thrombosis in free flap breast reconstruction: strategies for salvage after this real entity. Plast Reconstr Surg. 2012;129(1):8e–15e. doi: 10.1097/PRS.0b013e3182361f7f. [DOI] [PubMed] [Google Scholar]
- 54.Serletti JM, Moran SL, Orlando GS, O’Connor T, Herrera HR. Urokinase protocol for free-flap salvage following prolonged venous thrombosis. Plast Reconstr Surg. 1998;102(6):1947–1953. doi: 10.1097/00006534-199811000-00021. [DOI] [PubMed] [Google Scholar]
- 55.Noble S, Pasi J. Epidemiology and pathophysiology of cancer-associated thrombosis. British journal of cancer. 2010;102(Suppl 1):S2–9. doi: 10.1038/sj.bjc.6605599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Falati S, Gross P, Merrill-Skoloff G, Furie BC, Furie B. Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the mouse. Nature medicine. 2002;8(10):1175–1181. doi: 10.1038/nm782. [DOI] [PubMed] [Google Scholar]
- 57.Seo MH, Kim SM, Huan F, Myoung H, Lee JH, Lee SK. Analysis of Microvascular Free Flap Failure Focusing on the Microscopic Findings of the Anastomosed Vessels. The Journal of craniofacial surgery. 2015;26(7):2047–2051. doi: 10.1097/scs.0000000000002111. [DOI] [PubMed] [Google Scholar]
- 58.Johnson PC. Platelet-mediated thrombosis in microvascular surgery: new knowledge and strategies. Plast Reconstr Surg. 1990;86(2):359–367. doi: 10.1097/00006534-199008000-00032. [DOI] [PubMed] [Google Scholar]
- 59.Cho EH, Ligh C, Hodulik KL, Hollenbeck ST. Role of platelet inhibition in microvascular surgery. Journal of reconstructive microsurgery. 2014;30(9):589–598. doi: 10.1055/s-0034-1381955. [DOI] [PubMed] [Google Scholar]
- 60.Khouri RK, Cooley BC, Kenna DM, Edstrom LE. Thrombosis of microvascular anastomoses in traumatized vessels: fibrin versus platelets. Plast Reconstr Surg. 1990;86(1):110–117. doi: 10.1097/00006534-199007000-00017. [DOI] [PubMed] [Google Scholar]
- 61.Shah VP, Chegini HA, Vishneski SR, Weatherman RV, Blackmore PF, Dobrydneva Y. Tamoxifen promotes superoxide production in platelets by activation of PI3-kinase and NADPH oxidase pathways. Thrombosis research. 2012;129(1):36–42. doi: 10.1016/j.thromres.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 62.Dobrydneva Y, Weatherman RV, Trebley JP, Morrell MM, Fitzgerald MC, Fichandler CE, Chatterjie N, Blackmore PF. Tamoxifen stimulates calcium entry into human platelets. Journal of cardiovascular pharmacology. 2007;50(4):380–390. doi: 10.1097/FJC.0b013e31811ec748. [DOI] [PubMed] [Google Scholar]
- 63.Vitseva O, Flockhart DA, Jin Y, Varghese S, Freedman JE. The effects of tamoxifen and its metabolites on platelet function and release of reactive oxygen intermediates. The Journal of pharmacology and experimental therapeutics. 2005;312(3):1144–1150. doi: 10.1124/jpet.104.076315. [DOI] [PubMed] [Google Scholar]
- 64.Vemana HP, Karim ZA, Conlon C, Khasawneh FT. A critical role for the transient receptor potential channel type 6 in human platelet activation. PloS one. 2015;10(4):e0125764. doi: 10.1371/journal.pone.0125764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Varga-Szabo D, Braun A, Nieswandt B. Calcium signaling in platelets. Journal of thrombosis and haemostasis : JTH. 2009;7(7):1057–1066. doi: 10.1111/j.1538-7836.2009.03455.x. [DOI] [PubMed] [Google Scholar]
- 66.Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arteriosclerosis, thrombosis, and vascular biology. 2007;27(8):1687–1693. doi: 10.1161/atvbaha.107.141911. [DOI] [PubMed] [Google Scholar]
- 67.Tesselaar ME, Romijn FP, Van Der Linden IK, Prins FA, Bertina RM, Osanto S. Microparticle-associated tissue factor activity: a link between cancer and thrombosis? Journal of thrombosis and haemostasis : JTH. 2007;5(3):520–527. doi: 10.1111/j.1538-7836.2007.02369.x. [DOI] [PubMed] [Google Scholar]
- 68.Toth B, Liebhardt S, Steinig K, Ditsch N, Rank A, Bauerfeind I, Spannagl M, Friese K, Reininger AJ. Platelet-derived microparticles and coagulation activation in breast cancer patients. Thrombosis and haemostasis. 2008;100(4):663–669. [PubMed] [Google Scholar]
- 69.Trappenburg MC, van Schilfgaarde M, Bredewold EO, van Aalderen MC, Spronk HM, Ten Cate H, Leyte A, Terpstra WE. Elevated numbers and altered subsets of procoagulant microparticles in breast cancer patients using endocrine therapy. Thrombosis research. 2011;127(4):363–369. doi: 10.1016/j.thromres.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 70.Pritchard KI, Paterson AH, Paul NA, Zee B, Fine S, Pater J. Increased thromboembolic complications with concurrent tamoxifen and chemotherapy in a randomized trial of adjuvant therapy for women with breast cancer. National Cancer Institute of Canada Clinical Trials Group Breast Cancer Site Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1996;14(10):2731–2737. doi: 10.1200/JCO.1996.14.10.2731. [DOI] [PubMed] [Google Scholar]


