Abstract
Here, we show that high-dose γ-irradiation accompanied with syngeneic bone marrow transfer can confer complete protection against glaucoma in a mouse model. Because bone marrow genotype was unaltered by this procedure, it was not the causative agent. The neuroprotection is robust and highly reproducible. Glaucoma-prone DBA/2J mice received a single treatment at 5–8 weeks of age and were protected from glaucomatous retinal ganglion cell degeneration out to 14 months of age (oldest assessed). By 12–14 months, retinal ganglion cell degeneration is usually very severe and essentially complete in the majority of untreated DBA/2J mice. To assess reproducibility, three groups of mice were treated at different times, and the results were essentially the same each time. Considering all experiments, the vast majority of treated mice had no detectable glaucomatous neurodegeneration. A beneficial effect of treatment including high-dose radiation is unprecedented, and we are not aware of any other neuroprotective effects this substantial. Because of the robust protective effect, this treatment offers another tool for studying mechanisms of neuroprotection.
Keywords: neuroprotection, retinal ganlgion cell, mouse model
The glaucomas are a group of complex neurodegenerative diseases. As a consequence of this neurodegeneration, glaucoma patients exhibit a loss of retinal ganglion cells (RGCs), characteristic changes in the visual field, and degeneration of the optic nerve (for review, see refs. 1 and 2). Glaucoma has traditionally been viewed as a pressure-induced neurodegeneration, in which deleteriously high intraocular pressure (IOP) results in optic nerve damage over time. As a consequence, all major existing glaucoma therapeutics aim to manipulate a single event, lowering IOP. However, it is increasingly clear that the glaucomas are multifactorial diseases, and IOP is not the only important factor. Many individuals who have high IOP do not develop glaucoma, when followed over long periods of time, whereas others develop optic nerve damage despite normal IOP values (3, 41). Clearly, elucidating additional factors determining RGC and optic nerve head susceptibility to glaucomatous neurodegeneration would facilitate a fuller understanding of disease processes and guide efforts toward improved therapeutics (5).
Mouse studies are very useful for studying mechanisms contributing to multifactorial diseases and for testing potential treatments (6). Accordingly, our approach for studying the neurobiology of glaucoma has emphasized a naturally occurring mouse model of glaucoma, DBA/2J mice. DBA/2J mice develop an age-related form of hereditary glaucoma initiated by mutations in two genes, Tyrp1 and Gpnmb (7–9). Clinically, indications of DBA/2J glaucoma are first evident by a pigment-dispersing iris disease that involves melanosomal (8, 9) and inflammatory (10) components. As dispersed pigment from the iris disease accumulates within the aqueous humor drainage sites, DBA/2J mice develop an elevated IOP, which progressively insults RGCs and the optic nerve. By 10–12 months, the majority of DBA/2J mice have severe glaucoma evident by massive RGC loss and optic nerve damage.
Little is known about the mechanisms or molecular pathways that contribute to RGC degeneration in the glaucomas. As in other neurodegenerative diseases, the majority of effort has focused on apoptotic degeneration pathways (11, 12). Recently, there has been recognition that distinct degenerative processes exist within different parts of a neuron (13). The corollary is that if effective glaucoma neurotherapeutics are to be achieved, they must target both axonal and somal pathways (42). DBA/2J mice offer a powerful system for determining mechanisms of neurodegeneration and testing neuroprotective treatments.
Using DBA/2J mice, we have discovered a treatment that is profoundly neuroprotective and can completely prevent detectable glaucomatous degeneration of both the neuronal soma and their axons. This report documents the unexpected but overwhelming protective effect conferred by radiation treatment with syngeneic bone marrow transfer. This treatment offers another tool for studying neuroprotective processes. With increased understanding and refinement of the treatment protocol, this work may have important implications for the treatment, and possibly prevention of, multiple neurodegenerative diseases.
Materials and Methods
Animal Husbandry. DBA/2J mice were from our colony (Sj) that was derived from, and is periodically intermixed with, the stock colony of The Jackson Laboratory. All animals were treated according to protocols established by the Association for Research in Vision and Ophthalmology. All experimental protocols were approved by the Animal Care and Use Committee of The Jackson Laboratory. DBA/2J mice were fed a 6% fat (NIH31) diet ad libitum, and drinking water was acidified to a pH of 2.8–3.2. Mice were housed in cages containing white pine bedding and kept in a 21°C environment with a 14-h light and 10-h dark cycle.
Generation of Radiation-Induced Bone Marrow Chimeras. Bone marrow chimeras were generated as follows: 5- to 8-week-old female DBA/2J mice were irradiated with 1,000 rads of whole-body radiation. During treatment, mice were positioned on a slowly rotating platform to ensure uniform application. Radiation was applied from a 137Cs source in two equal doses of 500 rads spaced 3–4 h apart. The dose was applied at a rate of 132 rads/min. Shortly after the second radiation dose, mice received 200-μl i.v. injections (lateral tail vein) containing 5 × 106 T cell-depleted bone marrow cells. Donor mice in all experiments were 1.7–1.9 months old. Donor marrow was depleted of T lymphocytes with 10 μg/ml purified monoclonal antibodies to CD4 (GK1.5, The Jackson Laboratory Flow Cytometry Service) and CD8a (536.72, The Jackson Laboratory Flow Cytometry Service). Before injection, free antibodies were removed by centrifugation.
Clinical Slit-Lamp Analysis. DBA/2J mice develop a pigmentary form of glaucoma that involves iris atrophy and pigment dispersion. A slit lamp was used to determine whether the treatment altered the course of the disease. Eyes were examined with a slit-lamp biomicroscope and photographed through a ×40 objective lens. All exams viewed both the left and right eyes. All photographs were taken by using identical camera and light settings. Assessed phenotypes included: the degree of and pattern of pigment dispersion, the degree and pattern of iris atrophy, the degree and pattern of transillumination, and the depth of the anterior chamber. Details of the slit-lamp examination and evaluation criteria were described (7, 9, 14). The disease follows a characteristic clinical course that is very reproducible between populations of aged mice. We have successfully used this slit-lamp evaluation procedure to identify two mutant genes that underlie specific but different aspects of the overall iris phenotype, and to identify interventions that separately correct the effects of each of these genes (9, 10).
IOP Measurement. IOP was measured by using a method previously described in detail (15, 16). Briefly, mice were acclimatized to the procedure room environment for at least 1 week before measurement. To record IOP, mice were anesthetized by using intraperitoneal injection of ketamine (Ketalar, Parke-Davis, Paramus, NJ) and xylazine (Rompun, Phoenix Pharmaceutical, St. Joseph, MO). Because the IOPs of C57BL/6J are very consistent, C57BL/6J mice were interspersed with experimental mice during all experiments as a methodologic control to ensure proper equipment calibration and performance.
Optic Nerve Analysis. Optic nerve processing. Optic nerve cross sections were examined for glaucomatous damage by using a modified paraphenylenediamine (PPD) staining protocol to stain the myelin sheath of all axons, and the axoplasma of damaged axons (17). PPD stains all myelin sheaths, but differentially stains the axoplasm of sick or dying axons darkly. Optic nerves were fixed in situ in 0.8% paraformaldehyde, 1.2% gluteraldehyde, 0.08% phosphate buffer, pH 7.4 at 4°C. Sections of nerve between the orbit and chiasm were dissected free, processed, embedded in resin, sectioned, and stained with PPD as described (17). Each age group investigated contained left and right nerves. Stained sections were compared with identically processed sections from untreated DBA/2J mice at various disease stages.
Axon counting. Counts of normal appearing axons were performed by using established nonbiased counting methods. Before beginning axon counts, the optic nerve was outlined at ×100 magnification and its cross-sectional area was automatically calculated by using a computer program (metamorph, Version 4.6r9, Universal Imaging, Downingtown, PA). Magnification of the same nerve section was increased to ×1,000, and 20 ×1,000 fields were electronically collected, covering 80–90% of the nerve. The fields were spaced in a regular fashion across the entire nerve, taking care to avoid field overlap and not count the same area twice. The 20 collected images were stacked on the computer screen so that only the final image was visible to the operator. A rectangular box was then drawn near the center of the 20th image. The program (metamorph) then “cut” a rectangle centered at the same location in all 20 images. Because the operator could only see the top image, this action removed the possibility of unconscious operator bias and made the selection of axons to be counted random. Axons were counted manually and marked by using the computer. The program tracked the total area counted and the total axon count for all 20 images. The total counted area was >10% of the total nerve area. The final count was calculated and expressed as number of axons per optic nerve. We have used axon counting to quantify the number of axons in nerves of each damage level (see below). When performing this procedure, more than eight nerves of each level were randomly selected for counting. Additionally, to quantitatively assess the effects of treatment, axon counting was performed on randomly selected nerves from treated mice and compared with the values for young preglaucomatous strain-matched controls.
Damage level assignment. Because of the large number of mice, an optic nerve grading system was used to determine the level of glaucomatous damage in the 158 nerves analyzed in this study. The indicated damage levels are readily distinguishable upon inspection of the nerve without counting. Furthermore, axon counts on a randomly selected subset of DBA/2J nerves of each damage level indicate that the levels represent clearly distinct stages of disease. The damage level for each nerve was scored by taking into account several factors: the number of healthy axons remaining (compared with preglaucomatous DBA/2J nerves), the number of damaged axons, and the amount of scarring associated with gliosis. In many mild nerves, no axon loss/damage is detected. In other nerves, minor damage exists that is equivalent to that observed in similarly aged mice of various mouse strains that do not develop glaucoma (≤2% of axons appear damaged). Because the mild damage observed in some of these nerves also occurs in old mice of various strains, this mild stage of damage is not considered glaucomatous damage. The average axon count for nerves graded as mild is 50,888 ± 1, 441 (average ± SEM, n = 11). In moderate nerves, significant numbers of sick/degenerating axons are readily detected in many regions of the nerve, but the majority of remaining axons appear healthy. This stage is almost never seen in nonglaucomatous mice, and therefore, we consider this to be glaucomatous damage. The axon count for nerves graded moderate is clearly reduced (31,410 ± 2,199, n = 8, P < 0.001 compared with the counts for mild DBA/2J nerves). Nerves are classified as having severe glaucoma when the number of damaged axons closely approaches, or surpasses, the number of healthy axons. In fact, for the DBA/2J-untreated mice with severe glaucoma in this study, 82% of optic nerves were judged to have <5% healthy axons remaining and the other 18% of optic nerves were judged to have <50% healthy axons remaining. The average axon count for severe nerves is 5,454 ± 1,211 (n = 24, P < 0.001, compared with mild and moderate axon counts).
All nerves were scored by at least two “masked” investigators. Both investigators were unaware of the age of the mouse, or whether the nerve was from a treated or untreated animal. Furthermore, both investigators were unaware of the damage level assigned by the other investigator. Of the 158 nerves analyzed in this study, the investigators assigned the same damage level to ≈96% of the nerves. In the five cases where the two investigator's grades did not agree, a third investigator (also masked) analyzed the nerve. The third investigator's damage level always agreed with that of one of the first investigators'. The most commonly assigned damage level was used as the grade.
Histology and Retinal Flat Mounts. For retinal sections, whole eyes were removed and immersion-fixed in 0.8% paraformaldehyde, 1.2% gluteraldehyde, 0.08% phosphate buffer, pH 7.4, overnight at 4°C. Eyes were embedded in resin, sectioned, and stained with hematoxylin/eosin as described (7). Flat mounting was performed similar to that described by Stone (18). Briefly, eyes were marked for orientation, enucleated, and whole eyes were immersion-fixed in 4% paraformaldehyde in 0.1% phosphate buffer overnight at 4°C. Eyes were either processed immediately or stored in 0.4% paraformaldehyde in 0.1% phosphate buffer. Eyes were rinsed in PBS (pH 7.4) and the anterior chamber was removed. The resultant eye cup was incubated overnight in 0.3% Triton X-100 in PBS at 37°C. The neuronal retina was dissected free from retinal pigment epithelium and sclera. The free-floating retina was rinsed in PBS and then incubated overnight in 3% H202, 1% Na2HPO4 at room temperature. Retinas were rinsed in PBS and placed (RGC side up) onto a microscope slide. After air drying for 5–15 min (until translucent) retinas were flattened overnight under a coverslip with a 10-g weight placed on top. Retinas were then stained by using a brush for ≈1 min with 1% cresyl violet in water containing 2.5% (freshly added) acetic acid. Stained retinas were dehydrated, washed in xylene, and coverslipped.
Results
We previously determined that an inflammatory response contributes to the iris disease that induces IOP elevation in DBA/2J mice (10). As a control in experiments to assess the importance of bone marrow genotype in this iris disease, we performed syngeneic bone marrow transfers in which the genotype of the bone marrow was not changed (5-week-old DBA/2J mice were lethally irradiated with 1,000 rads of γ-irradiation and reconstituted with genetically identical DBA/2J bone marrow, hereafter referred to as treated). The mice were analyzed out to ages when the glaucoma is typically severe in this strain. Comparison of treated versus untreated DBA/2J mice found no difference in the iris disease. The dimensions of the anterior chamber became similarly enlarged in age- and sex-matched groups of treated and untreated mice, suggesting that IOP elevation occurred in both groups. Because the glaucoma inducing anterior chamber disease was unaltered, these groups of mice allowed the effects of treatment on glaucomatous RGC degeneration to be tested. The optic nerves of six treated mice were compared with control mice. With surprising robustness, treatment profoundly prevented optic nerve damage (n = 12/12 optic nerves had no detectable glaucomatous damage, data not shown).
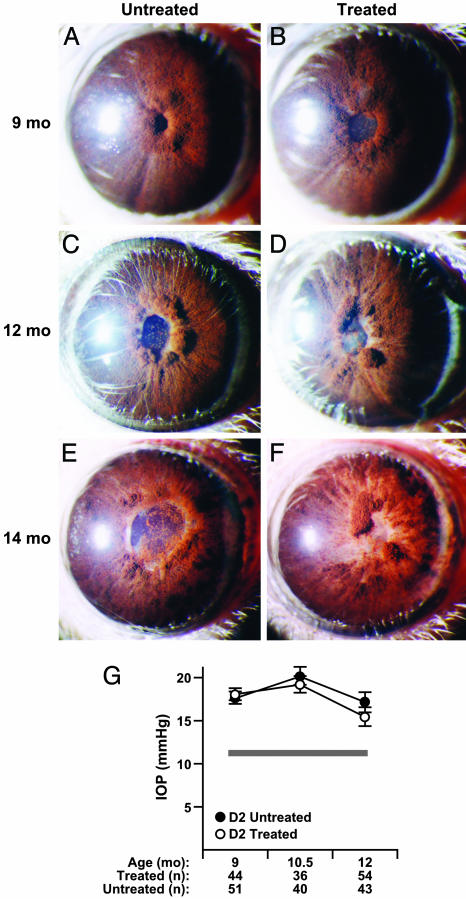
These initial experiments were not designed to study the pressure-induced neurodegeneration, but rather, the iris disease. Therefore, IOP was not directly measured. For this reason, the treatment was repeated on 45 more mice (consisting of two cohorts of 11 and 34 mice treated 3 months apart). The clinical phenotypes and IOP profiles of these cohorts of mice were carefully examined at multiple ages and compared with similarly housed, age-matched, untreated mice. IOP was monitored at three key ages during the period of glaucoma-inducing IOP elevation in this mouse strain. No differences were detected between the treated and untreated groups in the iris phenotype (Fig. 1 A–F). In both treated and untreated mice at all three glaucomatous ages examined, IOP was significantly elevated compared with young preglaucomatous DBA/2J mice (P < 0.001). Importantly, the degree of IOP elevation in treated mice was similar to that of untreated DBA/2J mice (P > 0.3 for two-factor ANOVA for treatment and age; Fig. 1G). This result indicates that RGCs of both the treated and untreated groups were exposed to similar pressure insults.
Fig. 1.
Clinical disease progression and glaucomatous insult is not influenced by radiation treatment. The overall clinical presentation of the iris disease is indistinguishable between treated and untreated groups. Typical images of mice of the indicated ages and treatment groups are shown. The only clinical difference in ocular phenotypes between untreated and treated cohorts was that all treated mice developed radiation induced lens opacities. (A and B) At 9 months, characteristic peripupillary swellings and dispersed pigment accumulations are evident in both treated and untreated mice. At this age, the degree of peripupillary iris atrophy (evident as white tissue adjacent to the pupil) varies from eye to eye in each treatment group. (C and D) At 12 months, dispersed pigment is clearly evident on the lens and across the surface of the iris. (E and F) At 14 months, there is advanced iris atrophy, which is not restricted to the peripupillary area. Full-thickness iris holes and severely atrophic areas that appear thin and depigmented occur in both groups. (G) IOP profiles showing that treatment did not change the glaucomatous IOP insult (mean ± SEM). The thickness of the gray line represents the mean IOP ± SEM (11.3 ± 0.25, n = 31) for DBA/2J mice at an age before ocular disease (3 months). The number of successful IOP recordings at each age are indicated.
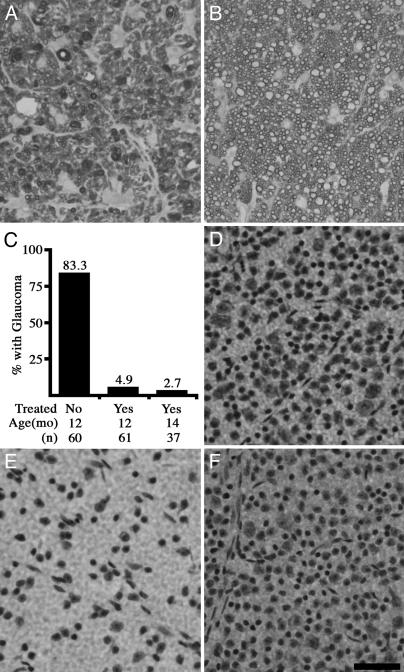
Treated and untreated mice were aged to 12 months, an age when the majority of DBA/2J eyes have severe glaucomatous damage (Fig. 2). Again, the treatment had an overwhelming protective effect and prevented detectable glaucomatous degeneration in the vast majority of nerves. Considering all experiments, the majority, 83%, of optic nerves from untreated 12-month-old DBA/2J mice had glaucomatous damage (Fig. 2), and 73% were characterized by severe glaucoma. Severe glaucoma is defined as very substantial reductions in the number of healthy axons and the presence of many sick and dying axons (see Materials and Methods). In contrast, only 5% of treated 12-month-old mice had any detectable glaucomatous damage, and only 3% had severe glaucoma. Numbers of nerves with each optic nerve grade were: 12-month untreated (62 total), 10 mild, 6 moderate, and 46 severe; 12-month treated (61 total), 58 mild, 1 moderate, and 2 severe. The mild stage occurs in normal mice with age and is not considered glaucoma (see Materials and Methods).
Fig. 2.
Treated mice are protected from glaucomatous neurodegeneration. (A–C) Optic nerves are stained with paraphenylenediamine to visualize the myelin sheath of all axons, and differentially darkly stain the axoplasm of damaged and dying axons. This is an extremely sensitive technique that allows for the detection of a single sick/dying axon in the optic nerve. (A) By 12 months, the majority of optic nerves from untreated DBA/2J mice have severe glaucoma, as defined by massive axon loss. (B) The vast majority of optic nerves from treated mice had no detectable glaucomatous damage, even out to 14 months. (C) A summary of the data from 12- and 14-month-old mice clearly demonstrates the protective effect of treatment, which prevents glaucomatous neurodegeneration in the vast majority of eyes. Because the results did not differ, the data from the experiments at independent times are combined. (D–F) Nissl-stained flat-mounted retinas from position-matched regions of the superior peripheral retina also demonstrate the profound protective effect of treatment (n = 5 flat mounted retinas per group). (D) Young DBA/2J mouse showing normal density of ganglion cell layer cells before glaucomatous damage. (E) Twelve-month-old untreated DBA/2J mouse, showing substantial reduction in the number of soma as a result of glaucoma. (F) Twelve-month-treated DBA/2J mouse with normal number of soma. (Scale bar, 50 μm.)
To further assess the duration of the protective effect, a subset of mice from one of the cohorts was aged to 14 months. In agreement with our findings at 12 months of age, treatment had conferred almost complete protection from glaucoma. At 14 months of age, only ≈3% of treated mice had detectable glaucomatous damage (Fig. 2C; 36 mild, 1 moderate, and 0 severe).
To explore the possibility of subtle axon loss in treated nerves that had no obvious glaucomatous damage, we counted the axons in the nerves of 10 randomly selected treated mice, and we compared them with the nerves of young preglaucomatous DBA/2J mice. Demonstrating the profound protective effect of treatment, no significant difference in axon number was detected (young preglaucomatous DBA/2J mice 51,554 ± 1,332, n = 8; mild treated DBA/2J mice, 48,625 ± 2,309, n = 10, P = 0.3). Finally, multiple other assays on a subset of eyes also demonstrated a striking prevention of glaucomatous damage. Treated mice had no obvious change in the number or morphology of somas in the RGC layer, whereas untreated mice had massive soma loss (Fig. 2 D–F). Retinal and optic nerve morphology also appeared normal in the treated mice, whereas nontreated DBA/2J mice had clear loss of RGC axons and optic nerve head atrophy (Fig. 3).
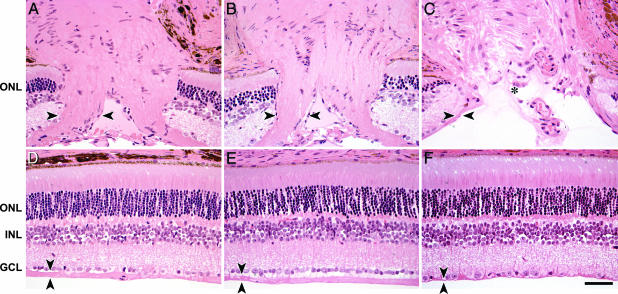
Fig. 3.
Treatment prevents glaucomatous optic nerve excavation. (A) The optic nerve heads of control nonglaucomatous DBA/2J mice include large numbers of axons, as evidenced by a thick nerve fiber layer, entering the optic nerve head (nerve fiber layer on left side of optic nerve head is marked by arrowheads). (B) The thickness of the nerve fiber layer in treated DBA/2J mice (14-month-old example) is indistinguishable from nonglaucomatous controls. (C) In contrast, untreated DBA/2J mice have severe axon loss, as evidenced by a very atrophied nerve fiber layer. Their optic nerve heads are also severely excavated (asterisk), a hallmark of glaucoma (12-month example). (D–F) Position-matched images of retinal cross sections. (D) Nonglaucomatous DBA/2J control mouse. (E) Treated DBA/2J mouse, 14 months old. (F) Untreated DBA/2J mouse, 12 months old. The nerve fiber layer (arrowheads) is of normal thickness in treated DBA/2J retina (compare D with E) and severely atrophied in the untreated glaucomatous DBA/2J retina (compare D with F). There is an obvious loss of somas in the ganglion cell layer (GCL) of the untreated DBA/2J mouse (F) but not in the treated DBA/2J mouse (E; compare both to control retina in D). ONL, outer nuclear layer; INL, inner nuclear layer. (Scale bar, 50 μm.)
Discussion
Here, we report a very robust and reproducible neuroprotective treatment. Using a mouse model of hereditary glaucoma, we show that treatment with large doses of γ-irradiation accompanied with syngeneic bone marrow reconstitution, resulted in almost all treated eyes having no detectable glaucomatous damage. The treatment conferred protection out to ages when the RGC degeneration is usually very severe and essentially complete in the majority of untreated mice. The mice were treated at ≈5 weeks of age and protected from glaucoma a full year later. Thus, the treatment has long-lasting benefit. By this statement, we mean that a single treatment at 5–8 weeks of age places the animals in a state that is resistant to glaucomatous neurodegeneration until they are at least 14 months old. Because the major IOP increase in DBA/2J mice occurs at ≈9 months of age, the neurodegeneration is at least delayed for 3–5 months after the neurodegenerative signals arise. We are unaware of any other neuroprotective treatment that confers such a profound protective effect. Comparison of untreated and treated cohorts of genetically identical mice that do and do not, respectively, develop IOP-induced glaucomatous neurodegeneration, will be a powerful approach for characterizing pathways that are necessary for the pressure-induced RGC degeneration.
The current experiments do not formally distinguish whether the neuroprotection is conferred by radiation, bone marrow transfer, or both. However, because bone marrow genotype was unaltered by the treatment, a role for radiation seems to be indicated. We have found no evidence that a protective effect of high-dose radiation has been previously experimentally tested. However, one recent associative study does suggest a protective effect of high-dose radiation without bone marrow transfer in a human population. In an epidemiological study of atomic bomb survivors, radiation appeared to be protective against glaucoma (19). Although more detailed studies of these human subjects are needed, together with our experiments, these data suggest that high-dose radiation is neuroprotective and that our finding is not unique to DBA/2J mice. Importantly, high-dose radiation may be neuroprotective against human disease and studies of other human populations exposed to large doses of radiation appear to be warranted.
It is important to distinguish our findings from those using low-dose radiation. For decades, potential protective effects of low-dose radiation have been reported to shield from a variety of stresses and insults (20). Because the effects are often small, difficult to reproduce, and nonamenable to mechanistic study, past studies of radiation-induced effects have often been controversial (21, 22). Of most relevance to our findings, a recent paper reported protective effects of low-dose radiation for RGCs damaged by mechanical crush or NMDA toxicity (23). Although interesting, the results were of modest effect. For example, one of the largest results (from an optic nerve crush experiment performed on Lewis rats) resulted in a change of RGC survival from 40.3 ± 1.8 to 114.4 ± 5.6 RGCs per mm2. As estimated by Kipnis et al. (23), this correlates to a rescue of ≈3,500 RGCs in the whole retina. This is a very modest effect, whether compared with the normal density of RGCs in Lewis rats (2,525 ± 368 RGCs per mm2; ref. 24), or the total number of RGCs typical of a healthy rat retina (97,000–131,000 RGCs; refs. 25 and 26). Our current results in an inherited glaucoma are clearly of much greater magnitude. We have obtained complete protection in the majority of animals in three independent experiments that were spaced in time.
We are unaware of any published work that would have predicted the robust protection conferred by this treatment. Significant future experiments are needed to determine the mechanism(s) of this protection. Some of the potential mechanisms, which are not mutually exclusive, are discussed below.
Neuronal Preconditioning. The radiation insult might change RGCs, making them much more resistant to the later insult induced by IOP elevation. RGC preconditioning in response to retinal ischemia has been well described (27–29), but typical responses have only been demonstrated over a time frame of hours to several days. In separate experiments, radiation-induced changes in neuronal gene expression potentially associated with cellular stress have also been observed (30).
Altered Immune Responses. Protective autoimmunity is reported to mediate protection against various neurodegenerative stresses, including experimentally induced IOP elevation (31, 32). In our experiments, the iris disease of the treated mice remains unaltered, even though inflammatory events are a required component of that disease (10). This finding indicates that ocular immune responses are at least not universally changed by the treatment.
Radiation-Sensitive Cell Types. Ablation of a radiation-sensitive cell type that is necessary to induce glaucomatous neurodegeneration could explain our findings. Although no cell types are obviously missing in treated eyes from old mice, this does not preclude the possibility that a rare but potent cell type is missing. Identification of such a cell type would be very important for understanding glaucoma and possibly other neurodegenerative conditions.
Trophic Factors. Radiation induces lens injury and some forms of lens injury are shown to activate macrophages so that they produce neuroprotective molecules (33–35). Interestingly, radiation treatment of tumors has been shown to induce tumor expression of multiple growth factors that secondarily inhibit apoptosis of neighboring endothelial cells (36, 37). A similar radiation induced response might exist within the retina or optic nerve.
Glial Activation. Altered glial responses to elevated IOP may be protective. It is possible that protective responses of glia are modulated after radiation exposure. Alternatively, high IOP may normally activate a noxious response of glia that damages RGCs, and this response may not occur in glia that are somehow changed by the treatment. Glial cells in the optic nerve have been suggested to contribute to glaucoma (5, 38, 39). If true, our treatment protocol may lead to increased understanding of relationships between glia and neurons in glaucoma.
Stem/Precursor Cells. Radiation treatment may result in some type of protective cell taking up residence in the eye (possibly in the retina, optic nerve, or their associated vasculature). Whether bone marrow transfer proves necessary for this treatment to work, this cell type may be bone marrow-derived. Relevantly, purified bone marrow-derived precursors of endothelial cells were recently shown to be neuroprotective in a model of retinal degeneration (40). If bone marrow transfer proves an important component of our treatment, there will be important implications for cellular therapies. This finding would imply that it is not always necessary to purify or administer rare individual cell types for therapeutic use. A person's own bone marrow might be therapeutically administered. The potential applicability of radiation to other forms of stem cell therapy would be equally exciting. Finally, although less likely, considering the reported effect of radiation without bone marrow transfer on human glaucoma (19), bone marrow administration by itself may allow stem/precursor cells to become protective.
In summary, high-dose radiation and bone marrow transfer treatment was never previously shown, to our knowledge, to have a beneficial effect for neurons, and our initial result was surprising. Given that the current treatment had substantial benefit, it will be very important to test its potency to shield against other models of glaucoma and other neurodegenerative diseases. This finding is especially important because, rather than undergoing delayed death, the neurons were saved until ages when the disease is typically at an end stage. Because the protection we discovered is robust, it is very tractable for mechanistic studies. If protective in people, refinement of dose, site of radiation, and time of treatment may one day make targeted radiation therapy for patients achievable. For that matter, better understanding of the mechanism behind this neuroprotection may even eliminate the need for radiation. In the interim, our discovery provides a powerful tool for basic research into mechanisms of neurodegeneration and neuroprotection.
Acknowledgments
We thank members of the S.W.M.J. laboratory P. Finger, and M. Osborne, for technical support. This work was supported by National Cancer Institute Grant CA34196 (to The Jackson Laboratory), National Eye Institute Grants F32EY07015 (to M.G.A.) and F32EY014515 (to R.T.L.), and the Canadian Institute of Health Research and the Canadian Stroke Network (D.B.G.). S.W.M.J. is an Investigator of The Howard Hughes Medical Institute.
Author contributions: M.G.A., R.T.L., D.B.G., and S.W.M.J. designed research; M.G.A., R.T.L., D.B.G., R.S.S., and S.W.M.J. performed research; M.G.A., R.T.L., D.B.G., R.S.S., and S.W.M.J. analyzed data; and M.G.A., R.T.L., and S.W.M.J. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: RGC, retinal ganglion cell; IOP, intraocular pressure.
References
- 1.Ritch, R., Shields, M. B. & Krupin, T. (1996) The Glaucomas (Mosby, St. Louis).
- 2.Weinreb, R. N. & Khaw, P. T. (2004) Lancet 363, 1711–1720. [DOI] [PubMed] [Google Scholar]
- 3.Heijl, A., Leske, M. C., Bengtsson, B., Hyman, L. & Hussein, M. (2002) Arch. Ophthalmol. 120, 1268–1279. [DOI] [PubMed] [Google Scholar]
- 4.Collaborative Normal-Tension Glaucoma Study Group (1998) Am. J. Ophthalmol. 126, 487–497. [DOI] [PubMed] [Google Scholar]
- 5.Wax, M. B. & Tezel, G. (2002) Mol. Neurobiol. 26, 45–55. [DOI] [PubMed] [Google Scholar]
- 6.John, S. W., Anderson, M. G. & Smith, R. S. (1999) J. Glaucoma 8, 400–412. [PubMed] [Google Scholar]
- 7.John, S. W. M., Smith, R. S., Savinova, O. V., Hawes, N. L., Chang, B., Turnbull, D., Davisson, M., Roderick, T. H. & Heckenlively, J. R. (1998) Invest. Ophthalmol. Visual Sci. 39, 951–962. [PubMed] [Google Scholar]
- 8.Chang, B., Smith, R. S., Hawes, N. L., Anderson, M. G., Zabaleta, A., Savinova, O., Roderick, T. H., Heckenlively, J. R., Davisson, M. T. & John, S. W. (1999) Nat. Genet. 21, 405–409. [DOI] [PubMed] [Google Scholar]
- 9.Anderson, M. G., Smith, R. S., Hawes, N. L., Zabaleta, A., Chang, B., Wiggs, J. L. & John, S. W. (2002) Nat. Genet. 30, 81–85. [DOI] [PubMed] [Google Scholar]
- 10.Mo, J. S., Anderson, M. G., Gregory, M., Smith, R. S., Savinova, O. V., Serreze, D. V., Ksander, B. R., Streilein, J. W. & John, S. W. (2003) J. Exp. Med. 197, 1335–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quigley, H. A. (1999) Prog. Retin. Eye Res. 18, 39–57. [DOI] [PubMed] [Google Scholar]
- 12.Nickells, R. W. (2004) Brain Res. Bull. 62, 439–446. [DOI] [PubMed] [Google Scholar]
- 13.Raff, M. C., Whitmore, A. V. & Finn, J. T. (2002) Science 296, 868–871. [DOI] [PubMed] [Google Scholar]
- 14.Smith, R. S., Hawes, N. L., Miller, J., Sundberg, J. P. & John, S. W. M. (2002) in Systematic Evaluation of the Mouse Eye: Anatomy, Pathology, and Biomethods, ed. Smith, R. S. (CRC, Boca Raton, FL), pp. 251–264.
- 15.Savinova, O. V., Sugiyama, F., Martin, J. E., Tomarev, S. I., Paigen, B. J., Smith, R. S. & John, S. W. (2001) BMC Genet. 2, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.John, S. W. M. & Savinova, O. V. (2002) in Systematic Evaluation of the Mouse Eye, ed. Smith, R. S. (CRC, Boca Raton, FL).
- 17.Smith, R. S., Zabeleta, A., John, S. W., Bechtold, L. S., Ikeda, S., Relyea, M. J., Sundberg, J. P., Kao, W. W.-Y. & Liu, C.-Y. (2002) in Systematic Evaluation of the Mouse Eye, ed. Smith, R. S. (CRC, Boca Raton, FL), pp. 265–297.
- 18.Stone, J. (1981) The Wholemount Handbook (Maitland Publishing, Sydney).
- 19.Yamada, M., Wong, F. L., Fujiwara, S., Akahoshi, M. & Suzuki, G. (2004) Radiat. Res. 161, 622–632. [DOI] [PubMed] [Google Scholar]
- 20.Hively, W. (2002) Discover 2002, 74–80. [Google Scholar]
- 21.Wolff, S. (1989) Science 245, 575, 621. [DOI] [PubMed] [Google Scholar]
- 22.Sagan, L. A. (1989) Science 245, 574, 621. [DOI] [PubMed] [Google Scholar]
- 23.Kipnis, J., Avidan, H., Markovich, Y., Mizrahi, T., Hauben, E., Prigozhina, T. B., Slavin, S. & Schwartz, M. (2004) Eur. J. Neurosci. 19, 1191–1198. [DOI] [PubMed] [Google Scholar]
- 24.Bakalash, S., Kipnis, J., Yoles, E. & Schwartz, M. (2002) Invest. Ophthalmol. Visual Sci. 43, 2648–2653. [PubMed] [Google Scholar]
- 25.Yang, J., Tezel, G., Patil, R. V. & Wax, M. B. (2000) Curr. Eye Res. 21, 981–985. [DOI] [PubMed] [Google Scholar]
- 26.Danias, J., Shen, F., Goldblum, D., Chen, B., Ramos-Esteban, J., Podos, S. M. & Mittag, T. (2002) Invest. Ophthalmol. Visual Sci. 43, 587–594. [PubMed] [Google Scholar]
- 27.Zhu, Y., Ohlemiller, K. K., McMahan, B. K. & Gidday, J. M. (2002) Invest. Ophthalmol. Visual Sci. 43, 1903–1911. [PubMed] [Google Scholar]
- 28.Roth, S. (2004) Brain Res. Bull. 62, 461–466. [DOI] [PubMed] [Google Scholar]
- 29.Roth, S., Li, B., Rosenbaum, P. S., Gupta, H., Goldstein, I. M., Maxwell, K. M. & Gidday, J. M. (1998) Invest. Ophthalmol. Visual Sci. 39, 777–785. [PubMed] [Google Scholar]
- 30.Cai, L., Cherian, M. G., Iskander, S., Leblanc, M. & Hammond, R. R. (2000) Int. J. Radiat. Biol. 76, 1009–1017. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz, M. (2004) Brain Res. Bull. 62, 481–484. [DOI] [PubMed] [Google Scholar]
- 32.Tezel, G. & Wax, M. B. (2004) Curr. Opin. Ophthalmol. 15, 80–84. [DOI] [PubMed] [Google Scholar]
- 33.Fischer, D., Pavlidis, M. & Thanos, S. (2000) Invest. Ophthalmol. Visual Sci. 41, 3943–3954. [PubMed] [Google Scholar]
- 34.Leon, S., Yin, Y., Nguyen, J., Irwin, N. & Benowitz, L. I. (2000) J. Neurosci. 20, 4615–4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin, Y., Cui, Q., Li, Y., Irwin, N., Fischer, D., Harvey, A. R. & Benowitz, L. I. (2003) J. Neurosci. 23, 2284–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moeller, B. J., Cao, Y., Li, C. Y. & Dewhirst, M. W. (2004) Cancer Cell 5, 429–441. [DOI] [PubMed] [Google Scholar]
- 37.Gorski, D. H., Beckett, M. A., Jaskowiak, N. T., Calvin, D. P., Mauceri, H. J., Salloum, R. M., Seetharam, S., Koons, A., Hari, D. M., Kufe, D. W. & Weichselbaum, R. R. (1999) Cancer Res. 59, 3374–3378. [PubMed] [Google Scholar]
- 38.Neufeld, A. H. & Liu, B. (2003) Neuroscientist 9, 485–495. [DOI] [PubMed] [Google Scholar]
- 39.Ahmed, F., Brown, K. M., Stephan, D. A., Morrison, J. C., Johnson, E. C. & Tomarev, S. I. (2004) Invest Ophthalmol. Visual Sci. 45, 1247–1258. [DOI] [PubMed] [Google Scholar]
- 40.Otani, A., Dorrell, M. I., Kinder, K., Moreno, S. K., Nusinowitz, S., Banin, E., Heckenlively, J. & Friedlander, M. (2004) J. Clin. Invest. 114, 765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leske, M. C. (1983) Am. J. Epidemiol. 118, 166–191. [DOI] [PubMed] [Google Scholar]
- 42.Whitmore, A., Libby, R. T. & John, S. W. M. Prog. Retin. Eye Res., in press. [DOI] [PubMed]