Abstract
Objectives
Lactoferrin (LF) is a breast milk glycoprotein with protective effects against neonatal infections, mainly in premature and low-birth-weight (LBW) neonates. The aims of this study were to determine LF concentration in breast milk of mothers of LBW infants during the first two months postpartum, and to identify the factors associated with LF concentration.
Study Design
Prospective study conducted as a part of an ongoing clinical trial in three Neonatal Units in Peru. We included 346 mothers of neonates with a birth weight <2000g. We measured LF concentration in four stages of lactation using a commercial enzyme-linked immunosorbent assay kit. Multivariate analysis was performed to assess the association between maternal and neonatal factors, and LF concentration.
Results
We collected 695 milk samples. LF mean concentration ± standard deviation was 14.92±7.96 mg/mL in colostrum (n=277), 10.73±5.67 in transitional milk (n=55), 10.34±6.27 at 1 month (n=259), and 8.52±6.47 at 2 months (n=104). There was a significant difference in LF concentration between different stages of lactation (p<0.001). Mothers with higher LF concentration in colostrum had higher values in the following two months. High maternal income and multiple gestation were significantly associated with higher LF levels; in contrast, maternal peri-partum infections and male neonatal gender were associated with lower LF levels.
Conclusions
LF concentration in breast milk of mothers of LBW infants was high and remained elevated even at 1 and 2 months postpartum. LF concentration in colostrum was higher in mothers with higher income and multiple pregnancies, and lower in mothers with peri-partum infections.
Keywords: Lactoferrin concentration, human milk, neonates, low-birth-weight
Introduction
Neonatal sepsis is one of the most important causes of death in infants, mainly in premature and low-birth-weight (LBW) neonates(1,2). Multiple interventions are being studied to reduce the morbidity of this serious infection and therefore, prevent its high mortality and negative effect on growth and long-term neurodevelopmental outcome(3–5). The beneficial effects of human milk against neonatal sepsis in these vulnerable populations are well established(6,7).
Lactoferrin (LF), a breast milk’s protective factor, is a glycoprotein with antimicrobial properties which is present not only in human breast milk, but also, in other body fluids(8). LF’s antimicrobial effect has been demonstrated in vitro and in animal models(9,10). Currently, several clinical trials around the world are investigating the effect of supplemental bovine or recombinant human LF for preventing neonatal infections(11). Three recent double-blinded, placebo-controlled, randomized clinical trials have been published regarding the protective effect of bovine LF supplementation in newborns: Manzoni in 472 very LBW infants in Italy(12), Akin in 50 very LBW infants or with gestational age below 32 weeks in Turkey(13), and Ochoa in 190 infants below 2500 grams at birth in Peru(14). These trials have been reviewed in a recent meta-analysis(15). In addition, two pilot studies conducted in the United States (16) and Canada (17) have been recently published.
LF is the second most abundant protein in breast milk; however, its concentration varies through lactation, and is associated with some maternal and neonatal factors(18). A recent systematic review describes the LF longitudinal changes in breast milk from mothers around the world(19). However, there are not published studies that include a large sample size with a standardized method for LF measurement, especially among breast milk of mothers of premature infants. The aims of this study were to determine the changes in LF concentrations during the first 2 months postpartum in breast milk of mothers of LBW infants and to identify the maternal or neonatal factors associated with these concentrations.
Methods
Study design and setting
We conducted a prospective study as a part of an ongoing randomized placebo-controlled trial of bovine LF supplementation for prevention of late-onset-sepsis in infants (NEOLACTO, NCT01525316). This study was carried out from May 2012 to September 2014 in the Neonatal Units of three tertiary care hospitals in Lima, Peru: “Hospital Cayetano Heredia”, Hospital Nacional Almenara” and “Hospital Nacional Sabogal”. The study was approved by the Institutional Review Boards (IRB) of the University of Texas Health Science Center at Houston, Universidad Peruana Cayetano Heredia, and the IRB of the 3 participating hospitals. Parents gave written informed consent to participate before the collection of clinical data and biological samples.
Study participants
We included 346 mothers of neonates with a birth weight <2000g. From the 414 infants enrolled in the NEOLACTO clinical trial, 37 mothers of multiple pregnancies (35 had twins and 2 had triplets), so they were only considered once. Milk samples from 29 mothers were not obtained or were out of the time frame, therefore, they were not included in the analysis. We included mothers whose infant’s birth weight was between 500 and 2000 grams within the first 72 hours after birth. We excluded mothers whose infants had gastrointestinal problems (n=7) that prevented oral intake (such as esophageal atresia, gastroschisis, duodenal atresia, small bowel obstruction, among others), mothers whose infants were born with severe neurological conditions (n=17) that profoundly affect the growth and development (such as trisomy 18, trisomy 21, anencephaly, myelomeningocele, among others), or family history of allergy to cow’s milk.
Data from the mother’s medical history (maternal age, parity, prenatal care, prenatal complications, use of antibiotics or steroids, anemia, socio-economic status, and mode of delivery) and neonatal characteristics (weeks’ gestation, gender, birth weight and Apgar score) were collected at enrollment.
Milk collection and processing
Sample collection was standardized to reduce bias and daily variability. The mothers were educated by qualified nurses for adequate milk collection. They collected an aliquot of 2–3 ml of milk in sterile polystyrene containers by manual extraction from either breast before breastfeeding. Samples were obtained according to the stages of lactation: colostrum (0–7 days), transitional milk (8–14 days), mature milk at 1 month (30±7 days), and at 2 months postpartum (60±7 days). If a sample was collected out of those time frames, it was not included in the analysis. They were immediately stored in coolers that contained an ice block at −4 C° for transportation to the laboratory.
Lactoferrin concentration measurement
First, the milk samples were centrifuged (13000 RPM for 15 minutes); then, the fat layer was removed and the milk serum was kept frozen at −70°C until LF quantification. We measured the LF concentration using a commercial enzyme-linked immunosorbent assay kit (ELISA) (Assaypro LLC, 30 Triad South Drive St., Charles, MO 63304, USA) according to the manufacturer's instructions. All the reagents were allowed to be warmed up to room temperature (25°C) before use. The plates were read by optical density at a wavelength of 450nm using the Microplate reader (BIOTEK, Synergy H1m Multi-Mode Microplate Reader). The quantitative analysis of samples was done using a Four Parameter Logistic Fit (http://www.myassays.com/welcome.aspx). The LF concentrations were reported as milligrams per milliliter (mg/mL).
We performed quality control assays to determine if our LF concentrations were reliable. Every sample was tested twice; when the second value differed more than 5mg/mL from the original, the sample was retested in order to verify an accurate LF concentration. To ensure the precision of the ELISA kit used (Assaypro), we randomly selected and retested 8 milk samples with a different ELISA kit (Abcam – Massachusetts, USA). In addition, we collected 6 milk samples (4 samples were collected at 1 month postpartum, and 2 samples at 2 months postpartum) and looked for variations according to the moment of milk extraction: at the beginning, middle or at the end of a lactation. Also, we measured LF concentrations in 9 samples from all milk expressed by emptying the entire breast (all milk), to compare with the concentrations obtained from an aliquot collected with our standard technique.
Statistical analysis
The data analysis was performed with Stata software version 8 (College Station, Texas, USA). Categorical variables were expressed in frequencies and percentages. Continuous variables were summarized using means (standard deviation [SD]) and/or medians (interquartile ranges [IQR]). Quantitative variables, such as maternal age, parity, monthly income, neonatal birth weight, gestational age and Apgar score were recoded into categorical variables using standard cut-off points. The comparison of LF concentration between different stages of lactation was done using linear regression to determine the slope (β), the r2 coefficient and the p-value. To compare changes in LF concentration between colostrum and mature milk at one month in the same woman we used a paired sample t test. The milk samples that were obtained as a quality control were analyzed by using linear regression.
We looked for associations between the LF concentration in colostrum or mature milk (at 1 month), and maternal/neonatal variables using linear regression with a logarithmic transformation of the dependent variable (LF concentration) to achieve homoscedasticity. The milk samples of mothers with multiple births were excluded in this analysis. All variables with a p-value of <0.1 in the bivariate analysis and all possible two-factor interaction terms were included in the multivariate analysis. For the multivariate analysis, statistical significance was defined as a p-value <0.05. Using a stepwise approach, non-significant variables were excluded until a final model was achieved. Only the variables with significant association were presented in the results.
Results
We included 346 mothers and collected 695 milk samples: 277 (39.9%) colostrum, 55 (7.9%) transitional milk, 259 (37.3%) mature milk at 1 month and 104 (14.9%) at 2 months postpartum.
Maternal and neonatal characteristics
The median maternal age (IQR) was 30 years (24 – 34); 278 (83.5%) had prenatal care and the median monthly income (IQR) was $333.3 (266.7 – 533.3) (Table 1). The main complications were cesarean delivery 274 (79.4%), preterm labor 157 (45.5%), preeclampsia 110 (31.8%) and maternal peri-partum infection 90 (28.0%). The newborns had a median gestational age (IQR) of 31 weeks (29 – 33), a median birth weight of 1415 grams, and a birth weight range between 570 to 2000 grams. 24.6% of infants were found to be small for gestational age.
Table 1.
Maternal and neonatal characteristics.
| Maternal/neonatal characteristics | n=346 |
|---|---|
| Maternal characteristics | |
| Maternal age, median (IQR) years | 30 (24–34) |
| Parity, median (IQR) | 1 (0–2) |
| Prenatal care, n/N (%) | 278/333 (83.5%) |
| Monthly income, median (IQR) USD | 333.3 (266.7–533.3) |
| Maternal anemia, n/N (%) | 16/345 (4.6%) |
| Delivery characteristics and complications | |
| Cesarean delivery, n/N (%) | 274/345 (79.4%) |
| Use of steroids, n/N (%) | 237/307 (77.2%) |
| Preterm labor, n/N (%) | 157/345 (45.5%) |
| Preeclampsia/eclampsia, n/N (%) | 110/345 (31.8%) |
| Peri-partum infection, n/N (%) | 90/321 (28.0%) |
| Premature rupture of membranes, n/N (%) | 80/333 (24.0%) |
| Multiple pregnancy, n/N (%) | 52/345 (15.1%) |
| Neonatal characteristics | |
| Male, n/N (%) | 175/294 (59.5%) |
| Gestational age, median (IQR) | 31 (29 – 33) |
| Birth weight, median (IQR) grams | 1415 (1130 – 1692) |
| Apgar score 1 min, median (IQR) | 8 (6–8) |
| Apgar score 5 min, median (IQR) | 9 (8–9) |
IQR: interquartile range
Lactoferrin concentration
LF mean concentration ± SD varied according to the lactation stage (Figure 1): colostrum 14.92±7.96 mg/mL, transitional milk 10.73±5.67 mg/mL, mature milk at 1 month 10.34±6.27 mg/mL and mature milk at 2 months 8.52±6.47 mg/mL (Table 2A). There was a significant difference in LF concentration between different stages of lactation (p<0.001) in all subjects and in the <1500g birth weight infants. LF concentration categorized by days postpartum based on Rai’s systematic review(19) is presented in Table 2B. Using a cutoff point of 28 days, we found a LF concentration of 13.73±7.71 mg/mL in milk samples collected up to 28 days postpartum (n=369), and a concentration of 9.89±6.48 in samples from 28 days onwards (n=326). Of interested, after 30 days postpartum, the mean ± SD of LF concentration was 9.57±6.48 mg/mL.
Figure 1.
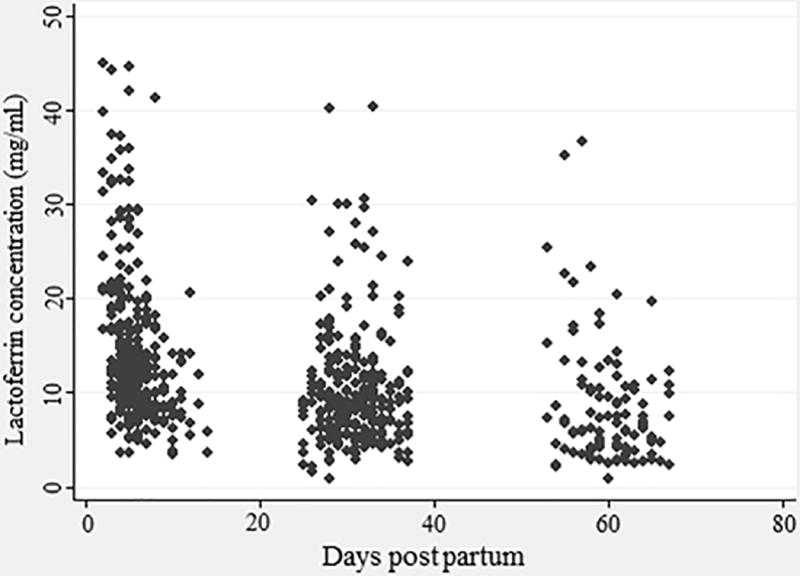
Lactoferrin concentration according to stage of lactation. n = 695 milk samples of mothers of low-birth-weight infants (< 2000g).
Table 2.
| A. Lactoferrin concentration according to birth weight and stage of lactation. | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Birth weight | Milk sample | n | Lactoferrin concentration (mg/mL) | |||
|
| ||||||
| Mean±SD | Median (IQR) | Min | Max | |||
| All subjects | Colostrum | 277 | 14.92±7.96 | 12.66 (9.64 – 17.94) | 3.57 | 44.99 |
| Transitional milk | 55 | 10.73±5.67 | 10.01 (7.24 – 13.37) | 3.52 | 41.19 | |
| Mature milk at 1 month | 259 | 10.34±6.27 | 8.98 (6.27 – 12.19) | 0.91 | 40.34 | |
| Mature milk at 2 months | 104 | 8.52±6.47 | 6.72 (4.19 – 10.65) | 0.92 | 36.76 | |
| 1500–2000g | Colostrum | 119 | 15.62±8.54 | 12.98 (9.90 – 17.30) | 3.57 | 44.99 |
| Transitional milk | 16 | 11.53±8.48 | 9.13 (7.35 – 12.31) | 5.41 | 41.19 | |
| Mature milk at 1 month | 111 | 9.94±6.50 | 8.18 (5.73 – 11.29) | 0.91 | 40.22 | |
| Mature milk at 2 months | 30 | 8.75±6.82 | 5.95 (3.80 – 13.20) | 0.92 | 25.34 | |
| <1500g | Colostrum | 158 | 14.40±7.47 | 12.42 (9.26 – 18.16) | 3.60 | 44.59 |
| Transitional milk | 39 | 10.40±4.11 | 10.34 (7.19 – 13.37) | 3.52 | 20.55 | |
| Mature milk at 1 month | 148 | 10.64±6.10 | 9.19 (6.47 – 12.84) | 2.12 | 40.34 | |
| Mature milk at 2 months | 74 | 8.43±6.37 | 7.00 (4.60 – 10.26) | 2.11 | 36.73 | |
| B. Lactoferrin concentration according to days postpartum* | |||||
|---|---|---|---|---|---|
|
| |||||
| Days postpartum | n | Lactoferrin concentration (mg/mL) | |||
|
|
|||||
| Mean±SD) | P50 (IQR) | ||||
| 0–5 days | 177 | 16.4±8.90 | 13.46 (10.35–20.46) | ||
| 6–10 days | 140 | 11.9±5.29 | 11.18 (8.43–14.08) | ||
| 11–30 days | 142 | 10.3±5.9 | 8.74 (6.40–12.38) | ||
| >30 days | 236 | 9.57±6.48 | 7.76 (5.19–11.78) | ||
IQR: interquartile range; SD: standard deviation
Based on the categorization of days postpartum described by Rai et al.(17)
When looking for correlation between different samples from the same woman, we found a significant positive correlation between LF concentration in colostrum and mature milk at 1 month postpartum, and between mature milk at 1 month and at 2 months postpartum (Suppl. figure 1).
Mothers with higher LF concentration in colostrum had higher values in the following stages of lactation, although the differences tend to disappear over the course of time (Figure 2). In those women who provided a sample of colostrum and mature milk at 1 month (n=203), there was a significant reduction of 5.38 mg/mL in the LF concentration (15.68 – 10.30 mg/mL respectively, p<0.001). The administration of supplemental bovine LF had no effect on LF concentration in human milk, measured at 1 month postpartum (p=0.09).
Figure 2.
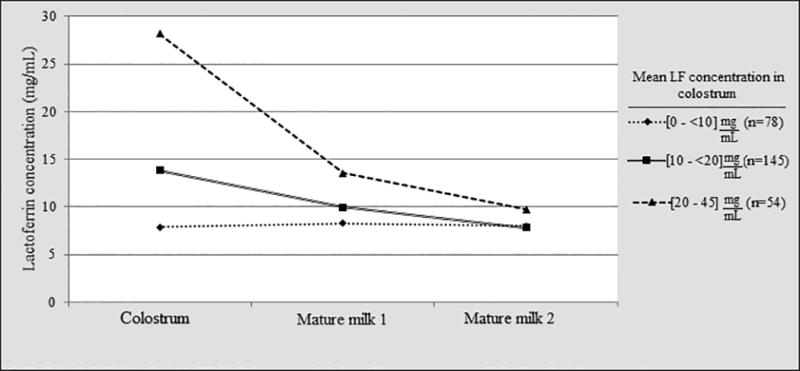
Variation in LF concentration in breast milk from each individual mother over the course of lactation. Data are presented as mean LF concentrations, and are classified in three groups per initial LF concentration in colostrum. Mothers with colostrum LF concentrations between 20 and 45 mg/mL (▲) maintained relative higher concentrations at 1 month, when compared to mothers with lower colostrum LF concentrations [10 to < 20 mg/mL (■) and 0 to < 10 mg/mL (◆)]. At two months, all mothers had similar LF concentration values.
As a quality control, we performed three additional comparisons to verify the accuracy of LF concentration. We compared LF level and its variation in the same woman using two different ELISA kits by a linear regression. We also compared LF concentration in milk samples collected from the same woman at different timings within an extraction: beginning, middle, end of lactation. Finally, LF concentration was also measured in 9 samples obtained from the same mother using 2 methods of extraction: aliquot collected by our standard method versus all milk expressed by emptying the entire breast. No significant differences were found among LF concentrations obtained by performing these three quality controls.
Association between LF concentration and maternal or neonatal characteristics
All the maternal and neonatal characteristics were analyzed in the bivariate analysis with the logarithmic transformation of LF concentration in colostrum and maternal milk at 1 month (Table 3). Significant associations were found between LF concentration and monthly income, peri-partum infections, and multiple pregnancy. These variables, in addition to prenatal care and preterm labor, were included in the multivariate analysis comparing the maternal or neonatal characteristics, with the logarithmic transformation of LF concentration, as in the bivariate analysis. Maternal income and multiple pregnancy were significantly associated with higher LF concentrations in colostrum. Maternal peri-partum infection was significantly associated with lower a LF concentration in colostrum (Table 4). Regarding the neonatal factors, neonatal gender and gestational age were included in the multivariate analysis. Male gender was associated with a significant reduction of LF concentration at 1 month postpartum (Table 4). No interaction terms were significant.
Table 3.
Bivariate analysis between maternal/neonatal characteristics and LF concentration in colostrum and mature milk at 1 month
| Maternal/neonatal characteristics | Δ LFc (mg/mL) |
Colostrum (Log LF) | Δ LFm1 (mg/mL) |
Mature milk 1 (Log LF) | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| β | p-value | β | p-value | |||
| Maternal characteristics | ||||||
| Maternal age ≥ 35 years | −0.437 | −0.01 | 0.91 | −0.164 | −0.01 | 0.87 |
| Parity ≥ 2 | −1.439 | −0.07 | 0.22 | −0.585 | −0.04 | 0.58 |
| Prenatal care ≥ 1 | +2.342 | +0.14 | 0.08* | −0.958 | −0.10 | 0.28 |
| Monthly income ≥ 300 USD | +2.561 | +0.17 | 0.01* | +0.304 | −0.01 | 0.86 |
| Maternal anemia | +1.7126 | +0.13 | 0.38 | −0.981 | −0.08 | 0.61 |
| Delivery characteristics and complications | ||||||
| Cesarean delivery | +0.114 | +0.03 | 0.72 | +0.435 | +0.05 | 0.58 |
| Use of steroids | +1.459 | +0.08 | 0.25 | −0.454 | −0.02 | 0.83 |
| Preterm labor | −2.079 | −0.11 | 0.06* | −0.923 | −0.05 | 0.49 |
| Preeclampsia/eclampsia | +0.121 | +0.02 | 0.80 | +0.990 | +0.10 | 0.19 |
| Peri-partum infection | −2.010 | −0.15 | 0.02* | −0.687 | −0.09 | 0.26 |
| Premature rupture of membranes | +1.094 | +0.09 | 0.19 | −0.589 | −0.04 | 0.59 |
| Multiple pregnancy | +2.752 | +0.16 | 0.04* | −1.080 | −0.09 | 0.34 |
| Neonatal characteristics | ||||||
| Male gender | −0.001 | +0.01 | 0.94 | −1.623 | −0.15 | 0.04* |
| Gestational age ≥ 30 weeks | +1.703 | +0.08 | 0.22 | −1.283 | −0.13 | 0.10* |
| Birth weight ≥ 1500 grams | +1.224 | +0.08 | 0.18 | −0.706 | −0.09 | 0.22 |
| Apgar score 1 min < 7 | −0.590 | −0.01 | 0.85 | +0.375 | +0.04 | 0.60 |
| Apgar score 5 min < 7 | −0.511 | −0.02 | 0.87 | −0.267 | +0.04 | 0.77 |
Variables included in the multivariate analysis.
Table 4.
Multivariate analysis between maternal/neonatal characteristics and logarithmic transformation of LF concentration in different stages of lactation.
| Maternal/neonatal characteristics |
Colostrum (Log LF) | Mature milk at 1 month (Log LF) | ||
|---|---|---|---|---|
|
| ||||
| β | p-value | β | p-value | |
| Monthly income ≥ 300 USD | +0.19 | 0.004 | - | - |
| Multiple pregnancy | +0.18 | 0.03 | - | - |
| Peri-partum infection | −0.19 | 0.008 | - | - |
| Male gender | - | - | −0.15 | 0.035 |
Discussion
This is the first study to present in detail the variation of lactoferrin concentration in milk from a large set of mothers of preterm newborns. We found high LF concentrations in breast milk of mothers of LBW infants. The mean of LF concentration decreases in time, from 15.68 mg/mL in colostrum to 10.30 mg/mL in mature milk at 1 month postpartum in those mothers who provided both samples. These results are consistent with previous studies, which have shown that LF levels decrease significantly in relation to the number of days postpartum (20–22). Our LF values are considerably higher than those previously reported; in general, it is considered that the concentration of LF is 5–6 mg/mL in colostrum, and 0.5–1 mg/mL after the first month of lactation(23,24). However, previous studies included mainly mothers of term infants and used different analytical methods to quantify LF concentration (radial immunodiffusion, immunoelectrophoresis, ELISA, and SDS-PAGE). Rai et al. published a systematic review on LF concentrations around the world including 52 studies in 25 countries(19). In “early milk” (<28 days lactation) they found a LF concentration ± SD of 4.91±0.31 mg/mL, and in “mature milk” (≥28 days lactation) it was 2.10±0.87 mg/mL. Using the same cutoff point in days postpartum, in our sample we found a LF concentration ± SD of 13.73±7.71 mg/mL in early milk, and 9.89±6.48 mg/mL in mature milk.
LF levels are higher in mothers of preterm neonates (23,25,26). When analyzing the data that included only preterm infants from eight previous studies, Rai et al. found the LF concentration in colostrum was 5.93±1.39 mg/mL and 3.55±0.90 mg/mL in mature milk(19). These values, although higher than studies in term infants, are lower than what we found. One possible explanation for this discrepancy is the lack of data on the gestational age or birth weight from infants of those mothers enrolled in the previous studies. Therefore, we cannot determine if the populations are comparable; our sample included mothers of infants with a median gestational age (IQR) of 31 (29–33) weeks and a median birth weight (IQR) of 1415 (1130–1692) grams, all were LBW infants. In addition, some limitations of previous studies in preterm infants were the relative small sample size, the use of different sample collections techniques, and different methods for LF measurement(19); all of which are the strengths of our study.
The elevated LF values found in our study may be related to the high proportion of mothers who underwent cesarean delivery (79.4%), or used steroids (77.2%), which may cause stress and a delay in breast milk secretion, and therefore, an increase in the LF concentration. However, none of those two variables showed statistical significance in the bivariate analysis (p=0.72 and p=0.25, respectively). This theory has been suggested by Tang et al., who proposed that steroid hormones may have an effect on LF expression(27). Thus, these results could probably be extrapolated only to areas in the world with similar delivery conditions, especially delivery of LBW infants. Of interest, although high LF levels in colostrum could be related to stressed delivery conditions, the groups of mothers with high LF levels in colostrum (> 20 mg/mL) continue producing higher LF concentrations at 1-month postpartum (≈ 14 mg/mL) (Figure 2).
Since the LF concentrations found in our study were high, we performed several quality control assays to verify the accuracy of these finding. First, we used two different commercial LF ELISA kits to compare the LF concentration in the same milk samples and found similar results. Then, we compared the concentration in milk samples obtained at 3 different timings within a milk extraction (beginning, middle, and end), with the hypothesis that probably the LF concentration was higher at the beginning of the milk breast extraction (the standard technique used in our study). However, we found similar concentrations in either middle or final timing of extraction when comparing with the levels obtained at the beginning. Finally, we compared the LF concentration in an aliquot (2–3 mL) taken out of the total breast extraction (40–50 mL of mature milk) versus our standard aliquot (2–3 mL) taken at the beginning of the milk extraction (our collection technique) from the same mother, and found no significant differences.
In relation to the factors that may influence the LF levels, the literature has contradictory findings. In colostrum, we found higher LF concentrations in mothers with higher monthly income, which is consistent to what was published by Hennart et al.(28) comparing mothers from rural and urban areas in Zaire. However, Sanchez-Pozo et al. found an opposite association in Spain with higher values in mothers of low socio-economic groups(29). We found higher LF levels in colostrum of mothers with multiple pregnancy (twins), contrary to what was previously published(28). On the other hand, we found an inverse relationship between maternal peri-partum infection and LF concentration in colostrum. Lönnerdal et al. showed similar results, higher LF levels in non-ill Peruvian mothers than ill mothers (urinary tract infection, chorioamnionitis, respiratory or skin infection)(20). Two different studies compared the LF concentration in amniotic fluid in mothers with choriamnionitis; both found an increased LF value in the amniotic fluid of infected mothers(30,31). Our lower LF concentrations in milk may be related to a “compensatory decrease” in breast milk due to a significant rise in the amniotic fluid; however, this is only a hypothesis that needs to be proven. Finally, we found a significant association between male neonates and reduced LF concentration in mature milk at 1 month. There are no previous studies that have analyzed this association. Our study did not find a significant correlation with other factors, such as daily fat intake(32), maternal parity(28,33), and ill-infants(34). Nevertheless, many geographical and socioeconomic factors may explain the differences found among studies. There are no large, multicenter and standardized studies to determine the factors associated with LF concentration in breast milk.
Our study has some limitations. First, we have not used the “gold standard” technique for breast milk collection, which is all the milk collected by total breast emptying during 24 hours; however, we have compared it with our technique (aliquot sample at the beginning of breastmilk extraction) in a small number of samples and found no significant differences. Second, not all of our samples were collected at the same time of the day. We know there are circadian differences in breast milk composition, and this could explain some of the heterogeneity on LF levels between samples at the same stage of lactation. Third, we have not collected information on some maternal factors such as nutritional status, diet, and signs of labor, which could affect the LF concentration. Fourth, we have a smaller number of milk samples at 2 months postpartum, because we started to collect these samples later on our trial due to high LF levels found at 1 month postpartum. Finally, we only included samples from Peruvian mothers, which may affect this study’s generalizability across other ethnicities. Nevertheless, this is, to our knowledge, the study with the largest number of milk samples collected (≈ 700) from mothers of LBW preterm infants with sequential samples up to 2 months postpartum. Future additional studies are needed to determine the protective effect of different LF concentrations in breast milk.
In conclusion, our study showed that LF concentrations in breast milk of mothers of LBW infants are high and remain elevated in the first two months postpartum. Peruvian mothers of LBW infants have higher LF values than previously reported for preterm neonates. LF concentration in colostrum was significantly higher in mothers with higher income and multiple pregnancies, and lower in mothers with peri-partum infections. Although LF concentrations decrease with days postpartum, preterm neonates continue to receive a high concentration of this protective factor during the critical first two months of life. Therefore, pediatricians and all healthcare personnel should continue to promote exclusive breast-feeding during this period.
Supplementary Material
Supplemental figure 1. Correlation between LF concentration in colostrum and mature milk at 1 month (A), and between mature milk at 1 and 2 months (B). Each dot represents each individual mother’s LF measurement in two consecutive stages of lactation. The slope (β), the r2 coefficient and the p-value for each correlation are described in the right upper corner of each graphic.
Acknowledgments
Funding Source: Funded by the National Institute of Child Health & Human Development (NICHD), grant number: R01-HD067694.
We are grateful to all the members of the NEOLACTO Research Group: Rospigliosi M, MD, Borda G, MD, Webb V, MD, Lino A, MD, Cama A, MD, Llanos R, MD, Chumbes O, MD, Cuba, L, MD, Tresierra J, MD, Chincaro C, MD, Alarcon W, MD, Bravo E, MD, Pacheco K, MD, Guillen D, MD, Medina P, MD, Tori A, MD, Rivas M, MD, Campos M, MD, Tucto L, RN, Suarez C, RN, Huanay M, RN, Rojas N, RN.
We also want to express our most sincere gratitude to the volunteer mothers, for their participation and commitment to the project.
Abbreviations
- ELISA
enzyme-linked immunosorbent assay
- IQR
interquartile ranges
- IRB
Institutional Review Board
- LF
lactoferrin
- LBW
low-birth-weight
- SD
standard deviation
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Clinical Trial Registration: Lactoferrin for Prevention of Sepsis in Infants (NEOLACTO), NCT01525316, web link: https://clinicaltrials.gov/ct2/show/NCT01525316
Conflict of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Stoll BJ. The global impact of neonatal infection. Clin Perinatol. 1997;24:1–21. [PubMed] [Google Scholar]
- 2.Shane AL, Stoll BJ. Neonatal sepsis: progress towards improved outcomes. J Infect. 2014;68(Suppl 1):S24–32. doi: 10.1016/j.jinf.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Ferreira RC, Mello RR, Silva KS. Neonatal sepsis as a risk factor for neurodevelopmental changes in preterm infants with very low birth weight. J Pediatr (Rio J) 2014;90:293–9. doi: 10.1016/j.jped.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 4.van Vliet EOG, de Kieviet JF, Oosterlaan J, van Elburg RM. Perinatal infections and neurodevelopmental outcome in very preterm and very low-birth-weight infants: a meta-analysis. JAMA Pediatr. 2013;167:662–8. doi: 10.1001/jamapediatrics.2013.1199. [DOI] [PubMed] [Google Scholar]
- 5.Dong Y, Speer CP. Late-onset neonatal sepsis: recent developments. Arch Dis Child Fetal Neonatal Ed. 2015;100:F257–63. doi: 10.1136/archdischild-2014-306213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Underwood MA. Human milk for the premature infant. Pediatr Clin North Am. 2013;60:189–207. doi: 10.1016/j.pcl.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furman L, Taylor G, Minich N, Hack M. The effect of maternal milk on neonatal morbidity of very low-birth-weight infants. Arch Pediatr Adolesc Med. 2003;157:66–71. doi: 10.1001/archpedi.157.1.66. [DOI] [PubMed] [Google Scholar]
- 8.Vogel HJ. Lactoferrin, a bird’s eye view. Biochem Cell Biol. 2012;90:233–44. doi: 10.1139/o2012-016. [DOI] [PubMed] [Google Scholar]
- 9.Embleton ND, Berrington JE, McGuire W, Stewart CJ, Cummings SP. Lactoferrin: Antimicrobial activity and therapeutic potential. Semin Fetal Neonatal Med. 2013;18:143–9. doi: 10.1016/j.siny.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Ochoa TJ, Cleary TG. Effect of lactoferrin on enteric pathogens. Biochimie. 2009;91:30–4. doi: 10.1016/j.biochi.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turin CG, Zea-Vera A, Pezo A, Cruz K, Zegarra J, Bellomo S, et al. Lactoferrin for prevention of neonatal sepsis. Biometals. 2014;27:1007–16. doi: 10.1007/s10534-014-9754-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manzoni P, Rinaldi M, Cattani S, Pugni L, Romeo MG, Messner H, et al. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial. JAMA. 2009;302:1421–8. doi: 10.1001/jama.2009.1403. [DOI] [PubMed] [Google Scholar]
- 13.Akin IM, Atasay B, Dogu F, Okulu E, Arsan S, Karatas HD, et al. Oral lactoferrin to prevent nosocomial sepsis and necrotizing enterocolitis of premature neonates and effect on T-regulatory cells. Am J Perinatol. 2014;31:1111–20. doi: 10.1055/s-0034-1371704. [DOI] [PubMed] [Google Scholar]
- 14.Ochoa TJ, Zegarra J, Cam L, Llanos R, Pezo A, Cruz K, et al. Randomized controlled trial of lactoferrin for prevention of sepsis in peruvian neonates less than 2500 g. Pediatr Infect Dis J. 2015;34:571–6. doi: 10.1097/INF.0000000000000593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pammi M, Abrams SA. Oral lactoferrin for the prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2015;2 doi: 10.1002/14651858.CD007137.pub4. CD007137. [DOI] [PubMed] [Google Scholar]
- 16.Sherman MP, Adamkin DH, Niklas V, Radmacher P, Sherman J, Wertheimer F, et al. Randomized Controlled Trial of Talactoferrin Oral Solution in Preterm Infants. J Pediatr. 2016;175:68–73.e3. doi: 10.1016/j.jpeds.2016.04.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrington KJ, Assaad M-A, Janvier A. The Lacuna Trial: a double-blind randomized controlled pilot trial of lactoferrin supplementation in the very preterm infant. J Perinatol. 2016;36:666–9. doi: 10.1038/jp.2016.24. [DOI] [PubMed] [Google Scholar]
- 18.Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am. 2013;60:49–74. doi: 10.1016/j.pcl.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rai D, Adelman AS, Zhuang W, Rai GP, Boettcher J, Lönnerdal B. Longitudinal changes in lactoferrin concentrations in human milk: a global systematic review. Crit Rev Food Sci Nutr. 2014;54:1539–47. doi: 10.1080/10408398.2011.642422. [DOI] [PubMed] [Google Scholar]
- 20.Lönnerdal B, Zavaleta N, Kusunoki L, Lanata CF, Peerson JM, Brown KH. Effect of postpartum maternal infection on proteins and trace elements in colostrum and early milk. Acta Paediatr. 1996;85:537–42. doi: 10.1111/j.1651-2227.1996.tb14081.x. [DOI] [PubMed] [Google Scholar]
- 21.Ronayne de Ferrer PA, Baroni A, Sambucetti ME, López NE, Ceriani Cernadas JM. Lactoferrin levels in term and preterm milk. J Am Coll Nutr. 2000;19:370–3. doi: 10.1080/07315724.2000.10718933. [DOI] [PubMed] [Google Scholar]
- 22.Broadhurst M, Beddis K, Black J, Henderson H, Nair A, Wheeler T. Effect of gestation length on the levels of five innate defence proteins in human milk. Early Hum Dev. 2015;91:7–11. doi: 10.1016/j.earlhumdev.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Goldman AS, Garza C, Nichols BL, Goldblum RM. Immunologic factors in human milk during the first year of lactation. J Pediatr. 1982;100:563–7. doi: 10.1016/s0022-3476(82)80753-1. [DOI] [PubMed] [Google Scholar]
- 24.Hamosh M. Bioactive factors in human milk. Pediatr Clin North Am. 2001;48:69–86. doi: 10.1016/s0031-3955(05)70286-8. [DOI] [PubMed] [Google Scholar]
- 25.Dawarkadas AM, Saha K, Mathur NB. A comparative study of cells and anti-microbial proteins in colostrum of mothers delivering pre- and full-term babies. J Trop Pediatr. 1991;37:214–9. doi: 10.1093/tropej/37.5.214. [DOI] [PubMed] [Google Scholar]
- 26.Montagne P, Cuillière ML, Molé C, Béné MC, Faure G. Immunological and nutritional composition of human milk in relation to prematurity and mother’s parity during the first 2 weeks of lactation. J Pediatr Gastroenterol Nutr. 1999;29:75–80. doi: 10.1097/00005176-199907000-00018. [DOI] [PubMed] [Google Scholar]
- 27.Teng CT. Lactoferrin gene expression and regulation: an overview. Biochem Cell Biol. 2002;80:7–16. doi: 10.1139/o01-215. [DOI] [PubMed] [Google Scholar]
- 28.Hennart PF, Brasseur DJ, Delogne-Desnoeck JB, Dramaix MM, Robyn CE. Lysozyme, lactoferrin, and secretory immunoglobulin A content in breast milk: influence of duration of lactation, nutrition status, prolactin status, and parity of mother. Am J Clin Nutr. 1991;53:32–9. doi: 10.1093/ajcn/53.1.32. [DOI] [PubMed] [Google Scholar]
- 29.Sanchez-Pozo A, Lopez Morales J, Izquierdo A, Martinez-Valverde A, Gil A. Protein composition of human milk in relation to mothers’ weight and socioeconomic status. Hum Nutr Clin Nutr. 1987;41:115–25. [PubMed] [Google Scholar]
- 30.Otsuki K, Yoda A, Saito H, Mitsuhashi Y, Toma Y, Shimizu Y, et al. Amniotic fluid lactoferrin in intrauterine infection. Placenta. 1999;20:175–9. doi: 10.1053/plac.1998.0368. [DOI] [PubMed] [Google Scholar]
- 31.Pacora P, Maymon E, Gervasi MT, Gomez R, Edwin SS, Yoon BH, et al. Lactoferrin in intrauterine infection, human parturition, and rupture of fetal membranes. Am J Obstet Gynecol. 2000;183:904–10. doi: 10.1067/mob.2000.108882. [DOI] [PubMed] [Google Scholar]
- 32.Leelahakul V, Tanaka F, Sinsuksai N, Vichitsukon K, Pinyopasakul W, Kido N, et al. Comparison of the protein composition of breast milk and the nutrient intake between Thai and Japanese mothers. Nurs Health Sci. 2009;11:180–4. doi: 10.1111/j.1442-2018.2009.00445.x. [DOI] [PubMed] [Google Scholar]
- 33.Prentice A, Prentice AM, Cole TJ, Whitehead RG. Determinants of variations in breast milk protective factor concentrations of rural Gambian mothers. Arch Dis Child. 1983;58:518–22. doi: 10.1136/adc.58.7.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breakey AA, Hinde K, Valeggia CR, Sinofsky A, Ellison PT. Illness in breastfeeding infants relates to concentration of lactoferrin and secretory Immunoglobulin A in mother’s milk. Evol Med Public Health. 2015;2015:21–31. doi: 10.1093/emph/eov002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure 1. Correlation between LF concentration in colostrum and mature milk at 1 month (A), and between mature milk at 1 and 2 months (B). Each dot represents each individual mother’s LF measurement in two consecutive stages of lactation. The slope (β), the r2 coefficient and the p-value for each correlation are described in the right upper corner of each graphic.


