Abstract
Pain, a critical component of host defense, is one hallmark of the inflammatory response. We therefore hypothesized that pain might be exacerbated by proinflammatory chemokines. To test this hypothesis, CCR1 was cotransfected into human embryonic kidney (HEK)293 cells together with transient receptor potential vanilloid 1 (TRPV1), a cation channel required for certain types of thermal hyperalgesia. In these cells, capsaicin and anandamide induced Ca2+ influx mediated by TRPV1. When CCR1:TRPV1/HEK293 cells were pretreated with CCL3, the sensitivity of TRPV1, as indicated by the Ca2+ influx, was increased ≈3-fold. RT-PCR analysis showed that a spectrum of chemokine and cytokine receptors is expressed in rat dorsal root ganglia (DRG). Immunohistochemical staining of DRG showed that CCR1 is coexpressed with TRPV1 in >85% of small-diameter neurons. CCR1 on DRG neurons was functional, as demonstrated by CCL3-induced Ca2+ ion influx and PKC activation. Pretreatment with CCL3 enhanced the response of DRG neurons to capsaicin or anandamide. This sensitization was inhibited by pertussis toxin, U73122, or chelerythrine chloride, inhibitors of Gi-protein, phospholipase C, and protein kinase C, respectively. Intraplantar injection of mice with CCL3 decreased their hot-plate response latency. That a proinflammatory chemokine, by interacting with its receptor on small-diameter neurons, sensitizes TRPV1 reveals a previously undescribed mechanism of receptor cross-sensitization that may contribute to hyperalgesia during inflammation.
Keywords: CCR1, chemokine receptor, inflammation, pain
Pain is a component of host defense that provides a warning to avoid potential further injury. Noxious stimuli, such as acid, heat, and chemical irritants, are detected by nociceptors, a group of specialized primary afferent neurons. One protein reported to transduce noxious thermal and chemical stimuli into nociceptor depolarization is the capsaicin (CAP)-heat- and proton-gated ion channel, transient receptor potential vanilloid 1 (TRPV1), also known as the vanilloid receptor (1, 2). Mice with a disruption in the trpv1 gene were incapable of sensing CAP, the main pungent ingredient in hot peppers, and showed little thermal hypersensitivity during inflammation (3). TRPV1 is mainly expressed in primary neurons whose cell bodies are located in dorsal root ganglia (DRG), trigeminal ganglia (TG), and nodose sensory ganglia (1). In these neurons, noxious stimuli evoke Na+ and Ca2+ ion flux and consequent membrane depolarization (4). The resulting action potentials are propagated to the central nervous system to evoke a sensation of pain.
Inflammation has been reported to enhance the perception of pain by decreasing the activation threshold and by increasing the excitability of nociceptive neurons. The up-regulation of TRPV1 signaling by several cellular inflammatory mediators has been reported. For example, prostaglandin E2 reduces TRPV1 desensitization by inducing PKA-mediated TRPV1 phosphorylation (5, 6). Bradykinin, ATP, and nerve growth factor all activate phospholipase C (PLC), which has been proposed to sensitize TRPV1 in at least three distinct ways: by initiating the production of endogenous TRPV1 agonists such as 12-HPETE (7); by removing PtdIns (4, 5) P2, a TRPV1 inhibitor (8); and by promoting TRPV1 phosphorylation by the PKC family of serine/threonine kinases (5, 9). At least two PKC subtypes have been implicated in TRPV1 sensitization. Prevention of PKCε recruitment to the plasma membrane of sensory neurons reduced bradykinin-evoked sensitization of heat-activated currents, whereas disruption of the gene encoding this isoform results in impairment of thermal and acid induced hyperalgesia in vivo (10). Activation of PKCα under acidic conditions activates TRPV1 and enhances its sensitivity to other stimuli (11). Down-regulation of this isoform in cultured sensory neurons has also been associated with a reduction in TRPV1 sensitization. Thus, TRPV1 is a pivotal target of proalgesic substances produced during inflammation.
Chemokines, a group of chemotactic cytokines, regulate immune responses by inducing leukocyte infiltration, enhancing angiogenesis, and facilitating host defense. CCL3 (macrophage inflammatory protein 1α), an 8-kDa chemokine originally purified from supernatant of endotoxin-stimulated murine macrophages, plays an important role in mediating inflammation and proliferation of hematopoietic stem cells (12). The interaction between chemokines and their receptors exhibits extensive redundancy. One receptor, such as CCR1, can be activated by several endogenous ligands, including CCL3, -5, and -7 (13). The converse is also true: CCL3 can activate several receptors, including CCR1, -3, and -5 (12). CCR1, extensively expressed on leukocytes, is a major receptor for CCL3. Upon ligand binding, activated CCR1 induces the dissociation of Gαiβγ protein, which in turn activates PLC to hydrolyze PtIns(4,5)P2 into IP3 and diacylglycerol (DAG). IP3 induces Ca2+ release through its receptor on the endoplasmic reticulum. Both DAG and Ca2+ activate PKC. Expression of chemokine receptors is not limited to immune cells. Our previous studies have shown that prior interaction of chemokines with their receptors desensitizes opioid receptors in the periauqaductal gray region of the CNS, resulting in hyperalgesia by depressing the analgesic action of opioid receptors (14). Recently, chemokine receptor activity has also been detected on DRG neurons (15). Because both PLC and PKC have been implicated in TRPV1 sensitization, we hypothesized that activation of chemokine receptors during inflammation may enhance the sensitivity of TRPV1, thereby promoting hyperalgesia.
Materials and Methods
Constructs and Cell Lines. All human embryonic kidney (HEK)293 cell lines were cultured in DMEM supplemented with 10% FCS (Cambrex, Walkersvile, MD), antibiotics, and selection drugs. The stable cell line, CCR1/HEK293, was established as described (16). Rat TRPV1 was subcloned into vector pcDNA3 to make pTRPV1. CCR1:TRPV1/HEK293 cells were obtained by transiently transfecting CCR1/HEK293 with pTRPV1 by using FuGENE 6 (Roche Diagnostics) according to the manufacturer's protocol. Briefly, CCR1/HEK293 cells were plated in a 75-cm2 flask the day before transfection. The next day, cells were incubated in 10 ml of fresh DMEM with 10% FCS for 1 hr. FuGENE 6 (30 μl) was added to 1 ml of DMEM followed by 10 μg of pTRPV1. The mixture was incubated at room temperature for 30 min and then added drop-wise onto the cell monolayer. The transfected cells were allowed to grow for 48 hr before use. Microscope-based Ca2+ ion imaging revealed that >80% of transfected cells were TRPV1-positive, based on CAP responsiveness.
DRG Culture. Isolation of DRG neurons and primary neuron culture were carried out as described with minor modifications Briefly, DRG (lumbar level, L2–5) were isolated from 4- to 6-week-old male rats or mice, incubated with collagenase (Sigma) at 37°C for 45 min, digested with 0.05% trypsin-EDTA (GIBCO), resuspended in complete medium (DMEM/F12/10% heat-inactivated horse serum/0.8% glucose/antibiotics), and plated on eight-well chambered cover glass slides (Nunc) that had been coated with laminin and poly(dl-ornithine) (Sigma). Cells were allowed to adhere to the bottom for 1 hr, and then fresh medium was added. Experiments were carried out within 6–10 hr of neuronal isolation.
Western Blotting Analysis. Western blotting analysis was performed as described with minor modifications (17). Briefly, CCR1:TRPV1/HEK293 cells were homogenized by sonication. After centrifugation at 12,000 × g for 5 min, the supernatant was discarded, and the pellet was dissolved in SDS/PAGE loading buffer (Invitrogen). The samples were centrifuged at 12,000 × g for 10 min and loaded on 8% or 10% Tris-glycine gels (Invitrogen). After electrophoresis and electrotransfer, the poly(vinylidene difluoride) membranes (Millipore) were probed with CCR1 antiserum (H-52, from Santa Cruz Biotechnology) or TRPV1 antiserum, followed by horseradish-peroxidase-labeled secondary antibody (Sigma). Band detection was achieved by using enhanced chemiluminescence (Pierce).
Ca2+ Flux Analysis by Fluorimeter- or Microscope-Based Ratiometric Imaging. Fluorimeter-based Ca2+ flux analysis was performed as described with minor modifications (16). CAP- (Sigma) or anandamide- (Ana) (Sigma) induced Ca2+ flux was measured at 25°C, and CCL3- (PeproTech, Rocky Hill, NJ) induced responses were measured at 37°C. In sensitization experiments, CCR1:TRPV1/HEK293 cells were pretreated with CCL3 at 28°C for 20 min before being loaded into the Perkin–Elmer luminescence spectrometer for assay at 25°C. Microscope-based ratiometric analysis was performed as described with minor modifications (1). Primary neurons from rat DRG were cultured in eight-well chambered cover glass slides (Nunc) for 6–10 h. For sensitization experiments, CCL3 was added after loading with FURA-2-acetomethoxy ester (Molecular Probes). Pertussis toxin (PTX) and chelerythrine chloride at 5 μM were added during FURA-2 loading, and U73122 was added 5 min before CCL3. Ratiometric Ca2+ imaging was performed by using a Nikon Eclipse TE200 fluorescence microscope equipped with a variable filter wheel (Sutter Instruments, Novato, CA), a Spot charge-coupled device camera, and a Nikon S Fluor ×40 objective lens. Dual images (340- and 380-nm excitation, 510 emission) were collected by using openlab system 3.14 (Improvision, Lexington, MA), and pseudocolor ratiometric images were monitored every 4 sec. The statistical analysis of Ca2+ responses was performed by using prism 3.0 (GraphPad, San Diego) and ANOVA.
RT-PCR, Immunohistochemistry, and Confocal Imaging-Based PKC Translocation Analysis. Primary neurons from rat DRG were isolated as described (3). Total RNA was extracted by TRIzol (Invitrogen), and RT-PCR was performed by using the Gene-Amp kit (Roche Diagnostics) according to the manufacturer's protocol. Immunohistochemical staining of rat DRG tissue was performed as described with minor modifications (3). Briefly, male Sprague–Dawley rats were fixed by perfusion with 4% formalin, and tissues were isolated, postfixed in formalin, cryoprotected in 30% sucrose, embedded in OCT compound (Ted Pella, Inc., Redding, CA), frozen, and sectioned on a cryostat (14 μm). Slide-mounted sections were then stained with either rabbit anti-TRPV1 antibody [(18), 1:300] or rabbit anti-CCR1 antibody (H-52 from Santa Cruz Biotechnology, diluted 1:200), washed, and stained with Cyanine 3-conjugated anti-rabbit secondary antibody. Double staining of CCR1 and TRPV1 was achieved by staining two tissue sections separately with anti-CCR1 and anti-TRPV1 antisera. The same areas of the two stained slices were compared with map identical cells. After fluorescence, images were recorded on a Nikon Eclipse TE200 fluorescence microscope equipped with a charge-coupled device camera, and the slides were counterstained with hematoxylin to permit total neuron counting (Vector Laboratories; H3401). Cell sizes were analyzed by using openlab system 3.14 (Improvision). The fluorescence intensity of each TIFF image was analyzed by using photoshop 6.0 (Adobe Systems, San Jose, CA), and a cell with a median intensity over the threshold of 130 units was defined as a positive cell. Alternate sections were skipped to prevent double-counting of immunopositive neurons. Frequency was defined as the ratio of positive cells in one particular size group to the total number of positive cells. Primary neurons from rat DRG were cultured for 24 h. Cells were starved in serum-free medium for 3 h and subjected to PKC translocation analysis as described (16). Cell harvesting and tissue perfusions were conducted by using protocols approved by the National Cancer Institute.
Behavioral Assays. Two groups of eight mice (BALB/c/M; 24–28 g; JAX) were tested on a 54°C hot plate (Ugo Basile, Varese, Italy) according to the method of Dewey et al. (19). A baseline response latency was obtained for each animal after two conditioning runs. One group of mice was then injected s.c. into the midplantar area of the left hind paw with 250 ng of CCL3 (2.5 μl of 100 μg/ml CCL3 in PBS); control animals received 2.5 μl of PBS by the same route. Each mouse was retested on the hot plate at +15 min and thereafter at 15-min intervals up to +90 min by using either jumping or hind-paw licking as the nociceptive endpoint and 30 s as the cutoff point. Mean response latencies were subjected to an analysis of variance by using pharmtoolspro software (McCary, Elkins Park, PA). Post hoc comparisons were made by the Newman–Keuls tests at each time point, again by using the same software. This study was approved by the Temple University (Philadelphia) Institutional Animal Care and Use Committee.
Statistical Analysis. The comparison of ligand-induced activation of TRPV1 between nontreated and CCL3-pretreated cells was carried out by two-way ANOVA by using prism 3.0 software (Fig. 1 C and D). Ca2+ responses in Figs. 3 B and C and 4 A–C were plotted and analyzed by using prism 3.0 and Student's t test analysis. Hot-plate data mean-response latencies were subjected to ANOVA by using pharmtoolspro software (McCary). Post hoc comparisons were made by Newman–Keuls tests at each time point.
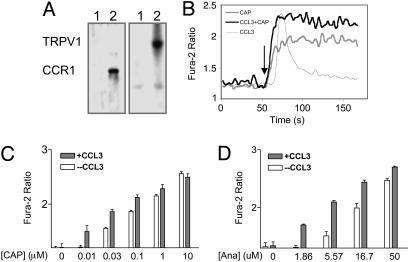
Fig. 1.
Activation of CCR1 enhances the Ca2+ ion channel activity of TRPV1 in CCR1:TRPV1/HEK293 cells. (A) Western blotting analysis of CCR1 and TRPV1 on CCR1:TRPV1/HEK293 cells. (Left) Probed with anti-CCR1 antibody; (Right) probed with anti-TRPV1 antibody. Lane 1 contained HEK cell lysates, and lane 2 contained CCR1:TRPV1/HEK293 cell lysates. (B) In CCR1:TRPV1/HEK293 cells, 100 ng/ml CCL3 induced a transient Ca2+ influx (thin gray line) CAP at 0.3 μM induced a more potent Ca2+ influx in cells pretreated with CCL3 at 100 ng (black line) than without CCL3 pretreatment (thick gray line). CAP or CCL3 was added at 50 s. (C) Dose-response curve of CAP-induced Ca2+ influx in the presence (dark bars) or absence (empty bars) of CCL3 pretreatment (n = 8), the error bar represents SEM. The data were analyzed by two-way ANOVA (P < 0.0286). (D) Dose–response curve of Ana-induced Ca2+ influx in the presence (dark bars) or absence (empty bars) of CCL3 pretreatment (n = 8) Error bars represent SEM. The data were analyzed by two-way ANOVA) (P < 0.0189).
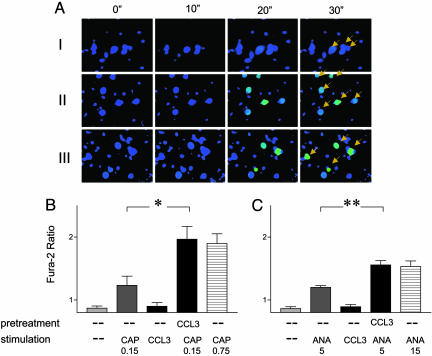
Fig. 3.
Pretreatment with CCL3 enhances the response of rat DRG neurons to CAP. (A) Time-lapse images from microscope-based ratiometric analysis of Ca2+ influxes. The green color reflects a high [Ca2+]i, whereas the blue color reflects low [Ca2+]i. CAP was added to the DRG neurons after the second sets of images. I, cells were stimulated with 0.15 μM CAP; II, cells were stimulated with 0.75 μM CAP; and III, cells were pretreated with 100 ng/ml CCL3 and then stimulated with 0.15 μM CAP. (B) Quantitative analysis of CCL3-induced sensitization of TRPV1 to CAP. Each bar represents an average of four experiments, including 8–20 responding cells. *, P < 0.01. (C) Quantitative analysis of CCL3-induced sensitization of TRPV1 to Ana. Each bar represents an average of four experiments, including 8–20 responding cells. **, P < 0.04.
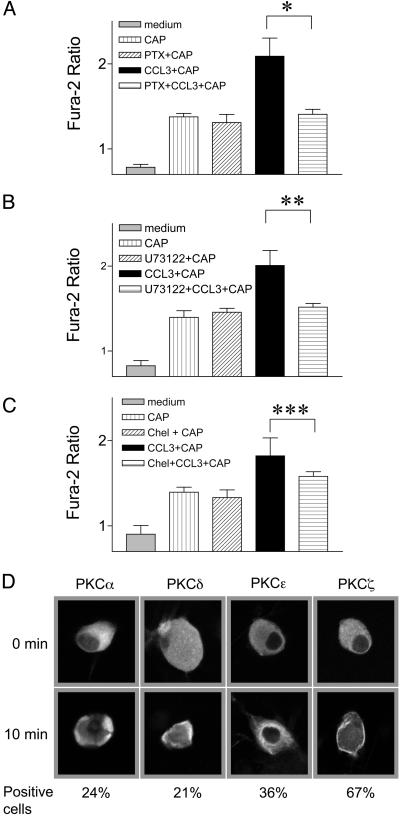
Fig. 4.
Molecular mechanism of CCL3-induced sensitization. (A) Pretreatment of DRG neurons with PTX impairs CCL3-induced sensitization of TRPV1. *, P < 0.011. (B) Pretreatment of DRG neurons with U73122 blocks CCL3-induced sensitization of TRPV1. **, P < 0.017. (C) Treatment with chelerythrine chloride impairs CCL3-induced sensitization of TRPV1. ***, P < 0.05. D CCL3 induces PKC translocation in a subpopulation of DRG neurons. All responses were evoked by using 0.15 μM CAP. Percent of cells exhibiting change in PKC isotype localization is indicated at bottom. Data in A–C were analyzed by using one-way ANOVA and are presented as mean ± SEM from four independent experiments.
Results
CCR1, one of the proinflammatory chemokine receptors, is expressed on activated T cells, monocytes, neutrophils, and eosinophils (20–23). We assessed the possibility that proinflammatory chemokine receptors may modulate TRPV1 function by cotransfecting HEK293 cells with CCR1 and TRPV1. The expression of TRPV1 and CCR1 proteins was confirmed by Western blotting analysis (Fig. 1 A). Bands of 95 kDa and 43 kDa, corresponding to TRPV1 and CCR1, respectively, appeared only in extracts from doubly transfected cells but not in those from control cells. When stimulated with CAP, doubly transfected cells responded with a persistent and concentration-dependent increase in [Ca2+]i, as indicated by an increase in the ratio of fura-2 emission at 340/380-nm excitation, demonstrating that TRPV1 in these cells is functional (Fig. 1 B and C). A TRPV1 antagonist, capsazepine, effectively inhibited CAP-induced Ca2+ influx in CCR1:TRPV1/HEK293 cells (Fig. 6, which is published as supporting information on the PNAS web site). CCL3 also induced a marked increase in [Ca2+]i, indicating that CCR1 in these cells was functional (Fig. 1B). In contrast to CAP-induced Ca2+ flux, the CCR1-induced Ca2+response was transient and rapidly adapting. Fig. 1B shows that, at 25°C, CAP at 0.3 μM elicited an increase in fura ratio from 1.25 ± 0.05 to 1.91 ± 0.09. When pretreated with CCL3 at 100 ng/ml for 20 min at 28°C, CCR1:TRPV1 cells responded to 0.3 μM CAP with a much higher change in fura ratio, reaching 2.31 ± 0.11 at 25°C (Fig. 1B). In fact, activation of CCR1 resulted in an increase in TRPV1 mediated Ca2+ influx and increased the sensitivity of TRPV1 to CAP by ≈3-fold (Fig. 1C) (P < 0.0286). The sensitization of TRPV1 by CCL3-CCR1 interaction was more evident when cells were stimulated with low concentrations of CAP (Fig. 1C). At saturating concentrations of CAP (10 μM), the sensitizing effect of CCL3 became undetectable. CCL3-induced sensitization of TRPV1 was further confirmed by an endogenous ligand of this channel, Ana. After CCL3 treatment, CCR1:TRPV1/HEK293 cells consistently responded to Ana at 1.86 μM with a clear persistent Ca2+ influx (Fig. 1D). [Ca2+]i in cells pretreated with CCL3 was significant higher in the presence of 5.57 or 16.7 μM Ana (P < 0.0189).
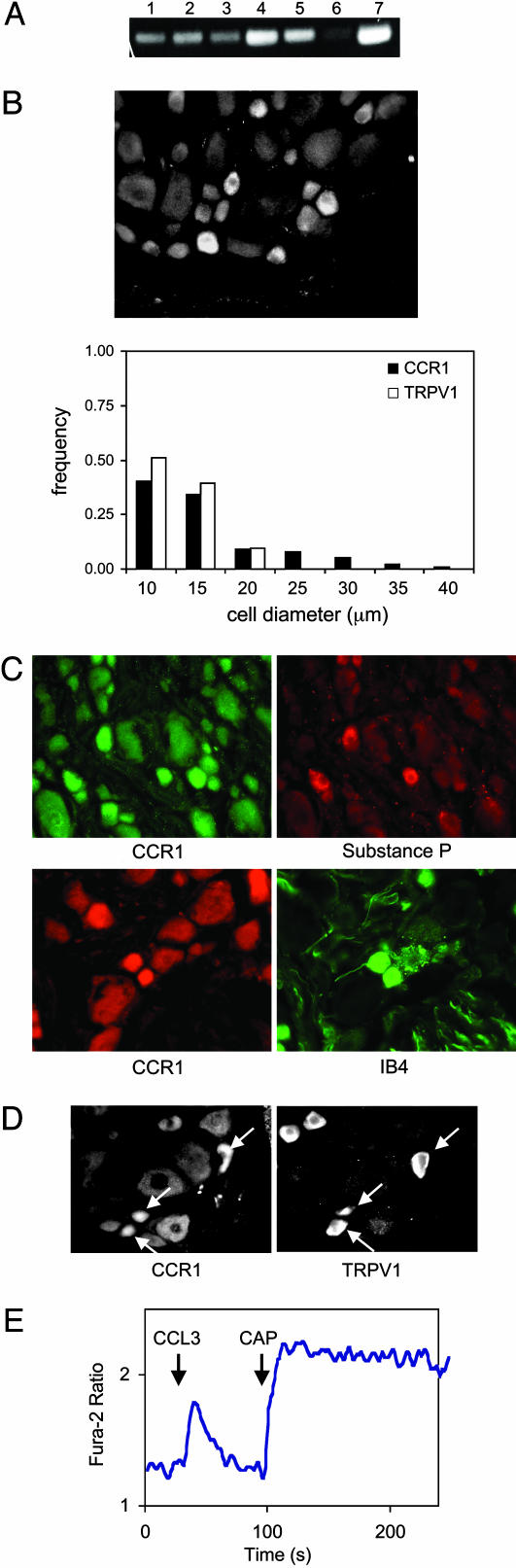
We next examined the possibility that chemokines might interact with TRPV1 in sensory neurons. RT-PCR analysis revealed significant expression of transcripts encoding IL1β receptors, TNF receptors, CCR5, CCR1, μ-opioid receptors, δ-opioid receptors, and TRPV1 in rat DRG (Fig. 2A). The expression of CCR1 in DRG was evaluated by using immunohistochemical staining (Fig. 2B). Among 344 cells analyzed, 62% were CCR1 positive. CCR1 immunostaining was apparently enriched in small- to medium-diameter neurons (Fig. 2B; see also Fig. 7, which is published as supporting information on the PNAS web site). Double staining of the same DRG sections revealed that all cells binding the isolectin IB4 (9/9) and all substance P immunoreactive cells (23/23) expressed CCR1, suggesting that nociceptive neurons expressed CCR1 (Fig. 2C). When two adjacent 14-μm tissue sections were examined, considerable overlap was detected in the expression of CCR1 and TRPV1 (Fig. 2D). The majority of TRPV1-positive neurons also expressed medium to high levels of CCR1. However, the subcellular expression pattern of CCR1 was not as polarized as that of TRPV1 (Fig. 2D).
Fig. 2.
Expression of functional CCR1 on TRPV1-positive DRG neuronal cells. (A) RT-PCR analysis of DRG (lane 1, IL1βR; lane 2, TNFR; lane 3, CCR5; lane 4, CCR1; lane 5, μ-opioid receptor; lane 6, δ-opioid receptor; and lane 7, TRPV1). (B) Immunohistochemical staining showing that CCR1 is preferentially expressed on small-diameter DRG neurons. Size-frequency distribution of CCR1- and TRPV1-immunoreactive neurons, derived from 10 nonconsecutive sections from one animal is at bottom. (C) Immunofluorescent costaining of CCR1 with substance P or FITC-conjugated IB4 on the same DRG section. (D) Immunohistochemical analysis of two adjacent tissue slices. (Left) Stained with anti-CCR1 antibody. (Right) Stained with anti-TRPV1. Both sections were probed with Cys-3-conjugated anti-rabbit secondary antibodies. (E) Microscope-based analysis of Ca2+ influx in a DRG neuron treated with 100 ng/ml CCL3 followed by 0.3 μM CAP.
We next evaluated the functional consequences of CCR1 and TRPV1 activation in primary DRG neurons. Both CAP and CCL3 were capable of eliciting Ca2+ flux in these cells, as measured by microscopic ratiometric imaging. Moreover, in 24 ± 5% cases, CCL3 induced Ca2+ flux in CAP-responding cells, indicative of a high expression level of chemokine receptors on these neurons (Fig. 2E). Because CCL3 interacts with three chemokine receptors, it is possible that some of the CCL3-induced Ca2+ influx is mediated by CCR5 or -3 on DRG cells. Whereas CAP evoked a prolonged increase in intracellular [Ca2+]i, the response to CCL3 was transient. To assess the capacity of CCL3 to sensitize TRPV1 in sensory neurons, we compared responses to CAP with and without CCL3 pretreatment. As shown in Fig. 3A Top, 42% of DRG neurons responded to 0.15 μM CAP with a low level of Ca2+ flux. Furthermore, 10 μM capsazepine blocked this activation (data not shown). The magnitude of Ca2+ flux in DRG neurons evoked by CAP was dose-dependent, as indicated by a greater Ca2+ flux induced by CAP at 0.75 μM (Fig. 3A Middle). Primary neurons preincubated with CCL3 for 20 min at 28°C responded to 0.15 μM CAP at 25°C with a robust Ca2+ flux, similar to that of naïve cells stimulated with 0.75 μM CAP (Fig. 3A Bottom). We did not detect a significant alteration in the percentage of CAP-responding neurons after CCL3 treatment. A quantitative comparison of CCL3-induced sensitization effects is presented in Fig. 3B. We also detected a slight enhancement of basal [Ca2+]i after CCL3 pretreatment, but the magnitude of sensitization was much greater than the increase in basal [Ca2+]i. Pretreatment with CCL3 produced no enhancement of the Ca2+ response to 10 μM CAP, indicating that the sensitization effects of CCL3 are more prominent at lower CAP concentrations. Pretreatment of neurons with CCL5 and CXCL8 resulted in similar enhanced response to CAP (Fig. 8A, which is published as supporting information on the PNAS web site). Furthermore, a combination of CCL3 and CXCL8 appeared to further enhance CAP-induced Ca2+ influx, suggesting that the presence of multiple chemokines at inflammatory sites may exacerbate nociception (Fig. 8A). The sensitization of TRPV1 was further examined by using an endogenous activator of this channel, Ana. Ana at 5 μM induced a modest Ca2+ response (Fig. 3C). Pretreatment with 100 ng/ml CCL3 for 20 min significantly enhanced [Ca2+]i to a level similar to that induced by 15 μM Ana. These results clearly indicated that chemokine treatments increased the sensitivity of native TRPV1.
We further assessed the signal transduction components that mediate the chemokine sensitization of TRPV1. Pretreatment of DRG neurons with PTX, a potent inhibitor of Gi protein, at 150 ng/ml for 90 min, did not affect CAP-induced Ca2+ flux, but abolished the sensitizing effects of CCL3 (P < 0.011) (Fig. 4A). Pretreatment of DRG neurons with 10 μM U73122, an inhibitor of PLC, also blocked the effects of CCL3 (Fig. 4B). Phosphorylation of TRPV1 by PKC has been reported to sensitize CAP-induced Ca2+ flux (9). As shown in Fig. 4D, CCL3 elicited the translocation of PKC α, δ, and ζ to the plasma membrane and PKCε to an intracellular compartment, indicating that CCL3 was capable of activating PKC in neurons. When primary neurons were treated with a potent PKC inhibitor, chelerythrine chloride, at 5 μM, the sensitizing effects of CCL3 were impaired (Fig. 4C, P < 0.035). These data suggest that CCL3 exerts its effects through a G protein-dependent PLC- and PKC-mediated signaling pathway.
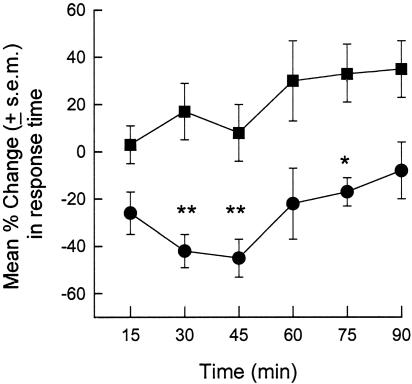
Finally, we examined the in vivo sensitizing effects of CCL3 in the mouse hot-plate test. The mean baseline latency for BALB/c/M mice to withdraw their paws from a 54°C hot plate was 17.9 ± 0.94 s (CCL3 group) and 17.5 ± 0.81 s (controls). Intraplantar injection of vehicle (PBS) induced a slight increase (maximum = 34.3 ± 13.5% at +90 min) in latency. In contrast, 41.2 ± 7.3% and 42.9 ± 8.6% decreases in hind-paw withdrawal latency occurred at +30 and +45 min, respectively, when mice were injected with 250 ng of CCL3 (Fig. 5). This effect subsided within 90 min. These in vivo hyperalgesic effects of CCL3 are consistent with our in vitro observations that CCL3 treatment enhances the sensitivity of TRPV1.
Fig. 5.
Intraplantar CCL3 enhances hot-plate sensitivity in the mouse. Change in hot-plate (54°C) escape latency is plotted at the indicated times after intraplantar injection of one hind paw with saline (filled squares) or CCL3 (250 ng, filled circles) (n = 8 mice per group) *, P < 0.05, **, P < 0.01, ANOVA followed by the Newman–Keuls test at each time point.
Discussion
Our data indicate that activation of CCR1 on DRG neurons is capable of enhancing the sensitivity of TRPV1 through a G protein-dependent signaling pathway. Previous studies have shown that CCL5, a ligand for CCR1, CCR3, CCR5, and CCR9, is capable of inducing increased [Ca2+]i in DRG neurons (15). The immunohistochemical analysis presented here clearly reveals that TRPV1-positive cells express CCR1. Furthermore, the responsiveness of sensory neurons to CCR1 was confirmed by Ca2+ flux and PKC translocation in the presence of CCL3, a chemokine that preferentially binds CCR1. Chemokine receptors regulate immune responses by mediating cell trafficking, differentiation, and angiogenesis. In the nervous system, chemokine receptors have been implicated in development and inflammation. Our finding that CCL3 sensitizes TRPV1-mediated signaling suggests a role for chemokine receptors, namely translating inf lammatory signals into nociceptor sensitization.
Chemokine receptors activate both G protein-dependent and independent pathways. We investigated the molecular mechanism of the CCR1-mediated sensitization process by using PTX, U73122, and chelerythrine chloride. That pretreatment with PTX blocks CCR1-mediated sensitization suggests that Gi or a related G protein is necessary. Our data further suggest the involvement of both PLC and PKC, downstream components of Gi signaling pathways. Previous studies have shown that both Ca2+-dependent and independent PKC isotypes are capable of sensitizing TRPV1 (10, 11). Thus, we propose a simple model to interpret the molecular mechanism of chemokine receptor-mediated sensitization of TRPV1. Accumulation of proinflammatory chemokines activates Gi-coupled chemokine receptors present on the neuronal cell surface. The consequent activation of PLC may then sensitize TRPV1 through PKC phosphorylation by the removal of a phospholipid inhibitor or by the production of an endogenous ligand (8, 11).
Our data provide a mechanism to explain how chemokines can induce hyperalgesia. Previous studies have identified a variety of inflammatory mediators, including BK, substance P, NGF, and prostaglandins, as being capable of enhancing pain sensation. However, the potential capacity of chemokines to sensitize peripheral pain receptors has not previously been well studied. Oh et al. (15) have shown that chemokines are capable of inducing transient calcium fluxes in certain DRG neurons, suggesting that such calcium responses may directly contribute to the sensation of pain. Abbadie et al. (24) showed that ccr2–/– mice exhibited an impaired neuropathic pain response but attributed this deficit to the lack of recruitment of CCR2-positive monocytes, macrophages, and microglia to injured nerve tissue (24). Our data clearly show that TRPV1 and CCR1 are coexpressed on small- to medium-diameter neurons in DRG, and that activation of CCR1 directly sensitizes TRPV1 through a G protein PKC- and PLC-dependent pathway in primary neurons.
In view of the fact that multiple chemokines accumulate during inflammation, it is surprising that CCL3 is sufficient to produce both in vitro effects on TRPV1 and in vivo effects on the sensation of pain. Indeed, our preliminary in vitro assays showed that a combination of CCL3 and CXCL8 induced a stronger sensitization of TRPV1 than either agent alone. Consequently, chemokines secreted in the course of inflammation appear to enhance the perception of pain by desensitization not only of central (CNS) opioid receptors (14) but also of peripheral transducers of noxious stimuli, such as TRPV1. We cannot determine the relative contribution of these two mechanisms at present. Presumably, both contribute to the increased perception of pain. The use of knockout mice may permit a clarification of this issue.
Supplementary Material
Acknowledgments
We thank Dr. O. M. Zack Howard for inspiring discussions and Mr. Edward Cho and Ali Deniz Guler for assistance with techniques. The research is supported by National Institutes of Health Grants T32DA07237 (to A.C.), DA-06650 (to A.C. and T.J.R.), DA14230 (to T.J.R.), DA-16544 (to T.J.R.), and DA-13429 (to A.C. and T.J.R.).
Author contributions: N.Z., S.I, A.C., R.S., T.J.R., M.C., and J.J.O. designed research; N.Z., S.I., A.C., R.S., J.M.W., T.J.R., and M.C. performed research; N.Z., A.C., J.M.W., T.J.R., M.C, and J.J.O. analyzed data; and N.Z., A.C., T.J.R., M.C., and J.J.O. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: TRPV1, transient receptor potential vanilloid 1; CAP, capsaicin; DRG, dorsal root ganglia; PLC, phospholipase C; Ana, anandamide; PTX, pertussis toxin.
References
- 1.Caterina, M. J., Schumacher, M. A., Tominaga, M., Rosen, T. A., Levine, J. D. & Julius, D. (1997) Nature 389, 816–824. [DOI] [PubMed] [Google Scholar]
- 2.Tominaga, M., Caterina, M. J., Malmberg, A. B., Rosen, T. A., Gilbert, H., Skinner, K., Raumann, B. E., Basbaum, A. I. & Julius, D. (1998) Neuron 21, 531–543. [DOI] [PubMed] [Google Scholar]
- 3.Caterina, M. J., Leffler, A., Malmberg, A. B., Martin, W. J., Trafton, J., Petersen-Zeitz, K. R., Koltzenburg, M., Basbaum, A. I. & Julius, D. (2000) Science 288, 306–313. [DOI] [PubMed] [Google Scholar]
- 4.Caterina, M. J. & Julius, D. (1999) Curr. Opin. Neurobiol. 9, 525–530. [DOI] [PubMed] [Google Scholar]
- 5.Bhave, G., Zhu, W., Wang, H., Brasier, D. J., Oxford, G. S. & Gereau, R. W. (2002) Neuron 35, 721–731. [DOI] [PubMed] [Google Scholar]
- 6.Hu, H. J., Bhave, G. & Gereau, R. W. (2002) J. Neurosci. 22, 7444–7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin, J., Cho, H., Hwang, S. W., Jung, J., Shin, C. Y., Lee, S. Y., Kim, S. H., Lee, M. G., Choi, Y. H., Kim, J., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 10150–10155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chuang, H. H., Prescott, E. D., Kong, H., Shields, S., Jordt, S. E., Basbaum, A. I., Chao, M. V. & Julius, D. (2001) Nature 411, 957–962. [DOI] [PubMed] [Google Scholar]
- 9.Vellani, V., Mapplebeck, S., Moriondo, A., Davis, J. B. & McNaughton, P. A. (2001) J. Physiol. 534, 813–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khasar, S. G., Lin, Y. H., Martin, A., Dadgar, J., McMahon, T., Wang, D., Hundle, B., Aley, K. O., Isenberg, W., McCarter, G., et al. (1999) Neuron 24, 253–260. [DOI] [PubMed] [Google Scholar]
- 11.Crandall, M., Kwash, J., Yu, W. & White, G. (2002) Pain 98, 109–117. [DOI] [PubMed] [Google Scholar]
- 12.Saeki, T. & Naya, A. (2003) Curr. Pharm. Des 9, 1201–1208. [DOI] [PubMed] [Google Scholar]
- 13.Menten, P., Wuyts, A. & Van Damme, J. (2002) Cytokine Growth Factor Rev. 13, 455–481. [DOI] [PubMed] [Google Scholar]
- 14.Szabo, I., Chen, X. H., Xin, L., Adler, M. W., Howard, O. M., Oppenheim, J. J. & Rogers, T. J. (2002) Proc. Natl. Acad. Sci. USA 99, 10276–10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oh, S. B., Tran, P. B., Gillard, S. E., Hurley, R. W., Hammond, D. L. & Miller, R. J. (2001) J. Neurosci. 21, 5027–5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang, N., Hodge, D., Rogers, T. J. & Oppenheim, J. J. (2003) J. Biol. Chem. 278, 12729–12736. [DOI] [PubMed] [Google Scholar]
- 17.Zhang, N., Long, Y. & Devreotes, P. N. (2001) Mol. Biol. Cell 12, 3204–3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tominaga, M., Wada, M. & Masu, M. (2001) Proc. Natl. Acad. Sci. USA 98, 6951–6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dewey, W. L., Harris, L. S., Howes, J. F. & Nuite, J. A. (1970) J. Pharmacol. Exp. Ther. 175, 435–442. [PubMed] [Google Scholar]
- 20.Broxmeyer, H. E., Cooper, S., Hangoc, G., Gao, J. L. & Murphy, P. M. (1999) J. Exp. Med. 189, 1987–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Domachowske, J. B., Bonville, C. A., Gao, J. L., Murphy, P. M., Easton, A. J. & Rosenberg, H. F. (2000) J. Immunol. 165, 2677–2682. [DOI] [PubMed] [Google Scholar]
- 22.Khan, I. A., Murphy, P. M., Casciotti, L., Schwartzman, J. D., Collins, J., Gao, J. L. & Yeaman, G. R. (2001) J. Immunol. 166, 1930–1937. [DOI] [PubMed] [Google Scholar]
- 23.Shang, X., Qiu, B., Frait, K. A., Hu, J. S., Sonstein, J., Curtis, J. L., Lu, B., Gerard, C. & Chensue, S. W. (2000) Am. J. Pathol. 157, 2055–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abbadie, C., Lindia, J. A., Cumiskey, A. M., Peterson, L. B., Mudgett, J. S., Bayne, E. K., DeMartino, J. A., MacIntyre, D. E. & Forrest, M. J. (2003) Proc. Natl. Acad. Sci. USA 100, 7947–7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.