Abstract
IMPORTANCE
Human papillomavirus (HPV)-related oropharyngeal squamous cell carcinoma (OPSCC) has a favorable prognosis, and p16 immunohistochemistry is a surrogate marker of high-risk HPV infection and strong prognosticator. Given this favorable prognosis, treatment de-escalation for p16-positive OPSCC is now being considered with the goal of decreasing treatment-associated morbidity without compromising tumor control. The role of adjuvant chemotherapy in this setting is becoming increasingly unclear.
OBJECTIVE
To compare survival between surgically managed patients with p16-positive OPSCC who received adjuvant chemoradiotherapy and patients who received adjuvant radiotherapy alone.
DESIGN, SETTING, AND PARTICIPANTS
This was a cohort study of patients with OPSCC diagnosed from June 1996 to June 2010, with follow-up through December 2014, at a single tertiary referral center. One hundred ninety-five surgically managed, p16-positive patients without a history of head and neck cancer or distant metastasis at time of diagnosis were included.
EXPOSURES
Patients were dichotomized into adjuvant radiotherapy and adjuvant chemoradiotherapy groups.
MAIN OUTCOMES AND MEASURES
Overall survival was the primary outcome, and disease-free survival was the secondary outcome. Propensity-weighted multivariate Cox proportional hazards analysis was conducted to quantify the effect of adjuvant chemotherapy on survival.
RESULTS
The study included 195 patients with p16-positive, surgically managed OPSCC. Median duration of follow-up was 87 months (interquartile range, 68–116 months). Ninety patients received adjunct chemoradiotherapy (mean age, 54.3 years), 88 patients received adjuvant radiotherapy (mean age, 56.4 years), and 17 patients received surgery alone. The 5-year overall survival rate for patients who received adjuvant chemoradiotherapy was 82% (95% CI, 73%–90%) and 84% (95% CI, 76%–91%) for patients who received adjuvant radiotherapy alone. The 5-year disease-free survival rate for patients who received adjuvant chemoradiotherapy was 79% (95% CI, 71%–88%) and 79% (95% CI, 70%–88%) for patients who received radiotherapy alone. After weighting cases by the inverse probability of receiving adjuvant chemotherapy and controlling for age, comorbidity, smoking, pathological T stage, and pathological N stage, the receipt of adjuvant chemotherapy was not significantly associated with disease-free survival (adjusted hazard ratio, 0.91; 95% CI, 0.59–1.42) but was associated with a statistically insignificant yet clinically meaningful increase in all-cause mortality (adjusted hazard ratio, 1.46; 95% CI, 0.91–2.33).
CONCLUSIONS AND RELEVANCE
Among patients with p16-positive OPSCC managed surgically with adjuvant radiotherapy, the addition of adjuvant chemotherapy provided no additional disease-free survival benefit and was associated with worse overall survival. These results should help inform future clinical trials aiming to deescalate treatment for p16-positive patients.
In the past 30 years, human papillomavirus (HPV) has been identified as a causative agent for a subset of oropharyngeal squamous cell carcinomas (OPSCCs).1,2 The prevalence of HPV-related OPSCC has risen dramatically over this time, and HPV-positive OPSCC now accounts for the most cases of OPSCC in the United States.3–6 HPV-positive OPSCC has a characteristic biological, pathological, and clinical profile with distinct risk factors.1,2,7–9 Importantly, HPV-positive OPSCC has a markedly improved prognosis compared with HPV negative OPSCC.1,10–13 Given the unique biology of HPV related tumors, p16 immunohistochemistry can be used as a surrogate marker of active, high-risk HPV infection because p16 is markedly overexpressed in tumor cells actively expressing HPVE6.14,15 p16 status is highly correlated with HPV status and has been shown to be a strong prognostic factor.13
Although HPV-positive OPSCC has proven to be a distinct disease entity with a superior prognosis, current OPSCC treatment protocols do not consider HPV status.16 Traditionally, OPSCC has required aggressive treatment that is associated with high morbidity and poor functional and quality-of-life outcomes.17 With the advent of transoral surgical approaches, intensity-modulated radiotherapy, and targeted systemic agents, such as cetuximab, treatment-associated morbidity has decreased but remains substantial. Given the favorable prognosis of HPV-positive OPSCC, many investigators have called for a deintensification of treatment for HPV-positive OPSCC with the goal of further minimizing treatment-associated morbidity without compromising tumor control.18 Many treatment de-escalation strategies have been proposed, including eliminating chemotherapy for selected patients, replacing platinum based agents with cetuximab, reducing the total radiation dose, and using a single modality approach, but currently there is no consensus on an optimal de-escalation strategy.19 The role of adjuvant chemotherapy in HPV-positive OPSCC has become particularly unclear, and few studies have adequately investigated the utility of chemotherapy in this setting.
In the present study, we evaluated the effect of adjuvant chemotherapy on survival in a cohort of surgically managed patients with p16-positive OPSCC. Patients who received adjuvant chemoradiotherapy were compared with patients who received adjuvant radiotherapy alone. We hypothesized that the addition of chemotherapy to the treatment regimen would not provide an overall survival (OS) or disease-free survival (DFS) benefit.
Methods
Patient Population
We assembled a cohort of patients with OPSCC diagnosed and treated at Barnes Jewish Hospital in St Louis, Missouri, between June 1996 and June 2010. Inclusion criteria consisted of pathologically confirmed, surgically managed p16- positive OPSCC. p16 Immunohistochemical analysis was conducted retrospectively by a single pathologist (J.S.L.) blinded to patient clinical characteristics and outcome following standard protocols as previously described.20 Patients with distant metastasis at the time of presentation or with a history of head and neck cancer were excluded. Patients were not compensated and did not provide written informed consent. This study was approved by Washington University School of Medicine’s Human Research Protection Office.
Data Acquisition
The Oncology Data Services tumor registry was used to obtain prospectively gathered demographic, clinicopathological, and treatment data. The electronic medical record was used to investigate and resolve any missing values or discrepancies. Vital status was updated through December 2014 using the electronic medical record, and the date of death was confirmed using the Social Security Death Index. Patients with missing values did not differ from patients with complete data in either OS or DFS. Since racial disparities exist in survival of head and neck cancer,21 we included patient-reported race in our analysis and classified it as white or nonwhite. Smoking history was assessed at the time of diagnosis. Patients who were current smokers or with any history of smoking were considered smokers for our analysis. Comorbid illness at the time of diagnosis was documented using the Adult Comorbidity Evaluation–2722 and was dichotomized in our analysis as “none to mild” vs “moderate to severe” comorbidity. Pathological tumor, nodal, and overall stage was recorded from surgical pathology reports in accordance with the American Joint Committee on Cancer Staging Manual, 7th edition, criteria.23 When pathology specimens were unavailable or insufficient, stage was determined using imaging, endoscopy, and physical examination findings.
Treatment
All patients received surgical treatment of the primary site consisting of local tumor excision, partial pharyngectomy, total pharyngectomy, or laryngopharyngectomy. Neck dissection was performed in patients with nodal metastases or those who desired elective dissection. Radiotherapy included conventional external beam radiation therapy or intensity-modulated radiation therapy. Chemotherapeutic regimens consisted of platinum- based agents, taxanes, or cetuximab administered either alone or in combination. Adjuvant radiotherapy or chemoradiotherapy was provided based on the recommendation of a multidisciplinary tumor board that considered the extent of nodal disease, the presence of extracapsular spread, margin positivity, performance status, metabolic criteria, and patient preference. When adjuvant chemoradiotherapy was indicated, it was administered concurrently. For our primary comparison, patients were grouped into adjuvant radiotherapy and adjuvant chemoradiotherapy groups.
Outcomes
Overall survival was the primary outcome measure and was defined as the duration from diagnosis to death from any cause. Disease-free survival was the secondary outcome measure and was defined as the duration from diagnosis to either death from any cause or the first documented recurrence. Recurrences were classified as either locoregional failure or distant metastasis and were considered only in patients who were declared disease-free following initial treatment. Patients who were never disease-free were not classified as having had a recurrence.
Statistical Analysis
Standard descriptive statistics were used to describe the cohort. Differences between treatment groups were explored using χ2 test or Fisher exact test for categorical data and independent samples t test for continuous data. The Kaplan- Meier method with the log-rank test and Cox proportional hazards (PH) analysis were used for univariate survival analysis. To account for differences between treatment groups at baseline, propensity scores were generated using binary logistic regression with the receipt of adjuvant chemotherapy as the dependent variable. Age, sex, race, comorbidity, smoking, pathological T stage, and pathological N stage were used for propensity score estimation. Patients were then weighted according to the inverse probability of receiving adjuvant chemotherapy, and following weighting, covariate balance was assessed between treatment groups. Multivariate, propensity-weighted Cox PH analysis was conducted to evaluate the independent effect of adjuvant chemotherapy on survival. The PH assumption was tested for all variables using log-minus-log plots, and variables significant at the α level of 0.1 in univariate analysis were included in the multivariate model. The effect of adjuvant chemotherapy on survival was presented as an adjusted hazard ratio (aHR) with 95% CI. Following our primary analysis, a sensitivity analysis was performed to determine the effect of a hypothetical unmeasured binary confounder on the aHR for adjuvant chemotherapy. WinPepi software (version 11.43) was used for sensitivity analysis,24 and SPSS software (version 22.0; SPSS Inc) was used for all other analyses. All tests of statistical significance were evaluated at the 2-sided α level of .05.
Results
Description of the Cohort
Our cohort consisted of 195 patients with p16-positive, surgically managed OPSCC. The median duration of follow-up among surviving patients was 87 months (interquartile range, 68–116 months). Ninety patients received adjuvant chemoradiotherapy, 88 patients received adjuvant radiotherapy, and 17 patients received surgery alone. The 17 patients who received surgery alone did not differ from patients who received adjuvant therapy in age, sex, race, comorbidity, smoking history, or pathological T stage but did differ in pathological N stage. Patients with stage N2B or greater nodal disease were more than 3 times as likely to receive adjuvant therapy (odds ratio [OR], 3.3; 95% CI, 1.2–9.3]). The 17 patients who received surgery alone were excluded from analyses comparing patients who received adjuvant radiotherapy vs adjuvant chemoradiotherapy. Baseline characteristics of the cohort stratified by adjuvant therapy modality are shown in Table 1. Nodal stage and overall stage were the only variables statistically significantly associated with adjuvant therapy modality; patients with stage N2B or greater nodal disease and patients with overall stage IV disease were significantly more likely to receive adjuvant chemoradiotherapy than radiotherapy alone (OR, 4.92; 95% CI, 2.51–9.66, and OR, 3.42; 95% CI, 1.43–8.17, respectively). Vital status at last follow-up and the number of documented recurrences in each treatment group are shown in Table 2.
Table 1.
Characteristics of the Cohort Stratified by Adjuvant Therapy Modality
| Characteristic | Adjuvant, No. (%) | |
|---|---|---|
| RT (n = 88) | CRT(n = 90) | |
| Age, mean (SD), y | 56.4 (9.2) | 54.3 (8.0) |
| Sex | ||
| Male | 79 (90) | 81 (90) |
| Female | 9 (10) | 9 (10) |
| Race | ||
| White | 87 (99) | 83 (92) |
| Nonwhite | 1 (1) | 7 (8) |
| Comorbidity | ||
| None | 41 (47) | 37 (41) |
| Mild | 34 (39) | 37 (41) |
| Moderate | 10 (11) | 10 (11) |
| Severe | 0 (0) | 6 (7) |
| Unknown | 3 (3) | 0 (0) |
| Smoking | ||
| Yes | 52 (59) | 58 (65) |
| No | 29 (33) | 29 (32) |
| Unknown | 7 (8) | 3 (3) |
| Pathological T stage | ||
| T1 | 33 (38) | 32 (36) |
| T2 | 37 (42) | 35 (39) |
| T3 | 8 (9) | 14 (15) |
| T4 | 8 (9) | 9 (10) |
| Unknown | 2 (2) | 0 (0) |
| Pathological N stage | ||
| N0 | 7 (8) | 0 (0) |
| N1 | 20 (23) | 8 (9) |
| N2A | 20 (23) | 9 (10) |
| N2B | 34 (38) | 46 (51) |
| N2C | 5 (6) | 22 (24) |
| N3 | 2 (2) | 5 (6) |
| Pathological overall stage | ||
| I, II | 5 (6) | 0 (0) |
| III | 17 (19) | 8 (9) |
| IV | 66 (75) | 82 (91) |
Abbreviations: CRT, chemoradiotherapy; RT, radiotherapy.
Table 2.
Vital Status and Documented Recurrences Stratified by Adjuvant Therapy Modality
| Characteristic | Adjuvant, No. (%) | Difference, % (95% CI)a | |
|---|---|---|---|
| RT (n = 88) | CRT (n = 90) | ||
| Vital status | |||
| Alive | 64 (73) | 70 (78) 5 | (−8 to 18) |
| Dead | 24 (27) | 20 (22) | |
| Locoregional failure | |||
| No | 86 (98) | 89 (99) | 1 (−4 to 7) |
| Yes | 2 (2) | 1 (1) | |
| Distant metastasis | |||
| No | 83 (94) | 82 (91) | 3 (−5 to 12) |
| Yes | 5 (6) | 8 (9) | |
Abbreviations: CRT, chemoradiotherapy; RT, radiotherapy.
Difference between adjuvant treatment groups.
Effect of Baseline Characteristics on Survival
The results of univariate and multivariate survival analyses are reported in Table 3. In univariate OS and DFS analyses, age, smoking, pathological T stage, and pathological N stage were significant prognosticators. After controlling for other variables, both age and smoking remained significant independent prognosticators in multivariate OS and DFS analyses. Pathological nodal stage N2C-N3 was not statistically significantly associated with OS in multivariate analysis (aHR, 1.25; 95% CI, 0.75–2.08) but was an independent statistically significant prognostic factor in DFS multivariate analysis (aHR, 2.13;95% CI, 1.34–3.40). Patients with moderate to severe comorbidity had a 1.9- fold increased risk of death from any cause (HR, 1.93; 95% CI, 0.99–3.79) and 1.9-fold increased risk of either death from any cause or recurrence (HR, 1.85; 95% CI, 0.98–3.51) in univariate analyses; however, these relationships vanished after controlling for other variables in multivariate analysis.
Table 3.
Univariate and Inverse Probability-Weighted Multivariate Survival Analysis
| Survival | Analysis | |
|---|---|---|
| Univariate, HR (95% CI) | Multivariate, aHR (95% CI)a | |
| Overall Survival | ||
| Age: continuous | 1.06 (1.03–1.10) | 1.06 (1.03–1.09) |
| Sex: male vs female | 2.03 (0.63–6.55) | |
| Race: white vs nonwhite | 1.12 (0.27–4.62) | |
| Comorbidity: Moderate to severe vs none to mild | 1.93 (0.99–3.79) | 0.92 (0.52–1.62) |
| Smoking: yes vs no | 4.30 (1.82–10.14) | 4.02 (1.94–8.30) |
| Pathological | ||
| T Stage:T3-T4 vs T1-T2 | 3.04 (1.70–5.43) | 1.38 (0.80–2.37) |
| N Stage: N2C-N3 vs N0-N2B | 2.06 (1.11–3.84) | 1.25 (0.75–2.08) |
| Adjuvant therapy: CRT vs RT | 1.05 (0.57–1.93) | 1.46 (0.91–2.33) |
| Disease-Free Survival | ||
| Age: continuous | 1.05 (1.02–1.08) | 1.04 (1.01–1.06) |
| Sex: male vs female | 1.81 (0.65–4.99) | |
| Race: white vs nonwhite | 1.37 (0.34–5.64) | |
| Comorbidity: Moderate to severe vs none to mild | 1.85 (0.98–3.51) | 0.77 (0.44–1.36) |
| Smoking: yes vs no | 3.42 (1.61–7.26) | 5.24 (2.55–10.78) |
| Pathological | ||
| T Stage: T3, T4 vs T1, T2 | 2.55 (1.47–4.43) | 1.31 (0.78–2.20) |
| N Stage: N2C-N3 vs N0-N2B | 1.87 (1.03–3.37) | 2.13 (1.34–3.40) |
| Adjuvant therapy: CRT vs RT | 0.93 (0.52–1.66) | 0.91 (0.59–1.42) |
Abbreviations: aHR, adjusted hazard ratio; CRT, chemoradiotherapy; HR, hazard ratio; RT, radiotherapy.
Inverse probability-weighted multivariate model included all variables significant in univariate analysis at the α level of 0.1 and adjuvant chemotherapy.
Effect of Adjuvant Chemotherapy on Survival
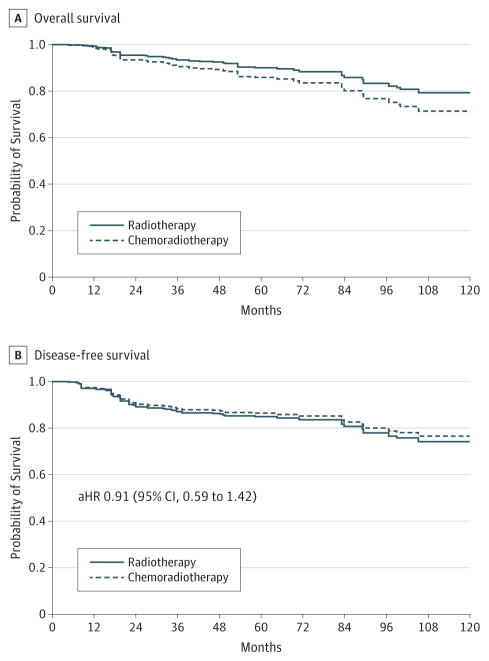
The 5-year OS rate for patients who received adjuvant chemoradiotherapy was 82% ( 95% CI, 73%–90%)and for patients who received adjuvant radiotherapy alone was 84% (95% CI, 76%–91%). Likewise, the 5-year DFS rate for patients who received adjuvant chemoradiotherapy was 79% (95% CI, 71%–88%) and for patients who received radiotherapy alone was 79% (95% CI, 70%–88%). After weighting cases by the inverse probability of receiving adjuvant chemotherapy and controlling for age, comorbidity, smoking, pathological T stage, and pathological N stage, the receipt of adjuvant chemotherapy was not associated with DFS (aHR, 0.91; 95% CI, 0.59–1.42) but was associated with a statistically insignificant yet clinically meaningful 46%increased risk of death from any cause (aHR, 1.46; 95% CI, 0.91–2.33). Inverse probability-weighted, adjusted survival curves illustrating the effect of adjuvant chemotherapy on survival are shown in the Figure.
Figure. Inverse Probability-Weighted, Adjusted Survival Curves for Adjuvant Therapy.
Survival curves are inverse probability-weighted and adjusted for age, comorbidity, smoking, pathological T stage, and pathological N stage. Adjusted hazard ratio, 1.46; 95% CI, 0.91–2.33. Because this figure is for a predicted model, numbers at risk at not presented.
The weighted, aHRs quantifying the relationship between adjuvant chemotherapy and OS and DFS were further adjusted in a sensitivity analysis of a hypothetical unmeasured binary confounder. The results of this sensitivity analysis are shown in Table 4. This analysis demonstrates the extent of confounding by an unmeasured variable that would have to exist to explain our results if adjuvant chemotherapy actually provides a significant survival benefit. For example, for adjuvant chemotherapy to significantly improve OS, an unmeasured binary confounder must have a hazard ratio (HR) for OS greater than 2 and must be substantially more prevalent in the adjuvant chemoradiotherapy group. For adjuvant chemotherapy to significantly improve DFS, an unmeasured binary confounder that has an HR for DFS of 2 must be approximately 50% more prevalent in the adjuvant chemoradiotherapy group.
Table 4.
The Effect of a Hypothetical Unmeasured Binary Confounder on the Adjuvant Chemotherapy Hazard Ratio
| UBC Prevalence, % | UBC HR for Overall Survival | Adjuvant Chemotherapy, HR (95% CI) Adjusted for UBCa | |
|---|---|---|---|
| CRT Group | RT Group | ||
| 50 | 0 | 4.00 | 0.58 (0.36–0.93) |
| 90 | 10 | 4.00 | 0.51 (0.32–0.82) |
| 50 | 0 | 3.00 | 0.73 (0.46–1.17) |
| 70 | 0 | 3.00 | 0.61 (0.38–0.97) |
| 90 | 10 | 3.00 | 0.63 (0.39–1.00) |
| 100 | 0 | 2.00 | 0.73 (0.46–1.17) |
| CRT Group | RT Group | UBC HR for Disease-Free Survival | Adjuvant Chemotherapy, HR (95% CI) Adjusted for UBCb |
| 20 | 0 | 3.00 | 0.65 (0.42–1.01) |
| 30 | 0 | 3.00 | 0.57 (0.37–0.89) |
| 30 | 10 | 3.00 | 0.68 (0.44–1.06) |
| 40 | 10 | 3.00 | 0.61 (0.39–0.95) |
| 40 | 0 | 2.00 | 0.65 (0.42–1.01) |
| 50 | 0 | 2.00 | 0.61 (0.39–0.95) |
| 50 | 10 | 2.00 | 0.67 (0.43–1.04) |
| 60 | 10 | 2.00 | 0.63 (0.41–0.98) |
Abbreviations: aHR, adjusted hazard ratio; CRT, chemoradiotherapy; HR, hazard ratio; RT, radiotherapy; UBC, unmeasured binary confounder.
Inverse probability-weighted aHR for the relationship between adjuvant chemotherapy and overall survival (aHR, 95% CI, 1.46; 95% CI, 0.91–2.33) after further adjustment for unmeasured binary confounder.
Inverse probability-weighted aHR for the relationship between adjuvant chemotherapy and disease-free survival (aHR, 0.91; 95% CI, 0.59–1.42) after further adjustment for unmeasured binary confounder.
Discussion
In our investigation of 195 surgically managed patients with p16-positive OPSCC, the addition of chemotherapy to the adjuvant treatment regimen was not found to improve DFS and was associated with worse OS. Because this is an observational study and treatment allocation was not random, confounding by indication25 may account for the observed lack of benefit of adjuvant chemoradiotherapy over radiotherapy alone. In this study, patients who received adjuvant chemoradiotherapy, compared with patients who received radiotherapy alone, had more severe pathological tumor status, which could account for worse outcome. To account for these differences between treatment groups, we calculated propensity scores and conducted the multivariate analyses after weighting cases by the inverse probability of receiving chemotherapy.
Propensity scores estimate the probability of receiving a treatment given measured baseline covariates and can be used to minimize or eliminate bias due to measured confounders.26 The goal of using propensity scores is to make the treatment groups as similar as possible and minimize bias with respect to measured covariates to allow for an unbiased comparison of treatment groups. Propensity scores, therefore, allow observational data to more closely approximate randomized clinical trial data. There are several methods for using propensity scores, but inverse probability of treatment weighting (IPTW) is a preferred method for survival analysis.27 With IPTW, patients who have a high probability of receiving the treatment that they ultimately receive are assigned the lowest weight. Conversely, patients who receive chemotherapy and have a low probability of receiving chemotherapy are weighted more heavily. Through the calculation of propensity scores with IPTW, treatment groups become balanced with respect to measured covariates, and bias is reduced in the comparison between treatment groups.
While the use of propensity scores minimizes bias due to measured confounders, unmeasured confounding still exists in observational research and can have a substantial effect on study results.28 Therefore, we conducted a sensitivity analysis to determine the extent of unmeasured confounding that would have to be present to explain our results if adjuvant chemotherapy does provide a significant survival benefit. Our sensitivity analysis indicates that for adjuvant chemotherapy to be significantly beneficial, a hypothetical, unmeasured binary confounder would have to be very strongly associated with survival and greatly disproportionately distributed between our treatment groups. This hypothetical confounder would have to have a greater effect on survival than comorbidity or TNM staging. The presence of such a variable is unlikely. Two variables that are often considered when selecting an adjuvant therapy regimen include extracapsular spread and surgical margin. While these variables have traditionally been prognostic, recent studies indicate that these variables are of limited prognostic value among surgically managed patients with p16-positive OPSCC. Extracapsular spread has poor interobserver and intraobserver reliability29 and is not associated with OS among surgically managed patients with p16- positive OPSCC.20,30,31 Likewise, surgical margin is a poor prognosticator among these patients.32 This is perhaps due to the relative few number of patients who have positive margins and the fact that many patients with positive margins undergo reresection. A limitation to our sensitivity analysis is that we examined the effect a single binary unmeasured confounding variable. It is possible that numerous unmeasured confounding variables exist, each of which biases our observed relationship between adjuvant chemotherapy and survival. Nonetheless, if adjuvant chemotherapy truly provides a survival benefit, the extent of unmeasured confounding required to explain our results would be substantial.
Our results highlight the superior prognosis of patients with p16-positive OPSCC regardless of treatment modality. Both of our treatment groups had 5-year OS rates greater than 80%, and both groups experienced few recurrences. Distant metastasis was the predominant pattern of failure in our cohort, which is consistent with other studies.18 Because distant failure is more common than local or regional failure in HPV-positive OPSCC, it would be logical to include a systemic chemotherapeutic agent in the treatment regimen. However, our chemoradiotherapy group did not experience fewer recurrences than our radiotherapy group, and DFS was not statistically significantly improved in the chemoradiotherapy group. These findings suggest that the addition of adjuvant chemotherapy in surgically managed, p16-positive OPSCC may be unnecessary.
The use of postoperative adjuvant chemoradiotherapy in locally advanced or high-risk OPSCC is the current standard of care according to the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology.33 This guideline is based on evidence from 2 large, multicenter randomized clinical trials.34,35 The NCCN considers the evidence in support of this guideline as category 1–the highest level of evidence. However, the methodology and analyses used in these studies and on which this guideline rests have recently come under critical appraisal.36 The epidemiology, clinical course, and prognosis of OPSCC have shifted in recent decades with the advent of HPV-related OPSCC, and the management of these patients must be reconsidered.
To our knowledge, our study is the largest and most thorough investigation of postoperative adjuvant chemotherapy among patients with p16-positive OPSCC. To date, no other studies examining the role of adjuvant chemotherapy in this setting have used propensity scores or included a formal sensitivity analysis. At our institution, Sinha et al31 investigated the effects of adjuvant chemotherapy on survival among surgically managed patients with p16-positive OPSCC and found no difference in survival between patients treated with adjuvant radiotherapy and those treated with adjuvant chemoradiotherapy. This was a smaller study examining the effects of adjuvant chemotherapy between 24 matched pairs who all exhibited extracapsular spread and who were treated with neck dissection and transoral laser microsurgery. Similarly, Kumar et al37 found no difference between adjuvant treatment groups among surgically managed, HPV-positive patients with OPSCC. O’Sullivan et al18 also reported no survival benefit with the addition of adjuvant chemotherapy, with the exception of patients with N2C nodal disease; however, this study investigated adjuvant chemotherapy among nonsurgically managed HPV-positive patients. Our results must be interpreted with caution because observational data have inherent biases. In the absence of randomized studies, our results provide evidence that surgically managed, p16-positive OPSCC may be adequately treated with adjuvant radiotherapy alone.
Limitations
A limitation of our study is the dichotomization of patients into 2 mutually exclusive adjuvant treatment groups. Patients were categorized as having received adjuvant radiotherapy or adjuvant chemoradiotherapy without consideration for total dose or fractionation of radiation received, type or dose of chemotherapeutic agent, or duration of adjuvant therapy. Given the diversity of treatment options, the wide array of patient and physician preferences, and the uniqueness of each patient’s disease, it is impossible to fully explore the nuances among different treatment modalities.
Conclusions
Because HPV-related OPSCC is increasingly being recognized as a unique disease entity, new treatment protocols are required to minimize treatment morbidity without sacrificing tumor control. We have shown that for p16-positive patients treated with surgery and adjuvant radiotherapy, the addition of postoperative chemotherapy to the treatment regimen provides no additional DFS benefit and may decrease overall survival. Our findings should inform future clinical trials that seek to optimize the management of p16-positive OPSCC.
Key Points.
Question
Is there a survival difference between patients with surgically managed, p16-positive oropharyngeal squamous cell carcinoma who receive adjuvant radiotherapy and patients who receive adjuvant chemoradiotherapy?
Findings
In this cohort study of 195 surgically managed, p16-positive patients, an inverse probability-weighted, multivariate analysis indicated that the receipt of adjuvant chemotherapy is not significantly associated with disease-free survival but is associated with a statistically insignificant yet clinically meaningful increase in all-cause mortality.
Meaning
Among patients with p16-positive oropharyngeal squamous cell carcinoma managed surgically with adjuvant radiotherapy, the addition of adjuvant chemotherapymay be unnecessary.
Acknowledgments
Funding/Support: This publication was supported by the Washington University Institute of Clinical and Translational Sciences grants UL1 TR000448 and TL1 TR000449 from the National Center for Advancing Translational Sciences.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest, and none were reported. Drs Kallogjeri and Piccirillo own stock and serve as consultants for potential MED, but the work is not related to the submitted manuscript. No other disclosures are reported.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr Piccirillo is the Editor of JAMA Otolaryngology–Head & Neck Surgery, but he was not involved in any of the decisions regarding review of the manuscript or its acceptance.
Additional Contributions: We thank the members of the Head and Neck Division of the Department of Otolaryngology–Head and Neck Surgery at Washington University for their role in the surgical management of the patients described herein.
Author Contributions: Dr Piccirillo had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Skillington, Kallogjeri, Piccirillo.
Acquisition, analysis, or interpretation of data: All Authors.
Drafting of the manuscript: Skillington, Piccirillo.
Critical revision of the manuscript for important intellectual content: Skillington, Kallogjeri, Lewis.
Statistical analysis: Skillington, Kallogjeri.
Administrative, technical, or material support: Kallogjeri.
Study supervision: Piccirillo.
Other—pathology support for diagnosis and patient outcome data collection and organization: Lewis.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92(9):709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 2.Andl T, Kahn T, Pfuhl A, et al. Etiological involvement of oncogenic human papillomavirus in tonsillar squamous cell carcinomas lacking retinoblastoma cell cycle control. Cancer Res. 1998;58(1):5–13. [PubMed] [Google Scholar]
- 3.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26(4):612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 4.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stein AP, Saha S, Yu M, Kimple RJ, Lambert PF. Prevalence of human papillomavirus in oropharyngeal squamous cell carcinoma in the United States across time. Chem Res Toxicol. 2014;27(4):462–469. doi: 10.1021/tx500034c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole L, Polfus L, Peters ES. Examining the incidence of human papillomavirus-associated head and neck cancers by race and ethnicity in the U.S., 1995–2005. PLoS One. 2012;7(3):e32657. doi: 10.1371/journal.pone.0032657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hess CB, Rash DL, Daly ME, et al. Competing causes of death and medical comorbidities among patients with human papillomavirus-positive vs human papillomavirus-negative oropharyngeal carcinoma and impact on adherence to radiotherapy. JAMA Otolaryngol Head Neck Surg. 2014;140(4):312–316. doi: 10.1001/jamaoto.2013.6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillison ML. Human papillomavirus-associated head and neck cancer is a distinct epidemiologic, clinical, and molecular entity. Semin Oncol. 2004;31(6):744–754. doi: 10.1053/j.seminoncol.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 9.Gillison ML, D’ Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100(6):407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 10.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 11.Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007;121(8):1813–1820. doi: 10.1002/ijc.22851. [DOI] [PubMed] [Google Scholar]
- 12.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rischin D, Young RJ, Fisher R, et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol. 2010;28(27):4142–4148. doi: 10.1200/JCO.2010.29.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klussmann JP, Gultekin E, Weissenborn SJ, et al. Expression of p16 protein identifies a distinct entity of tonsillar carcinomas associated with human papillomavirus. Am J Pathol. 2003;162(3):747–753. doi: 10.1016/S0002-9440(10)63871-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H, Sun R, Lin H, Hu WH. P16INK4A as a surrogate biomarker for human papillomavirus associated oropharyngeal carcinoma: consideration of some aspects. Cancer Sci. 2013;104(12):1553–1559. doi: 10.1111/cas.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samuels SE, Eisbruch A, Beitler JJ, et al. Management of locally advanced HPV-related oropharyngeal squamous cell carcinoma: where are we? Eur Arch Otorhinolaryngol. 2015;273(10):2877–2894. doi: 10.1007/s00405-015-3771-x. [DOI] [PubMed] [Google Scholar]
- 17.Lee MK, Nalliah RP, Kim MK, et al. Prevalence and impact of complications on outcomes in patients hospitalized for oral and oropharyngeal cancer treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112(5):581–591. doi: 10.1016/j.tripleo.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 18.O’Sullivan B, Huang SH, Siu LL, et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol. 2013;31(5):543–550. doi: 10.1200/JCO.2012.44.0164. [DOI] [PubMed] [Google Scholar]
- 19.Masterson L, Moualed D, Liu ZW, et al. De-escalation treatment protocols for human papillomavirus-associated oropharyngeal squamous cell carcinoma: a systematic review and meta-analysis of current clinical trials. Eur J Cancer. 2014;50(15):2636–2648. doi: 10.1016/j.ejca.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Lewis JS, Jr, Carpenter DH, Thorstad WL, Zhang Q, Haughey BH. Extracapsular extension is a poor predictor of disease recurrence in surgically treated oropharyngeal squamous cell carcinoma. Mod Pathol. 2011;24(11):1413–1420. doi: 10.1038/modpathol.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gourin CG, Podolsky RH. Racial disparities in patients with head and neck squamous cell carcinoma. Laryngoscope. 2006;116(7):1093–1106. doi: 10.1097/01.mlg.0000224939.61503.83. [DOI] [PubMed] [Google Scholar]
- 22.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL., Jr Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291(20):2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 23.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A., III . AJCC Cancer Staging Manual. 7. New York, NY: Springer; 2010. [Google Scholar]
- 24.Abramson JH. WINPEPI updated: computer programs for epidemiologists, and their teaching potential. Epidemiol Perspect Innov. 2011;8(1):1. doi: 10.1186/1742-5573-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salas M, Hofman A, Stricker BH. Confounding by indication: an example of variation in the use of epidemiologic terminology. Am J Epidemiol. 1999;149(11):981–983. doi: 10.1093/oxfordjournals.aje.a009758. [DOI] [PubMed] [Google Scholar]
- 26.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 27.Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med. 2013;32(16):2837–2849. doi: 10.1002/sim.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fewell Z, Davey Smith G, Sterne JA. The impact of residual and unmeasured confounding in epidemiologic studies: a simulation study. Am J Epidemiol. 2007;166(6):646–655. doi: 10.1093/aje/kwm165. [DOI] [PubMed] [Google Scholar]
- 29.Lewis JS, Jr, Tarabishy Y, Luo J, et al. Inter- and intra-observer variability in the classification of extracapsular extension in p16 positive oropharyngeal squamous cell carcinoma nodal metastases. Oral Oncol. 2015;51(11):985–990. doi: 10.1016/j.oraloncology.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geiger JL, Lazim AF, Walsh FJ, et al. Adjuvant chemoradiation therapy with high-dose versus weekly cisplatin for resected, locally-advanced HPV/p16-positive and negative head and neck squamous cell carcinoma. Oral Oncol. 2014;50(4):311–318. doi: 10.1016/j.oraloncology.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Sinha P, Lewis JS, Jr, Piccirillo JF, Kallogjeri D, Haughey BH. Extracapsular spread and adjuvant therapy in human papillomavirus-related, p16-positive oropharyngeal carcinoma. Cancer. 2012;118(14):3519–3530. doi: 10.1002/cncr.26671. [DOI] [PubMed] [Google Scholar]
- 32.Haughey BH, Sinha P. Prognostic factors and survival unique to surgically treated p16+ oropharyngeal cancer. Laryngoscope. 2012;122(suppl 2):S13–S33. doi: 10.1002/lary.23493. [DOI] [PubMed] [Google Scholar]
- 33. [Accessed January 1, 2016];NCCN Clinical Practice Guidelines in Oncology. Version 1.2015. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 34.Bernier J, Domenge C, Ozsahin M, et al. European Organization for Research and Treatment of Cancer Trial 22931. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350(19):1945–1952. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 35.Cooper JS, Pajak TF, Forastiere AA, et al. Radiation Therapy Oncology Group 9501/Intergroup. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350(19):1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 36.Sinha P, Piccirillo JF, Kallogjeri D, Spitznagel EL, Haughey BH. The role of postoperative chemoradiation for oropharynx carcinoma: a critical appraisal of the published literature and National Comprehensive Cancer Network guidelines. Cancer. 2015;121(11):1747–1754. doi: 10.1002/cncr.29242. [DOI] [PubMed] [Google Scholar]
- 37.Kumar B, Cipolla MJ, Old MO, et al. Surgical management of oropharyngeal squamous cell carcinoma: survival and functional outcomes. Head Neck. 2016;38(suppl 1):E1794–E1802. doi: 10.1002/hed.24319. [DOI] [PubMed] [Google Scholar]