Abstract
T cells patrol our bodies preventing pathogenic infections and malignant cell outgrowth. However, T cells must be properly controlled because aberrant or persistent T cell responses can damage tissues and contribute to autoimmune diseases and other chronic inflammatory diseases including metabolic syndrome. One regulatory mechanism utilized in immune cells is immunometabolic regulation, which ensures immune cells properly respond to systemic and peripheral metabolic cues. Recent work has suggested that deregulated metabolism in tumor cells creates a microenvironmental barrier for mounting effective anti-tumor immune responses. Here, we discuss how tumor cells evade immunosurveillance by modulating metabolic checkpoints in immune cells and discuss how memory T cells could provide effective anti-tumor responses by sustaining metabolic fitness and longevity.
Introduction
Tumor-infiltrating lymphocytes can play critical roles in regulating tumor outgrowth [1,2] and numerous forms of immunotherapies are now under investigation to enhance anti-tumor immune responses. Approaches such as vaccination and adoptive T cell transfer can expand the tumor-specific T cell population, but often in solid tumors, such beneficial responses are hindered by the immunosuppressive tumor microenvironment (TME) [2–6]. Programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) checkpoint blockade have been shown to successfully boost host anti-tumor immunity in a restricted portion of patients [2,7]. Intriguingly, this indicates that it is possible to subdue immunosuppressive features of the TME. Therefore, understanding in detail the mechanisms exploited by tumor cells to negate anti-tumor responses is essential for developing new interventions to work alone or with current cancer immunotherapies.
Tumor cells display abnormal metabolic activities; consuming nutrients and generating metabolites differently compared to non-proliferative normal tissues and benign lesions [1,8]. These metabolic alterations, including Warburg glycolysis and de novo fatty acid synthesis, support the unrestricted proliferation and survival of tumor cells [8]. However, these intrinsically beneficial metabolic changes in tumor cells can also have extrinsic effects and modulate neighboring cell behavior through nutrient competition and exchanges of metabolic intermediates [9–14]. Details on how particular metabolic processes regulate immune cell function are covered elsewhere in this issue. Therefore, this piece will focus on how altered metabolic states of the tumor cells act extrinsically to influence the types of tumor infiltrating immune cells, their metabolic activities and functions, and how targeting T cell memory pathways could be an attractive strategy to sustain anti-tumor immunity.
Anti-tumor immune responses are impaired by the altered nutrient state of the tumor microenvironment (TME)
Acidosis and lactic acid in the TME
Otto Warburg discovered that, in normoxic conditions, rapidly growing tumor cells produce copius amounts of lactic acid from glucose, a process known as the Warburg glycolysis [15]. This process allows tumor cells to accumulate the metabolic intermediates, such as nucleotides and amino acids, to support macromolecule production necessary for cell division [15,16]. However, the lactic acids produced by tumor cells during this process acidify the TME and both the lower pH or the direct effects of lactate/lactic acid can modulate the neighboring immune cells in the TME [17–19]. Particularly in hypoxic areas in tumors where the low diffusion rates of oxygen further enhances tumor cell glycolysis and TME acidosis [16,20–22]. Lactic acids are co-transported with the proton concentration gradient through the monocarboxylate transporter (MCT). Therefore, MCT inhibitors, acting upon tumor cells, may neutralize acidosis and prevent lactic acid transport into T cells, thereby improving T cell functions [18,19]. However, it is possible that MCT inhibitors could also impair anti-tumor T cell responses because the reduced dissipation of lactic acids within activated T cells could raise intracellular pH to toxic levels [14,23,24]. Lactic acid is directly suppressive to T cell effector functions such as IFNγ and Granzyme B production [18,24,25] Lactate can also induce VEGF production in tumor associated macrophages that promotes angiogenesis and tumor growth [14] Intriguingly, the mobility of CD8+ and CD4+ T cells could be differentially suppressed by lactic acid and sodium lactate due to the expression of T cell subtype-specific transporters [18]. Additionally, a recent report showed that tumors with low lactate dehydrogenase A (LDHA) activity were more infiltrated by T and NK cells [24] These findings suggest that increased lactic acid production by tumors aides in tumor evasion by hindering T cell mobility and effector functions in the TME. Thus, targeting lactic acids and lactate in the tumor microenvironment may be an attractive strategy to unleash T cell and macrophage anti-tumor responses.
Glucose and amino acids deprivation in the tumor microenvironment impairs anti-tumor immunity
Effector T cells utilize aerobic glycolysis and increased amino acid metabolism to support proliferation and production of effector molecules by modulating signaling cascades and transcriptional and translational events [26]. However, oncogenic mutations and hypoxia profoundly enhance the rate and ability of tumor cells to uptake glucose [15], which could in turn, restrict glucose availability to infiltrating T cells and other neighboring cells, such as antigen-presenting and endothelial cells. Glucose restriction in T cells promotes T cell dysfunction and impairs calcium signaling, mechanistic target of rapamycin (mTOR) activity, translation of effector molecules, and survival through various metabolic regulations [10,26–29]. Moreover, PD-1 signal suppresses glycolytic metabolism whilst stimulating fatty acid oxidation in T cells to further prevent T cells from achieving the metabolic demands required to sustain anti-tumor responses [30,31]. Recent studies support the notion that tumor infiltrating T cells fail to acquire sufficient glucose in certain types of tumors and that tumor cells displaying higher glycolytic activity are prone to escape immunosurveillance [12,13]. Intriguingly, boosting glycolytic activity by stabilizing HIF1α through PHD- or VHL-deficiency or metabolic rewiring tumor-specific T cells by overexpressing phosphoenolpyruvate carboxykinase 1 (PCK1) improved anti-tumor T cell activities [12,32,33]. These findings reveal ways by which the glycolytic activity of tumor cells can facilitate immune evasion. Thus, targeting oncogenic mutations that sustain anabolic metabolism in tumor cells (such as oncogenic BrafV600E in melanoma or EGF receptor tyrosine kinase in head and neck squamous cell carcinoma and non-small-cell lung cancer), might indirectly lead to improved T cell anti-tumor responses and metabolic fitness in part due to the suppressive effects of these drugs on tumor cell glycolysis [34–36]. Interestingly, the expression of ligands for co-inhibitory receptors, such as PD-L1 and B7-H3, could enhance Warburg glycolysis and glucose uptake rates in tumor cells [13,37,38]. In these studies, targeting these ligands of co-inhibitory receptors could suppress glycolytic activity of tumor cells. Therefore, in addition to relief the inhibitory signal in T cells PD-1 blockade may synergize with treatments targeting ligands of co-inhibitory receptors to boost the metabolic fitness of infiltrating T cells.
Amino acid metabolism is also highly regulated in the TME by tumor cells and other immunoregulatory cells. For example, tumor cells, M2-like TAMs and myeloid-derived suppressor cells (MDSCs) often have increased Arginase 1 (Arg1) and indoleamine-2,3-dioxygenase (IDO) activity that metabolize arginine and tryptophan, key amino acids necessary for T cell receptor signaling, effector functions, proliferation and survival [26,39–44]. Additionally, IDO catabolizes tryptophan into kynurenine [45], a metabolite that promotes CD4+ Treg differentiation and immunosuppression of effector T cells [46–48]. Given the immunosuppressive nature of Arg1 and IDO, inhibitors of Arg1 and IDO could mount effective anti-tumor T cell responses and suppress tumor progression in murine tumor models and how these inhibitors unleash host anti-tumor responses in patients are undergoing intensively investigations in multiple clinical trails [49]. In addition, increased glutaminolysis in tumor cells is critical to replenish metabolites through anaplerosis, which could result in deprivation of glutamine to tumor infiltrating T lymphocytes. Since glutamine metabolism in T cells controls T cell activation and self-renewal by enhancing mTOR activation, protein O-GlcNAcylation, and is also a key substrate for the formation of 2-ketoglutarate (2-KG) and S-2-hydroxyglutarate (S-2-HG) that regulate effector T cell functions and differentiation [50–52], inhibiting glutaminolysis and glutamine transporters in tumor cells might induce anti-tumor responses and synergize with other cancer immunotherapies. Taken together, these findings indicate that the TME could also suppress immunisurveillance by quenching amino acid availability to T cells.
Lipid metabolism: a new metabolic regulator for immune evasion
Prolific lipogenesis via de novo fatty acid synthesis and fatty acid uptake are other hallmarks of transformed cells that promote tumor progression, metastasis, and resistance to cellular stress [53,54]. Indeed, targeting lipogenesis in tumor cells suppresses tumor progression [53,55–57], but it remains unclear whether increased lipid synthesis in tumor cells impacts the neighboring cells in the TME. It is possible that free fatty acids accumulate in the TME by tumor cell necrosis, increased lipolysis of TAGs by lysophosphatidyl lipase (LPL) or local adipocytes may enhance the persistence or survival of lipid-oxidizing CD4+ Tregs and MDSCs, decrease antigen-presentation ability of dendritic cells, and/or suppress the effector functions of cytotoxic CD8+ and TH1 CD4+ T cells [27,58,59]. These findings suggest that lipid metabolism in tumors could be a regulator for anti-tumor immunity. Intriguingly, emerging evidence revealed that short-chain fatty acids (SCFAs) could promote Tregs formation but middle- and long-chain fatty acids support differentiation of Th1 and Th17 cells in gut [60]. Thus, we postulate that in the TME, tumor cells may disarm T cell anti-tumor immunity by feeding unique fatty acids to reorient their effector functions and differentiation states. Taken together, it will be critical for future research to determine how immune cells adapt their immune functions in response to particular species of lipid and lipid composition, both in vitro and in vivo.
Superior anti-tumor immunity induced by tumor-specific memory T cells
Developing long-lived immunity to cancer is an ultimate goal for effective cancer therapy and may reduce incidence of recurrence in patients. Given that adoptive cell transfer (ACT) is a strategy to select and manipulate cellular activities of tumor-specific CTLs, ACT becomes a platform to understand the qualitative difference of the in vitro expanded tumor-specific CTLs. Interestingly, in contrast to transferring effector CD8+ T cells that have a limited lifespan, the same amount of memory CD8+ T cells elicit stronger anti-tumor responses and give prolonged protection for tumor recurrence [2]. These findings suggest that a promising anti-tumor strategy would be to manipulate the memory phenotype of tumor-specific CD8+ T cells during the in vitro expansion of transferred T cells. In contrast to utilizing anabolic metabolism in effector CD8+ T cells, memory CD8+ T cells prefer to engage catabolic metabolism, including increased oxidative metabolism and fatty acid oxidation, to support metabolic demands [26,61–63]. Therefore, it may be possible to force the generation of memory CD8+ T cells by manipulating the metabolic pathways during in vitro T cell expansion. Indeed, several recent studies have shown that treating T cells with 2-deoxyglucose or an Akt inhibitor produces memory-like T cell populations with stronger anti-tumor responses and survival potential [64,65]. In addition, increasing L-Arginine availability could increase T cell mitochondrial respiration in an in vitro culture and these T cells display memory phenotypes and superior anti-tumor responses [66]. These findings lend credence to the idea that tailoring metabolic program of transferred T cells may elicit different anti-tumor responses, and further indicates that memory CD8+ T cells may adapt their metabolic demands to the nutrient composition in the TME. In support of this possibility, a recent study using chimeric antigen receptor (CAR) T-cells discovered that the newest generation of CAR allows T cells to increase growth and survival by engaging a metabolic phenotype seen in memory CD8+ T cells [67]. These studies indicate that metabolically modulated adoptively transferred T cells, including endogenous and CAR T cells, can achieve longer-lasting and possibly more potent anti-tumor responses; however, it remains unclear why memory CD8+ T cells can perform better in the TME. Since mitochondrial activity and fatty acid oxidation support the metabolic demands of memory T cells [61–63], we postulate that these properties may augment the persistence and proliferative capacity of memory T cells in the TME. Indeed, declined mitochondrial content and inability to engage mitochondrial biogenesis in tumor infiltrating CD8+ T cells was recently shown to result in metabolic insufficiency and poor anti-tumor responses [68]. Intriguingly, overexpressing peroxisome proliferator-activated receptor gamma co-activator 1-alpha (PGC1α) in tumor antigen-specific CD8+ T cells could enhance their anti-tumor responses by promoting mitochondrial biogenesis [68]. Since increasing mitochondrial content is a metabolic hallmark of memory T cells, it is likely that overexpression of PGC1α could skew activated CD8+ T cells into a memory-like phenotype. In addition to mitochondrial content, memory T cells have been reported to display fused mitochondrial structure and promoting mitochondrial fusion in T cells could lead to a memory-like phenotype and stronger anti-tumor activities [69]. Interestingly, the formation of tubular and fused network of mitochondria has been shown to allow fibroblasts to adapt their metabolic demands during nutrient starvation [70]. Thus, in addition to anabolic metabolism, the mitochondrial fitness and activity in memory T cells might also play critical roles to sustain their survival and proliferation in the TME (Figure 1). Altogether, these findings indicate that targeting metabolic regulations of memory T cells could be applied in cell-based immunotherapies to elicit superior anti-tumor responses and long-lasting protection for tumor recurrence. However, an important direction for future studies is to determine if and how memory T cells and secondary effector T cells derived from memory T cells could resist the metabolic crisis that can occur in the TME.
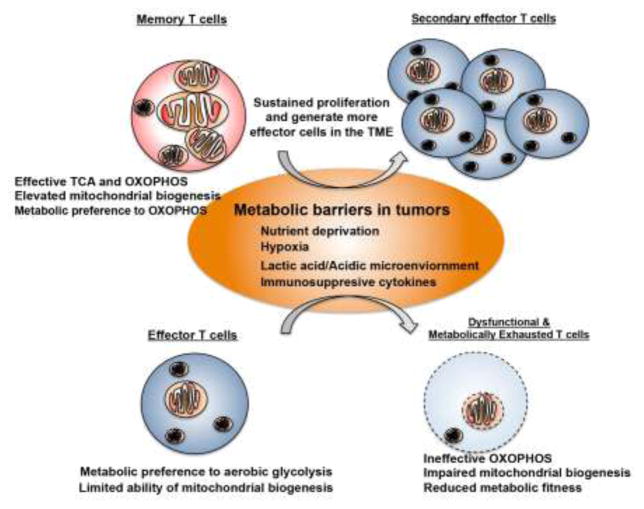
Figure 1. Mitochondria-mediated metabolic support in memory T cell anti-tumor immunity.
The metabolic stresses in the tumor microenvironment, including nutrient deprivation, hypoxia, acidic environment and immunosuppressive cytokine milieu, suppress anti-tumor responses and metabolic fitness in effector T cells. These microenviornmental barriers could affect mitochondrial mass and activity that lead to impaired TCA cycle and changes in bioenergetic programm. However, memory T cells might maintain their anti-tumor immunity by resisting to these microenvironmental barriers or sustaining their mitochondrial mass and functions.
Conclusions and future directions
We are at a remarkable point in the new era of immunotherapy and cancer immunology, and as we deepen our understanding of immunometabolism by elucidating how particular metabolic pathways and nutrients influence T cell differentiation, function and trafficking, this may reveal new types of drugs and/or treatments that could be combined with other forms of immunotherapy. Even the handful of recent reports described herein offers several clinically-viable possibilities for new cancer treatments that could be implemented in the very near future. Considering memory T cells provide superior anti-tumor immunity, it is critical to determine how metabolic pathway(s) of memory T cells support their survival, function and proliferative capacity in the TME. It would also be critical to examine how the TME impairs the mitochondrial fitness of tumor infiltrating T cells and determine how mitochondria of T cells could orchestrate desired anti-tumor responses. The knowledge gained from these studies would serve as a springboard for tailoring T cell-based cancer immunotherapies.
Highlights.
Metabolic stress in tumors impairs T cell anti-tumor immunity.
Memory T cells could be optimal cell type to sustain metabolic fitness in tumors.
Skewing memory T cell formation is a promising strategy in adoptive cell therapy.
Mitochondrial metabolism may be critical to sustain T cell tumoricidal functions.
Acknowledgments
P.-C. H. is supported in part by SNSF project grant (31003A_163204), Harry J. Lloyd Charitable Foundation, and Anna Fuller Fund. P.-C. H. is also a recipient of Melanoma Research Alliance Young Investigator Award. S.M.K. was supported by NIH RO1CA195720, R01CA196660, P50CA196530 Yale SPORE in Lung Cancer (PI, Herbst, R), the MRA Team Science Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klemm F, Joyce JA. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol. 2015;25:198–213. doi: 10.1016/j.tcb.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baitsch L, Fuertes-Marraco SA, Legat A, Meyer C, Speiser DE. The three main stumbling blocks for anticancer T cells. Trends Immunol. 2012;33:364–372. doi: 10.1016/j.it.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Baitsch L, Baumgaertner P, Devevre E, Raghav SK, Legat A, Barba L, Wieckowski S, Bouzourene H, Deplancke B, Romero P, et al. Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients. J Clin Invest. 2011;121:2350–2360. doi: 10.1172/JCI46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature. 2015;523:231–235. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 7.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boroughs LK, DeBerardinis RJ. Metabolic pathways promoting cancer cell survival and growth. Nat Cell Biol. 2015;17:351–359. doi: 10.1038/ncb3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *9.Ho PC, Liu PS. Metabolic communication in tumors: a new layer of immunoregulation for immune evasion. J Immunother Cancer. 2016;4:4. doi: 10.1186/s40425-016-0109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *10.Chang CH, Pearce EL. Emerging concepts of T cell metabolism as a target of immunotherapy. Nat Immunol. 2016;17:364–368. doi: 10.1038/ni.3415. Reference 9 and 10 describe that tumor cells suppress T cell anti-tumor responses in the tumor microenvironment by restrciting glucose to the tumor infiltrating T cells, a new way to evade immunosurveillance. These studies further show that metabolic interventions, including metabolic rewiring and targeting, are able to rejuvenate anti-tumor responses of infiltrating T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghesquiere B, Wong BW, Kuchnio A, Carmeliet P. Metabolism of stromal and immune cells in health and disease. Nature. 2014;511:167–176. doi: 10.1038/nature13312. [DOI] [PubMed] [Google Scholar]
- 12.Ho PC, Bihuniak JD, Macintyre AN, Staron M, Liu X, Amezquita R, Tsui YC, Cui G, Micevic G, Perales JC, et al. Phosphoenolpyruvate Is a Metabolic Checkpoint of Anti-tumor T Cell Responses. Cell. 2015;162:1217–1228. doi: 10.1016/j.cell.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang CH, Qiu J, O’Sullivan D, Buck MD, Noguchi T, Curtis JD, Chen Q, Gindin M, Gubin MM, van der Windt GJ, et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell. 2015;162:1229–1241. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. 2012;21:297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 17.Robey IF, Baggett BK, Kirkpatrick ND, Roe DJ, Dosescu J, Sloane BF, Hashim AI, Morse DL, Raghunand N, Gatenby RA, et al. Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res. 2009;69:2260–2268. doi: 10.1158/0008-5472.CAN-07-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haas R, Smith J, Rocher-Ros V, Nadkarni S, Montero-Melendez T, D’Acquisto F, Bland EJ, Bombardieri M, Pitzalis C, Perretti M, et al. Lactate Regulates Metabolic and Pro-inflammatory Circuits in Control of T Cell Migration and Effector Functions. PLoS Biol. 2015;13:e1002202. doi: 10.1371/journal.pbio.1002202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, Gottfried E, Schwarz S, Rothe G, Hoves S, et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109:3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 20.DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guillaumond F, Leca J, Olivares O, Lavaut MN, Vidal N, Berthezene P, Dusetti NJ, Loncle C, Calvo E, Turrini O, et al. Strengthened glycolysis under hypoxia supports tumor symbiosis and hexosamine biosynthesis in pancreatic adenocarcinoma. Proc Natl Acad Sci U S A. 2013;110:3919–3924. doi: 10.1073/pnas.1219555110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hensley CT, Faubert B, Yuan Q, Lev-Cohain N, Jin E, Kim J, Jiang L, Ko B, Skelton R, Loudat L, et al. Metabolic Heterogeneity in Human Lung Tumors. Cell. 2016;164:681–694. doi: 10.1016/j.cell.2015.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murray CM, Hutchinson R, Bantick JR, Belfield GP, Benjamin AD, Brazma D, Bundick RV, Cook ID, Craggs RI, Edwards S, et al. Monocarboxylate transporter MCT1 is a target for immunosuppression. Nat Chem Biol. 2005;1:371–376. doi: 10.1038/nchembio744. [DOI] [PubMed] [Google Scholar]
- 24.Brand A, Singer K, Koehl GE, Kolitzus M, Schoenhammer G, Thiel A, Matos C, Bruss C, Klobuch S, Peter K, et al. LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell Metab. 2016;24:657–671. doi: 10.1016/j.cmet.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Droge W, Roth S, Altmann A, Mihm S. Regulation of T-cell functions by L-lactate. Cell Immunol. 1987;108:405–416. doi: 10.1016/0008-8749(87)90223-1. [DOI] [PubMed] [Google Scholar]
- 26.MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu Rev Immunol. 2013;31:259–283. doi: 10.1146/annurev-immunol-032712-095956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG, Rathmell JC. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finlay DK, Rosenzweig E, Sinclair LV, Feijoo-Carnero C, Hukelmann JL, Rolf J, Panteleyev AA, Okkenhaug K, Cantrell DA. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J Exp Med. 2012;209:2441–2453. doi: 10.1084/jem.20112607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cham CM, Gajewski TF. Glucose availability regulates IFN-gamma production and p70S6 kinase activation in CD8+ effector T cells. J Immunol. 2005;174:4670–4677. doi: 10.4049/jimmunol.174.8.4670. [DOI] [PubMed] [Google Scholar]
- 30.Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patsoukis N, Bardhan K, Chatterjee P, Sari D, Liu B, Bell LN, Karoly ED, Freeman GJ, Petkova V, Seth P, et al. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat Commun. 2015;6:6692. doi: 10.1038/ncomms7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doedens AL, Phan AT, Stradner MH, Fujimoto JK, Nguyen JV, Yang E, Johnson RS, Goldrath AW. Hypoxia-inducible factors enhance the effector responses of CD8(+) T cells to persistent antigen. Nat Immunol. 2013;14:1173–1182. doi: 10.1038/ni.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clever D, Roychoudhuri R, Constantinides MG, Askenase MH, Sukumar M, Klebanoff CA, Eil RL, Hickman HD, Yu Z, Pan JH, et al. Oxygen Sensing by T Cells Establishes an Immunologically Tolerant Metastatic Niche. Cell. 2016;166:1117–1131. e1114. doi: 10.1016/j.cell.2016.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ho PC, Meeth KM, Tsui YC, Srivastava B, Bosenberg MW, Kaech SM. Immune-based antitumor effects of BRAF inhibitors rely on signaling by CD40L and IFNgamma. Cancer Res. 2014;74:3205–3217. doi: 10.1158/0008-5472.CAN-13-3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumai T, Matsuda Y, Oikawa K, Aoki N, Kimura S, Harabuchi Y, Celis E, Kobayashi H. EGFR inhibitors augment antitumour helper T-cell responses of HER family-specific immunotherapy. Br J Cancer. 2013;109:2155–2166. doi: 10.1038/bjc.2013.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen N, Fang W, Zhan J, Hong S, Tang Y, Kang S, Zhang Y, He X, Zhou T, Qin T, et al. Upregulation of PD-L1 by EGFR Activation Mediates the Immune Escape in EGFR-Driven NSCLC: Implication for Optional Immune Targeted Therapy for NSCLC Patients with EGFR Mutation. J Thorac Oncol. 2015;10:910–923. doi: 10.1097/JTO.0000000000000500. [DOI] [PubMed] [Google Scholar]
- 37.Lim S, Liu H, Madeira da Silva L, Arora R, Liu Z, Phillips JB, Schmitt DC, Vu T, McClellan S, Lin Y, et al. Immunoregulatory Protein B7-H3 Reprograms Glucose Metabolism in Cancer Cells by ROS-Mediated Stabilization of HIF1alpha. Cancer Res. 2016;76:2231–2242. doi: 10.1158/0008-5472.CAN-15-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nunes-Xavier CE, Karlsen KF, Tekle C, Pedersen C, Oyjord T, Hongisto V, Nesland JM, Tan M, Sahlberg KK, Fodstad O. Decreased expression of B7-H3 reduces the glycolytic capacity and sensitizes breast cancer cells to AKT/mTOR inhibitors. Oncotarget. 2016;7:6891–6901. doi: 10.18632/oncotarget.6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siska PJ, Rathmell JC. T cell metabolic fitness in antitumor immunity. Trends Immunol. 2015;36:257–264. doi: 10.1016/j.it.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee GK, Park HJ, Macleod M, Chandler P, Munn DH, Mellor AL. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology. 2002;107:452–460. doi: 10.1046/j.1365-2567.2002.01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 42.Taheri F, Ochoa JB, Faghiri Z, Culotta K, Park HJ, Lan MS, Zea AH, Ochoa AC. L-Arginine regulates the expression of the T-cell receptor zeta chain (CD3zeta) in Jurkat cells. Clin Cancer Res. 2001;7:958s–965s. [PubMed] [Google Scholar]
- 43.Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, Delgado A, Correa P, Brayer J, Sotomayor EM, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;64:5839–5849. doi: 10.1158/0008-5472.CAN-04-0465. [DOI] [PubMed] [Google Scholar]
- 44.Murray PJ, Wynn TA. Obstacles and opportunities for understanding macrophage polarization. J Leukoc Biol. 2011;89:557–563. doi: 10.1189/jlb.0710409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Platten M, Wick W, Van den Eynde BJ. Tryptophan catabolism in cancer: beyond IDO and tryptophan depletion. Cancer Res. 2012;72:5435–5440. doi: 10.1158/0008-5472.CAN-12-0569. [DOI] [PubMed] [Google Scholar]
- 46.Baban B, Chandler PR, Sharma MD, Pihkala J, Koni PA, Munn DH, Mellor AL. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol. 2009;183:2475–2483. doi: 10.4049/jimmunol.0900986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan Y, Zhang GX, Gran B, Fallarino F, Yu S, Li H, Cullimore ML, Rostami A, Xu H. IDO upregulates regulatory T cells via tryptophan catabolite and suppresses encephalitogenic T cell responses in experimental autoimmune encephalomyelitis. J Immunol. 2010;185:5953–5961. doi: 10.4049/jimmunol.1001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185:3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adams JL, Smothers J, Srinivasan R, Hoos A. Big opportunities for small molecules in immuno-oncology. Nat Rev Drug Discov. 2015;14:603–622. doi: 10.1038/nrd4596. [DOI] [PubMed] [Google Scholar]
- 50.Sinclair LV, Rolf J, Emslie E, Shi YB, Taylor PM, Cantrell DA. Control of aminoacid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat Immunol. 2013;14:500–508. doi: 10.1038/ni.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swamy M, Pathak S, Grzes KM, Damerow S, Sinclair LV, van Aalten DM, Cantrell DA. Glucose and glutamine fuel protein O-GlcNAcylation to control T cell self-renewal and malignancy. Nat Immunol. 2016;17:712–720. doi: 10.1038/ni.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tyrakis PA, Palazon A, Macias D, Lee KL, Phan AT, Velica P, You J, Chia GS, Sim J, Doedens A, et al. S-2-hydroxyglutarate regulates CD8+ T-lymphocyte fate. Nature. 2016;540:236–241. doi: 10.1038/nature20165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Currie E, Schulze A, Zechner R, Walther TC, Farese RV., Jr Cellular fatty acid metabolism and cancer. Cell Metab. 2013;18:153–161. doi: 10.1016/j.cmet.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 55.Zitvogel L, Kroemer G. Targeting dendritic cell metabolism in cancer. Nat Med. 2010;16:858–859. doi: 10.1038/nm0810-858. [DOI] [PubMed] [Google Scholar]
- 56.Flaveny CA, Griffett K, El-Gendy Bel D, Kazantzis M, Sengupta M, Amelio AL, Chatterjee A, Walker J, Solt LA, Kamenecka TM, et al. Broad Anti-tumor Activity of a Small Molecule that Selectively Targets the Warburg Effect and Lipogenesis. Cancer Cell. 2015;28:42–56. doi: 10.1016/j.ccell.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Svensson RU, Parker SJ, Eichner LJ, Kolar MJ, Wallace M, Brun SN, Lombardo PS, Van Nostrand JL, Hutchins A, Vera L, et al. Inhibition of acetyl-CoA carboxylase suppresses fatty acid synthesis and tumor growth of non-small-cell lung cancer in preclinical models. Nat Med. 2016;22:1108–1119. doi: 10.1038/nm.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cubillos-Ruiz JR, Silberman PC, Rutkowski MR, Chopra S, Perales-Puchalt A, Song M, Zhang S, Bettigole SE, Gupta D, Holcomb K, et al. ER Stress Sensor XBP1 Controls Anti-tumor Immunity by Disrupting Dendritic Cell Homeostasis. Cell. 2015;161:1527–1538. doi: 10.1016/j.cell.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Herber DL, Cao W, Nefedova Y, Novitskiy SV, Nagaraj S, Tyurin VA, Corzo A, Cho HI, Celis E, Lennox B, et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med. 2010;16:880–886. doi: 10.1038/nm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haghikia A, Jorg S, Duscha A, Berg J, Manzel A, Waschbisch A, Hammer A, Lee DH, May C, Wilck N, et al. Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine. Immunity. 2015;43:817–829. doi: 10.1016/j.immuni.2015.09.007. [DOI] [PubMed] [Google Scholar]
- *61.Cui G, Staron MM, Gray SM, Ho PC, Amezquita RA, Wu J, Kaech SM. IL-7-Induced Glycerol Transport and TAG Synthesis Promotes Memory CD8+ T Cell Longevity. Cell. 2015;161:750–761. doi: 10.1016/j.cell.2015.03.021. This paper establish that AQP9-mediated glycerol uptake is a critical event for supporting triglyceride synthesis in memory T cells. This event supports IL7-mediated survival and self-renewal of memory T cells by modulating fatty acid metabolism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *62.van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, Pearce EJ, Pearce EL. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. This is the first study to show that memory CD8+ T cells display increased mitochondrial content and dependency on oxidative metabolism. It further uncover fatty acid oxidation is the major metabolic pathway to support memory T cell development and survival. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *63.O’Sullivan D, van der Windt GJ, Huang SC, Curtis JD, Chang CH, Buck MD, Qiu J, Smith AM, Lam WY, DiPlato LM, et al. Memory CD8(+) T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity. 2014;41:75–88. doi: 10.1016/j.immuni.2014.06.005. This paper unveil that memory T cells utilize fatty acid oxidation to support their development. It also showes that memory T cells utilize glycolytic pathway to support fatty acid synthesis instead of taking up fatty acids from extracellular environment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **64.Sukumar M, Liu J, Ji Y, Subramanian M, Crompton JG, Yu Z, Roychoudhuri R, Palmer DC, Muranski P, Karoly ED, et al. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J Clin Invest. 2013;123:4479–4488. doi: 10.1172/JCI69589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **65.Crompton JG, Sukumar M, Roychoudhuri R, Clever D, Gros A, Eil RL, Tran E, Hanada K, Yu Z, Palmer DC, et al. Akt inhibition enhances expansion of potent tumor-specific lymphocytes with memory cell characteristics. Cancer Res. 2015;75:296–305. doi: 10.1158/0008-5472.CAN-14-2277. Reference 64 and 65 describe that inhibiting glycolytic pathway and Akt kinase activity during in vitro expansion of tumor-specific T cells could yield memory-like populations. Tumor-specific T cells generated by these strategies are able to provide superior and durable anti-tumor responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Geiger R, Rieckmann JC, Wolf T, Basso C, Feng Y, Fuhrer T, Kogadeeva M, Picotti P, Meissner F, Mann M, et al. L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity. Cell. 2016;167:829–842. e813. doi: 10.1016/j.cell.2016.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kawalekar OU, O’Connor RS, Fraietta JA, Guo L, McGettigan SE, Posey AD, Jr, Patel PR, Guedan S, Scholler J, Keith B, et al. Distinct Signaling of Coreceptors Regulates Specific Metabolism Pathways and Impacts Memory Development in CAR T Cells. Immunity. 2016;44:380–390. doi: 10.1016/j.immuni.2016.01.021. [DOI] [PubMed] [Google Scholar]
- *68.Scharping NE, Menk AV, Moreci RS, Whetstone RD, Dadey RE, Watkins SC, Ferris RL, Delgoffe GM. The Tumor Microenvironment Represses T Cell Mitochondrial Biogenesis to Drive Intratumoral T Cell Metabolic Insufficiency and Dysfunction. Immunity. 2016;45:374–388. doi: 10.1016/j.immuni.2016.07.009. This paper shows that tumor infiltrating T lymphocytes fail to sustain their metabolic fitness due to impaired mitchondrial biogenesis. It further provides eveidence that overexpression of PGC1α in adoptively transferred tumor-specific T cells results in stronger metabolic fitness and anti-tumor immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Buck MD, O’Sullivan D, Geltink RIK, Curtis JD, Chang CH, Sanin DE, Qiu J, Kretz O, Braas D, van der Windt GJW, et al. Mitochondrial Dynamics Controls T Cell Fate through Metabolic Programming. Cell. 2016;166:63–76. doi: 10.1016/j.cell.2016.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J. Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc Natl Acad Sci U S A. 2011;108:10190–10195. doi: 10.1073/pnas.1107402108. [DOI] [PMC free article] [PubMed] [Google Scholar]