Abstract
Sox2 is a well-established neuronal stem cell-associated transcription factor that regulates neural development and adult neurogenesis in vertebrates, and is one of the critical genes used to reprogram differentiated cells into induced pluripotent stem cells. We examined if Sox2 was involved in the early reprogramming-like events that Müller glia undergo as they upregulate many pluripotency- and neural stem cell-associated genes required for proliferation in light-damaged adult zebrafish retinas. In the undamaged adult zebrafish retina, Sox2 is expressed in Müller glia and a subset of amacrine cells, similar to other vertebrates. Following 31 hours of light damage, Sox2 expression significantly increased in proliferating Müller glia. Morpholino-mediated knockdown of Sox2 expression resulted in decreased numbers of proliferating Müller glia, while induced overexpression of Sox2 stimulated Müller glia proliferation in the absence of retinal damage. Thus, Sox2 is necessary and sufficient for Müller glia proliferation. We investigated the role of Wnt/β-catenin signaling, which is a known regulator of sox2 expression during vertebrate retinal development. While β-catenin 2, but not β-catenin 1, was necessary for Müller glia proliferation, neither β-catenin paralog was required for sox2 expression following retinal damage. Sox2 expression was also necessary for ascl1a (neurogenic) and lin28a (reprogramming) expression, but not stat3 expression following retinal damage. Furthermore, Sox2 was required for Müller glial-derived neuronal progenitor cell amplification and expression of the pro-neural marker Tg(atoh7:EGFP). Finally, loss of Sox2 expression prevented complete regeneration of cone photoreceptors. This study is the first to identify a functional role for Sox2 during Müller glial-based regeneration of the vertebrate retina.
Keywords: Müller glia, regeneration, Sox2, neuronal progenitor cell, β-catenin
INTRODUCTION
Adult zebrafish can regenerate a multitude of tissues and organ systems following damage, including the brain, spinal cord and retina (Vihtelic and Hyde, 2000; Fausett and Goldman, 2006; Bernardos et al., 2007; Kassen et al., 2007; Kroehne et al., 2011; Dias et al., 2012). Retinal regeneration in zebrafish is driven by damage-induced reprogramming of Müller glia that divide to produce a population of neuronal progenitor cells (NPCs), which continue to proliferate and migrate to the site of neuronal damage and differentiate into the neuronal types that were lost (Vihtelic and Hyde, 2000; Fimbel et al, 2007; Yurco and Cameron, 2007; Ramachandran et al, 2010; Ramachandran et al, 2011; Nelson et al, 2012; Wan et al, 2012).
It is likely that Müller glia reprogramming and cell cycle reentry are the rate limiting steps for zebrafish retinal regeneration. Not surprisingly, many of the genes required for somatic cell reprogramming to produce induced pluripotent stem cells (iPSCs; Takahashi et al., 2006), such as sox2, klf4, oct4, myc and nanog, are also expressed in the damaged zebrafish retina (Ramachandran et al., 2010). However, their spatial expression pattern and potential functional role in Müller glia reprogramming during retinal regeneration has not been examined.
Of these reprogramming factors, Sox2 (Sry-box 2) is particularly interesting, as it is expressed in the developing vertebrate nervous system from its earliest origins (Okuda et al., 2006) and is widely considered an adult neural stem cell (NSC) marker in neurogenic regions of the adult brain (Favaro et al., 2009). In the developing vertebrate retina, Sox2 maintains both the neurogenic and gliogenic potential of retinal progenitor cells in a dose-dependent manner (Taranova et al., 2006; Agathocleous et al., 2009).
In the mature vertebrate retina, Sox2 is expressed in the Müller glia and a subset of amacrine cells (Taranova et al., 2006; Lin et al., 2009; Surzenko et al., 2013). Müller glia in the postnatal mouse retina require Sox2 to prevent their terminal mitotic division (Surzenko et al., 2013). Conditional ablation of Sox2 in Müller glia causes loss of radial morphology, loss of quiescence and global disruption of retinal morphology (Surzenko et al., 2013). Maintaining these progenitor qualities in Müller glia is the fundamental basis of retinal regeneration in zebrafish.
We report a key role for Sox2 in Müller glia reprogramming during zebrafish retinal regeneration. While basal Sox2 expression is maintained in amacrine cells and Müller glia of the mature zebrafish retina, Sox2 expression significantly increased in reprogrammed Müller glia as they began proliferating in the regenerating retina. Further, Sox2 expression was necessary, and ectopic Sox2 was sufficient, for Müller glia proliferation. Sox2 expression was also required for maximal ascl1a expression, most likely through induction of lin28a-dependent repression of let7 miRNA biogenesis. In addition, Sox2 was required for amplification of Müller glial-derived NPCs and expression of the pro-neural marker atoh7 in late-stage NPCs. These data demonstrate a key role for Sox2 in regulating Müller glia-dependent regeneration of retinal neurons in zebrafish and provide a foundation for future comparative studies with the damaged mammalian retina.
MATERIALS AND METHODS
Zebrafish maintenance and light-lesion protocol
Wild-type AB, albinob4, albinob4; Tg(gfap:EGFP)nt11 (Kassen et al., 2007), Tg(hsp70l:sox2)x21 (Millimaki et al., 2010), and Tg(atoh7:EGFP)rw021 (Masai et al., 2003; Fimbel et al., 2007) zebrafish lines were maintained in the Center for Zebrafish Research at the University of Notre Dame Freimann Life Science Center. Adult zebrafish used for these studies were between 6–12 months old (4–5 cm) and maintained under a standard 14 hour light-10 hour dark cycle at 28.5°C (Westerfield, 1993). Rod and cone cell death was induced according to established protocols (Vihtelic and Hyde, 2000; Vihtelic et al., 2006). Briefly, adult fish were dark adapted for 14 days, then transferred to clear polycarbonate tanks placed between four fluorescent bulbs (15,000–20,000 lux) for up to 4 days. At various time points during or after light treatment, fish were euthanized by anesthetic overdose of 0.2% 2-phenoxyethanol and eyes were enucleated for further processing. All experimental protocols were approved by the animal use committee at the University of Notre Dame and are in compliance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
Heat shock
Adult Tg(hsp70l:sox2) x21 transgenic zebrafish and wild-type siblings were genotyped using the following primers: sox2 R (5′-CTTCAGCTCGGTTTCCATCATG-3′) and hsp70l F (5′-CTCCTCTCAATGACAGCTG-3′). Fish were heat shocked daily at 38°C for two to four days. Fish were transferred to 3-inch diameter polycarbonate tubes (3–4 fish per tube) with mesh screen bottoms in a circulating water bath. Water temperature was set to 28°C and gradually ramped up to 38°C over the course of 30 minutes. Fish were maintained at 38°C for one hour before being transferred back to plastic tanks filled with 38°C water. Water temperature was allowed to cool slowly to ambient temperature before being placed back on the system.
Pharmacological treatment
Injections of RO4929097 and recombinant zebrafish TNFα were performed as previously described (Conner et al., 2014). Briefly, adult AB zebrafish were injected intraperitoneally with 25 μL of 1 mM RO4929097 using a 30-gauge beveled needle. Recombinant TNFα (0.5–1 μL at ~1 mg/mL concentration; Conner et al., 2014) was intravitreally injected into left eyes with a Hamilton syringe (World Precision Instruments) and a 30-gauge blunt end needle after using a sapphire blade (World Precision Instruments) to cut a small hole in the cornea. Control fish were intraperitoneally injected with 10% DMSO and left eyes injected with Ni-NTA elution buffer (50 mM Na2HPO4, 300 mM NaCl, 250 mM imidazole, pH 8.0; used to purify the recombinant TNFα). Injections were carried out every 12 hours for three days.
Injection and electroporation of morpholinos into adult zebrafish retinas
Morpholino-mediated knockdown of protein expression in adult zebrafish retinas was performed as previously described (Thummel et al., 2008; Thummel et al., 2011). Briefly, a 1 mM solution of lissamine-tagged morpholinos was intravitrially injected into the left eye of dark-adapted albino zebrafish prior to the initiation of light treatment. In a subset of experiments, electroporated Tg(gfap:EGFP) zebrafish retinas were maintained under standard light-dark cycle (14 hours/10 hours) for 2 to 5 days. The following morpholinos (MOs) were used in this study: standard control MO (GeneTools; Philomath, OR), ctnnb1 MO (5′-ATCAAGTCAGACTGGGTAGCCATGA-3′; previously validated by Gingerich et al., 2005), ctnnb2 MO (5′-AGCCATCGTTGCGTCAATCCTTTAG -3′; Xiong et al., 2006; Bellipanni et al., 2006), sox2 MO3 (5′-GAAAGTCTACCCCACCAGCCGTAAA-3′; Kamachi et al., 2008), ascl1a MO (5′-ATCTTGGCGGTGATGTCCATTTCGC-3′; Ramachandran et al., 2010), lin28a MO (5′-TGAGATGCGGATTTGCCGGGGGCAT-3′; Ramachandran et al., 2010). All the morpholinos used in this study were previously validated to confirm that they knockdown the expression of the desired target protein. Platinum plate electrode tweezers (CUY650-P3, Protech International Inc., San Antonio, TX) were used to deliver two 50 ms pulses (75 V with a 1 second pause between pulses) to the left eye using a CUY21 Square Wave Electroporator (Protech International Inc.). Fish were allowed to recover and were returned to the dark or placed in constant light for various periods of time.
Immunofluorescence
Fish were euthanized in 0.2% 2-phenoxyethanol. Eyes were enucleated and fixed in either 4% paraformaldehyde/1× PBS or in 9:1 ethanolic formaldehyde (Vihtelic and Hyde, 2000). After fixation in 4% PFA, eyes were briefly rinsed with 1× PBS, then washed 3 × 5 minutes in 5% sucrose/1× PBS. Eyes fixed in 9:1 were rehydrated through an ethanol series (90%, 80%, 70%, 50% v/v ethanol in water) and then washed with 5% sucrose as described above. Eyes were incubated in 30% sucrose/1× PBS at 4°C overnight. The next day, eyes were transferred to a 2:1 mixture of tissue freezing media (TFM, Triangle Biomedical Sciences, Durham, NC) and 30% sucrose/1× PBS and incubated at 4°C overnight. Eyes were then embedded in 100% TFM and frozen at −80°C. Cryosections (12–14 μm) were prepared and stored at −80°C.
Frozen retinal sections were dried at 55°C for 20 minutes before rehydrating in 1× PBS for 20 minutes. Sections were blocked for one hour in blocking buffer (1× PBS, 4% normal goat serum, 0.4% Triton X-100, 1% DMSO). Primary antibodies were diluted in blocking buffer, applied to sections and incubated overnight at 4°C. The primary antibodies used in these studies and their dilutions were as follows: mouse monoclonal anti-PCNA antibody (1:1000; P8825, clone PC10, Sigma-Aldrich, St. Louis, MO), goat anti-Sox2 polyclonal antiserum (1:100; AF2018, R&D Systems, Minneapolis, MN), rabbit anti-β-catenin polyclonal antiserum (1:100; ab6302, Abcam, Cambridge, MA), rabbit anti-GFP polyclonal antiserum (1:500; ab6556, Abcam), mouse anti-HuC/D monoclonal antibody (1:250; A21271, clone 16A11, Life Technologies, Carlsbad, CA), mouse anti-zpr-1 monoclonal antibody (1:200; ZIRC, Eugene, OR), rabbit anti-Blue opsin polyclonal antiserum (1:500; Vihtelic et al., 1999), rabbit anti-UV opsin antiserum (1:500; Vihtelic et al., 1999), and mouse anti-glutamine synthetase monoclonal antibody (1:500; MAB302, clone GS-6, Millipore, Billerica, MA). Sections were washed 3 × 10 minutes in PBST (1× PBS with 0.5% Tween-20). Fluorescent-labeled secondary antibodies were diluted 1:500 in PBST and applied to sections for 1 hour at room temperature. DAPI was routinely added to the secondary antibody solution at a concentration of 5 μg/mL to label nuclei. Secondary antibodies used in these studies were as follows: goat anti-mouse IgG or goat anti-rabbit IgG conjugated to Alexa Fluor 488, 568, 594, or 647; chicken anti-goat IgG conjugated to Alexa Fluor 488 (Jackson Immunoresearch Laboratories). Following secondary antibody incubation, sections were washed 3 × 10 minutes in PBST and once for 5 minutes in 1× PBS. Slides were mounted with glass coverslips and VECTASHIELD (Vector Laboratories, Burlingame, CA).
In experiments utilizing goat anti-Sox2 polyclonal antiserum, the blocking buffer was prepared with an equivalent amount of normal chicken serum rather than normal goat serum. If the goat anti-Sox2 polyclonal antiserum was used for co-labeling with either rabbit or mouse antibodies, the secondary antibody staining was carried out in two steps. First, chicken anti-goat Alexa Fluor 488 was incubated for 1 hour and washed as described above for secondary antibody staining. This process was then repeated with a goat anti-rabbit and/or goat anti-mouse Alexa Fluor antibodies required to detect the additional primary antibodies.
To detect Sox2, HuC/D and PCNA (in retinas fixed in 4% PFA) antigen retrieval was required (Raymond et al, 2006; Nelson et al, 2012; Nelson et al, 2013). Coplin jars were filled with citrate buffer (10 mM citrate pH 6.0 with 0.1% Tween-20) and placed in boiling water baths. Warmed slides were placed directly into the hot citrate solution and incubated for 20 minutes, covered. Coplin jars were then removed from water baths and allowed to cool gradually at room temperature (~45 minutes). Slides were then briefly rinsed 3 × 2 minutes in 1× PBS and transferred to block as described above.
EdU labeling and detection
To label proliferating cells, we intraperitoneally injected 30–50 μL of 1 mg/ml EdU (5-ethynyl-2′-deoxyuridine) into adult zebrafish one hour before fixation at either 0, 8, 16, 20, 25, or 31 hours of constant light. Frozen sections were prepared as described above. For EdU detection, we utilized the Click-iT EdU Alexa Fluor 647 Imaging Kit (Invitrogen) as previously described (Conner et al., 2014; Lahne et al., 2015) prior to starting the immunohistochemistry.
In other experiments, we injected EdU four hours prior to the first heat shock of adult Tg(hsp70l:sox2) x21 transgenic zebrafish to label any Müller glia involved in persistent rod neurogenesis (Fig. 8K). Retinas were examined at 2 days post-heat shock for EdU incorporation as described above.
Figure 8. Ectopic Sox2 expression does not induce cell death or expansion of non-quiescent Müller glia.
Tg(hsp70l:sox2)x21 transgenic zebrafish or wild-type (WT) sibling control zebrafish were exposed to one hour of heat shock daily at 38°C for either two or four days. Retinal sections were analyzed for cell death using TUNEL assays (A–J). DNase I-treated AB control retinal sections displayed uibiquitous TUNEL-positive cells throughout cell nuclei in the retina (E and J). TUNEL-positive cells were not observed in either Tg(hsp70l:sox2) (C–D, H–I) or wild-type (A–B, F–G) sibling control zebrafish at either time point analyzed. Tg(hsp70l:sox2) transgenic zebrafish were injected with EdU 4 hours prior to beginning the 2 day heat shock regimen (K). Retinal sections revealed no PCNA-positive cells that were also EdU-positive (L–N). The occasional EdU-positive cells in the ONL (L–N, arrowhead) likely represent cycling rod precursor cells. Retinas were stained with DAPI to detect the nuclear layers (F–J, N). TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; PCNA, proliferating cell nuclear antigen; EdU, 5-ethynyl-2′-deoxyuridine; GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Scale bars in panels A and L are 25 μm and are the same for panels B–J and M–N, respectively.
TUNEL
Terminal deoxynucleotidyl transferase-mediated biotinylated dUTP nick end labeling (TUNEL) was performed using the ApoAlert DNA Fragmentation Assay kit (Clonetech, Mountain View, CA) as previously described (Bailey et al., 2010; Nelson et al., 2013). Biotinylated dNTPs were detected by using Alexa Fluor 488-conjugated streptavidin (Life Technologies) diluted 1:200 in PBS for 20 minutes at room temperature. Slides were mounted with glass coverslips and VECTASHIELD (Vector Laboratories, Burlingame, CA).
Immunoblot Analysis
Total protein lysates were obtained by pooling 5–10 adult dorsal retinas per treatment and homogenizing in protein extraction buffer (1× PBS, 1% Triton-X-100, protease inhibitors; 10 μL per retina; Roche Applied Science, Indianapolis, IN) using a polypropylene micro pestle. Lysates were incubated for one hour on ice then centrifuged briefly to remove Triton-X-100-insoluble debris and the supernatant was collected and stored at −80°C. Protein concentration was determined using Bradford assay (Life Technologies) and 10 μg of lysate was combined with Novex 2× tris-glycine-SDS sample buffer and 10× sample reducing agent (Life Technologies). Samples were heated at 95°C for 5 minutes and then electrophoresed through Novex 4%–12% tris-glycine gels (Life Technologies). Proteins were transferred to Hybond-P PVDF membrane (GE Healthcare, Piscataway, NJ) and then blocked for 1 hour at room temperature blocking buffer composed of PBST (1× PBS with 0.1% Tween-20) with 5% (w/v) nonfat dry milk. Membranes were probed with the following antibodies diluted in blocking buffer overnight at 4°C: rabbit anti-Sox2 polyclonal antiserum (1:2000; GTX124477, GeneTex), mouse anti-glutamine synthetase monoclonal antibody (1:1000; MAB302, clone GS-6, Millipore), rabbit anti-GFAP polyclonal antiserum (1:5000; Z0334, Dako, Carpinteria, CA), and mouse anti-actin monoclonal antibody (1:5000, clone AC-40, Sigma-Aldrich).
Membranes were washed three times for 5 minutes, then once for 15 minutes in PBST before incubating with ECL HRP-conjugated secondary antibodies (1:10,000, GE Healthcare) diluted in blocking buffer. Membranes were washed as before and then developed using the ECL Prime kit (GE Healthcare). Exposed film was imaged using a Carestream Gel Logic 2200 Pro imaging station (Carestream Health, Rochester, NY). Actin was used as a loading control for all blots.
Quantitative Real-Time PCR
RNA was prepared from light treated retinas as previously described (Nelson et al., 2012; Nelson et al., 2013). Briefly, 8–10 dorsal retinas per time point or treatment group were dissected and pooled. RNA was extracted using TRIzol reagent (Life Technologies) and purified RNA was DNase treated using the Turbo DNA-free Kit (Life Technologies). Total cDNA was generated from 1 μg of RNA using qScript cDNA SuperMix (Quanta Biosciences, Gaithersburg, MD).
Reactions were assembled using PerfeCta SYBR Green SuperMix (low ROX; Quanta Biosciences). For most targets, 25 ng of cDNA was sufficient for amplification within an optimal Ct range. For 18S controls, 0.25 ng of cDNA were used. The gene specific primers for these studies (Table 1) were used at a final concentration of 300 nM. Reactions were carried out and data acquired using the ABI 7500 Fast Real-Time PCR system (Applied Biosystems, Foster City, CA). Cycling conditions were as follows: 2 minutes at 50°C,10 minutes at 95°C, 40 cycles of 15 seconds at 95°C and 1 minute at 60°C, with data collection occurring after extension for each cycle. Dissociation curve analysis verified that single products were produced with each set of the primer pairs used in these experiments. For each gene examined by qRT-PCR, cDNA from each time point or treatment was run in triplicate and the median Ct value was normalized against the 18S rRNA Ct. The comparative ΔΔCt method was used for data analysis, with 0 hours of light serving as the reference time point and the 18S rRNA as the reference gene to generate a log2-fold difference in gene expression levels (Johnson et al., 2000; Vong et al., 2003). For knockdown experiments measuring gene expression among different treatments at the same time point, data are presented in linear fold-change.
Table 1.
qRT-PCR primers.
| Gene Name | Primers for qRT-PCR |
|---|---|
| sox2 | F: 5′-GCTCCAGTACAACTCCATGAC-3′ |
| R: 5′-CTGCGAATAGGACATGCTGTAG-3′ | |
| ctnnb1 | F: 5′-CAAGAGCAAGTAGCAGACATCG-3′ |
| R: 5′-CTGTGTGGAAGGTATCTGCATG-3′ | |
| ctnnb2 | F: 5′-AACTACCAGGACGATGCAGAG-3′ |
| R: 5′-CTTTATTCACCACCACCTGGTC-3′ | |
| stat3 | F: 5′-GAGGAGGCGTTTGGCAAA-3′ |
| R: 5′-TGTGTCAGGGAACTCAGTGTCTG-3′ | |
| ascl1a | F: 5′-GCCAGACGGAACGAGAGAGA -3′ |
| R: 5′-AGGGTTGCAAAGCCGTTG-3′ | |
| lin28 | F: 5′-TAACGTGCGGATGGGCTTCGGATTTCTGTC-3′ |
| R: 5′-ATTGGGTCCTCCACAGTTGAAGCATCGATC-3′ | |
| 18s | F: 5′-TCGGCTACCACATCCAAGGAAGGCAGC-3′ |
| R: 5′-TTGCTGGAATTACCGCGGCTGCTGGCA-3′ |
Confocal Imaging and Statistical Analysis
Confocal imaging was performed with a Nikon A1R laser scanning confocal microscope. Low-intensity signals were enhanced and background signals were reduced in representative images using the levels function in Adobe Photoshop (Adobe Systems, San Jose, CA). Levels were adjusted identically to all layers within a panel and to all panels in a figure. Red-green color scheme images were converted to magenta-green using Adobe Photoshop as described previously (Montgomery et al., 2010).
To compare Sox2 expression levels at 36 hours of constant light treatment to Sox2 levels in undamaged retinas, acquisition settings were first set to maximize the dynamic range at the higher expressing time point (36 hours) and maintained for the undamaged retinas. To maintain consistency between eyes and experimental groups, only retinal sections encompassing or immediately adjacent to, the optic nerve were utilized. Quantification of all markers was performed on 5 μm thick confocal z-stacks from the middle of the 12–14 μm sections. All images were acquired across a 350 μm region of the central/dorsal retina that consistently received the same degree of damage and reliably resulted in comparable subsequent high levels of Müller glia proliferation (Thomas et al., 2012). Retinas from ≥ 5 different individual fish were examined for every control and experimental group at each time point in this study.
Statistical significance was determined between control and experimental groups of all experiments using either a two-tailed Student’s t-test (when analyzing two data points) or ANOVA (with Tukey’s post-hoc test) where appropriate. For either statistical test, p-values less than 0.05, 0.01, and 0.005 were considered significant, highly significant, and very highly significant, respectively. The text states either the ANOVA p value (if it is not significant) or the Tukey’s post-hoc p value (if the ANOVA p value is significant), with both the ANOVA and Tukey’s post-hoc p values stated in the figure legend. In some cases (Figs. 9 and 10), qRT-PCR data is given for both 0 and 31 hours. In these cases, the 0 hour timepoint is shown to reveal the extent of increased expression at 31 hours in the control. Thus, the statistical analysis is solely between the control and morphant values at 31 hours and a Student’s t-test was used. The statistical test used in each analysis is stated in the corresponding Figure legend.
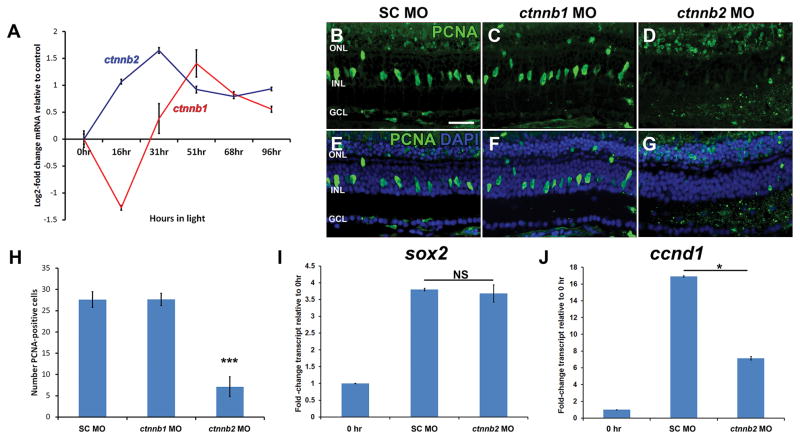
Figure 9. β-catenin 2 is required for Müller glia proliferation, but does not regulate sox2 expression.
RNA was isolated from dark-adapted albino zebrafish retinas either immediately prior to (0 hr) or following 16, 31, 51, 68 or 96 hours of constant light treatment. qRT-PCR revealed differential expression of ctnnb2 and ctnnb1 (A). Adult albino zebrafish retinas were injected and electroporated with either standard control morpholino (SC MO), anti-ctnnb1 morpholino (ctnnb1 MO), or anti-ctnnb2 morpholino (ctnnb2 MO) immediately prior to light treatment. After 31 hours of constant light, multiple PCNA-positive cells were observed in the INL of SC morphant (B and E) and ctnnb1 morphant (C and F) retinas, while significantly fewer PCNA-positive cells were observed in the INL of ctnnb2 morphant retinas (D, G, H; ANOVA p = 4.23 × 10−8, Tukey’s post-hoc test p = 0.001, n = 9). Retinas were stained with DAPI to detect the nuclear layers (E–G). RNA isolated after 31 hours of constant light was analyzed by qRT-PCR and revealed a similarly large increase in sox2 expression in both SC and ctnnb2 morphant retinas relative to the 0 hour control, but not a significant difference between the SC and ctnnb2 morphant at 31 hours (I; Student’s t-test p = 0.07, n = 3). To independently assay the quality of the mRNA, qRT-PCR confirmed cyclin D1 (ccnd1) expression was significantly reduced in ctnnb2 morphant retinas relative to SC morphant after 31 hours of light (J; Student’s t-test p = 3.11 × 10−7, n = 3). PCNA, proliferating cell nuclear antigen; GCL, ganglion cell layer; INL, inner nuclear layer; NS, not significant; ONL, outer nuclear layer. Scale bar in panel B is 25 μm and is the same for panels C–G.
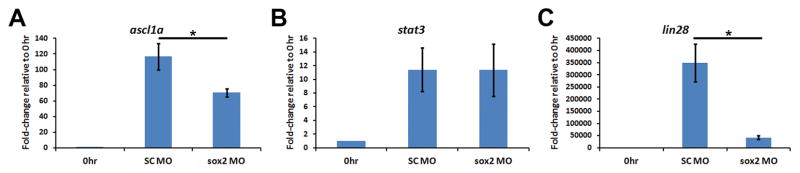
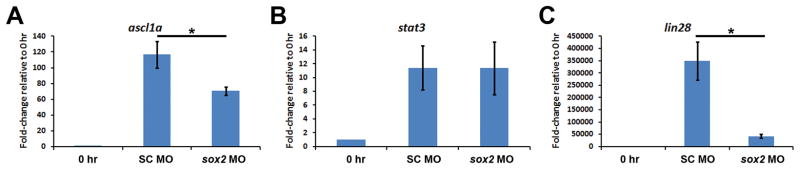
Figure 10. Sox2 regulates ascl1a and lin28a, but not stat3, expression in the regenerating zebrafish retina.
Adult albino zebrafish eyes were either uninjected, or injected and electroporated with either standard control morpholino (SC MO) or anti-sox2 morpholino (sox2 MO) immediately prior to the onset of light treatment. Total RNA was isolated from retinas after 31 hours of constant light and expression of ascl1a, stat3, and lin28a were assayed via qRT-PCR. Knockdown of Sox2 expression had no effect on stat3 expression (B; Student’s t-test, p > 0.9, n = 3). In contrast, expression of both ascl1a (A; Student’s t-test, p < 0.0001, n = 3) and lin28a (C; Student’s t-test, p = 0.001, n = 3) decreased significantly in sox2 morphant retinas relative to SC morphant retinas. The 0 hour control is shown for each transcript to simply demonstrate the extent of increased gene expression in the control at 31 hours.
Morphometric Measurements
To determine the average thickness of rod outer segments (ROS), confocal images of retinal sections labeled for rhodopsin were divided into thirds laterally. Linear measurements of the Rhodopsin-positive outer segments (roughly, from the outer surface of the cone nuclei to the end of Rhodopsin signal) were taken at the center of each third of the frame. These measurements were averaged for each retinal section (n = 10 retinas per treatment).
Rod nuclei were counted along a central 100 μm length of the ONL (~30% of the field of view for one confocal image).
RESULTS
Sox2 expression in adult zebrafish retinas recapitulates that of other vertebrates
Expression of Sox2 in the developing vertebrate retina has been widely studied in rodent, chick, amphibians and fish (Van Raay et al., 2005; Taranova et al., 2006; Lin et al., 2009; Agathocleous et al., 2009; Meyers et al., 2012). In addition, Sox2 expression persists into adulthood in a subpopulation of amacrine cells localized in the inner nuclear and ganglion cell layers and all Müller glia in most vertebrates (Taranova et al., 2006; Lin et al., 2009; Surzenko et al., 2013). While this Sox2 expression pattern is also present in juvenile zebrafish (Meyers et al., 2012), expression of sox2 RNA was not detected in the adult retina (Ramachandran et al., 2010).
To examine Sox2 expression in the adult zebrafish retina, undamaged albino; Tg(gfap:EGFP) zebrafish retinal sections were co-labeled with antibodies against Sox2, EGFP to label the Müller glia (based on the transgenic line expressing EGFP from the glial fibrillary acidic protein promoter (Kassen et al., 2007)), and the amacrine and ganglion cell marker HuC/D (Fig. 1A–E). Sox2 expression was detected in both the INL and GCL. A subset of Sox2-positive cells coexpressed with HuC/D-positive amacrine cells in the INL and displaced amacrine cells in the GCL (Fig. 1A–C; arrows). Additionally, Sox2 expression was detected in a population of HuC/D-negative INL cells with fusiform-shaped nuclei (Fig. 1A–C; arrowheads), indicative of Müller glia. These fusiform-shaped INL nuclei co-labeled for Sox2 and the Müller glial marker EGFP in the Tg(gfap:EGFP) transgenic line, confirming that they were Müller glia (Fig. 1A, D, E; arrowheads). Thus, Sox2 expression in the adult zebrafish retina recapitulates that of other vertebrates. It is worth noting that, despite Sox2 expression in the ciliary marginal zone (CMZ) of embryonic and juvenile zebrafish retinas (Meyers et al., 2012), we did not detect Sox2 expression in the CMZ of the adult retina (data not shown).
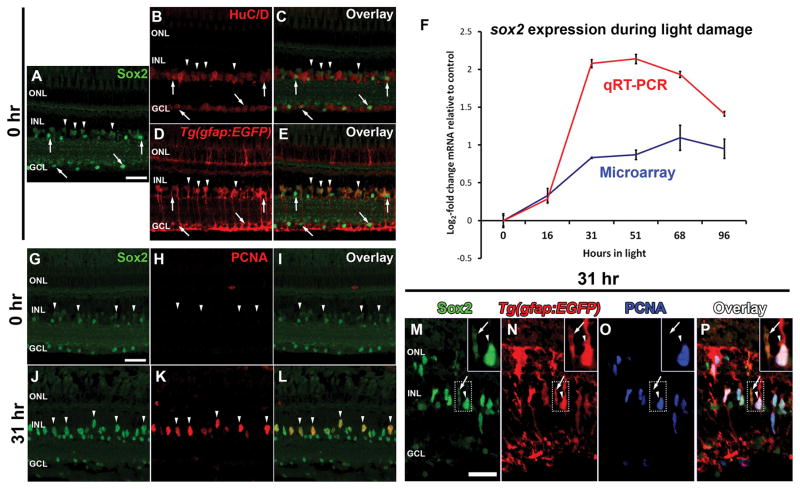
Figure 1. Sox2 expression is dynamic in the regenerating zebrafish retina.
Cryosections of undamaged adult albino;Tg(gfap:EGFP) zebrafish retinas were prepared and labeled with anti-Sox2 antiserum (A–E) and either the amacrine and ganglion cell marker HuC/D (B,C) or anti-EGFP to label Müller glia (D,E). Sox2 co-labeled with HuC/D-positive round nuclei in the INL and GCL (A–C; arrows) and fusiform Müller glia nuclei in the INL (A, D, E, arrowheads). RNA was isolated from adult zebrafish retinas at 0, 16, 31, 51, 68 and 96 hours of constant intense light. Total cDNA was prepared and sox2 expression analyzed via qRT-PCR (F). After 31 hours of constant light, sox2 expression increased > 2 log2-fold, and remained elevated throughout the remainder of the time course. This increased sox2 expression is consistent with our previous microarray data (Kassen et al., 2007; F). Retinal cryosections from undamaged (0 h, G–I) or 31 hour light-damaged eyes (J–L) revealed low levels of Sox2 expression in Müller glia of the undamaged retina (G and I, arrowheads) and greatly increased expression in PCNA-positive INL cells following 31 hours of light (J–L, arrowheads). The acquisition settings for panels G–L were equivalent and set for an optimal dynamic range for Sox2 expression at the 31 hour time point (J and L). Co-labeling of Sox2, PCNA and EGFP in Tg(gfap:EGFP) transgenic zebrafish retinas (M–P), which express EGFP in the Müller glia, revealed elevated Sox2 expression in PCNA-positive Müller glia compared to PCNA-negative Müller glia (arrowheads and arrows, respectively) after 31 hours of light. Inset corresponds to the boxed region in each panel and contains both a PCNA-positive and PCNA-negative Müller glia (arrowhead and arrow, respectively). qRT-PCR, quantitative real-time polymerase chain reaction; PCNA, proliferating cell nuclear antigen; GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Scale bars in panels A, G and M are 25 μm and are the same for panels B–E, H–L and N–P, respectively.
Müller glia upregulate Sox2 expression following retinal damage
Because Sox2 expression persists in the Müller glia of adult zebrafish retinas and Sox2 is widely considered an adult neural stem cell marker, we hypothesized that Sox2 may be required for Müller glia reprogramming and proliferation in the damaged zebrafish retina. Using qRT-PCR, sox2 expression increased dramatically by 31 hours of light damage (Fig. 1F). This time point corresponds to the initiation of Müller glia proliferation (Kassen et al., 2007). The elevated sox2 expression profile through 96 hours of light treatment is similar to our previous microarray study (Fig. 1F; Kassen et al., 2007), as well as previous reports in the stab-lesioned retina (Ramachandran et al., 2010).
We investigated the spatial pattern of increased Sox2 protein expression in the light-damaged retina. The pattern of Sox2 expression in the undamaged retina at 0 hours of constant light (Fig. 1G–I) recapitulated what we described previously (Fig. 1A). At 0 hours, we also did not detect any INL cells that labeled with the proliferation marker PCNA (Fig. 1H, I). After 31 hours of constant light, increased Sox2 expression was detected in a population of fusiform-shaped, PCNA-positive INL nuclei, relative to the weak expression observed in the fusiform-shaped INL nuclei of the undamaged retina (Fig. 1J–L vs G–I; arrowheads, respectively). At 31 hours, the fusiform-shaped nuclei expressing increased levels of Sox2 and PCNA also co-labeled with GFP in Tg(gfap:EGFP) transgenic zebrafish retinas (Fig. 1M–P; arrowhead). Importantly, non-proliferating (PCNA-negative) Müller glia displayed basal levels of Sox2 expression (Fig. 1M–P, insert, arrow). Together, these data suggest that Sox2 expression is maintained at low levels in amacrine cells and Müller glia in the undamaged retina, and increases in reprogrammed Müller glia that are proliferating in response to light-induced photoreceptor cell death.
Sox2 expression is required for Müller glia proliferation
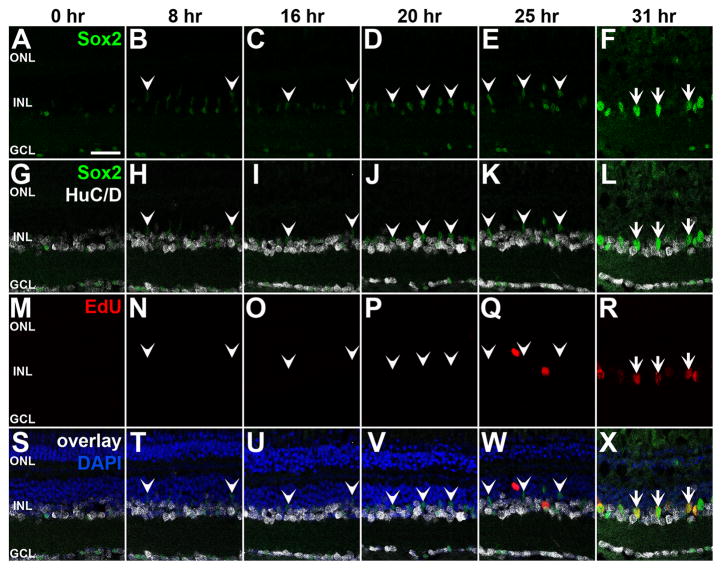
To determine whether increased Sox2 levels preceded Müller glia reentering the cell cycle, we placed dark-adapted albino zebrafish into constant intense light and intraperitoneally injected EdU 1 hour before eyes were collected at 0, 8, 16, 20, 25 or 30 hours of constant intense light-treatment. Retinal sections revealed that Sox2 expression was slightly elevated at 8 and 16 hours of constant light relative to the 0 hour timepoint and further increased at 20 hours in HuC/D-negative INL cells (Fig. 2, arrowheads). In contrast, EdU labeling was not detected until 25 hours of constant light (Fig. 2). Thus, Sox2 expression increased prior to EdU labeling, further suggesting that Sox2 may be required for Müller glia proliferation in the light-damaged retina.
Figure 2. Sox2 expression increases in the light-damaged retina prior to Müller glia proliferation.
Dark-adapted adult albino zebrafish were placed in constant intense light and one hour before eyes were fixed at 0, 8, 16, 20, 25 and 31 hours zebrafish were intravitreally injected with 1 mg/ml of EdU. Cryosections were labeled for Sox2 (A–L, S–X), HuC/D (G–L, S–X), EdU (M–X) and DAPI (S–X). Sox2 protein expression in HuC/D-negative cells (i.e. Müller glia) began to increase at 20 hours of light-treatment compared to undamaged retinas (0 h) and continued to increase in expression at subsequent timepoints, while EdU was only incorporated into cells beginning at 25 hours of light-treatment. Notably, the EdU-positive cells were Sox2-positive and HuC/D-negative, consistent with them representing Müller glia. Arrowheads mark Sox2-positive cells that are HuC/D-negative and EdU-negative, while arrows identify Sox2-positive cells that are HuC/D-negative and EdU-positive. PCNA, proliferating cell nuclear antigen; GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Scale bar in A, 25 μm and is the same for panels B–X.
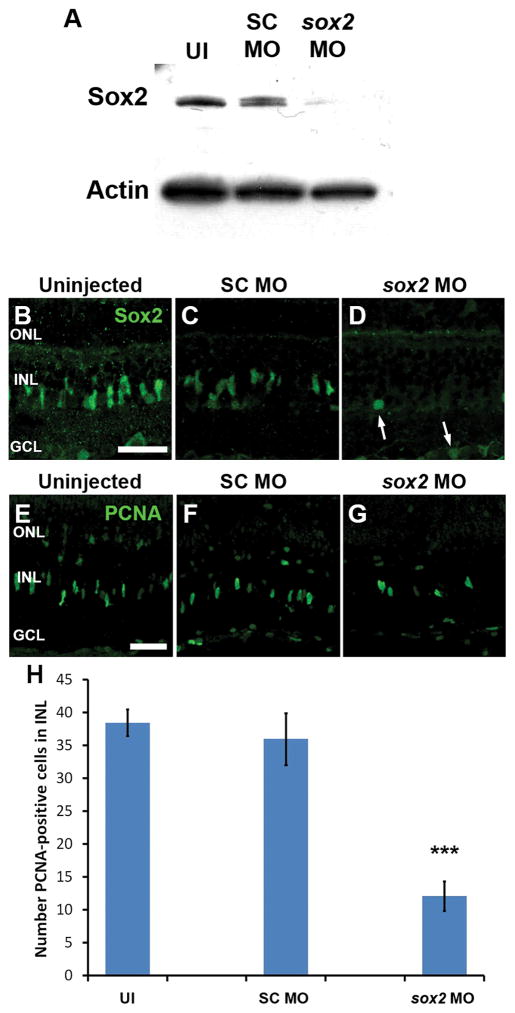
To test whether Sox2 expression is required for Müller glia proliferation, we electroporated morpholinos to knockdown Sox2 expression in adult retinas immediately prior to initiating light damage (Thummel et al., 2008). The anti-sox2 morpholino was previously shown to knockdown both endogenous Sox2 expression and a luciferase-based reporter harboring the sox2 5′-UTR to a greater extent than any other translation-blocking anti-sox2 morpholino (Kamachi et al., 2008). Western blot analysis revealed a global reduction in Sox2 expression at 72 hours postfertilization in protein lysates from sox2 morphant embryos (sox2 MO) relative to either uninjected (UI) or standard control morphant (SC MO) embryos (Fig. 3A). Thus, we confirmed that this morpholino effectively targeted and knocked down endogenous Sox2 expression. Similarly, Sox2 expression was greatly reduced in the central-dorsal region of sox2 MO retinas after 31 hours of constant light relative to either uninjected or SC MO control retinas (Fig. 3B–D), confirming the successful Sox2 knockdown in the adult zebrafish retina. The few remaining Sox2-positive cells observed in the sox2 MO retinas were generally circular nuclei in either the INL or GCL (Fig. 3D; arrows), which are likely amacrine cells, rather than the fusiform-shaped Müller glia nuclei.
Figure 3. Sox2 is required for Müller glia proliferation in the light-damaged zebrafish retina.
Immunoblots were performed on protein lysates prepared from 72 hours postfertilization (hpf) embryos that were either uninjected (UI), or injected with standard control morpholino (SC MO) or anti-sox2 morpholino (sox2 MO) at the 1–4 cell stage (A). These blots revealed a dramatic reduction of Sox2 expression in the sox2 MO embryos compared to controls. Actin was used as a loading control. Adult eyes were either uninjected or injected and electroporated with SC MO or sox2 MO immediately prior to 36 hours of constant light damage. Retinal sections were labeled with either Sox2 antibodies (B–D) or PCNA antibodies (E–G). Sox2 expression was dramatically knocked down in the majority of the Müller glia and amacrine cells in sox2 MO retinas (D) relative to control retinas (B, C). Very few round Sox2-positive nuclei were observed in sox2 MO retinas (D, arrows). The sox2 MO retinas (G, H) also contained significantly fewer PCNA-positive cells relative to either control (E–F and H; ANOVA analysis, p = 6.68 × 10−8, Tukey’s post-hoc test p = 0.001, n ≥ 9). PCNA, proliferating cell nuclear antigen; GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer, UI, uninjected. Scale bars in panels B and E are 25 μm and are the same for panels C–D and F–G, respectively.
To assess the effect of Sox2 knockdown on Müller glia proliferation, we labeled uninjected, SC MO, and sox2 MO retinal sections with PCNA (Fig. 3E–G). Following 31 hours of light, uninjected and SC MO retinas contained an average of 38.5 ± 2.0 and 36.0 ± 3.9 PCNA-positive cells per 350 μm of retinal section, respectively (Fig. 3E, F, and H). In contrast, sox2 MO retinas displayed significantly fewer PCNA-positive cells per section (12.1 ± 2.3; Tukey’s post-hoc test p = 0.001 relative to both controls, n ≥ 9; Fig. 3G–H). Thus, Sox2 expression is required for Müller glia proliferation following retinal damage.
One possible explanation for reduced Müller glia proliferation following Sox2 knockdown is a reduction in photoreceptor cell death. To determine if reducing Sox2 expression was neuroprotective, we performed TUNEL analysis of uninjected, SC MO, and sox2 MO retinal sections following 16 hr of light (the peak of photoreceptor cell death; Nelson et al., 2013). We observed equivalent numbers of TUNEL-positive photoreceptors in uninjected, SC MO, and sox2 MO retinas (42.8 ± 6.4, 36.4 ± 3.5, and 37.6 ± 2.9, respectively; Fig. 4; ANOVA p = 0.589, n = 5). These data demonstrate that the reduced number of proliferating Müller glia in light-damaged sox2 MO retinas is not due to reduced photoreceptor cell death.
Figure 4. Sox2 knockdown does not affect light-induced photoreceptor cell death.
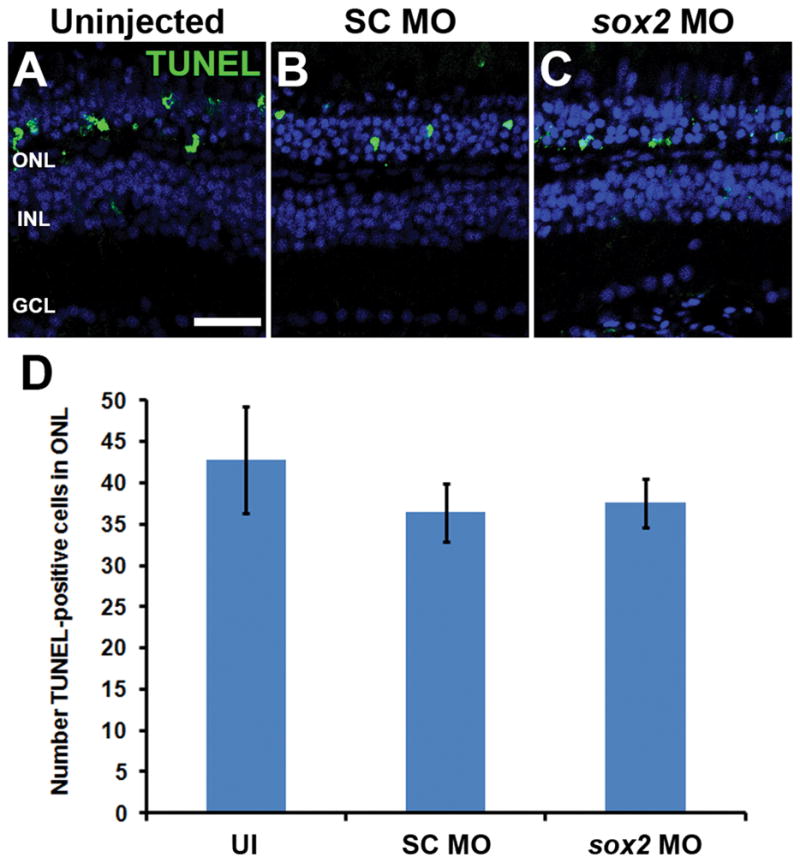
Adult albino zebrafish eyes were either uninjected (UI) or injected and electroporated with either standard control morpholino (SC MO) or anti-sox2 morpholino (sox2 MO). After 16 hours of constant light treatment, retinal cryosections were analyzed for cell death by TUNEL. The TUNEL signal in the ONL of uninjected, SC morphant and sox2 morphant retinas (A–C) was not statistically different (D; ANOVA p = 0.589, n = 5). Retinas were stained with DAPI to detect the nuclear layers (A–C). TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer, UI, uninjected. Scale bar in panel A is 25 μm and is the same for panels B and C.
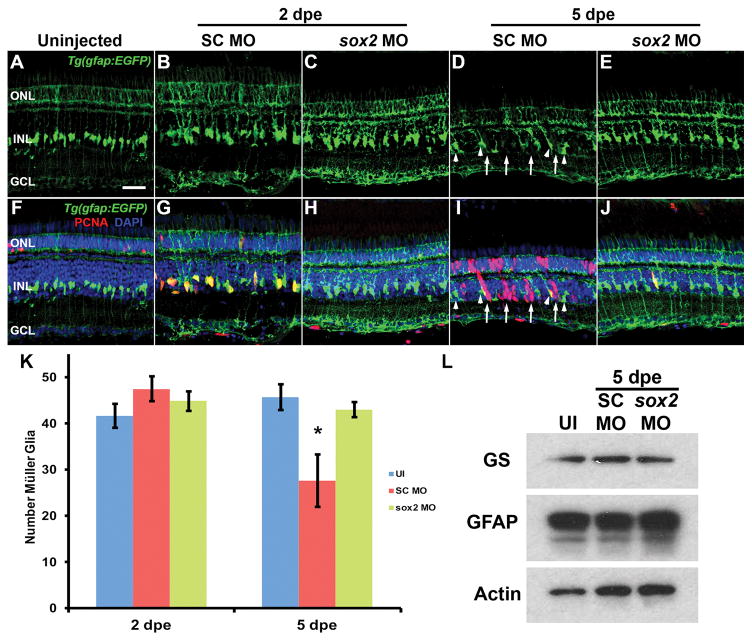
It is also possible that reduced Sox2 expression could decrease the number of Müller glia, which would result in fewer proliferating Müller glia. For example, conditional knockout of Sox2 expression in P0 mouse retinas resulted in the Müller glia losing their radial morphology, reentering the cell cycle and terminally dividing with a loss in the number of Müller glia (Surzenko et al., 2013). To determine if Sox2 is required to maintain Müller glia in the adult zebrafish retina, we knocked down Sox2 expression in Tg(gfap:EGFP) retinas and allowed them to recover for either 2 or 5 days under standard light conditions. At 2 days post electroporation (dpe), there was no significant difference in the number of Müller glia present in uninjected, SC MO or sox2 MO retinas (Fig. 5A–C, K; ANOVA p = 0.293, n = 5). At 5 dpe, the number of Müller glia (Fig. 5K, red bars) in sox2 MO retinas remained indistinguishable from uninjected retinas (Fig. 5A, E, K; ANOVA p = 0.874), while there was a significant reduction in the number of GFP-positive Müller glia in the SC MO retinas at 5 dpe (Fig. 5D, K, red bars; Tukey’s post-hoc test p < 0.024, n = 5). This is most likely due to the inherent damage caused by electroporation, which stimulates a regeneration response. The SC MO retina lacked the regular pattern of GFP-positive Müller glia, due to a subset of Müller glia reentering the cell cycle and producing PCNA-positive neuronal progenitor cell clusters (Fig. 5I). The location of these clusters coincided with the gaps in the regular pattern of GFP-positive Müller glia (Fig. 5D, I, arrows). This damage response is also evident by the presence of PCNA-positive Müller glia in the SC MO retina at 2 dpe (Fig. 5G) and the hypertrophied GFP-positive Müller glial processes in all the electroporated retinas (Fig. 5B–E, G–J) relative to the uninjected retinas. These data demonstrated that knockdown of Sox2 expression did not reduce the number of Müller glia. We independently confirmed that there was not a global reduction in the number of Müller glia by examining the expression of the Müller glia proteins, Glutamine Synthetase (GS) and Glial Acidic Fibrillary Protein (GFAP) on immunoblots. At 5 dpe, we detected relatively equivalent amounts of GS, GFAP, and actin control protein in uninjected (UI), SC MO or sox2 MO retinas (Fig. 5L). Taken together, the reduced number of proliferating Müller glia observed in light-damaged sox2 morphant retinas is most likely due to disrupting an endogenous Sox2-dependent Müller glia signaling pathway and not a global reduction in the number of Müller glia.
Figure 5. Sox2 is not required to maintain Müller glia identity in the undamaged zebrafish retina.
Eyes of adult albino;Tg(gfap:EGFP) zebrafish were either uninjected (A, F) or injected and electroporated with either the Standard Control morpholino (SC MO; B, D, G, I) or anti-sox2 morpholino (sox2 MO; C, E, H, J) and placed back in a standard light-dark cycle to recover. Retinal sections were immunolabeled for GFP (A–J) and PCNA (F–J) after either 2 (B, C, G, H) or 5 days post electroporation (dpe, D, E, I, J). At both time points, no differences in the morphology of Müller glia were observed between sox2 morphant retinas, SC morphant retinas and uninjected control retinas. There was no significant difference between the number of Müller glia in all three groups at 2 dpe (K; ANOVA p = 0.293, n = 5). At 5 dpe, the SC morphant retinas contained significantly fewer GFP-positive Müller glia relative to both the uninjected control and sox2 morphant retinas (K; ANOVA p = 0.0025 and Tukey’s post-hoc test p = 0.006 between UI and SC MO, p = 0.023 for SC MO and sox2 MO, n = 5), due to a subset of Müller glia reentering the cell cycle and producing clusters of PCNA-positive neuronal progenitor cells in the sox2 morphant (I, arrows; arrowheads indicate PCNA-negative GFP-positive Müller glia). The damage response is also evident by the presence of PCNA-positive Müller glia in the SC MO retina at 2 dpe (G) and the hypertrophied GFP-positive Müller glial processes in all the electroporated retinas (B–E, G–J) relative to the uninjected retinas. Retinas were stained with DAPI to detect the nuclear layers (F–J). At 5 dpe, immunoblots of retinal lysates confirmed that there was not a global reduction in the number of Müller glia based on the relatively equivalent expression of the Müller glia proteins, Glutamine Synthetase (GS) and Glial Acidic Fibrillary Protein (GFAP) in uninjected (UI), SC MO or sox2 MO retinas (L). PCNA, proliferating cell nuclear antigen; GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer, UI, uninjected. Scale bar in panel A is 25 μm and is the same for panels B–J.
Sox2 overexpression is sufficient to induce Müller glia proliferation without retinal damage
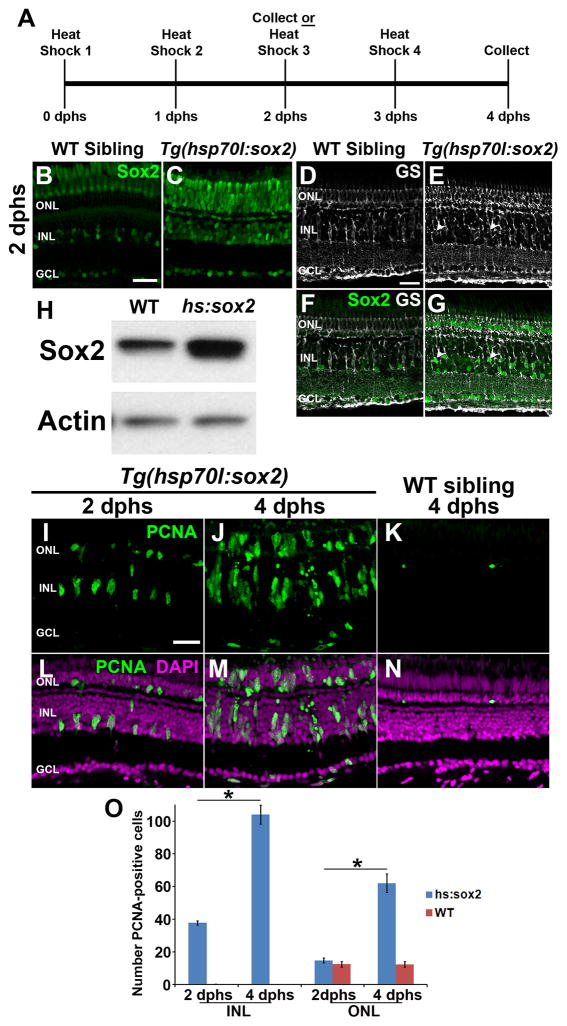
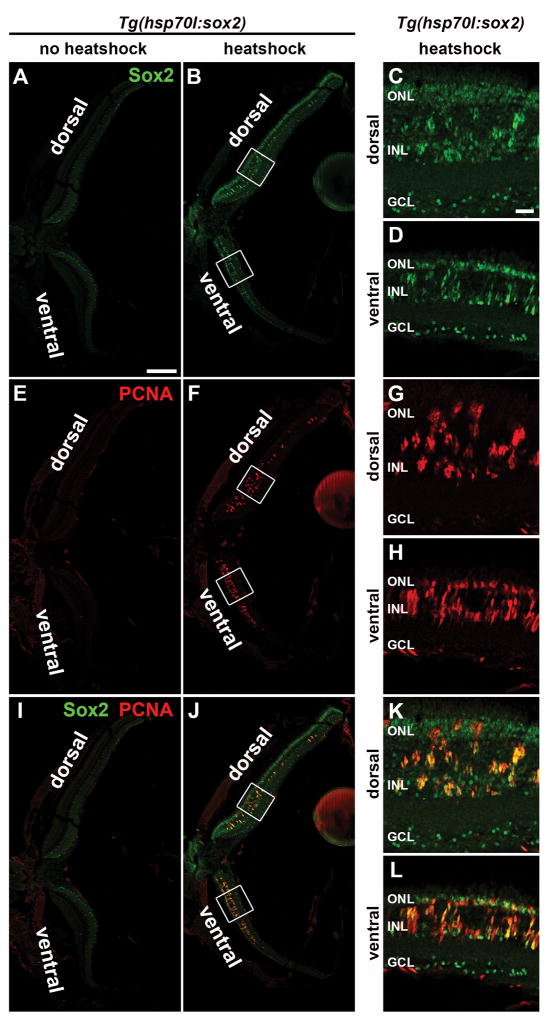
Because Sox2 expression increases following retinal damage and is required for Müller glia proliferation, we hypothesized that ectopically elevating Sox2 expression above the basal levels observed in the undamaged retina could induce Müller glia proliferation, even in the absence of damage. To test this hypothesis, we utilized the Tg(hsp70l:sox2)x21 transgenic zebrafish line (Millimaki et al., 2010), which ubiquitously overexpresses Sox2 upon heat shock. Adult Tg(hsp70l:sox2)x21 fish and wild-type siblings were subjected to heat shock at 38°C for one hour per day for either two or four days. Eyes were collected for analysis at 2 and 4 days post initial heat shock (dphs; Fig. 6A). Retinal cryosections revealed increased Sox2 expression throughout the retina of Tg(hsp70l:sox2)x21 fish at 2 dphs (Fig. 6C), while heat-shocked wild-type siblings maintained the expected Sox2 basal expression pattern of undamaged retinas (Fig. 6B). Additionally, immunolabeling retinal cryosections for glutamine synthetase to identify Müller glia (Fig. 6D–G) revealed that Tg(hsp70l:sox2)x21 fish possessed increased Sox2 expression in Müller glia (Fig. 6G, arrowheads), while the wild-type siblings possessed only basal Sox2 expression (Fig. 6F). Finally, Western blot analysis confirmed elevated levels of Sox2 protein in Tg(hsp70l:sox2)x21 retinal lysates persisted 24 hours after the second heat shock relative to identically heat-shocked wild-type sibling controls (Fig. 6H). However, the increased Sox2 expression was not uniform across the retina, with the majority of the increased Sox2 expression detected in the central dorsal (Fig. 7B, C, J, and K) and central ventral retina (Fig. 7B, D, J, and L), with expression decreasing towards the dorsal and ventral margins. Thus, heat shocking the Tg(hsp70l:sox2)x21 transgenic fish resulted in increased expression of Sox2 across the retina, including the Müller glia.
Figure 6. Overexpression of Sox2 can induce Müller glia proliferation in the undamaged zebrafish retina.
Tg(hsp70l:sox2)x21 transgenic zebrafish or wild-type (WT) sibling control zebrafish were exposed to one hour of heat shock daily at 38°C for either two or four days (A). After 2 days post-initial heat shock (2 dphs), retinal sections were immunolabeled for Sox2 (B, C, F and G) and the Müller glia marker, glutamine synthetase (GS, D–G). Sox2 expression in undamaged wild-type siblings (B) was low while Tg(hsp70l:sox2)x21 retinas exhibited strong ubiquitous expression (C). Co-labeling with glutamine synthetase revealed increased expression of Sox2 in Müller glia in Tg(hsp70l:sox2)x21 retinas (E,G) compared to wild-type sibling (D, F). Immunoblots of protein lysates confirmed that increased Sox2 expression in Tg(hsp70l:sox2)x21 retinas persisted 24 hours after the second heat shock relative to WT sibling retinas that were identically heat shocked (H). Immunolabeling of Tg(hsp70l:sox2)x21 retinas at 2 dphs (I and L) and 4 dphs (J and M) revealed significantly greater numbers of PCNA-positive cells from 2 dphs to 4 dphs in both the INL (O; ANOVA p = 8.85 × 10−16, Tukey’s post-hoc test p = 0.001, n = 4) and ONL cells (O; ANOVA p = 5.72 × 10−10, Tukey’s post-hoc test p = 0.001 n = 4) Retinas were stained with DAPI to detect the nuclear layers (L–N). dpHS, day post heatshock; GCL, ganglion cell layer; GS, glutamine synthetase, INL, inner nuclear layer; ONL, outer nuclear layer; PCNA, proliferating cell nuclear antigen; WT, wildtype. Scale bars in panels B, D, and I are 25 μm and are the same for panels C, E–G, and J–N, respectively.
Figure 7. Heat-shock-induced ectopic expression in Tg(hsp70l:sox2)x21 zebrafish of Sox2 and PCNA across the entire retinal section.
Single confocal images of a retinal section from a Tg(hsp70l:sox2)x21 zebrafish that was either not heat-shocked (A, E, I) or heat shocked daily at 38°C for 3 days (B, F, J). Retinal cryosections were immunolabeled for Sox2 (A–D and I–L) and PCNA (E–L). The non-heat shocked fish exhibited a low basal level of Sox2 expression across the retina, while the heat-shocked Tg(hsp70l:sox2)x21 fish exhibited increased levels of Sox2 expression that were non-uniform across the retina, with higher levels in the central dorsal and ventral retina and decreased expression towards the retinal margins. Additionally, PCNA expression was restricted to the regions containing increased Sox2 expression in the INL of both the ventral and central dorsal retina. The boxed regions in B, F, and J represent the magnified dorsal and ventral images in C, D, G, H, K, and L. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. The scale bar in A, 200 μm and is the same for B, E, F, I, and J. The scale bar in C, 25 μm and is the same for D, G, H, K, and L.
To determine if the overexpression of Sox2 was sufficient to induce Müller glia proliferation in the undamaged retina, we immunostained Tg(hsp70l sox2)x21 retinal sections for PCNA. At 2 dphs, individual PCNA-positive cells were observed in the INL of Tg(hsp70l:sox2)x21 retinas (Fig. 6I and L), but rarely, if ever, in controls (data not shown). At 4 dphs, large clusters of PCNA-positive cells were present in the INL and appeared to be migrating to the ONL in Tg(hsp70l:sox2)x21 retinas (Fig. 6J and M), similar to the Müller glia-derived neuronal progenitor cells observed in the light-damaged retina. In contrast, very few PCNA-positive cells were present in the INL of heat-shocked wild-type siblings at 4 dphs (Fig. 6K, N and O). The number of PCNA-positive cells in heat-shocked Tg(hsp70l:sox2)x21 retinas increased significantly from 2 to 4 dphs in both the INL (37.8 ± 1.3 and 109 ± 5.9, Tukey’s post-hoc test p = 0.001, n = 4; Fig. 6O) and the ONL (14.8 ± 1.5 and 62.0 ± 5.7, Tukey’s post-hoc test p = 0.001 n = 4; Fig. 6O). Interestingly, this heat shock-induced proliferation was localized predominantly to retinal regions where ectopic Sox2 expression was detected in the heat-shocked retina (Fig. 7B–D, F–H, and J–L). Furthermore, nearly all of the PCNA-positive cells exhibited increased levels of Sox2 expression. Though we did detect some disruption in outer segment morphology in Tg(hsp70l:sox2)x21 retinas, particularly at 4 dphs, when large clusters of proliferating cells were observed migrating into the ONL, we did not detect elevated TUNEL-labeling at either 2 dphs or 4 dphs (Fig. 8A–J). Together, these data suggest that ectopic Sox2 overexpression is sufficient to induce proliferation in a subset of Müller glia in the undamaged retina.
We next investigated the source of the proliferating Müller glia in the heat-shocked Tg(hsp70l:sox2)x21 retinas. Zebrafish exhibit persistent retinal neurogenesis throughout the life of the fish, with slowly cycling Müller glia throughout the central retina continually producing new rod photoreceptors (Otteson and Hitchcock, 2003). It is possible that overexpressing Sox2 might cause hyperproliferation of the Müller glia involved in persistent neurogenesis to generate more progenitors rather than inducing additional Müller glia to reenter the cell cycle. To test this, we injected EdU four hours prior to the first heat shock to label any Müller glia currently in the process of persistent rod neurogenesis (Fig. 8K). If Sox2 overexpression expands these rare proliferating Müller glia, we would expect to see many EdU-positive/PCNA-positive cells following Sox2 overexpression. At 2 dphs, we observed few, if any, EdU-positive cells in the INL in both Tg(hsp70l:sox2)x21 (Fig. 8M–N) and control retinas (not shown), consistent with thymidine-analog tracing studies in this short timeframe (Otteson et al., 2001). More importantly, we never observed EdU-positive cells among the Sox2-induced proliferating INL cells at 2 dphs (Fig. 8L–N). These data demonstrate that Sox2 overexpression induces many Müller glia to reenter the cell cycle that are not actively involved in persistent neurogenesis.
β-catenin 2 is required for Müller glia proliferation, but not for the expression of sox2
Several studies recently showed that Wnt/β-catenin directly regulates sox2 expression in the developing vertebrate retina (Agathocleous et al. 2009, Meyers et al. 2012). Additionally, canonical Wnt signaling is a key regulator of Müller glia proliferation in the regenerating adult zebrafish retina following stab lesion (Ramachandran et al. 2011). There are two β-catenin (ctnnb) paralogs in zebrafish, ctnnb1 and ctnnb2. Interestingly, Ramachandran et al. (2011) observed little-to-no change in expression of either ctnnb paralog following the retinal stab injury. However, microarray data previously obtained in our lab (Kassen et al., 2007) revealed differential expression of the two genes following light damage. We performed qRT-PCR to determine how the two ctnnb paralogs were temporally regulated during the initial regeneration response to light damage. Consistent with the microarray data, we observed increased ctnnb2 gene expression very early in the regeneration response, prior to the increased ctnnb1 expression (Fig. 9A).
Because ctnnb1 exhibited little or no increase in expression by 31 hours of constant light, while ctnnb2 expression increased by 31 hours of constant light, it is likely that ctnnb2 accounts for the increased β-catenin protein expression in proliferating Müller glia following either light damage (data not shown) or a stab lesion (Ramachandran et al., 2011). Based on these expression profiles, we hypothesized that β-catenin 2, but not β-catenin 1, may play a role in Müller glia proliferation and increased Sox2 expression. To determine the individual contribution of both β-catenin proteins to Müller glia proliferation, we knocked down both proteins independently prior to constant light treatment (Fig. 9B–G). Both the ctnnb1 and ctnnb2 morpholinos were previously validated to confirm that they knockdown the expression of the desired target protein (Gingerich et al., 2005; Xiong et al., 2006; Bellipanni et al., 2006). We quantified the number of PCNA-positive INL cells and found significantly fewer proliferating Müller glia in ctnnb2 MO retinas (7.1 ± 2.3; Tukey’s post-hoc test p = 0.001, n = 9, Fig. 9D and G–H) relative to either the SC MO or ctnnb1 MO retinas after 31 hours of light (27.6 ± 1.8 and 27.7 ± 1.5, respectively; Fig. 9B–C, E–F, and H). These data confirm that β-catenin 2, but not β-catenin 1, is required for Müller glia proliferation in the light-damaged zebrafish retina.
We then examined whether β-catenin 2 regulates sox2 expression in the light-damaged retina, as it does in neuronal progenitor cells during retinal development. Using qRT-PCR, we observed no difference in sox2 levels in ctnnb2 MO retinas relative to the SC MO control retinas after 31 hours of constant light (3.68 ± 0.0.3 and 3.80 ± 0.04, respectively; p = 0.07, n = 3) Fig. 9I). In contrast, expression of cyclin D1 (ccnd1), a canonical β-catenin target gene, was significantly reduced in ctnnb2 MO retinas compared to SC MO controls (7.15 ± 0.1 and 16.9 ± 0.1, respectively; p = 3.11 × 10−7, n = 3; Fig. 9J) and served as an internal control for our qRT-PCR dataset. Thus, β-catenin 2 is required for Müller glia proliferation in the light-damaged retina, but it does not act via regulating sox2 expression.
Sox2 regulates well-characterized Müller glia reprogramming factors
Ascl1a and Stat3 are two well-characterized transcription factors required for Müller glial reprogramming/proliferation in the regenerating zebrafish retina (Kassen et al., 2007; Fausett et al., 2008; Ramachandran et al., 2010; Nelson et al., 2012; Nelson et al., 2013; Wan et al., 2014) and morpholino-mediated knockdown of either protein results in reduced expression of the other (Nelson et al., 2012). Transcription of both genes (ascl1a and stat3) increases rapidly following retinal damage. To determine if Sox2 regulates expression of either transcription factor, we performed qRT-PCR to analyze ascl1a and stat3 expression in sox2 MO retinas. We observed a significant reduction in ascl1a expression after 31 hours of light treatment in the sox2 MO retinas relative to the SC MO retinas (SC MO: 116.5 ± 16.8-fold change; sox2 MO: 70.6 ± 5.2-fold change; p < 0.0001, n = 3; Fig. 10A). Interestingly, stat3 expression was unaffected in the sox2 MO retinas relative to the SC MO retinas (p > 0.9, n = 3; Fig. 10B). This represents the first report whereby a loss of ascl1a occurs without affecting stat3 expression.
In cultured human NSCs, SOX2 maintains LIN28 expression by recruiting chromatin remodeling factors to the LIN28 locus and LIN28 expression prevents biogenesis of mature let7 miRNA family members that directly destabilize MASH1 (the mammalian ortholog of ascl1a) mRNA (Cimadamore et al., 2013). Thus, in cultured NSCs, SOX2 indirectly regulates MASH1 expression through LIN28. In zebrafish, Lin28a is also required for Müller glia reprogramming and proliferation in the injured retina (Ramachandran et al., 2010; Nelson et al., 2012). To investigate whether Sox2 regulates lin28a expression, we analyzed lin28a expression in sox2 MO retinas using qRT-PCR. After 31 hours of constant light, we observed a significant reduction of lin28a expression in sox2 MO retinas compared to the SC MO retinas (SC MO: 3.5 × 105-fold change; sox2 MO: 4.2 × 104-fold change; p = 0.001, n = 3; Fig. 10C). This extremely large increase in lin28a expression in the light-damaged SC MO retina is due to very low levels of lin28a expression in the undamaged retina (average lin28a CT = 37.5 ± 0.219) relative to the light-damaged SC MO retina (average lin28a CT = 20.0 ± 0.926), which results in a very small denominator in calculating the fold-change. Based on Ramachandran et al. (2010), it is likely that this reduced lin28a expression is responsible for the reduced ascl1a expression in the sox2 MO retina.
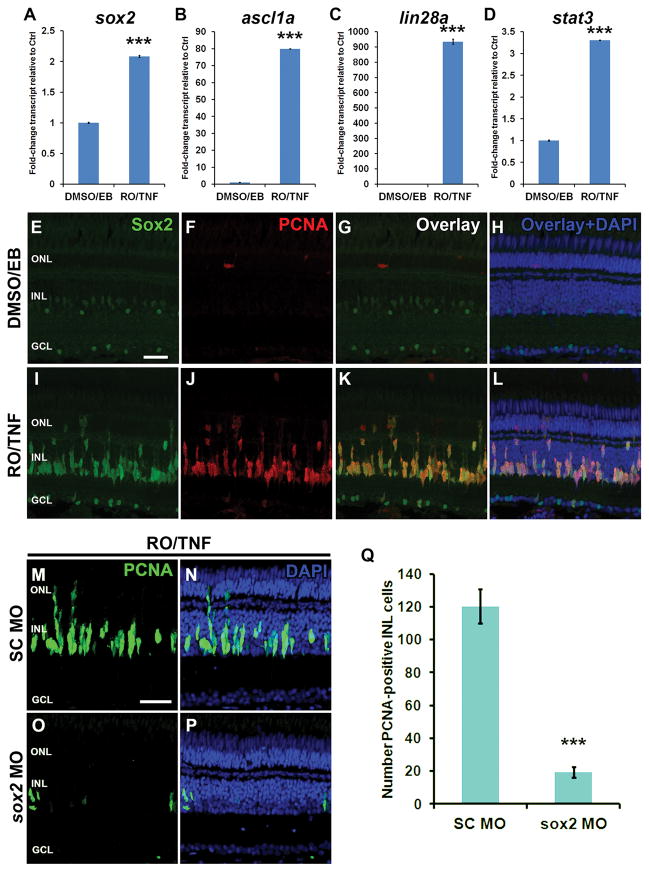
Increased Sox2 expression is required for Müler glia proliferation in undamaged TNFα/Notch inhibitor-treated retinas
Conner et al. (2014) demonstrated that concurrent exposure to recombinant zebrafish TNFα and RO4929097, a γ-secretase inhibitor, induced a high percentage of Müller glia to begin proliferating in the absence of retinal damage. We used qRT-PCR to investigate whether this damage-independent model of Müller glia proliferation induced increased sox2 expression similar to what was observed in the light-damaged retina (Fig. 11). After 3 days of TNFα and RO4929097 coinjections, we observed significant increases in expression of sox2, ascl1a, lin28a and stat3 (RO/TNF: Student’s t-tests: sox2 p = 6.4 × 10−7, ascl1a p = 5.9 × 10−9, lin28a p = 3.3 × 10−6, stat3 p = 2.5 × 10−6, n=3; Fig. 11A–D) relative to coinjection of DMSO and elution buffer controls (DMSO/EB; Fig. 11A–D). Additionally, Sox2 expression was more robustly expressed in PCNA-positive INL cells of RO4929097/TNFα coinjected undamaged retinas (Fig. 11I–L) compared to the basal Sox2 expression observed in DMSO/EB-exposed undamaged controls (Fig. 11E–H). Because the increased expression of the regeneration-associated genes (sox2, ascl1a, lin28a, stat3) in TNFα and RO4929097 coinjected undamaged retinas was analogous to the expression pattern in the light-damaged retinas (Fig. 10, comparing 0 and 31 hour controls), we hypothesized that Sox2 was also required to induce Müller glia proliferation in this undamaged model. We injected and electroporated either standard control or anti-sox2 morpholinos immediately prior to the first of three daily TNFα/RO4929097 coinjections and assayed for PCNA expression. While the SC MO retinas exhibited large numbers of PCNA-positive cells (Fig. 11M,N,Q; 120.4 ± 10.4), the sox2 MO retinas (Fig. 11O, P) displayed significantly fewer PCNA-positive cells (Fig. 11Q; 19.3 ±3.3, p = 8.5×10−7, n = 7). Together, these data demonstrate that Sox2 is a crucial factor regulating Müller glia proliferation, even in the absence of retinal damage.
Figure 11. Sox2 regulates Müller glia proliferation in the undamaged retina following TNFα exposure and Notch inhibition.
Adult AB zebrafish were intraperitoneally (ip) injected with the γ-secretase inhibitor RO4929097 (RO) and left eyes were intravitreally injected with recombinant zebrafish soluble TNFα (TNF). Control fish were injected ip with 10% DMSO and left eyes were intravitreally injected with the Ni-NTA native elution buffer (EB) used for TNFα purification. Injections were carried out every 12 hours for 3 days. RNA was purified from left eyes and the sox2 (A), ascl1a (B), lin28a (C), or stat3 (D) gene expression levels were determined by qRT-PCR. Compared to control eyes (DMSO/EB), expression of all four genes increased following treatment with RO4929097 and TNFα (RO/TNF; Student’s t-tests, sox2 p = 6.4 × 10−7, ascl1a p = 5.9 × 10−9, lin28a p = 3.3 × 10−6, stat3 p = 2.5 × 10−6, n=3). Immunocytochemistry confirmed that the RO4929097 and TNFα treatment (RO/TNF) was sufficient to induce increased Sox2 protein expression in PCNA-positive cells relative to buffer (DMSO/EB) injected eyes (E–L). Immediately prior to the RO4929097 and TNFα or control injections, either standard control morpholino (SC MO) or anti-sox2 morpholino (sox2 MO) were injected and electroporated into eyes. After the three-day injection regimen, retinal sections were labeled with anti-PCNA antibodies (M–P). The sox2 morphant retina (O–Q) possessed significantly fewer PCNA-positive cells relative to the SC morphant retinas (M, N, Q; Student’s t-test, p = 8.5×10−7, n = 7). Retinas were stained with DAPI to detect the nuclear layers (H, L, N, P). PCNA, proliferating cell nuclear antigen; GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Scale bars in panels E and M are 25 μm and are the same for panels F–L and N–P, respectively.
Sox2 is required for NPC amplification and induction of proneural gene expression
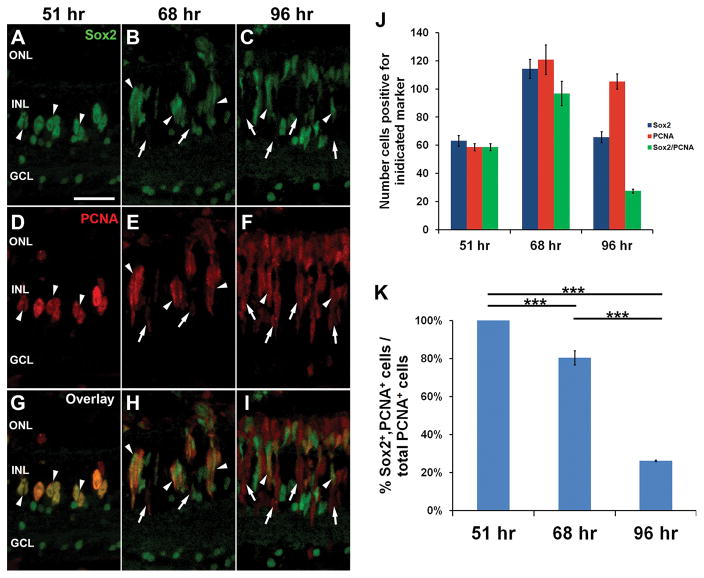
While the most dramatic increase in sox2 expression was observed between 16 and 31 hours of light, sox2 expression remained elevated at 51 and 68 hours of light before beginning to decrease at 96 hours (Fig. 1F). We examined the spatial expression of Sox2 at these later time points and discovered that nearly all PCNA-positive neuronal progenitor cells (NPCs) at 51 hours also expressed Sox2 (Fig. 12A,D,G, arrowheads). Quantification of the number of INL cells expressing Sox2, PCNA or both (Sox2 + PCNA; Fig. 12J) confirmed that all the PCNA-positive cells also expressed Sox2 (Fig. 12K). After 68 hours of light (Fig. 12B,E,H), there was a significantly lower percentage of PCNA-positive cells that co-expressed Sox2 (Fig. 12J,K, 80 ± 3.7%; Tukey’s post-hoc test p = 0.001, n = 5). At 96 hours of light (Fig. 12C,F,I), the percentage of PCNA-positive cells that co-expressed Sox2 was significantly reduced further (Fig. 12J,K; 26 ± 0.29%; Tukey’s post-hoc test p = 0.001, n = 5) relative to either 51 or 68 hours of constant light. Together, these data that indicate late-stage NPCs may downregulate Sox2 expression, possibly prior to committing to a neuronal fate.
Figure 12. Sox2 expression dynamics in early and late-stage neuronal progenitor cells.
Dark-adapted adult albino zebrafish were exposed to constant intense light for either 51, 68 or 96 hours and retinal cryosections were labeled with antibodies to Sox2 (A–C, G–I) and PCNA (D–I). After 51 hours of constant light, many small clusters of INL cells were observed expressing both PCNA and Sox2 (A,D,G, arrowheads). After 68 hours, most PCNA-positive cells in the INL still expressed Sox2 (B,E,H, arrowheads), but some cells within these clusters did not express Sox2 (B,E,H, arrows). At 96 hours, few cells expressed both markers (C,F,I, arrowheads), as the majority of PCNA-positive cells did not express Sox2 (C,F,I, arrows). The number of INL cells expressing Sox2, PCNA, or those expressing both markers (J) and the percentages of all of the PCNA-positive INL cells that also express Sox2 at each time point was determined (K). We observed a significant reduction in the percentage of PCNA-positive cells that also express Sox2 from 51 hours through 96 hours of light treatment (K; ANOVA p = 3.71 × 10−11, for 51 to 68 hours, Tukey’s post-hoc test p = 0.001, n = 5, for 68 to 96 hours, Tukey’s post-hoc test p = 0.001, n = 5). PCNA, proliferating cell nuclear antigen; GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Scale bar in panel A is 25 μm and is the same for panels B–I.
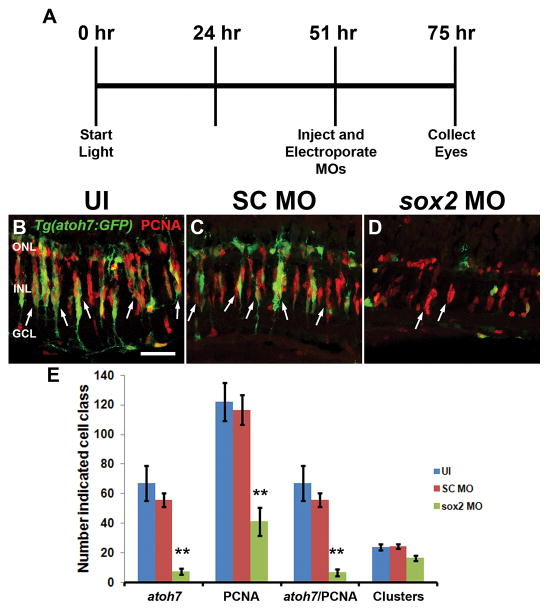
Because Sox2 was expressed in nearly all proliferating NPCs at 51 hours of light, we hypothesized that Sox2 may be required for NPC amplification. We placed dark-adapted albino;Tg(atoh7:EGFP) transgenic fish in constant intense light for 51 hours (a time point following Müller glia division, but preceding NPC amplification). We then intravitreally injected and electroporated either Standard Control or sox2 morpholinos and then returned the fish to constant light for another 24 hours (75 total hours of light; Fig. 13A). This experimental design permitted normal Müller glia proliferation prior to knocking down Sox2 expression to examine the subsequent effect on both NPC proliferation and expression of the Tg(atoh7:EGFP) transgene. Compared to the large number of PCNA-positive NPCs observed in uninjected and SC MO retinas (Fig. 13B–C, E; 122.0 ± 12.9 and 116.6 ± 10.2, respectively), sox2 MO retinas displayed significantly fewer PCNA-positive cells (Fig. 13D–E, 41.2 ± 0.5, Tukey’s post-hoc test p < 0.01, n ≥ 5). In addition, the uninjected and SC MO retinas contained a marginally significant reduction in the number of PCNA-positive cell clusters (Fig. 13E; 23.8 ± 2.2 and 24.4 ± 1.7, respectively) than the sox2 MO retinas (Fig. 13E; 16.6 ± 1.8, Tukey’s post-hoc test p = 0.06, n ≥ 5). Because each cluster likely corresponds to the amplification of a single Müller glia-derived NPC, the reduced number of PCNA-positive NPCs, along with the slightly reduced number of PCNA-positive clusters, is consistent with Sox2 being required for the amplification of Müller glia-derived NPCs.
Figure 13. Sox2 regulates NPC amplification and neuronal commitment.
Following 51 hours of constant intense light treatment, adult albino; Tg(atoh7:EGFP) zebrafish eyes were either uninjected (UI) or injected and electroporated with either standard control morpholino (SC MO) or anti-sox2 morpholino (sox2 MO). Fish were placed back into constant light for an additional 24 hours (75 hours of total light exposure) before eyes were collected for analysis (A). Retinal sections were labeled with antibodies to GFP and PCNA (B–D). The sox2 morphant retinas (D, E) contained significantly fewer PCNA-positive cells in the INL relative to either uninjected or SC morphant retinas (B–C, E; ANOVA p = 5.19 × 10−4, Tukey’s post-hoc test p < 0.01, n ≥ 5). Additionally, significantly fewer EGFP-positive cells (atoh7) were present in the sox2 morphant retinas compared to either uninjected or SC morphant retinas (E; ANOVA p = 5.20 × 10−4, Tukey’s post-hoc test p < 0.01, n = 5). There was also a slight, but marginally significant decrease in the number of PCNA-positive cell clusters (B–D, arrows; one cluster is indicative of one initial Müller glial division) in sox2 morphants relative to either uninjected or SC morphant retinas (E; ANOVA p = 0.04, Tukey’s post-hoc test p = 0.06, n ≥ 5). PCNA, proliferating cell nuclear antigen; GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer, UI, uninjected. Scale bar in panel B is 25 μm and is the same for panels C and D.
In neural stem/progenitor cells of the developing amphibian retina and mammalian hippocampus, Sox2 regulates expression of proneural genes (Amador-Arjona et al., 2015) or their transcriptional targets (Agathocleous et al., 2009). Using the above protocol, we examined if Sox2 regulated induction of the atoh7 proneural gene in the regenerating albino;Tg(atoh7:EGFP) transgenic retina. In uninjected and Standard Control morphant retinas, a subset of NPCs expressed EGFP at 75 hours of light treatment, indicative of proneural atoh7 expression (Fig. 13B–C, E; 67.3 ± 11.8 and 56 ± 4.7 EGFP-positive cells, respectively). In contrast, significantly fewer EGFP-positive cells were observed in sox2 MO retinas (Fig. 13D–E; 7.6 ± 2.1, Tukey’s post-hoc test p < 0.01, n = 5), indicating that Sox2 expression is required for inducing NPC proliferation and/or expression of an atoh7 transgene.
Sox2 is required for optimal regeneration of photoreceptors
To determine whether Sox2 is required for photoreceptor regeneration, the light-damaged (4 days of constant light treatment) fish were recovered on a normal light/dark cycle for 21 days. Labeling for the rod outer segment (ROS) marker Rhodopsin revealed generally normal rod morphology in sox2 MO retinas (Fig. 14B) relative to SC MO controls (Fig. 14A). Further, there was not a significant difference in the number of rod nuclei per 100 μm of the ONL (Fig. 14G) in sox2 MO (56 ± 2.81) and SC MO (63.4 ± 2.70; p = 0.0735, n = 10) retinas. Similarly, there was no significant difference in the ROS length (Fig. 14H) between sox2 MO retinas (29.1 ±1.78 μm) and SC MO (32.7 ± 1.47 μm; p = 0.136, n = 10) retinas. The only rod metric that showed any significant difference between treatments was ONL thickness (Fig. 14H; 13.0 ± 0.389 μm in SC MO vs 10.8 ± 0.668 μm in sox2 MO; p = 0.0127, n = 10). Thus, it appeared that knockdown of Sox2 expression did not significantly affect the regeneration of rod photoreceptors.
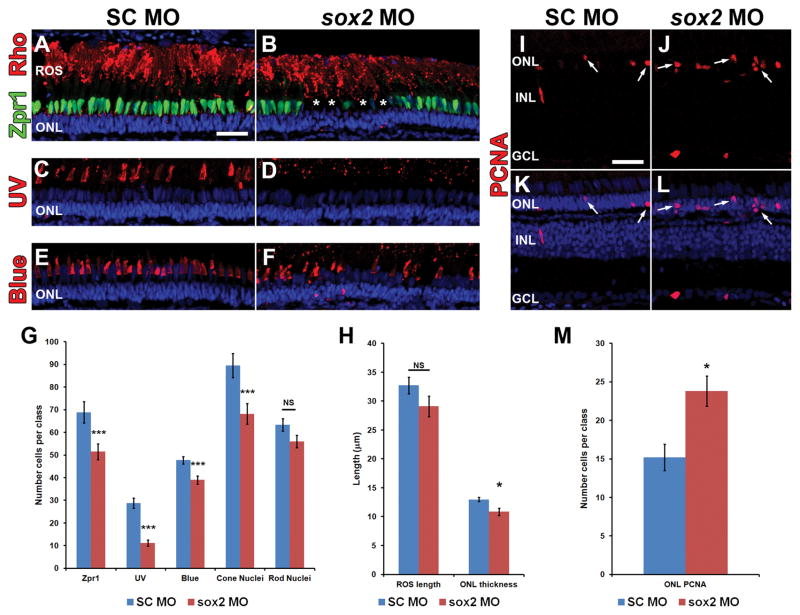
Figure 14. Sox2 is required for optimal regeneration of cones following light-damage.
Adult albino zebrafish retinas were injected and electroporated with either standard control morpholino (SC MO) or anti-sox2 morpholino (sox2 MO) immediately prior to light treatment. Following the standard four days of constant light, fish were returned to a normal light/dark cycle to recover. After 21 days of recovery, retinal sections were prepared and labeled with photoreceptor markers Zpr1 (A–B, green), Rhodopsin (A–B, red), UV opsin (C–D, red), and Blue opsin (E–F, red). Fewer cones of all classes were observed in sox2 morphant retinas (B, D, F) compared to controls (A, C, E). Cones were often missing in small patches throughout the sox2 morphant retinas (B, asterisk). Rhodopsin labeled rod outer segments (ROS) appeared to be slightly shorter in length in sox2 morphant retinas (B) compared to controls (A). Quantification of photoreceptor markers (G) indicated a significant reduction in the numbers of cells expressing Zpr1 (Student’s t-test, p = 0.009, n = 10), Blue opsin (Student’s t-test, p = 0.003, n ≥ 8), and UV opsin (Student’s t-test, p = 8.37 × 10−6, n = 8), and cone nuclei (Student’s t-test, p = 0.007, n = 10) in sox2 morphants relative to control retinas. While the average thickness of the ONL was significantly reduced in sox2 morphant retinas compared to controls (H; Student’s t-test, p = 0.0127, n = 10), the length of ROS (H; Student’s t-test, p = 0.136, n = 10) and number of rod nuclei per 100 μm (G; Student’s t-test, p = 0.074, n = 10) was not statistically altered between groups. Retinas labeled with antibodies to PCNA (I–L, arrows), displayed a significant increase in ONL proliferation (M; Student’s t-test, p = 0.011, n = 10) in sox2 morphant retinas compared to controls. Retinas were stained with DAPI to detect the nuclear layers (A–F, K, L). PCNA, proliferating cell nuclear antigen; GCL, ganglion cell layer; INL, inn and I are 25 μm and are the same for panels B–F and J–L.
We also analyzed the number of Zpr-1-positive double cones (Fig. 14A–B), UV opsin cones (Fig. 14C–D), blue opsin cones (Fig. 14E–F) and DAPI-positive cone nuclei (Fig. 14K–L). Overall, fewer cones of all classes were observed in sox2 MO retinas compared to SC MO control retinas which is reflected by the reduced number of DAPI-labeled cone nuclei (Fig 14G; 89.5 ± 5.27 in SC MO relative to 68.2 ± 4.55 in sox2 MO; p = 6.73 × 10−3, n = 10). Quantification of the specific cone classes (Fig. 14G) revealed a reduction in UV cones (28.8 ± 2.20 in SC MO relative to 11.1 ± 1.36 in sox2 MO; p = 8.37 × 10−6, n = 8). While smaller, significant reductions were also observed for Zpr-1 double cones (68.9 4.74 for SC MO relative to 51.5 ± 3.54 for sox2 MO; p = 8.72 × 10−3, n = 10). Similarly, fewer blue opsin long single cones were present in sox2 MO relative to SC MO retinas (47.8 ± 1.60 in SC MO compared to 39.0 ± 1.83 in sox2 MO; p = 2.99 × 10−3, n ≥ 8. Overall, these data suggest that Sox2 is required for complete regeneration of cones in light-damaged retinas.
This reduced regeneration of cones could be due to deficient Sox2 expression in the Müller glia, while the rods could regenerate from the resident rod precursor cells (Thummel et al., 2010). To determine if rod precursors persisted at 21 days post light treatment in sox2 MO retinas relative to SC MO control retinas, we labeled retinal cryosections for PCNA expression (Fig. 14I–L). We detected significantly more PCNA-positive cells in the ONL of sox2 MO retinas (23.8 ± 2.44; Fig. 14M) compared to SC MO retinas (15.2 ± 1.82; p = 0.0112, n = 10). Altogether, these data support the hypothesis that Sox2 is required for Müller glia-dependent regeneration of cone photoreceptors, while rods can regenerate via ONL-based rod precursor cells.
DISCUSSION
Sox2 is a well-established neuronal stem cell associated transcription factor required for: 1) maintaining pluripotency in ESCs (Kopp et al., 2008), 2) reprogramming somatic cells to generate iPSCs (Takahashi et al., 2006), iNSCs, or direct conversion of non-neuronal cells to functional neurons (Karow et al., 2012; Heinrich et al., 2014), and 3) regulation of neural development (Okuda et al., 2006; Taranova et al., 2006; Agathocleous et al., 2009) and adult neurogenesis (Favaro et al., 2009). Because zebrafish Müller glia respond to retinal damage by undergoing a reprogramming-like response and reentering the cell cycle to generate a pool of NPCs that replenish the lost retinal neurons, we examined the role of Sox2 in regenerating the light-damaged zebrafish retina. Upon light-induced photoreceptor cell death, Sox2 expression increased significantly and was required for Müller glia proliferation. Additionally, forced overexpression of Sox2 induced Müller glia proliferation in the absence of retinal damage, revealing that Sox2 is necessary and sufficient to stimulate Müller glia proliferation. Sox2 also regulated the expression of the neurogenic and reprogramming factors ascl1a and lin28a, respectively, following retinal damage. Interestingly, Sox2 was not required for stat3 expression. This is the first situation in which we observed a reduction of ascl1a that does not induce a concomitant reduction in stat3 expression. Finally, Sox2 expression was required for the continued proliferation of the Müller glia-derived neuronal progenitor cells and their commitment to a neuronal lineage, as well as optimal regeneration of cone photoreceptors.
Sox2 is also required to maintain the progenitor state and neuronal competence of retinal progenitor cells (RPCs) during vertebrate retinal development (Taranova et al., 2006; Agathocleous et al., 2009). In fish and frogs, Sox2 expression in RPCs is regulated by Wnt/β-catenin signaling (Agathocleous et al., 2009; Meyers et al., 2012). Several studies recently established a critical role for Wnt signaling during zebrafish retinal regeneration (Ramachandran et al., 2011; Wan et al., 2014). We determined that expression of the ctnnb2 paralog increased very early following retinal damage and the encoded β-catenin 2, but not β-catenin 1, was required for Müller glia proliferation. However, neither β-catenin paralog regulated sox2 expression in the light-damaged retina.
Two well-characterized genes required for zebrafish Müller glia reprogramming, ascl1a or stat3, both increase in expression and appear to be similarly regulated in the damaged retina (Faussett et al., 2006; Ramachandran et al., 2010; Nelson et al., 2012; Nelson et al., 2013; Wan et al., 2014). While morpholino-mediated knockdown of Sox2 expression significantly reduced ascl1a expression, it did not affect stat3 expression. Previously, we demonstrated that morpholino-mediated knockdown of Ascl1a expression significantly reduced Stat3 expression (Nelson et al., 2012). This discrepancy may be explained by the Sox2 knockdown resulting in a less severe decrease in Ascl1a expression compared to the morpholino-mediated knockdown of Ascl1a expression. Alternatively, it is possible that the loss of Sox2 still allows the pathway upstream of Stat3 to function, up to the point where it converges with Ascl1a. Regardless, Sox2 represents the first signaling molecule that differentially regulates the early expression of Stat3 and Ascl1a in the light-damaged zebrafish retina, which suggests that distinct signaling pathways are required for Müller glia-dependent retinal regeneration.
We also observed that morpholino-mediated Sox2 knockdown drastically reduced lin28a expression. In cultured human NSCs, SOX2 maintains LIN28 expression by recruiting chromatin remodeling factors to the LIN28 locus and LIN28 expression prevents biogenesis of mature let7 miRNA family members that directly destabilize MASH1 (the mammalian ortholog of ascl1a) mRNA (Cimadamore et al., 2013). Thus, in cultured NSCs, SOX2 indirectly regulates MASH1 expression through LIN28. Lin28a is also required for zebrafish Müller glia reprogramming (Ramachandran et al., 2010) and is necessary for Ascl1a expression (Nelson et al., 2012). Ramachandran et al. (2010) also demonstrated that Lin28a is required to antagonize let7 miRNA biogenesis and let7 suppressed zebrafish Ascl1a expression in HEK293 cells in a dose-dependent manner. These observations, along with the results of the current study, suggest Müller glia reprogramming may utilize a conserved Sox2/Lin28a/let7/Ascl1a pathway to generate NSCs in the regenerating zebrafish retina. Further studies will be required to determine if the Sox2-dependent loss of lin28a expression directly impacts expression of let7 family members. Alternatively, Sox2 may directly regulate the expression of Ascl1a and Lin28a, while Lin28a/let7 subsequently regulates the expression of Ascl1a. Furthermore, to accurately understand the role Sox2 plays in Müller glia reprogramming, more elaborate experiments will be required to identify additional transcriptional targets of Sox2. For example, Sox2 chromatin immunoprecipitation experiments of regenerating zebrafish retinas, as well as RNA sequencing following both Sox2 knockdown and overexpression, will yield a wealth of Sox2 target candidates.
Sox2 also plays a role during later stages of zebrafish retinal regeneration. Expression of sox2 remained elevated during early periods of NPC amplification, which correlated with nearly all proliferating NPCs expressing Sox2 after 51 hours of constant light (Fig. 12). Subsequent amplification of these NPCs was dependent on Sox2 (Fig. 12). The slightly reduced number of NPC clusters in the sox2 morphant (Fig. 12), suggests that a small number of Müller glia reenter the cell cycle after the 51 hour timepoint when the morpholino was injected and electroporated, which would reduce the total number of PCNA-positive cells in the sox2 morphant relative to the controls. However, this slight reduction in the number of proliferating Müller glia is insufficient to account for the entire reduction in the number of PCNA-positive NPCs relative to the controls and is consistent with Sox2 being required for NPC amplification. As NPCs began to commit to a neuronal fate, the number of Sox2-positive cells began to decrease (Fig. 12). Interestingly, the knockdown of Sox2 expression in the NPCs resulted in decreased numbers of GFP-positive cells in the Tg(atoh7:GFP) transgenic line (Fig. 13), suggesting that expression of the atoh7:GFP reporter was dependent on Sox2 expression. Alternatively, it is possible that Sox2 is required for NPC proliferation/commitment and loss of Sox2 expression prevents the NPCs from reaching the point in commitment where they would normally express atoh7. In this case, Sox2 would indirectly regulate atoh7 expression through its effect on NPC commitment rather than directly regulating atoh7 transcription. This interplay between Sox2 and proneural factors, such as Atoh7, in neural stem/progenitor cells is beginning to be elucidated in other systems. In general, Sox2 is required for the induction of proneural circuits, which then feedback to repress Sox2 expression and promote neuronal differentiation (Agathocleous et al., 2009; Hagey and Muhr, 2014; Amador-Arjona et al., 2015). Thus, it is likely that Sox2 regulates NPC proliferation and/or expression of proneural factors, such as atoh7, in late-stage Müller glia-derived NPCs during zebrafish retinal regeneration.
The use of morpholinos in this study allowed us to conditionally knockdown the expression of Sox2 in the adult retina. It is very likely that a sox2 loss-of-function mutation would result in either significant developmental abnormalities and/or death, which would have precluded these studies in the adult retina. However, the electroporation procedure itself induces some retinal damage, which prevents us from studying the role of Sox2 prior to the damage stimulus. This is critical because it would reveal the function of the basal Sox2 expression in the Müller glia and amacrine cells and allow us to draw parallels to the conditional knockout phenotype observed in postnatal mouse Müller glia (Surzenko et al., 2013). Conditional knockout of sox2 in the adult zebrafish Müller glia could reveal if Sox2 is required for Müller glia maintenance, retinal cytoarchitecture, and retinal function as described in the postnatal mouse retina (Bachleda et al., 2016). Loss of Sox2 could alter these basal Müller glial functions, which may be required for Müller glia-derived neuronal regeneration through an unknown mechanism. To this end, recent advances in nuclease-based genome editing may make it possible to someday generate conditional sox2 alleles in zebrafish to address such important questions.
In conclusion, Sox2 is necessary and ectopically sufficient for Müller glia reprogramming and proliferation in the regenerating zebrafish retina. Müller glia in both the undamaged mammalian and zebrafish retina maintain Sox2 expression into adulthood, but the former cannot provide the robust regenerative response observed in the fish. It is unclear what mechanisms result in increased expression of Sox2 in the Müller glia of light-damaged zebrafish, but this may be a critical mechanism that prevents Müller glia-dependent regeneration, in the damaged mammalian retina. Additionally, comparative studies between Sox2 targets and Sox2 expression dynamics in both the damaged mammalian and zebrafish retinas will be invaluable to increase our understanding of where the mammalian regeneration response fails and provide novel therapeutic targets to stimulate Müller glia to regenerate the human retina.
CONCLUSIONS
Sox2 is necessary and sufficient for Müller glia proliferation, a key early event for regeneration of the damaged zebrafish retina. Subsequently, Sox2 is required for amplification of the Müller glia-derived neuronal progenitor cells and their commitment to a neuronal lineage.
Supplementary Material
Cryosections of adult zebrafish retinas were prepared after 36 hours of constant light and labeled with two separate Sox2 antibodies (A–C). These antibodies were raised against different antigens in either goat (A; Green; R&D) or rabbit (B; Red; GeneTex). Overlaying both channels revealed an identical expression pattern between the antibodies. ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. Scale bar in panel A is 25 μm and is the same for panels B–C.
HIGHLIGHTS.
Sox2 is required for Müller glia proliferation in the damaged zebrafish retina.
Sox2 is sufficient for Müller glia proliferation in the absence of damage.
Sox2 is required for Müller glia-derived neuronal progenitor cell proliferation.
Sox2 is required for neuronal progenitor cell commitment to a neuronal lineage.
Acknowledgments
We are grateful for the outstanding help provided by the Freimann Life Sciences technicians and their care and husbandry of the zebrafish. We also appreciate the members of the Hyde lab for thoughtful discussions. We appreciate the support of William Archer and NDIIF. This study was supported by the National Eye Institute of NIH to DRH (grant numbers R01-EY018417 and R01-EY024519), the Center for Zebrafish Research and the Center for Stem Cells and Regenerative Medicine, University of Notre Dame, Notre Dame, IN.
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
References
- Agathocleous M, Iordanova I, Willardsen MI, Xue XY, Vetter ML, Harris WA, Moore KB. A directional Wnt/beta-catenin-Sox2-proneural pathway regulates the transition from proliferation to differentiation in the Xenopus retina. Development. 2009;136:3289–3299. doi: 10.1242/dev.040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amador-Arjona A, Cimadamore F, Huang CT, Wright R, Lewis S, Gage FH, Terskikh AV. SOX2 primes the epigenetic landscape in neural precursors enabling proper gene activation during hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2015;112:1936–45. doi: 10.1073/pnas.1421480112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachleda AR, Pevny LH, Weiss ER. Sox2-deficient Muller glia disrupt the structural and functional maturation of the mammalian retina. IOVS. 2016;57:1488–1499. doi: 10.1167/iovs.15-17994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellipanni G, Varga M, Maegawa S, Imai Y, Kelly C, Myers AP, Chu F, Talbot WS, Weinberg ES. Essential and opposing roles of zebrafish β-catenins in the formation of dorsal axial structures and neurectoderm. Development. 2006;133:1299–1309. doi: 10.1242/dev.02295. [DOI] [PubMed] [Google Scholar]
- Bernardos RL, Barthel LK, Meyers JR, Raymond PA. Late-stage neuronal progenitors in the retina are radial Muller glia that function as retinal stem cells. J Neurosci. 2007;27:7028–7040. doi: 10.1523/JNEUROSCI.1624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimadamore F, Amador-Arjona A, Chen C, Huang CT, Terskikh AV. SOX2-LIN28/let-7 pathway regulates proliferation and neurogenesis in neural precursors. Proc Natl Acad Sci U S A. 2013;110:3017–3026. doi: 10.1073/pnas.1220176110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner C, Ackerman KM, Lahne M, Hobgood JS, Hyde DR. Repressing notch signaling and expressing TNFalpha are sufficient to mimic retinal regeneration by inducing Muller glial proliferation to generate committed progenitor cells. J Neurosci. 2014;34:14403–14419. doi: 10.1523/JNEUROSCI.0498-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias TB, Yang YJ, Ogai K, Becker T, Becker CG. Notch signaling controls generation of motor neurons in the lesioned spinal cord of adult zebrafish. J Neurosci. 2012;32:3245–3252. doi: 10.1523/JNEUROSCI.6398-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausett BV, Goldman D. A role for alpha1 tubulin-expressing Muller glia in regeneration of the injured zebrafish retina. J Neurosci. 2006;26:6303–6313. doi: 10.1523/JNEUROSCI.0332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausett BV, Gumerson JD, Goldman D. The proneural basic helix-loop-helix gene ascl1a is required for retina regeneration. J Neurosci. 2008;28:1109–1117. doi: 10.1523/JNEUROSCI.4853-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaro R, Valotta M, Ferri AL, Latorre E, Mariani J, Giachino C, Lancini C, Tosetti V, Ottolenghi S, Taylor V, Nicolis SK. Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nat Neurosci. 2009;12:1248–1256. doi: 10.1038/nn.2397. [DOI] [PubMed] [Google Scholar]
- Fimbel SM, Montgomery JE, Burket CT, Hyde DR. Regeneration of inner retinal neurons after intravitreal injection of ouabain in zebrafish. J Neurosci. 2007;27:1712–1724. doi: 10.1523/JNEUROSCI.5317-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gringerich JL, Westfall TA, Slusarski DC, Pelegri F. Hecate, a zebrafish maternal effect gene, affects dorsal organizer induction and intracellular calcium transient frequency. Dev Bio. 2005;286:427–439. doi: 10.1016/j.ydbio.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Hagey DW, Muhr J. Sox2 acts in a dose-dependent fashion to regulate proliferation of cortical progenitors. Cell Rep. 2014;9:1908–1920. doi: 10.1016/j.celrep.2014.11.013. [DOI] [PubMed] [Google Scholar]
- Heavner WE, Andoniadou CL, Pevny LH. Establishment of the neurogenic boundary of the mouse retina requires cooperation of SOX2 and WNT signaling. Neural Dev. 2014;9:27-8104-9-27. doi: 10.1186/1749-8104-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich C, Bergami M, Gascon S, Lepier A, Vigano F, Dimou L, Sutor B, Berninger B, Gotz M. Sox2-mediated conversion of NG2 glia into induced neurons in the injured adult cerebral cortex. Stem Cell Reports. 2014;3:1000–1014. doi: 10.1016/j.stemcr.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde DR, Godwin AR, Thummel R. In vivo electroporation of morpholinos into the regenerating adult zebrafish tail fin. J Vis Exp. 2012;(61) doi: 10.3791/3632. pii: 3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MR, Wang K, Smith JB, Heslin MJ, Diasio RB. Quantitation of dihydropyrimidine dehydrogenase expression by real-time reverse transcription polymerase chain reaction. Anal Biochem. 2000;278:175–184. doi: 10.1006/abio.1999.4461. [DOI] [PubMed] [Google Scholar]
- Kamachi Y, Okuda Y, Kondoh H. Quantitative assessment of the knockdown efficiency of morpholino antisense oligonucleotides in zebrafish embryos using a luciferase assay. Genesis. 2008;46:1–7. doi: 10.1002/dvg.20361. [DOI] [PubMed] [Google Scholar]
- Karow M, Sanchez R, Schichor C, Masserdotti G, Ortega F, Heinrich C, Gascon S, Khan MA, Lie DC, Dellavalle A, Cossu G, Goldbrunner R, Gotz M, Berninger B. Reprogramming of pericyte-derived cells of the adult human brain into induced neuronal cells. Cell Stem Cell. 2012;11:471–476. doi: 10.1016/j.stem.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Kassen SC, Ramanan V, Montgomery JE, Burket TC, Liu CG, Vihtelic TS, Hyde DR. Time course analysis of gene expression during light-induced photoreceptor cell death and regeneration in albino zebrafish. Dev Neurobiol. 2007;67:1009–1031. doi: 10.1002/dneu.20362. [DOI] [PubMed] [Google Scholar]
- Kopp JL, Ormsbee BD, Desler M, Rizzino A. Small increases in the level of Sox2 trigger the differentiation of mouse embryonic stem cells. Stem Cells. 2008;26:903–911. doi: 10.1634/stemcells.2007-0951. [DOI] [PubMed] [Google Scholar]
- Kroehne V, Freudenreich D, Hans S, Kaslin J, Brand M. Regeneration of the adult zebrafish brain from neurogenic radial glia-type progenitors. Development. 2011;138:4831–4841. doi: 10.1242/dev.072587. [DOI] [PubMed] [Google Scholar]
- Lahne M, Li J, Marton RM, Hyde DR. Actin-cytoskeleton- and Rock-mediated INM are required for photoreceptor regeneration in the adult zebrafish retina. J Neurosci. 2015;35:15612–15634. doi: 10.1523/JNEUROSCI.5005-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YP, Ouchi Y, Satoh S, Watanabe S. Sox2 plays a role in the induction of amacrine and Muller glial cells in mouse retinal progenitor cells. Invest Ophthalmol Vis Sci. 2009;50:68–74. doi: 10.1167/iovs.07-1619. [DOI] [PubMed] [Google Scholar]
- Masai I, Lele Z, Yamaguchi M, Komori A, Nakata A, Nishiwaki Y, Wada H, Tanaka H, Nojima Y, Hammerschmidt M, Wilson SW, Okamoto H. N-cadherin mediates retinal lamination, maintenance of forebrain compartments and patterning of retinal neurites. Development. 2003;130:2479–2494. doi: 10.1242/dev.00465. [DOI] [PubMed] [Google Scholar]
- Meyers JR, Hu L, Moses A, Kaboli K, Papandrea A, Raymond PA. beta-catenin/Wnt signaling controls progenitor fate in the developing and regenerating zebrafish retina. Neural Dev. 2012;7:30. doi: 10.1186/1749-8104-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millimaki BB, Sweet EM, Riley BB. Sox2 is required for maintenance and regeneration, but not initial development, of hair cells in the zebrafish inner ear. Dev Biol. 2010;338:262–269. doi: 10.1016/j.ydbio.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery JE, Parsons MJ, Hyde DR. A novel model of retinal ablation demonstrates that the extent of rod cell death regulates the origin of the regenerated zebrafish rod photoreceptors. J Comp Neurol. 2010;518:800–814. doi: 10.1002/cne.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CM, Ackerman KM, O’Hayer P, Bailey TJ, Gorsuch RA, Hyde DR. Tumor Necrosis Factor-Alpha Is Produced by Dying Retinal Neurons and Is Required for Muller Glia Proliferation during Zebrafish Retinal Regeneration. J Neurosci. 2013;33:6524–6539. doi: 10.1523/JNEUROSCI.3838-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CM, Gorsuch RA, Bailey TJ, Ackerman KM, Kassen SC, Hyde DR. Stat3 defines three populations of Muller glia and is required for initiating maximal muller glia proliferation in the regenerating zebrafish retina. J Comp Neurol. 2012;520:4294–4311. doi: 10.1002/cne.23213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda Y, Yoda H, Uchikawa M, Furutani-Seiki M, Takeda H, Kondoh H, Kamachi Y. Comparative genomic and expression analysis of group B1 sox genes in zebrafish indicates their diversification during vertebrate evolution. Dev Dyn. 2006;235:811–825. doi: 10.1002/dvdy.20678. [DOI] [PubMed] [Google Scholar]
- Otteson DC, D’Costa AR, Hitchcock PF. Putative stem cells and the lineage of rod photoreceptors in the mature retina of the goldfish. Dev Biol. 2001;232:62–76. doi: 10.1006/dbio.2001.0163. [DOI] [PubMed] [Google Scholar]
- Otteson DC, Hitchcock PF. Stem cells in the teleost retina: persistent neurogenesis and injury-induced regeneration. Vision Res. 2003;43:927–936. doi: 10.1016/s0042-6989(02)00400-5. [DOI] [PubMed] [Google Scholar]
- Ramachandran R, Fausett BV, Goldman D. Ascl1a regulates Muller glia dedifferentiation and retinal regeneration through a Lin-28-dependent, let-7 microRNA signalling pathway. Nat Cell Biol. 2010;12:1101–1107. doi: 10.1038/ncb2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Zhao XF, Goldman D. Ascl1a/Dkk/beta-catenin signaling pathway is necessary and glycogen synthase kinase-3beta inhibition is sufficient for zebrafish retina regeneration. Proc Natl Acad Sci U S A. 2011;108:15858–15863. doi: 10.1073/pnas.1107220108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond PA, Barthel LK, Bernardos RL, Perkowski JJ. Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC Dev Biol. 2006;6:36. doi: 10.1186/1471-213X-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surzenko N, Crowl T, Bachleda A, Langer L, Pevny L. SOX2 maintains the quiescent progenitor cell state of postnatal retinal Muller glia. Development. 2013;140:1445–1456. doi: 10.1242/dev.071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Taranova OV, Magness ST, Fagan BM, Wu Y, Surzenko N, Hutton SR, Pevny LH. SOX2 is a dose-dependent regulator of retinal neural progenitor competence. Genes Dev. 2006;20:1187–1202. doi: 10.1101/gad.1407906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JL, Nelson CM, Luo X, Hyde DR, Thummel R. Characterization of multiple light damage paradigms reveals regional differences in photoreceptor loss. Exp Eye Res. 2012;97:105–116. doi: 10.1016/j.exer.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel R, Enright JM, Kassen SC, Montgomery JE, Bailey TJ, Hyde DR. Pax6a and Pax6b are required at different points in neuronal progenitor cell proliferation during zebrafish photoreceptor regeneration. Exp Eye Res. 2010;90:572–582. doi: 10.1016/j.exer.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel R, Kassen SC, Enright JM, Nelson CM, Montgomery JE, Hyde DR. Characterization of Muller glia and neuronal progenitors during adult zebrafish retinal regeneration. Exp Eye Res. 2008;87:433–444. doi: 10.1016/j.exer.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel R, Kassen SC, Montgomery JE, Enright JM, Hyde DR. Inhibition of Muller glial cell division blocks regeneration of the light-damaged zebrafish retina. Dev Neurobiol. 2008;68:392–408. doi: 10.1002/dneu.20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raay TJ, Moore KB, Iordanova I, Steele M, Jamrich M, Harris WA, Vetter ML. Frizzled 5 signaling governs the neural potential of progenitors in the developing Xenopus retina. Neuron. 2005;46:23–36. doi: 10.1016/j.neuron.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Vihtelic TS, Doro CJ, Hyde DR. Cloning and characterization of six zebrafish photoreceptor opsin cDNAs and immunolocalization of their corresponding proteins. Vis Neurosci. 1999;16:571–585. doi: 10.1017/s0952523899163168. [DOI] [PubMed] [Google Scholar]
- Vihtelic TS, Hyde DR. Light-induced rod and cone cell death and regeneration in the adult albino zebrafish (Danio rerio) retina. J Neurobiol. 2000;44:289–307. doi: 10.1002/1097-4695(20000905)44:3<289::aid-neu1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Vihtelic TS, Soverly JE, Kassen SC, Hyde DR. Retinal regional differences in photoreceptor cell death and regeneration in light-lesioned albino zebrafish. Exp Eye Res. 2006;82:558–575. doi: 10.1016/j.exer.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Vong QP, Chan KM, Cheng CH. Quantification of common carp (Cyprinus carpio) IGF-I and IGF-II mRNA by real-time PCR: differential regulation of expression by GH. J Endocrinol. 2003;178:513–521. doi: 10.1677/joe.0.1780513. [DOI] [PubMed] [Google Scholar]
- Wan J, Ramachandran R, Goldman D. HB-EGF is necessary and sufficient for Muller glia dedifferentiation and retina regeneration. Dev Cell. 2012;22:334–347. doi: 10.1016/j.devcel.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J, Zhao XF, Vojtek A, Goldman D. Retinal injury, growth factors, and cytokines converge on beta-catenin and pStat3 signaling to stimulate retina regeneration. Cell Rep. 2014;9:285–297. doi: 10.1016/j.celrep.2014.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. The zebrafish book: a guide for the laboratory use of zebrafish (Brachydanio rerio) Eugene, OR: University of Oregon Press; 1993. [Google Scholar]
- Xiong B, Rui Y, Zhang M, Shi K, Jia S, Tian T, Yin K, Huang H, Lin S, Zhao X, Chen Y, Chen Y-G, Lin S-C. Tob1 controls dorsal development of zebrafish embryos by antagonizing maternal β-catenin transcriptional activity. Dev Cell. 2006;11:225–238. doi: 10.1016/j.devcel.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Yurco P, Cameron DA. Cellular correlates of proneural and Notch-delta gene expression in the regenerating zebrafish retina. Vis Neurosci. 2007;24:437–443. doi: 10.1017/S0952523807070496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cryosections of adult zebrafish retinas were prepared after 36 hours of constant light and labeled with two separate Sox2 antibodies (A–C). These antibodies were raised against different antigens in either goat (A; Green; R&D) or rabbit (B; Red; GeneTex). Overlaying both channels revealed an identical expression pattern between the antibodies. ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. Scale bar in panel A is 25 μm and is the same for panels B–C.