Abstract
Oxidative injuries, such as those related to reactive oxygen species (ROS), have been implicated in various retinal and optic nerve disorders. Many ROS detection methods have been developed. Although widely utilized, many of these methods are useful only in post mortem tissues, or require relatively expensive equipment, or involve intraocular injection. In the present study, we demonstrated and characterized a chemiluminescent probe L-012 as a noninvasive, in vivo ROS detection agent in the mouse retina. Using optic nerve crush (ONC) and retinal ischemia/reperfusion (I/R) as mouse injury models, we show that L-012 produced intensive luminescent signals specifically in the injured eyes. Histological examination showed that L-012 administration was safe to the retina. Additionally, compounds that reduce tissue superoxide levels, apocynin and TEMPOL, decreased injury-induced L-012 chemiluminescence. The decrease in L-012 signals correlated with their protective effects against retinal I/R-induced morphological and functional changes in the retina. Together, these data demonstrate the feasibility of a fast, simple, reproducible, and non-invasive detection method to monitor in vivo ROS in the retina. Furthermore, the results also show that reduction of ROS is a potential therapeutic approach for protection from these retinal injuries.
Introduction
Mitochondrial respiration is the primary source for production of reactive oxygen species (ROS), including superoxide. Ordinarily endogenous antioxidant enzymes inside or outside the mitochondria degrade these highly reactive molecules. However, excessive levels of ROS can overwhelm these intrinsic defense mechanisms leading to damage including oxidative and nitrative modifications to cellular proteins, lipid peroxidation, DNA damage, and ultimately neuronal death (Manzanero et al., 2013; Bryan et al., 2012). Mitochondrial dysfunction and increased ROS generation have been linked to numerous CNS neurodegenerative conditions, such as amyotrophic lateral sclerosis, Parkinson’s disease, Alzheimer’s disease, and cognitive declines observed in normal aging (Emerit et al., 2004). In addition to their pathogenic and pathophysiological roles, however, ROS is also believed to contribute to physiological processes regulating cell fate, stress responses, vascular tone, and wound healing.
Retinal neurons are particularly susceptible to oxidative stress because of the high levels of oxygen consumption, glucose oxidation, and polyunsaturated fatty acids in the retina (Arden and Sivaprasad, 2011). Unsurprisingly, ROS has been demonstrated to be involved in many retinopathies and optic neuropathies, such as age-related macular degeneration (Winkler et al., 1999), retinopathy of prematurity (Wilkinson-Berka et al., 2013), diabetic retinopathy (Wilkinson-Berka et al., 2013), retinal ischemia (Osborne et al., 2004), optic nerve trauma (Kanamori et al., 2010), and glaucoma (Aslan et al., 2008). Generation of ROS following ischemic injury, more specifically during reperfusion (Kuriyama et al., 2001), is amongst the earliest pathogenic changes leading to immune cell infiltration and neuron apoptosis (Bonne et al., 1998). Therefore, ROS represents a prime therapeutic target against injury caused by retinal ischemia.
While several strategies for removal of ROS have been developed, in vivo monitoring of ROS is technically challenging. Current detection methods include fluorescent probes such as dihydroethidium (DHE), spectrophotometric measurements, electron spin resonance spectroscopy, spin traps, and markers for oxidation and nitration of proteins, lipids, and DNA. These are all useful for assessment of oxidative stress in cultured cells, ex vivo biopsy, or post mortem tissue samples, but not for in vivo applications (Halliwell and Whiteman, 2004). Techniques developed for in vivo measurement of ROS include magnetic resonance imaging (Berkowitz et al., 2015; Berkowitz et al., 2016), which requires specialized equipment not available to many laboratories, or intravitreal injection of detecting agents (Rayner et al., 2016), which limits the ability to perform repeated, frequent evaluations. Prunty and colleagues successfully used a sensitive ROS-activated fluorescent probe for in vivo imaging of retinal oxidative stress (Prunty et al., 2015), but this requires the animals to be kept in total darkness for 48 h to prevent photo-bleaching while the probe is accumulated in the eye, which severely limits its usefulness in detecting ROS changes shortly after injury or experimental manipulation. It is desirable to develop a technique that will overcome these limitations: requirement of relatively expensive equipment, intraocular injection, or a 48-h accumulation period.
Recent studies have demonstrated that L-012 (8-amino-5-chloro-7-phenylpyrrido [3,4-d]-pyridazine-1, 4 (2H, 3H) dione), a highly sensitive chemiluminescent probe (Nishinaka et al., 1993), can be used to detect subcutaneous ROS activity by in vivo luminescent imaging (Kielland et al., 2009; Zhou et al., 2012). Because of the ability for light to penetrate through the transparent media of the eye, we hypothesized that L-012 chemiluminescence from the retina could be detected by non-invasive in vivo imaging in mouse models of ocular injury. In the present study, we characterized and optimized the use of L-012 as a non-invasive in vivo method to assess temporal accumulation of ROS in the mouse retina following retinal ischemia/reperfusion (I/R) and optic nerve crush (ONC) injuries. Furthermore, we demonstrate the selectivity of L-012 through rapid modulation of luminescence, and the neuroprotective potential of early detection using two selective inhibitors of superoxide accumulation.
Materials and Supplies
Animals
Female C57BL/6J mice (9- to 12-week old) were purchased from Jackson Laboratory (Bar Harbor, ME, USA) and Guangdong Animal Experimental Center (Guangzhou, Guangdong, China). All animals were maintained and handled in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. The University of North Texas Health Center and Shenzhen Eye Hospital Institutional Animal Care and Use Committees approved the research protocol prior to initiation of the study.
Equipment
In vivo detection of L-012 signal required a small animal imaging system (IVIS Lumina XR™; Caliper LifeSciences, Hopkinton, MA, USA), which consists an enclosed, dark chamber with a warming pad to maintain animal body temperature. Luminescence signals were imaged by a CCD camera and were analyzed using the Living Image Software™ (PerkinElmer, Waltham, MA, USA).
Images of retina cross-sections were acquired using a Nikon Eclipse Ti inverted microscope (Nikon, Melville, NY, USA) containing the CRi Nuance FX multispectral imaging system (Caliper LifeSciences). Fluorescence was evaluated by equipping the microscope with a Semrock (Rochester, NY, USA) BrightLine TRITC-A-NTE filter cube (exciter: FF01-542/20-25, emitter: FF01-620/52-25, dichroic: FF570-Di01-25×36). Fluorescence was quantified using ImageJ software (NIH, Bethesda, MD, USA).
Electroretinography (ERG) was conducted using the HMsERG system (Ocuscience, Rolla, MO, USA). Amplitude and implicit times of waveforms were measured and analyzed in ERGView v4.380R software (Ocuscience).
Materials
L-012 was purchased from Wako Chemicals (catalog number: 120-04891; Richmond, VA, USA). Other materials and their respective suppliers are as follows: ketamine (Henry Schein Animal Health, Dublin, OH, USA), xylazine (Akorn, Lake Forest, IL, USA), acepromazine (Henry Schein Animal Health), isoflurane (Henry Schein Animal Health), Alcaine® ophthalmic solution (0.5% proparacaine HCl; Alcon, Fort Worth, TX, USA), Bacitracin zinc with polymixin B sulfate ophthalmic ointment (Akorn), 1% tropicamide ophthalmic solution (Bausch & Lomb Pharmaceuticals Inc., Claremont, CA, USA), Artificial Tears Solution™ (Rugby, Rockville Center, NY, USA), Gonak™ lubricant eye drop (Akorn), OxiSelect™ protein carbonyl ELISA Kit (Cell Biolabs, San Diego, CA, USA), Tissue-Tek OCT (Sakura Finetek, Torrance, CA, USA), ProlongGold anti-fade reagent with DAPI (Molecular Probes, Life Technologies, Grand Island, NY, USA), Dihydroethidium (DHE) (Thermo Fisher Scientific, Waltham, MA, USA), TEMPOL (4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl; Sigma-Aldrich, St. Louis, MO, USA), Apocynin (4-hydroxy-3-methoxyacetophenone; Sigma-Aldrich), Evans blue (Sigma-Aldrich).
Detailed Methods
Mouse models of injury
Crush of the mouse ON was conducted as previously reported (Liu et al., 2014; Choudhury et al., 2015). Briefly, mice were anesthetized by intraperitoneal injection of ketamine and xylazine (100 and 10 mg/kg, respectively). Topical anesthesia of the eye was achieved by topical administration of Alcaine® ophthalmic solution. The left ON of mice in the crush group was exposed intraorbitally through a small window made between the surrounding muscles and vascular plexus, and crushed approximately 1 mm posterior to the globe with a self-closing forceps for 10 s. In the sham surgery group the left ON was exposed similarly but not crushed. Bacitracin zinc with polymixin B sulfate ophthalmic ointment was topically administered post surgery to prevent ocular infection.
Retinal I/R was induced as described previously (Kim et al., 2013; Nashine et al., 2014; Silverman et al., 2016). Briefly, mice were anesthetized with a ketamine/xylazine/acepromazine cocktail (100/10/3 mg/kg, intraperitoneally) followed by cannulation of the anterior chamber with a 30-gauge needle connected to a reservoir filled with sterile PBS. The reservoir was elevated to generate an intraocular pressure (IOP) of 120 mmHg for 1 h to induce retinal ischemia. Afterwards, the cannula was removed and retinal circulation was allowed to resume.
In vivo L-012 chemiluminescence imaging
Retinal ROS detection by the chemiluminescent probe L-012 was captured using the small animal in vivo imaging system. L-012 dissolved in sterilized distilled water was administered intraperitoneally using an insulin syringe with a 31-gauge needle at the indicated dose in a volume of 100 μL. (Notes: Attempts to use saline or PBS to dissolve L-012 at ≥15 mg/mL often led to precipitation. Aqueous solution of L-012 has been shown to be stable for more than 4 h at 37°C (Zielonka et al., 2013). Frozen stocks of L-012 aqueous solution kept at −20°C were stable for at least 6 months.) Animals were anesthetized by isoflurane inhalation during the recording procedure. Prior to imaging, pupils were dilated by 1% tropicamide ophthalmic solution, the cornea was moistened with Artificial Tears Solution™, and their body temperatures were maintained at 37°C. Mice were examined for acceptability into the study prior to imaging as defined by a set of inclusion criteria including full pupil dilation and absence of cataract or cornea abnormalities as observed with an ophthalmoscope. Initially, mice were placed supine to visualize and compare light signals from both eyes simultaneously (Figure 1A). For signal quantitation, mice were placed on their side with the injured eye oriented towards the camera (Figure 1B). Images of chemiluminescence were acquired sequentially and summed in 5-min exposure intervals, from 15 to 45 min after L-012 injection. Light emission was quantified as photons/s/cm2/steradian. Data were analyzed using the Living Image Software™.
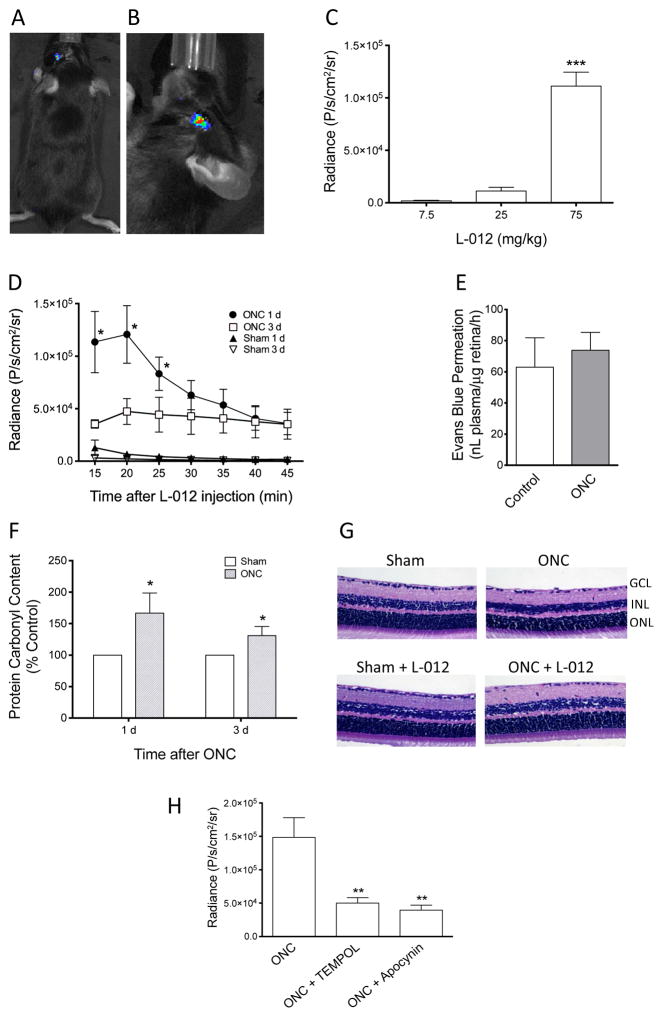
Figure 1. Characterization of L-012 chemiluminescence in mouse optic nerve crush-induced retina injury.
(A) L-012 chemiluminescence was detected in the injured (left) mouse eyes following ONC. No chemiluminescence in the contralateral (right) sham control eye. (B) For subsequent quantifications, animals were positioned so that the injured eye was facing the camera. Radiance is shown as pseudocolor overlay with photo image of the mouse head. (C) Relationship of L-012 dose and radiance at 20 min after L-012 injection, 1 d post ONC. ***: P<0.001 vs the 7.5 mg/kg group by one-way ANOVA and Bonferroni’s test (n=5). (D) Time courses of L-012 chemiluminescence. Quantification of radiance was obtained from 15 min to 45 min, in 5-min exposure intervals, after L-012 injection (75 mg/kg, ip) at 1 d and 3 d after ONC. *: P<0.05 vs the respective sham control groups (n=5) by unpaired Student’s t-test. The data points 3 d after ONC were not statistically different. (E) Evaluation of blood-retina barrier function by Evans blue retina permeation assay. At 1 d after ONC, mice were injected with Evans blue intravenously. Levels of Evans blue in retinas in the ONC-injured and its contralateral sham control eyes were assayed and compared 2 h later. No statistically significant difference (P>0.05) was detected by paired Student’s t-test (n=4). (F) Quantification of ONC induced retinal oxidative damage at 1 d and 3 d post-crush by protein carbonyl ELISA. *: P<0.05 vs the respective sham control groups (n=6) by unpaired Student’s t-test. (G) Histological evaluation of retinal cross sections of sham or ONC mice, with or without multiple L-012 injections (75 mg/kg, ip, at 1 d, 3 d, 5 d, and 7 d post-crush). Mice were euthanized and retina samples collected at 7 d after sham or ONC operation. Other than a reduced cell density in the ganglion cell layer in the ONC groups, no additional abnormality was observed. L-012 did not appear to damage the retina. Three animals from each group were assessed. GCL: ganglion cell layer, INL: inner nuclear layer, ONL: outer nuclear layer. (H) Effect of TEMPOL (100 mg/kg, ip, 15 min prior to L-012 injection) and apocynin (50 mg/kg, ip, 15 min prior to L-012 injection) on the L-012 (75 mg/kg, ip) signal at 1 d post-ONC. Radiance values at 20 min after L-012 injection are shown. **: P<0.01 vs the ONC group by one-way ANOVA and Bonferroni’s test (n=5). All data are shown as mean ± SEM.
Ex vivo correlation of L-012 imaging signals
Detection of ROS in the retina by L-012 was validated by a retinal protein carbonyl assay and by DHE staining. As markers of protein oxidation, retinal soluble protein carbonyl groups were quantified by the OxiSelect™ protein carbonyl ELISA Kit. At the indicated time points after injury, retinas were rapidly dissected from both eyes of euthanized mice. Each retina was placed in 200 μL PBS and homogenized on ice. The protein concentration of the supernatant was assayed and diluted to 10 μg/mL by PBS dilution. Samples in duplicates (100 μL each) were reacted with dinitrophenylhydrazine (DNPH), followed by an anti-DNPH antibody and HRP-conjugated secondary antibody. The quantity of protein carbonyl groups in retinal samples was determined by comparing their colorimetric absorbance values with that of an oxidized BSA standard curve.
Additionally, the superoxide indicator DHE was used to detect superoxide contents in the retina. Enucleated mouse eyes without fixation were submerged in Tissue-Tek OCT and snap frozen in methylbutane placed in liquid nitrogen. Eyes were cryo-sectioned at a thickness of 10 μm, placed on Superfrost Plus microscope slides (Thermo Fisher Scientific), and allowed to dry at room temperature for 1 h. Sections were gently washed in PBS followed by incubation with 5 μM DHE at 37°C for 25 min. Sections were then washed once in PBS and mounted with ProlongGold anti-fade reagent with DAPI. Images were taken using a Nikon Eclipse Ti inverted microscope equipped with a Semrock BrightLine TRITC-A-NTE filter cube (exciter: FF01-542/20-25, emitter: FF01-620/52-25, dichroic: FF570-Di01-25×36) and the CRi Nuance FX multispectral imaging system. Fluorescence was quantified using ImageJ.
Experimental manipulation of ROS level
Compounds known to decrease ROS levels were tested in the two models of ocular injury. TEMPOL, a superoxide dismutase (SOD)-mimetic and ROS scavenger (Thiemermann, 2003; Thaler et al., 2011), was dissolved in PBS and administered intraperitoneally (100 mg/kg in 100 μL). Apocynin, a selective reversible NADPH oxidase (NOX) inhibitor that efficiently reduces ROS production in various experimental models (Stefanska and Pawliczak, 2008; Haruta et al., 2009), was dissolved in PBS and 0.7% DMSO at a concentration of 50 mg/kg in 100 μL, and administered intraperitoneally.
Histology evaluation of the retina
Mice were euthanized immediately following their final live imaging session. Eyes were enucleated, fixed in 4% paraformaldehyde at 4°C overnight, paraffin embedded, and sectioned at a thickness of 5 μm. Retina cross-sections were evaluated for morphological changes after H&E staining. For thickness measurements, entire retinas were imaged, from ora serrata to ora serrata through the optic nerve head, and thicknesses of the inner plexiform layer (IPL), inner nuclear layer (INL), outer nuclear layer (ONL), and the whole retina including all layers were measured using calibrated calipers in ImageJ (Liu et al., 2014). Three sections were randomly selected per retina and four cross sectional measurements (at two peripheral and two central locations) of each cross section were recorded. The mean thickness values from the 12 measurements from each retina are reported.
Electroretinography
The abilities of TEMPOL and apocynin to protect against loss of visual function resulting from retinal I/R were determined. Mice were pretreated with either vehicle (PBS), TEMPOL, or apocynin 30 min prior to injury, and daily administration for 7 d after I/R. Visual function was measured using scotopic flash electroretinography (ERG) once weekly over a 4-week period (Kim et al., 2013). Mice were dark adapted overnight, anesthetized with 2% isoflurane, and connected to the HMsERG system. Body temperature was maintained at 37°C. A ground electrode was placed subcutaneously by the tail and reference electrodes were inserted under each eye. Both eyes were dilated using 1% tropicamide, and corneas were anesthetized topically with 0.5% proparacaine. Silver-thread electrodes were placed across the apex of the cornea and held in place with Gonak™ lubricant eye drop coated contact lenses. Eyes were exposed to a series of light flashes at increasing intensities (0.1, 0.3, 1, 3, 10, and 25 cd.s/m2). Amplitude and implicit times of waveforms were measured and analyzed in ERGView v4.380R software.
Evaluation of blood-retina barrier function: Evans blue extravasation
Blood-retina barrier function was evaluated using the sensitive method of Evans blue extravasation into the retina (Xu et al., 2001). Briefly, Evans blue dye (45 mg/kg) was injected into the tail vein of mice. After the dye circulated in the blood for 2 h, animals were anesthetized by ketamine/xylazine/acepromazine cocktail (100/10/3 mg/kg, intraperitoneally), and then 0.4–0.5 mL of blood was collected through the left ventricle and centrifuged at 12,000xg for 15 min at 4°C. The plasma was diluted 100x with formamide. After blood collection, mice were perfused via the left ventricle with PBS followed by 1% paraformaldehyde (approximately 5 mL). Retinas were then dissected from enucleated eyes and placed in pre-weighed tubes. They were vacuum-dried for 1 h and weighed. In each sample, 120 μL formamide was added, incubated for 18 h at 75°C for dye extraction, and centrifuged at 12,000xg for 30 min at 4°C. The Evans blue dye contents in blood and retina supernatants were assayed by comparing OD620 nm with a standard curve. Blood-retina barrier permeability was calculated as “Evans blue in retina sample/dry weight of retina sample/Evans blue concentration in plasma/circulation time (2 h)”, and has a unit of “nL (of plasma)/μg (of retina dry weight)/h”.
Statistical analysis
Data were analyzed using Prism software (Graphpad, San Diego, CA, USA). One-way ANOVA followed by Bonferroni’s test for multiple comparisons was used to compare differences among three or more groups. Paired or unpaired (as specified in the figure legends) Student’s t-test was used for comparison between two groups. A p value of <0.05 was regarded as statistically significant. Data are presented as mean ± SEM.
Results
Optic nerve injury increased L-012 chemiluminescence in the retina
L-012 chemiluminescence has recently been identified as a highly sensitive method for non-invasive in vivo detection for ROS in systemic inflammation (Kielland et al., 2009; Zhou et al., 2012). We tested if this method can be used to detect ROS accumulation in the retina after optic nerve or retinal injury. Figure 1A shows that 1 d after ONC in the mouse, intraperitoneal injection of L-012 (75 mg/kg) produced a time-dependent, transient increase in the chemiluminescence signal. As expected, the signal was detected only in the ONC eye, while no detectable signal was observed in the contralateral sham control eye. For quantification, mice were oriented with their studied eye facing towards the camera to allow maximal visualization. Figure 1B shows such configuration when a signal of an injured eye was collected. Results of preliminary studies also demonstrated that pupil size affected the signal intensity. Constriction of the mouse pupil by topical administration of pilocarpine significantly reduced the signal, whereas the chemiluminescence intensity was maximal when the pupil was fully dilated (data not shown). These results indicate that the signal originated posterior to the iris, likely from the retina. For subsequent studies, 1% tropicamide ophthalmic solution was administered to produce mydriasis prior to L-012 imaging.
To optimize the dose of L-012 that provided maximal dynamic range of retinal ROS signal, we compared the peak chemiluminescence intensities of three concentrations (7.5, 25, and 75 mg/kg, ip) of the compound at 1 d after ONC. As shown in Figure 1C, the signal intensity depended on the dose of L-012, and 75 mg/kg generated the maximal signal (p < 0.001 vs the lower doses). Thus, this dose of L-012 was used in the following studies.
The retinal L-012 chemiluminescence was time-dependent in two aspects; it was dependent on the time after L-012 injection during each imaging session, and the time after ONC. As dictated by the compound’s pharmacokinetic characteristics, peak retinal signal was typically observed at 20 min after L-012 injection, which faded in later time points (Figure 1D). The temporal profile after ONC shows that L-012 signal was clearly detectable at 1 d and 3 d after ON injury, with 1 d being the most prominent. At 1 d after ONC, significant differences (p < 0.05) of light intensities existed between the crushed and sham groups at time points of 15, 20, 25 min. At 3 d after ONC, the luminescence was significantly lower; signals at 15 and 20 min 3 d post-ONC were significantly (p < 0.05) lower than those at 1 d (Figure 1D). Interestingly, the L-012 signal at 4 h, 5 d, or 7 d after ONC was below the detection limit of the imager (data not shown). These data suggest that ROS accumulation produced by ONC was highest approximately 1 d after injury, and decreased thereafter.
Optic nerve injury did not significantly affect blood-retina barrier function
Theoretically, increase in L012 signal in the retina could be the result of injury-induced change in blood-retina barrier function. We used a previously reported, sensitive technique to evaluate this barrier function. As shown in Figure 1E, at 1 d after ONC, no significant change of dye permeation into the retina was detected compared to the contralateral uninjured eye, indicating that under the current study conditions, ONC did not significantly affect blood-retina barrier function, and the change in L012 signal was not a result of breakdown of this barrier.
Optic nerve injury increased protein carbonylation in the retina
To further confirm that the increase in L-012 signal in the eye correlated to accumulation of ROS, we assessed retinal protein carbonylation. Excessive accumulation of ROS leads to oxidative damage. A common modification of proteins by oxidative damage is the addition of carbonyl groups to amino acid side chains (Dalle-Donne et al., 2006). Detection of retinal protein carbonyl contents by ELISA provides a quantitative measurement of retinal oxidative damage. There was a significant increase in retina soluble protein carbonyl contents at 1 d and 3 d after ONC compared with that of sham control at the same time points. Similar to the in vivo imaging results, the carbonylation levels were higher at 1 d relative to 3 d after ON injury (Figure 1F), although the magnitudes of changes did not perfectly match those of L-012 signals. These results indicate a correlation between in vivo imaging findings and post mortem biochemical oxidation assessments, and suggest that the L-012 chemiluminescence is a useful technique for in vivo measurement of retinal oxidative damage. It should be noted that only RGCs are damaged by ONC injury, and total retinal extracts were used for this ELISA experiment, further demonstrating the sensitivity of this imaging technique.
L-012 treatment did not affect retina morphology
Mice treated with multiple L-012 injections did not show any observable gross abnormalities, either behaviorally or anatomically. After four L-012 intraperitoneal injections (75 mg/kg), one injection every other day, mouse retina sections showed no morphological evidence of retinal abnormalities compared to control animals that did not receive L-012 treatment (Figure 1G). Mice with ONC with or without L-012 treatment had lower density of cells in the ganglion cell layer (GCL) as previously demonstrated (Liu et al., 2014; Choudhury et al., 2015). These observations agree with previously published reports from others that L-012 is safe and indicate that it did not cause gross toxicity to retinal tissues.
Superoxide reduction decreased optic nerve injury-induced retinal L-012 chemiluminescence
TEMPOL, a SOD-mimetic and superoxide scavenger, and apocynin, a selective NOX inhibitor, have both been shown to effectively and significantly reduce in vivo levels of superoxide following tissue injury (Lu et al., 2013; Zhang et al., 2012; Dohare et al., 2014). To evaluate the potential molecular involvement of L-012 chemiluminescence with ROS at 1 d after ONC, the peak time to observe ROS accumulation, TEMPOL (100 mg/kg) or apocynin (50 mg/kg) were administered intraperitoneally 15 min prior to L-012 injection and live imaging. Under these conditions, both TEMPOL and apocynin significantly (p < 0.01) lowered the L-012 signal. L-012 signal intensity decreased to 33.7% of vehicle after TEMPOL treatment and 26.7% after apocynin treatment (Figure 1H). Since apocynin inhibits the conversion of O2 to superoxide, and TEMPOL is an effective superoxide scavenger, these findings indicate that reactive oxygen species, especially superoxide, in the retina account for the majority of the L-012 chemiluminescence associated with ON injury.
Retinal ischemia/reperfusion increased L-012 chemiluminescence and retinal superoxide
To explore the involvement of ROS and the potential usefulness of L-012 in other retina injury models, retinal I/R injury was also examined. Similar to ONC, I/R injury produced significant L-012 chemiluminescence in the mouse retina (Figure 2B), compared to the control eye (Figure 2A). Again similar to ONC, the emission intensity was highest at 1 d after I/R injury when compared to subsequent days (Figure 2C).
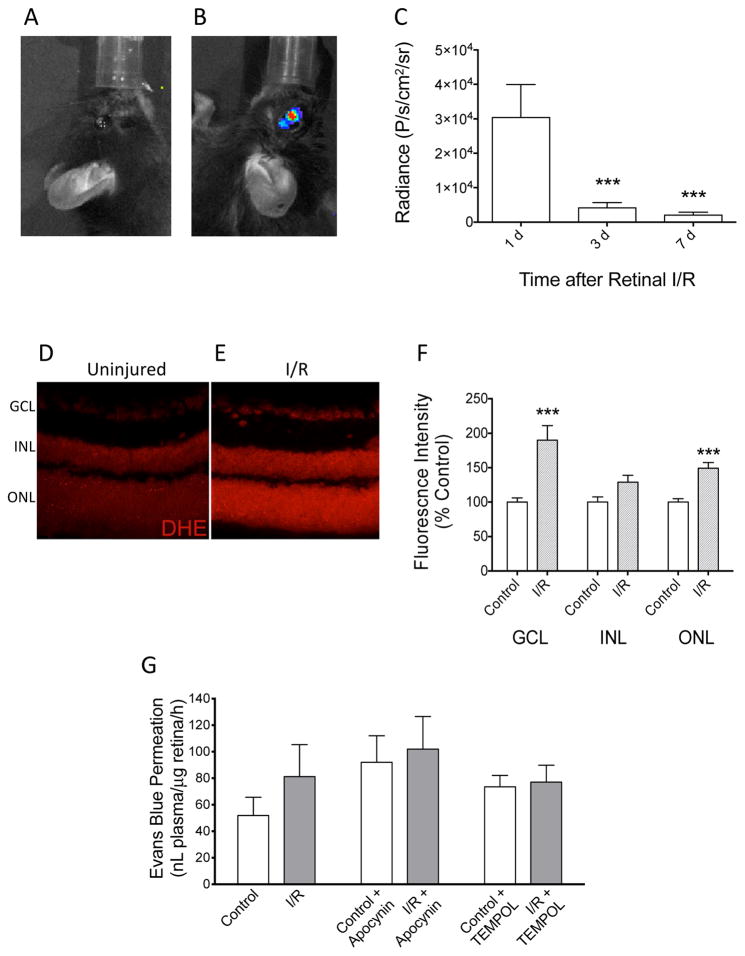
Figure 2. Retinal accumulation of ROS after ischemia/reperfusion.
(A) No chemiluminescence in the contralateral control eye. (B) L-012 chemiluminescence was detected in the injured (left) mouse eyes following retinal I/R. (C) Chemiluminescence signal at 20 min after L-012 injection (75 mg/kg, ip) at indicated time points after retinal I/R. ***: P<0.001 vs the 1 d group by one-way ANOVA and Bonferroni’s test (n=7). (D, E, F) Post mortem detection of superoxide in retina with DHE. Representative images of retina cross sections from control (D) and I/R (E) groups labeled with DHE. Retina samples were collected at 24 h after I/R. (F) Differences in fluorescence intensities were quantified in the three nucleated layers of the retina. Superoxide was observed to be significantly increased in the ganglion cell layer (GCL) and outer nuclear layer (ONL), but not the inner nuclear layer (INL). ***: P<0.001 vs control by unpaired Student’s t-test (n=4). Data are shown as mean ± SEM. (G) Evaluation of blood-retina barrier function by Evans blue retina permeation assay. At 1 d after I/R, mice were injected with Evans blue intravenously. In groups with apocynin (50 mg/kg) or TEMPOL (100 mg/kg) treatments, the drugs were administered intraperitoneally 15 min prior to Evans blue injection. Levels of Evans blue in retinas of the I/R-injured and contralateral control eyes were assayed 2 h after Evans blue injection. No statistically significant difference (P>0.05) was detected by paired Student’s t-test (No drug treatment groups: n=10; Apocynin-treated groups, n=6; TEMPOL-treated groups, n=7).
We utilized DHE, a superoxide indicator, to verify the presence of superoxide in retina cross-sections after I/R. Without DHE labeling, no fluorescence was observed under identical study conditions (data not shown). There was a baseline DHE labeling in the uninjured retinas, while DHE labeling in retinas 24 h post I/R showed an increased signal. Representative images of control and injured retinas are shown in Figures 2D & 2E. The most dramatic changes appeared to be in the ganglion cell layer (GCL), where cell bodies of retinal ganglion cells and displaced amacrine cells are located, and outer nuclear layer (ONL), where photoreceptor cells are located. Fluorescent intensity was quantified in all nucleated layers of the retina to further identify the increases in superoxide levels. There were statistically significant increases (p<0.001) in both the GCL and ONL in comparison to uninjured retinas (Figure 2F). Levels of superoxide after I/R in the inner nuclear layer (INL), where somas of bipolar, Müller, horizontal, and amacrine cells are present, appeared higher than that of control retinas, but the change did not reach statistical significance (Figure 2F).
Changes in L-012 chemiluminescence reflected changes in retinal superoxide level. Similarly, we confirmed that, by retinal Evans blue extravasation, the potential change of blood-retinal barrier was not an important contributory factor to the enhancement of this L-012 signal. As seen in Figure 2G, I/R showed a trend of small, but not significant, increase in Evans blue extravasation, which could not explain the dramatic increase in the L-012 signal. In addition, apocynin or tempol did not affect Evans blue extravasation, while they significantly suppressed ocular L-012 signal (Figure 3A).
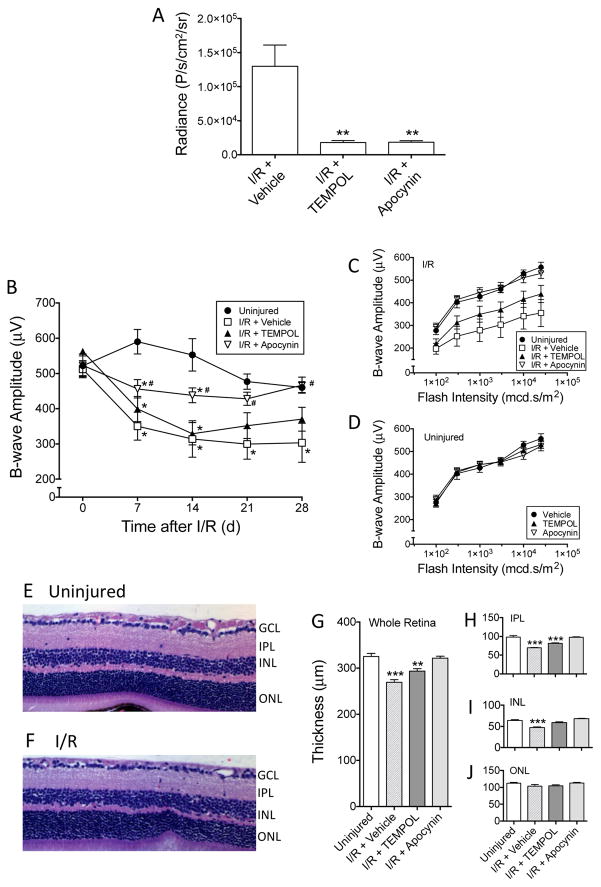
Figure 3. Protective effects of TEMPOL and apocynin against retina ischemia/reperfusion-induced damge.
(A) L-012 (75 mg/kg, ip) chemiluminescence was significantly reduced by TEMPOL (100 mg/kg, ip, 15 min prior to L-012 injection) and apocynin (50 mg/kg, ip, 15 min prior to L-012 injection) at 1 d post-injury. Radiance values at 20 min after L-012 injection are shown. **: P<0.01 vs the “I/R + Vehicle” group by one-way ANOVA and Bonferroni’s test (n=5). (B) Reduction of superoxide was protective against I/R-induced loss of visual function. Scotopic flash ERG was measured weekly following retinal I/R. The mice were treated with either vehicle, TEMPOL (100 mg/kg, ip), or apocynin (50 mg/kg, ip) 30 min prior to injury, then once daily from 1 d to 7 d. The time course plot shows b-wave amplitudes in response to a flash intensity of 3,000 mcd.s/m2 in each treatment group. While all three I/R groups displayed signifcant loss of b-wave amplitudes compared to the uninjured control group as early as 7 d, apocynin produced significant protection compared to the “I/R + Vehicle” group. TEMPOL treatment demonstrated a trend of protection, but the improvement was not significantly different from the “I/R + Vehicle” group. *: P<0.05 vs “Uninjured” group, and #: P<0.05 vs “I/R + Vehicle” group, by one-way ANOVA followed by Bonferroni post hoc test (n=6-7). (C) b-wave amplitudes in response to all flash intensities of the same animals in Figure 3B at 28 d post-injury. Amplitudes of apocynin-treated mice were similar to the uninjured eyes, while TEMPOL produced a trend of improvement compared to the “I/R + Vehicle” group. (D) TEMPOL and apocynin did not affect b-wave amplitudes of the contralateral uninjured eyes. At 28 d, the mice were euthanized and their retinas sectioned and H&E stained. L-012 did not affect the gross morphology of the mouse retina (E) & (F). However, I/R significantly decreased thicknesses of the whole retina (G), inner plexiform layer (IPL)(H), and inner nuclear layer (INL)(I), but not outer nuclear layer (ONL)(J). Apocynin was significantly protective against retinal thinning (G–J). It appeared that retina layers of the TEMPOL-treated mice were thicker than those of the “I/R + Vehicle” group, the differences were typically not significant (G–J). **: P<0.01, ***: P<0.001 vs Uninjured group by one-way ANOVA followed by Bonferroni post hoc test (n=6–7). Data are shown as mean ± SEM.
Superoxide reduction decreased retinal ischemia/reperfusion-induced L-012 chemiluminescence
Similar to results observed in the ONC model, 1 d after retinal I/R injury, both TEMPOL and apocynin significantly (p<0.01) suppressed chemiluminescent signals in the eye compared to vehicle-treated animals (Figure 3A). No statistical differences were detected between treatments with these two compounds.
Superoxide reduction was neuroprotective against retinal ischemic injury
Given the significant reduction in L-012 signals following TEMPOL and apocynin treatments, we hypothesized that early treatment targeting the reduction of ROS would protect animals against morphological and functional damage resulting from retinal I/R injury. Mice were pretreated with either TEMPOL (100 mg/kg, ip) or apocynin (50 mg/kg, ip) 30 min prior to ischemia, followed by a daily administration of the respective compound for 7 d post injury. To evaluate retinal functional changes, scotopic flash ERG readings with different flash intensities were assessed weekly for 28 d. Using the b-wave responses at 3,000 mcd.s/m2 as an example, retinal I/R caused a time-dependent decrease in ERG amplitudes compared to uninjured eyes (Figure 3B), which corroborates our previous findings (Kim et al., 2013; Nashine et al., 2014). Interestingly, TEMPOL treatment moderately, but not significantly, improved b-wave amplitudes compared to vehicle (Figure 3B). In contrast, animals receiving apocynin recorded b-wave amplitudes that were significantly (p<0.05) higher than that of vehicle-treated I/R-injured mice at all time points. The ERG response of the apocynin treatment group at 28 d was similar to that of the uninjured eyes, although they were significantly lower in earlier time points (Figure 3B). The same conclusion can be derived from examining the ERG responses to different flash intensities at the 28 d time point. In I/R-injured eyes, b-wave amplitudes were significantly decreased to approximately half of those of uninjured eyes in all flash intensities (Figure 3C). While TEMPOL treatment partially protected against the insult-induced ERG reduction, apocynin produced complete protection; mice treated with apocynin demonstrated b-wave amplitudes were indistinguishable from those of uninjured mice (Figure 6C). Treatment with TEMPOL or apocynin did not affect ERG responses in uninjured eyes (Figure 3D).
In addition to functional abnormalities, retinal I/R also induces morphological changes in the retina. We showed previously that these injuries caused a significant thinning of the IPL and INL, contributing to the thinning of the whole retina (Kim et al., 2013; Nashine et al., 2014). In the present study, we confirmed these findings. Careful measurement of retinal cross-sections (Figure 3E, 3F) from all treatment groups at 28 d after I/R indicated significant (p<0.001) thinning of the whole retina (269.5 ± 5.8 μm) in the vehicle-treated I/R group compared to uninjured eyes (325.3 ± 6.9 μm) (Figure 3G). The thinning was most prominent in the IPL and INL, but not ONL (Figure 3H–3J). Similar to our ERG results, while treatment with TEMPOL partially protected against the I/R-induced thinning, treatment with apocynin completely protected against the injury-induced changes (Figure 3G–3I). Both compounds did not significantly affect ONL thickness (Figure 3J) or thicknesses of all layers in uninjured retinas (data not shown).
Potential Pitfalls and Trouble Shooting
We have successfully demonstrated a method for non-invasive in vivo imaging of ROS production using L-012 chemiluminescence in the eyes of mice following retinal I/R and ONC injuries. We confirmed that L-012 detects superoxide in the retina through the use of ex vivo assays and modulation of luminescence with two separate superoxide inhibitors. Finally, we showed that treatment with apocynin is a viable neuroprotective strategy, capable of significantly rescuing against functional and morphological deficits resulting from retinal I/R injury.
This method is simple to perform, and as shown by the data, produces relatively reproducible results. This method can be used to monitor ROS changes in other ocular injury models. However, the imaging time point needs to be optimized according to ROS production in each injury model. The major limitation of the current detection method is that it did not produce an image with sufficiently high resolution to pinpoint the regional or cellular location of signals in the retina. We have tried to use the Micron IV™ Retinal Imaging Microscope (Phoenix Research Labs, Pleasanton, CA, USA) to observe an ONC-injured retina in a L-012-treated mouse, but were unable to detect a clear signal, likely due to issues related to signal intensity and detection sensitivity. Furthermore, in order to optimize the signal detection, it is obviously important to minimize any optical interference between the retina and the CCD camera. Thus, before image acquisition, we carefully examined the mouse eyes using an ophthalmoscope to ensure that the pupil was fully dilated and there was no cataract or cornea abnormality. It also is essential to position the mouse head correctly so that the eye is directly facing the camera, and to keep the cornea moist during the imaging procedure. We also would like to caution future users that it is important to minimize bleeding during or after surgery. Bleeding may activate neutrophils, which produce large amounts of superoxide and other free radicals, thus generating increased background noise added to the injury-induced signal. However, this theoretically would occur in both the injury group and sham-operated group, which is an important control. In addition, given that activated neutrophils could potentially confound the results of ex vivo assays and histology, the chemiluminescent assessment is a good way of screening potential outliers. Selected important points for best practice are listed in Table 1.
Table 1.
Selected Important Points for Best Practices
|
Additional Discussion
Numerous studies have previously established the significance of L-012 in the detection of ROS in cell culture (Daiber et al., 2004; Sohn et al., 1999; Nishinaka et al., 1993). However, only recently has the sensitivity of this luminol derivative been tested for the subcutaneous detection of reactive oxygen and nitrogen species. Injection of inflammatory stimulants such as lipopolysaccharides, ethanol, polylactide microspheres, polyethylene glycol particles, and Compound 48/80 were all identified in vivo to increase ROS as detected by L-012 chemiluminescence (Hu et al., 2015; Kielland et al., 2009; Zhou et al., 2012). Given these successes, we decided to test this compound’s sensitivity in a previously unstudied tissue, the retina, using injury models known to increase ROS. Only injured eyes produced a detectable luminescent signal following systemic administration of L-012. From this we can conclude that L-012 is capable of crossing the blood-retina barrier, previously unknown prior to this study. This finding opens the possibility for observation of superoxide in the retina and perhaps in the brain using models of focal or global ischemia. While other barriers to ROS detection in the brain exist, primarily the skull, the ability to cross blood retina barriers presents for the first time a noninvasive in vivo method of superoxide measurement in the CNS.
To confirm the detection of superoxide in the retina, we employed DHE fluorescence in retinal cross sections (Figure 2C). Accumulation of ROS including superoxide has been described following ischemic injury in different tissues (Osborne et al., 2004; Mehta et al., 2007; Fraser, 2011). Several of these studies have measured superoxide in situ using DHE. Likewise, in the retina, DHE has been used to visualize increased superoxide in mouse models of diabetic retinopathy, retinopathy of prematurity, uveitis, and retinal ischemia (Du et al., 2013; Sasaki et al., 2009; Stevenson et al., 2010; Edgar et al., 2015). Our studies also revealed a significant increase in DHE signals between control and ischemic eyes at 24 h post injury, corresponding to our in vivo findings with L-012.
To further identify that the signal detected during live imaging was due to accumulation of superoxide generation, we tested apocynin and TEMPOL. Both compounds were previously shown to reduce L-012 chemiluminescence following LPS treatment (Kielland et al., 2009), and as expected both significantly reduced luminescence in the injured eyes. Recently it was proposed that L-012 does not directly react with superoxide, but rather forms an endoperoxide, which then becomes excited. It was further proposed that apocynin inhibits luminescence by inhibiting the production of this end product rather than reducing superoxide level (Zielonka et al., 2013). However, Kielland et al (Kielland et al., 2009) observed that the nitric oxide synthase inhibitor, NG-nitro-L-arginine methyl ester (L-NAME), almost completely eliminated L-012 luminescence when compared to apocynin, contradicting the proposed endoperoxide involvement. Along with superoxide, L-012 is known to react with peroxynitrite in cell culture (Daiber et al., 2004). While we have not evaluated the presence of reactive nitrogen species (RNS) following retinal injury, L-NAME has previously been shown to preserve retinal morphology but fails to rescue ERG b-wave amplitudes after retinal ischemia (Ju et al., 2000; Ostwald et al., 1995). In comparison, we found both TEMPOL and apocynin eliminated as much as 85% of the L-012 luminescence detected during peak activity in vivo. Thus, our findings indicate that most of the retinal L-012 signal originated from the accumulation of ROS rather than RNS. However, we cannot rule out the possibility that the remaining signal may be a result of the reaction of L-012 with RNS. Future studies combining NOX and nitric oxide synthase inhibitors may shed light on the contribution of RNS in these ocular injuries.
To evaluate the contribution of ROS in injury-induced retinopathy, we tested whether either TEMPOL or apocynin is sufficient to provide protection against ischemic-induced damage. Previous studies have demonstrated that free radicals play an important role in the development of retinal I/R-induced injury, and the treatment with free radical scavengers, such as superoxide dismutase and catalase reduce the severity of reperfusion damage in various animals (Szabo et al., 1991; Nayak et al., 1993; Yamamoto et al., 1994; Chen and Tang, 2011). Activation of NF-E2-related factor 2 (Nrf2), a major regulator of antioxidant response in the cell, is protective against retinal I/R injury (Wei et al., 2011; He et al., 2014; Pan et al., 2014; Xu et al., 2015). Treatment with many other antioxidants, for example, EGB 761, extract of Ginkgo biloba, lutein, quercetin, vitamin E, crocin, crocetin, and resveratrol have all been shown to be at least partially protective against this retinal insult (Szabo et al., 1991; Dilsiz et al., 2006; Ishizuka et al., 2013; Qi et al., 2013; Vin et al., 2013; Arikan et al., 2015; Chen et al., 2015).
We found that, consistent with its effect in reducing I/R injury-induced L-012 chemiluminescence, apocynin produced statistically significant protection in both visual function and retinal morphology. These findings corroborate previously published results showing the involvement of NOX and in that expression of NOX-1 was significantly increased in retinal ganglion cells following retinal ischemia, thereby increasing their susceptibility to apoptosis (Dvoriantchikova et al., 2012). Furthermore, genetic deletion of Nox genes protects the retina from neovascularization, microglial activation and infiltration, and neuronal death (Wilkinson-Berka et al., 2014; Chan et al., 2013; Yokota et al., 2011). Apocynin also has previously been shown to reduce DHE fluorescence (Fujita et al., 2012; Yokota et al., 2011) and minimize neovascularization (Al-Shabrawey et al., 2005; Al-Shabrawey et al., 2008) after retinal ischemia. However, to our knowledge, our study is the first to demonstrate both functional and morphological protection from retinal ischemia using this NOX inhibitor. In contrast, TEMPOL showed a trend of protection, but produced less and often statistically insignificant benefits (Figures 3B–3J). As a superoxide scavenger, TEMPOL has been shown to increase neuronal survival, decrease apoptosis biomarkers, and improve neuromotor score following cerebral ischemia (Chiarotto et al., 2014; Deng-Bryant et al., 2008; Dohare et al., 2014). The reason for its lack of significant protective effect in our model is not clear. Nonetheless, we speculate that the transient increase in superoxide levels in the tissue between its production and elimination by scavenging may be sufficient to initiate the pathological changes in the retina. Increased TEMPOL concentrations may lead to better protection. Clarification awaits further investigation.
In conclusion, we have established and characterized the use and safety of the chemiluminescent compound L-012 for non-invasive in vivo measurement of superoxide generation following two different retinal injuries. Using this early detection as guidance for treatment, we were able to significantly rescue loss of visual function and preserve retinal morphology against ischemic injury with the NOX inhibitor apocynin. We propose L-012 as a viable tool for diagnostic detection of superoxide in other animal models of retinal injury. Clinically, further improvement of this technique may prove to be a useful tool for examining and diagnosing patients following ischemic events or other retinal disorders to prevent or minimize pathophysiological changes due to oxidative stress.
HIGHLIGHTS.
ROS production during retinal injury can be imaged in mouse eyes in vivo using L-012.
L-012 luminescence in vivo corresponded to ROS detection ex vivo.
ROS inhibitors blocked L-012 retinal injury-induced luminescence and protected the retinas from injury.
Acknowledgments
This research was supported by a grant from the US National Eye Institute (R21EY02463) and a grant from the National Natural Science Foundation of China (NNSF #81200688). We would like to thank Sandra Maansson for technical assistance. Drs. Hong Weng and Yi-Ting Tsai were involved in the initial stage of the imaging study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Shabrawey M, Bartoli M, El-Remessy AB, Platt DH, Matragoon S, Behzadian MA, Caldwell RW, Caldwell RB. Inhibition of NAD(P)H oxidase activity blocks vascular endothelial growth factor overexpression and neovascularization during ischemic retinopathy. Am J Pathol. 2005;167(2):599–607. doi: 10.1016/S0002-9440(10)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shabrawey M, Rojas M, Sanders T, Behzadian A, El-Remessy A, Bartoli M, Parpia AK, Liou G, Caldwell RB. Role of NADPH oxidase in retinal vascular inflammation. Invest Ophthalmol Vis Sci. 2008;49(7):3239–3244. doi: 10.1167/iovs.08-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arden GB, Sivaprasad S. Hypoxia and oxidative stress in the causation of diabetic retinopathy. Curr Diabetes Rev. 2011;7(5):291–304. doi: 10.2174/157339911797415620. [DOI] [PubMed] [Google Scholar]
- Arikan S, Ersan I, Karaca T, Kara S, Gencer B, Karaboga I, Hasan Ali T. Quercetin protects the retina by reducing apoptosis due to ischemia-reperfusion injury in a rat model. Arq Bras Oftalmol. 2015;78(2):100–104. doi: 10.5935/0004-2749.20150026. [DOI] [PubMed] [Google Scholar]
- Aslan M, Cort A, Yucel I. Oxidative and nitrative stress markers in glaucoma. Free Radic Biol Med. 2008;45(4):367–376. doi: 10.1016/j.freeradbiomed.2008.04.026. [DOI] [PubMed] [Google Scholar]
- Berkowitz BA, Bredell BX, Davis C, Samardzija M, Grimm C, Roberts R. Measuring In Vivo Free Radical Production by the Outer Retina. Invest Ophthalmol Vis Sci. 2015;56(13):7931–7938. doi: 10.1167/iovs.15-18420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz BA, Lewin AS, Biswal MR, Bredell BX, Davis C, Roberts R. MRI of Retinal Free Radical Production With Laminar Resolution In Vivo. Invest Ophthalmol Vis Sci. 2016;57(2):577–585. doi: 10.1167/iovs.15-18972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonne C, Muller A, Villain M. Free radicals in retinal ischemia. Gen Pharmacol. 1998;30(3):275–280. doi: 10.1016/s0306-3623(97)00357-1. [DOI] [PubMed] [Google Scholar]
- Bryan N, Ahswin H, Smart N, Bayon Y, Wohlert S, Hunt JA. Reactive oxygen species (ROS)--a family of fate deciding molecules pivotal in constructive inflammation and wound healing. Eur Cell Mater. 2012;24:249–265. doi: 10.22203/ecm.v024a18. [DOI] [PubMed] [Google Scholar]
- Chan EC, van Wijngaarden P, Liu GS, Jiang F, Peshavariya H, Dusting GJ. Involvement of Nox2 NADPH oxidase in retinal neovascularization. Invest Ophthalmol Vis Sci. 2013;54(10):7061–7067. doi: 10.1167/iovs.13-12883. [DOI] [PubMed] [Google Scholar]
- Chen B, Tang L. Protective effects of catalase on retinal ischemia/reperfusion injury in rats. Exp Eye Res. 2011;93(5):599–606. doi: 10.1016/j.exer.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Chen L, Qi Y, Yang X. Neuroprotective effects of crocin against oxidative stress induced by ischemia/reperfusion injury in rat retina. Ophthalmic Res. 2015;54(3):157–168. doi: 10.1159/000439026. [DOI] [PubMed] [Google Scholar]
- Chiarotto GB, Drummond L, Cavarretto G, Bombeiro AL, de Oliveira AL. Neuroprotective effect of tempol (4 hydroxy-tempo) on neuronal death induced by sciatic nerve transection in neonatal rats. Brain Res Bull. 2014;106:1–8. doi: 10.1016/j.brainresbull.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Choudhury S, Liu Y, Clark AF, Pang IH. Caspase-7: a critical mediator of optic nerve injury-induced retinal ganglion cell death. Mol Neurodegener. 2015;10(1):40. doi: 10.1186/s13024-015-0039-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiber A, Oelze M, August M, Wendt M, Sydow K, Wieboldt H, Kleschyov AL, Munzel T. Detection of superoxide and peroxynitrite in model systems and mitochondria by the luminol analogue L-012. Free Radic Res. 2004;38(3):259–269. doi: 10.1080/10715760410001659773. [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I, Aldini G, Carini M, Colombo R, Rossi R, Milzani A. Protein carbonylation, cellular dysfunction, and disease progression. J Cell Mol Med. 2006;10(2):389–406. doi: 10.1111/j.1582-4934.2006.tb00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng-Bryant Y, Singh IN, Carrico KM, Hall ED. Neuroprotective effects of tempol, a catalytic scavenger of peroxynitrite-derived free radicals, in a mouse traumatic brain injury model. J Cereb Blood Flow Metab. 2008;28(6):1114–1126. doi: 10.1038/jcbfm.2008.10. [DOI] [PubMed] [Google Scholar]
- Dilsiz N, Sahaboglu A, Yildiz MZ, Reichenbach A. Protective effects of various antioxidants during ischemia-reperfusion in the rat retina. Graefes Arch Clin Exp Ophthalmol. 2006;244(5):627–633. doi: 10.1007/s00417-005-0084-6. [DOI] [PubMed] [Google Scholar]
- Dohare P, Hyzinski-Garcia MC, Vipani A, Bowens NH, Nalwalk JW, Feustel PJ, Keller RW, Jr, Jourd’heuil D, Mongin AA. The neuroprotective properties of the superoxide dismutase mimetic tempol correlate with its ability to reduce pathological glutamate release in a rodent model of stroke. Free Radic Biol Med. 2014;77:168–182. doi: 10.1016/j.freeradbiomed.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Veenstra A, Palczewski K, Kern TS. Photoreceptor cells are major contributors to diabetes-induced oxidative stress and local inflammation in the retina. Proc Natl Acad Sci U S A. 2013;110(41):16586–16591. doi: 10.1073/pnas.1314575110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoriantchikova G, Grant J, Santos AR, Hernandez E, Ivanov D. Neuronal NAD(P)H oxidases contribute to ROS production and mediate RGC death after ischemia. Invest Ophthalmol Vis Sci. 2012;53(6):2823–2830. doi: 10.1167/iovs.12-9526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar KS, Matesanz N, Gardiner TA, Katusic ZS, McDonald DM. Hyperoxia depletes (6R)-5,6,7,8-tetrahydrobiopterin levels in the neonatal retina: implications for nitric oxide synthase function in retinopathy. Am J Pathol. 2015;185(6):1769–1782. doi: 10.1016/j.ajpath.2015.02.021. [DOI] [PubMed] [Google Scholar]
- Emerit J, Edeas M, Bricaire F. Neurodegenerative diseases and oxidative stress. Biomed Pharmacother. 2004;58(1):39–46. doi: 10.1016/j.biopha.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Fraser PA. The role of free radical generation in increasing cerebrovascular permeability. Free Radic Biol Med. 2011;51(5):967–977. doi: 10.1016/j.freeradbiomed.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Fujita T, Hirooka K, Nakamura T, Itano T, Nishiyama A, Nagai Y, Shiraga F. Neuroprotective effects of angiotensin II type 1 receptor (AT1-R) blocker via modulating AT1-R signaling and decreased extracellular glutamate levels. Invest Ophthalmol Vis Sci. 2012;53(7):4099–4110. doi: 10.1167/iovs.11-9167. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 2004;142(2):231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruta M, Bush RA, Kjellstrom S, Vijayasarathy C, Zeng Y, Le YZ, Sieving PA. Depleting Rac1 in mouse rod photoreceptors protects them from photo-oxidative stress without affecting their structure or function. Proc Natl Acad Sci U S A. 2009;106(23):9397–9402. doi: 10.1073/pnas.0808940106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Pan H, Chang RCC, So KF, Brecha NC, Pu M. Activation of the Nrf2/HO-1 Antioxidant Pathway Contributes to the Protective Effects of Lycium Barbarum Polysaccharides in the Rodent Retina after Ischemia-Reperfusion-Induced Damage. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0084800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XT, Ding C, Zhou N, Xu C. Quercetin protects gastric epithelial cell from oxidative damage in vitro and in vivo. Eur J Pharmacol. 2015;754:115–124. doi: 10.1016/j.ejphar.2015.02.007. [DOI] [PubMed] [Google Scholar]
- Ishizuka F, Shimazawa M, Umigai N, Ogishima H, Nakamura S, Tsuruma K, Hara H. Crocetin, a carotenoid derivative, inhibits retinal ischemic damage in mice. Eur J Pharmacol. 2013;703(1–3):1–10. doi: 10.1016/j.ejphar.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Ju WK, Kim KY, Park SJ, Park DK, Park CB, Oh SJ, Chung JW, Chun MH. Nitric oxide is involved in sustained and delayed cell death of rat retina following transient ischemia. Brain Res. 2000;881(2):231–236. doi: 10.1016/s0006-8993(00)02816-x. [DOI] [PubMed] [Google Scholar]
- Kanamori A, Catrinescu MM, Kanamori N, Mears KA, Beaubien R, Levin LA. Superoxide is an associated signal for apoptosis in axonal injury. Brain. 2010;133(9):2612–2625. doi: 10.1093/brain/awq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielland A, Blom T, Nandakumar KS, Holmdahl R, Blomhoff R, Carlsen H. In vivo imaging of reactive oxygen and nitrogen species in inflammation using the luminescent probe L-012. Free Radic Biol Med. 2009;47(6):760–766. doi: 10.1016/j.freeradbiomed.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Kim BJ, Braun TA, Wordinger RJ, Clark AF. Progressive morphological changes and impaired retinal function associated with temporal regulation of gene expression after retinal ischemia/reperfusion injury in mice. Mol Neurodegener. 2013;8:21. doi: 10.1186/1750-1326-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H, Waki M, Nakagawa M, Tsuda M. Involvement of oxygen free radicals in experimental retinal ischemia and the selective vulnerability of retinal damage. Ophthalmic Res. 2001;33(4):196–202. doi: 10.1159/000055670. [DOI] [PubMed] [Google Scholar]
- Liu Y, McDowell CM, Zhang Z, Tebow HE, Wordinger RJ, Clark AF. Monitoring retinal morphologic and functional changes in mice following optic nerve crush. Invest Ophthalmol Vis Sci. 2014;55(6):3766–3774. doi: 10.1167/iovs.14-13895. [DOI] [PubMed] [Google Scholar]
- Lu Q, Yang Y, Villar VA, Asico L, Jones JE, Yu P, Li H, Weinman EJ, Eisner GM, Jose PA. D5 dopamine receptor decreases NADPH oxidase, reactive oxygen species and blood pressure via heme oxygenase-1. Hypertens Res. 2013;36(8):684–690. doi: 10.1038/hr.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanero S, Santro T, Arumugam TV. Neuronal oxidative stress in acute ischemic stroke: sources and contribution to cell injury. Neurochem Int. 2013;62(5):712–718. doi: 10.1016/j.neuint.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Mehta SL, Manhas N, Raghubir R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev. 2007;54(1):34–66. doi: 10.1016/j.brainresrev.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Nashine S, Liu Y, Kim BJ, Clark AF, Pang IH. Role of C/EBP Homologous Protein in Retinal Ganglion Cell Death After Ischemia/Reperfusion Injury. Invest Ophthalmol Vis Sci. 2014;56(1):221–231. doi: 10.1167/iovs.14-15447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak MS, Kita M, Marmor MF. Protection of rabbit retina from ischemic injury by superoxide dismutase and catalase. Invest Ophthalmol Vis Sci. 1993;34(6):2018–2022. [PubMed] [Google Scholar]
- Nishinaka Y, Aramaki Y, Yoshida H, Masuya H, Sugawara T, Ichimori Y. A new sensitive chemiluminescence probe, L-012, for measuring the production of superoxide anion by cells. Biochem Biophys Res Commun. 1993;193(2):554–559. doi: 10.1006/bbrc.1993.1659. [DOI] [PubMed] [Google Scholar]
- Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res. 2004;23:91–147. doi: 10.1016/j.preteyeres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Ostwald P, Goldstein IM, Pachnanda A, Roth S. Effect of nitric oxide synthase inhibition on blood flow after retinal ischemia in cats. Invest Ophthalmol Vis Sci. 1995;36(12):2396–2403. [PubMed] [Google Scholar]
- Pan H, He M, Liu R, Brecha NC, Yu AC, Pu M. Sulforaphane protects rodent retinas against ischemia-reperfusion injury through the activation of the Nrf2/HO-1 antioxidant pathway. PLoS One. 2014;9(12):e114186. doi: 10.1371/journal.pone.0114186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prunty MC, Aung MH, Hanif AM, Allen RS, Chrenek MA, Boatright JH, Thule PM, Kundu K, Murthy N, Pardue MT. In Vivo Imaging of Retinal Oxidative Stress Using a Reactive Oxygen Species-Activated Fluorescent Probe. Invest Ophthalmol Vis Sci. 2015;56(10):5862–5870. doi: 10.1167/iovs.15-16810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Chen L, Zhang L, Liu WB, Chen XY, Yang XG. Crocin prevents retinal ischaemia/reperfusion injury-induced apoptosis in retinal ganglion cells through the PI3K/AKT signalling pathway. Exp Eye Res. 2013;107:44–51. doi: 10.1016/j.exer.2012.11.011. [DOI] [PubMed] [Google Scholar]
- Rayner CL, Bottle SE, Gole GA, Ward MS, Barnett NL. Real-time quantification of oxidative stress and the protective effect of nitroxide antioxidants. Neurochem Int. 2016;92:1–12. doi: 10.1016/j.neuint.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Ozawa Y, Kurihara T, Noda K, Imamura Y, Kobayashi S, Ishida S, Tsubota K. Neuroprotective effect of an antioxidant, lutein, during retinal inflammation. Invest Ophthalmol Vis Sci. 2009;50(3):1433–1439. doi: 10.1167/iovs.08-2493. [DOI] [PubMed] [Google Scholar]
- Silverman SM, Kim BJ, Howell GR, Miller J, John SW, Wordinger RJ, Clark AF. C1q propagates microglial activation and neurodegeneration in the visual axis following retinal ischemia/reperfusion injury. Mol Neurodegener. 2016;11:24. doi: 10.1186/s13024-016-0089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn HY, Gloe T, Keller M, Schoenafinger K, Pohl U. Sensitive superoxide detection in vascular cells by the new chemiluminescence dye L-012. J Vasc Res. 1999;36(6):456–464. doi: 10.1159/000025688. [DOI] [PubMed] [Google Scholar]
- Stefanska J, Pawliczak R. Apocynin: molecular aptitudes. Mediators Inflamm. 2008;2008:106507. doi: 10.1155/2008/106507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson L, Matesanz N, Colhoun L, Edgar K, Devine A, Gardiner TA, McDonald DM. Reduced nitro-oxidative stress and neural cell death suggests a protective role for microglial cells in TNFalpha −/− mice in ischemic retinopathy. Invest Ophthalmol Vis Sci. 2010;51(6):3291–3299. doi: 10.1167/iovs.09-4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo ME, Droy-Lefaix MT, Doly M, Braquet P. Free radical-mediated effects in reperfusion injury: a histologic study with superoxide dismutase and EGB 761 in rat retina. Ophthalmic Res. 1991;23(4):225–234. doi: 10.1159/000267107. [DOI] [PubMed] [Google Scholar]
- Thaler S, Fiedorowicz M, Grieb P, Wypych Z, Knap N, Borowik T, Zawada K, Kaminski J, Wozniak M, Rejdak R, Zrenner E, Schuettauf F. Neuroprotective effects of tempol acyl esters against retinal ganglion cell death in a rat partial optic nerve crush model. Acta Ophthalmol. 2011;89(7):e555–560. doi: 10.1111/j.1755-3768.2011.02180.x. [DOI] [PubMed] [Google Scholar]
- Thiemermann C. Membrane-permeable radical scavengers (tempol) for shock, ischemia-reperfusion injury, and inflammation. Crit Care Med. 2003;31(1 Suppl):S76–84. doi: 10.1097/00003246-200301001-00011. [DOI] [PubMed] [Google Scholar]
- Vin AP, Hu H, Zhai Y, Von Zee CL, Logeman A, Stubbs EB, Jr, Perlman JI, Bu P. Neuroprotective effect of resveratrol prophylaxis on experimental retinal ischemic injury. Exp Eye Res. 2013;108:72–75. doi: 10.1016/j.exer.2012.11.022. [DOI] [PubMed] [Google Scholar]
- Wei Y, Gong J, Yoshida T, Eberhart CG, Xu Z, Kombairaju P, Sporn MB, Handa JT, Duh EJ. Nrf2 has a protective role against neuronal and capillary degeneration in retinal ischemia-reperfusion injury. Free Radic Biol Med. 2011;51(1):216–224. doi: 10.1016/j.freeradbiomed.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson-Berka JL, Deliyanti D, Rana I, Miller AG, Agrotis A, Armani R, Szyndralewiez C, Wingler K, Touyz RM, Cooper ME, Jandeleit-Dahm KA, Schmidt HH. NADPH oxidase, NOX1, mediates vascular injury in ischemic retinopathy. Antioxid Redox Signal. 2014;20(17):2726–2740. doi: 10.1089/ars.2013.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson-Berka JL, Rana I, Armani R, Agrotis A. Reactive oxygen species, Nox and angiotensin II in angiogenesis: implications for retinopathy. Clin Sci (Lond) 2013;124(10):597–615. doi: 10.1042/CS20120212. [DOI] [PubMed] [Google Scholar]
- Winkler BS, Boulton ME, Gottsch JD, Sternberg P. Oxidative damage and age-related macular degeneration. Mol Vis. 1999;5:32. [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Qaum T, Adamis AP. Sensitive blood-retinal barrier breakdown quantitation using Evans blue. Invest Ophthalmol Vis Sci. 2001;42(3):789–794. [PubMed] [Google Scholar]
- Xu Z, Cho H, Hartsock MJ, Mitchell KL, Gong J, Wu L, Wei Y, Wang S, Thimmulappa RK, Sporn MB, Biswal S, Welsbie DS, Duh EJ. Neuroprotective role of Nrf2 for retinal ganglion cells in ischemia-reperfusion. J Neurochem. 2015;133(2):233–241. doi: 10.1111/jnc.13064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto F, Hiroi K, Honda Y. Effects of intravenous superoxide dismutase and catalase on electroretinogram in the cat postischemic retina. Ophthalmic Res. 1994;26(3):163–168. doi: 10.1159/000267408. [DOI] [PubMed] [Google Scholar]
- Yokota H, Narayanan SP, Zhang W, Liu H, Rojas M, Xu Z, Lemtalsi T, Nagaoka T, Yoshida A, Brooks SE, Caldwell RW, Caldwell RB. Neuroprotection from retinal ischemia/reperfusion injury by NOX2 NADPH oxidase deletion. Invest Ophthalmol Vis Sci. 2011;52(11):8123–8131. doi: 10.1167/iovs.11-8318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QG, Laird MD, Han D, Nguyen K, Scott E, Dong Y, Dhandapani KM, Brann DW. Critical role of NADPH oxidase in neuronal oxidative damage and microglia activation following traumatic brain injury. PLoS One. 2012;7(4):e34504. doi: 10.1371/journal.pone.0034504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Tsai YT, Weng H, Tang L. Noninvasive assessment of localized inflammatory responses. Free Radic Biol Med. 2012;52(1):218–226. doi: 10.1016/j.freeradbiomed.2011.10.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielonka J, Lambeth JD, Kalyanaraman B. On the use of L-012, a lumino-based chemiluminescent probe, for detecting superoxide and identifying inhibitors of NADPH oxidase: a reevaluation. Free Radic Biol Med. 2013;65:1310–1314. doi: 10.1016/j.freeradbiomed.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]