Abstract
Eph-ephrin bidirectional signaling is essential for eye lens transparency in humans and mice. Our previous studies in mouse lenses demonstrate that ephrin-A5 is mainly expressed in the anterior epithelium, where it is required for maintaining the anterior epithelial monolayer. In contrast, EphA2 is localized in equatorial epithelial and fiber cells where it is essential for equatorial epithelial and fiber cell organization and hexagonal cell shape. Immunostaining of lens epithelial and fiber cells reveals that EphA2 and ephrin-A5 are also co-expressed in anterior fiber cell tips, equatorial epithelial cells and newly formed lens fibers, although they are not precisely colocalized. Due to this complex expression pattern and the promiscuous interactions between Eph receptors and ephrin ligands, as well as their complex bidirectional signaling pathways, cataracts in ephrin-A5(−/−) or EphA2(−/−) lenses may arise from loss of function or abnormal signaling mechanisms. To test whether abnormal signaling mechanisms may play a role in cataractogenesis in ephrin-A5(−/−) or EphA2(−/−) lenses, we generated EphA2 and ephrin-A5 double knockout (DKO) mice. We compared the phenotypes of EphA2(−/−) and ephrin-A5(−/−) lenses to that of DKO lenses. DKO lenses displayed an additive lens phenotype that was not significantly different from the two single KO lens phenotypes. Similar to ephrin-A5(−/−) lenses, DKO lenses had abnormal anterior epithelial cells leading to a large mass of epithelial cells that invade into the underlying fiber cell layer, directly resulting in anterior cataracts in ephrin-A5(−/−) and DKO lenses. Yet, similar to EphA2(−/−) lenses, DKO lenses also had abnormal packing of equatorial epithelial cells with disorganized meridional rows, lack of a lens fulcrum and disrupted fiber cells. The DKO lens phenotype rules out abnormal signaling by EphA2 in ephrin-A5(−/−) lenses or by ephrin-A5 in EphA2(−/−) lenses as possible cataract mechanisms. Thus, these results indicate that EphA2 and ephrin-A5 do not form a lens receptor-ligand pair, and that EphA2 and ephrin-A5 have other binding partners in the lens to help align differentiating equatorial epithelial cells or maintain the anterior epithelium, respectively.
Introduction
Bidirectional signaling mediated by Eph receptor tyrosine kinases and membrane-anchored ephrins is a major form of cell-contact-dependent communication that plays important functions in a broad range of cell-cell recognition events, including axon pathfinding, early segmentation and organ morphogenesis (Arvanitis and Davy, 2008; Himanen et al., 2007; Kullander and Klein, 2002). Eph receptors mediate forward signaling in one cell while ephrin ligands transmit reverse signaling in an adjacent cell (Davy et al., 1999; Holland et al., 1996). In addition, Ephs and ephrins can also signal independently of each other (Barquilla and Pasquale, 2015). The Eph protein family includes 14 different members, divided into EphA (1 to 8 and 10) and EphB (1 to 4 and 6) classes. Ephrin ligands are divided into ephrin-A (1 to 5) and ephrin-B (1 to 3). EphAs preferentially bind to glycosyl-phosphatidylinositol (GPI)-anchored ephrin-A ligands while EphBs bind to transmembrane ephrin-B ligands. Each receptor can interact with multiple ligands and vice versa. Cross interactions between EphAs and ephrin-Bs or EphBs and ephrin-As can also occur (Himanen et al., 2004; Takemoto et al., 2002). Complementary or overlapping expression of Ephs and ephrins suggests diverse functions in development and in maintaining homeostasis (Poliakov et al., 2004).
Cataracts, defined as any opacity in the lens, remain the leading cause of blindness in the world (Asbell et al., 2005). Recent work, including our studies, show that Eph-ephrin bidirectional signaling is needed to establish and/or maintain life-long lens transparency. EphA2 and ephrin-A5 mutations are linked to congenital and age-related cataracts in humans and mice (Celojevic et al., 2015; Cheng et al., 2013; Cheng and Gong, 2011; Cooper et al., 2008; Jun et al., 2009; Kaul et al., 2010; Lin et al., 2014; Masoodi et al., 2012; Park et al., 2012; Shi et al., 2012; Shiels et al., 2008; Sundaresan et al., 2012; Tan et al., 2011; Zhang et al., 2009). EphA2 mutations cause human congenital dominant (Dave et al., 2013; Li et al., 2016; Park et al., 2012; Zhang et al., 2009) and recessive cataracts (Kaul et al., 2010), and non-synonymous SNPs in the EphA2 and ephrin-A5 genes have been linked to human age-related cataracts (Dave et al., 2013; Lin et al., 2014; Masoodi et al., 2012; Shiels et al., 2008; Sundaresan et al., 2012; Tan et al., 2011). In mice, loss of EphA2 or ephrin-A5 lead to distinct lens phenotypes, consistent with the different lens cell types in which they are predominantly expressed (Cheng et al., 2013; Cheng and Gong, 2011; Cooper et al., 2008; Jun et al., 2009; Shi et al., 2012; Son et al., 2013). The eye lens contains two major cell types, epithelial cells, which form a monolayer covering the anterior surface of the lens, and fiber cells which form the bulk of the lens (Piatigorsky, 1981). Ephrin-A5 is predominantly expressed in the quiescent anterior lens epithelial cells, while EphA2 is in differentiating epithelial cells at the lens equator and in fiber cells (Cheng et al., 2013; Cheng and Gong, 2011; Cooper et al., 2008; Jun et al., 2009; Son et al., 2013). There is no obvious subcellular colocalization of ephrin-A5 and EphA2 in the lens (Cheng and Gong, 2011).
Loss of ephrin-A5 leads to abnormal localization of E-cadherin and β-catenin in anterior epithelial cells along with epithelial-mesenchymal-transition (EMT) and aberrant expression of α-smooth muscle actin in these epithelial cells (Cheng and Gong, 2011). The severity of the cataract phenotype depends on the strain background of the mice. In the C57BL6 background, ephrin-A5 knockout [(−/−)] lenses often develop anterior cataracts that are directly correlated with the disruption of the anterior epithelial monolayer, but have no obvious defects in fiber cells (Cheng and Gong, 2011). In contrast, mixed background (129/SV/C57BL6) ephrin-A5(−/−) mice displayed severe fiber cell degeneration, nuclear cataracts and lens rupture (Biswas et al., 2016; Cooper et al., 2008; Son et al., 2013).
EphA2(−/−) is associated with disruption of the actin cytoskeleton, cell shape and perturbed organization of equatorial lens epithelial cells (Cheng et al., 2013), along with misaligned and disorganized fiber cells (Cheng et al., 2013; Cheng and Gong, 2011; Jun et al., 2009; Shi et al., 2012). The cataract phenotype varies depending on how the knockout mouse line was generated and the mouse strain background. EphA2(−/−) mice generated by a gene trapping method in the FVB/NJ background develop cortical cataracts that progress to whole cataracts and lens rupture with age (Jun et al., 2009). This severe and progressive cataract phenotype is associated with an increased stress response reflected by elevated Hsp27 levels in EphA2(−/−) lenses (Jun et al., 2009). In contrast, EphA2(−/−) mice created by targeted gene disruption in the C57BL6 background develop mild nuclear cataracts (Cheng et al., 2013). Our work shows that EphA2 likely signals through Src and cortactin to recruit actin to the vertices of hexagonal equatorial epithelial cells, controlling the alignment of meridional rows and hexagonal packing of fiber cells (Cheng et al., 2013). Abnormal fiber cell packing in the absence of EphA2 likely results in changes in refractive index and fiber cell orientation (Shi et al., 2012), leading to nuclear cataracts (Cheng et al., 2013). The variable phenotypes of ephrin-A5(−/−) and EphA2(−/−) lenses in different strain backgrounds suggest that genetic modifiers affect cataract severity and influence whether defects are primarily in epithelial vs. fiber cells. We will focus this study on mice in the C57BL6 background.
The distinct knockout lens phenotypes in the C57BL6 mouse background (Cheng et al., 2013; Cheng and Gong, 2011) had led us to the assumption that EphA2 and ephrin-A5 function in different cell types via separate pathways using other partners in the lens. On the other hand, the variable cataract phenotypes in different genetic backgrounds led others to propose that EphA2 and ephrin-A5 are a receptor-ligand pair in the lens, but with no direct evidence to support this hypothesis (Cooper et al. PNAS, 2008; Shi et al., IOVS, 2011; Son et al. Mol Vis, 2013). However, both of these interpretations are likely over-simplified. Due to the complex nature of bidirectional Eph-ephrin signaling, the loss of a receptor or ligand can trigger either loss of function or gain of function mechanisms in the same or neighboring cells, resulting in cataractogenesis. Therefore, cataracts in single knockout ephrin-A5(−/−) or EphA2(−/−) lenses may arise from at least three possible mechanisms: 1) the loss of the receptor or ligand disrupts normal signaling in lens epithelial cells (loss of function), 2) the receptor or ligand that normally interacts with ephrin-A5 or EphA2, respectively, binds to new partners in the knockout, leading to abnormal signaling to affect epithelial cell phenotypes or 3) in the case of ephrin-A5(−/−) lenses, the uncoupled receptor may autophosphorylate leading to non-ligand-mediated receptor clustering, self-activation and EMT (abnormal non-canonical signaling) (Barquilla et al., 2016; Miao et al., 2009; Yang et al., 2011; Zhou et al., 2015). Thus, to clarify whether EphA2 and ephrin-A5 are a lens receptor-ligand pair leading to KO cataract phenotypes due to abnormal signaling mechanisms, we used a genetic approach. We also have carefully re-examined the localization of ephrin-A5 and EphA2 in the lens and determined the phenotype and cell morphology of GFP-positive (GFP+) EphA2 and ephrin-A5 double knockout (DKO) lenses. Our results indicate that EphA2 and ephrin-A5 do not function in the same pathways in the lens and therefore are not a lens receptor-ligand pair.
Material and Methods
Mice
Mouse care and breeding were performed in accordance with an approved animal protocol (UC Berkeley Animal Care and Use Committee) and with the National Institutes of Health guide for the care and use of laboratory animals. All mice were maintained in the C57BL/6J background with wild-type Bfsp2 (CP49) genes and one copy of the GFP transgene. Non-GFP and GFP+ wild-type (WT), ephrin-A5(−/−) and EphA2(−/−) mice were generated and maintained as previously described (Cheng et al., 2013; Cheng and Gong, 2011; Frisen et al., 1998; Okabe et al., 1997). GFP+ EphA2(−/−) ephrin-A5(−/−) DKO mice were generated by intercrossing EphA2(−/−) and GFP+ ephrin-A5(−/−) mice and then intercrossing GFP+ and non-GFP double heterozygous offspring. GFP+ mouse pups were screened using a handheld UV lamp. A standard PCR method (Gong et al., 1997) with previously described primers were used for genotyping (Cheng et al., 2013; Cheng and Gong, 2011; Frisen et al., 1998).
Immunostaining lens capsule flat mounts
Lens capsule flat mounts with attached epithelial and peripheral fiber cells were prepared from postnatal day 21 (P21) non-GFP WT mouse lenses as previously described (Cheng et al., 2013; Cheng and Gong, 2011). Briefly, freshly dissected lenses were flash fixed in cold 100% methanol for 45 seconds. Lens capsules (along with attached epithelial and peripheral fiber cells) was gently dissected away from the fiber cell mass using radial cuts. Capsules were blocked (10% normal donkey serum and 0.3% Triton X-100) for 1 hour at room temperature before staining with goat anti-EphA2 (R&D Systems) and rabbit anti-ephrin-A5 (Invitrogen) primary antibody overnight at 4°C. Primary antibody incubation was followed washing and then incubation in appropriate fluorescent secondary antibodies (Jackson ImmunoResearch Laboratories) for 2 hours at room temperature. Lens capsules were then washed and flat mounted with DAPI VectorShield mounting medium (Vector Laboratories, Inc.). Z-stack images were collected by a Zeiss LSM700 confocal microscope, and 3D images were reconstructed from z-stack data collected at 0.38 μm steps using the ZEN software. Antibody specificity was tested previously using knockout tissues (Cheng and Gong, 2011).
GFP+ Lens Imaging
P21 mouse lenses were dissected from enucleated eyeballs and immediately immersed in 1X PBS. Lens pictures were acquired with a Leica MZ16 dissecting scope using a digital camera. Images of GFP+ lens epithelial and fiber cells with mosaic GFP expression pattern (Cheng et al., 2008; Shestopalov and Bassnett, 2003) were collected as previously described (Cheng et al., 2013; Cheng and Gong, 2011). Briefly, freshly dissected lenses were maintained in phenol-red free DMEM at 37°C on the microscope stage for imaging. Confocal images were acquired using a Zeiss LSM700 confocal microscope. Z-stack images of anterior lens epithelium and lens equator were collected with 0.5μm z-steps and analyzed using the ZEN 2010 software. At least six lenses of each genotype from three different mice were examined.
Lens Whole Mount Staining
Whole mount lens staining for cell membranes using rhodamine-conjugated wheat germ agglutinin (WGA, Vector Laboratories, Inc.) and for nuclei using DAPI VectorShield mounting medium (Vector Laboratories, Inc.) was performed as previously described (Cheng et al., 2013). Briefly, a small posterior opening was made in enucleated P21 eyeballs, and eyeballs were fixed in freshly made 4% paraformaldehyde for 30 minutes on ice. Eyeballs were then gently washed in 1X PBS twice and then incubated in 1X PBS at room temperature overnight. After overnight incubation, lenses were dissected from the eyes and then permeablized and blocked with 3% BSA, 3% normal goat serum, 0.3% Triton X-100 for 15 minutes at room temperature. Then, lenses were incubated for 30 minutes at room temperature in DAPI VectorShield mounting medium. Following this incubation lenses were washed with 1X PBS and then incubated in WGA for 30 minutes at room temperature. Finally lenses were washed again in 1X PBS before imaging as described above. Staining was repeated at least 3 times, and representative results are shown.
Results
EphA2 and ephrin-A5 are co-expressed in equatorial epithelial cells, the anterior tips of elongating fiber cells and in newly formed secondary fibers
We first determined the localization of EphA2 and ephrin-A5 proteins in lens epithelial and fiber cells by immunolabeling lens capsule flat mounts using specific antibodies previously validated using KO tissue (Cheng and Gong, 2011). As we had shown previously (Cheng and Gong, 2011), ephrin-A5 immunostaining signals are in small puncta in anterior epithelial cells and EphA2 is present in the anterior fiber cell tips (Fig. 1A). However, closer examination in a 3D reconstruction of a z-stack reveals that small ephrin-A5 puncta are also present in the anterior tips of lens fibers (Fig. 1B, arrows). While there is no obvious colocalization of the ephrin-A5 and EphA2 immunostaining signals, the two proteins are co-expressed in anterior fiber cell tips and in neighboring cells. Thus, it is possible that ephrin-A5-bearing anterior epithelial cells may interact with EphA2-bearing fiber cells to launch a bidirectional signaling pathway.
Fig. 1.
Immunostaining of anterior and equatorial epithelial and fiber cells from lens capsule flat mounts of P21 wild-type lenses for ephrin-A5 (red) and EphA2 (green). (A) Single optical sections (XY views) show that anterior epithelial cells have punctate ephrin-A5 staining signals and no EphA2 staining. In contrast, anterior fiber cells have EphA2 staining along the cell membrane with a few ephrin-A5 puncta. Scale bar, 10 μm. (B) 3D reconstruction of z-stacks (XZ view) shows ephrin-A5 punctate signals are concentrated near the basal and lateral cell membranes of anterior epithelial cells (EC) with some staining also at the apical junction between anterior epithelial cells and the tips of elongating fiber cells (F, arrows). EphA2 is predominately expressed in lens fiber cells. This staining pattern in anterior epithelial and fiber cells is consistent with our previously published data (Cheng and Gong, 2011). (C) Single optical sections show that ephrin-A5 and EphA2 are both present in equatorial epithelial cells organized into meridional rows and in the newly added secondary fiber cells. The EphA2 staining pattern is consistent with our previous results (Cheng et al., 2013). Scale bar, 10 μm. D) 3D reconstruction of z-stacks (YZ view) showing the bow region of the lens reveals the EphA2 and ephrin-A5 staining signals are present in equatorial epithelial cells (EC) and newly formed fibers (F). Arrows point to ephrin-A5 puncta in lens fiber cells.
We also examined the localization of ephrin-A5 and EphA2 in equatorial epithelial cells and newly formed lens fiber cells in lens capsule flat mounts. We find that EphA2 staining is predominantly at the membrane of equatorial epithelial and fiber cells (Fig. 1C), consistent with our previous data (Cheng et al., 2013). Interestingly, we find that ephrin-A5 puncta are also present in equatorial epithelial cells (Fig. 1C), and a 3D reconstruction at the bow region of the lens reveals small puncta of ephrin-A5 in newly formed fiber cells (Fig. 1D, arrows). There is no obvious colocalization of the EphA2 and ephrin-A5 staining signals in these cells. Thus, in addition to anterior fiber cell tips, EphA2 and ephrin-A5 are co-expressed in equatorial epithelial cells and newly formed fiber cells (Fig. 2A). Although there is no apparent colocalization of EphA2 and ephrin-A5 immunostaining signals, we cannot rule out that these two proteins may facilitate signaling between anterior epithelial cells and fiber cell tips, or between neighboring equatorial epithelial or fiber cells where they are co-expressed.
Fig. 2.
(A) Cartoon of ephrin-A5 (red) and EphA2 (green) localization in lens epithelial and fiber cells. Not drawn to scale. (B) There are three possible mechanisms for cataracts in ephrin-A5(−/−) lenses. Loss of ephrin-A5 leads to EMT in anterior epithelial cells (EC) due to disruption of bidirectional signaling (loss of function). It would be unlikely in this loss of function mechanism for EphA2 to be the receptor due to the divergent phenotypes between EphA2(−/−) and ephrin-A5(−/−) lenses. Alternatively, the ephrin-A5(−/−) lens phenotype might be a consequence of abnormal signaling due to EphA2 binding to a different ligand or self-activating through non-canonical autophosphorylation. (C) In EphA2(−/−) lenses, disorganization of equatorial epithelial cells (EC) may be caused by loss of function. In the loss of function mechanism, ephrin-A5 is unlikely to be the ligand interacting with EphA2 because of the vast differences in EphA2(−/−) versus ephrin-A5(−/−) lens phenotypes. However, it is possible that in the absence of EphA2, ephrin-A5 interacts with another receptor causing abnormal signaling in equatorial epithelial cells.
Our previous work had presumed that a loss of function mechanism was the explanation for the cataract phenotypes, and due to the divergent single KO phenotypes, we suggested that EphA2 and ephrin-A5 have other partners in the lens. However, the fact that ephrin-A5 and EphA2 are co-expressed in some cell populations in the lens necessitates a reevaluation of the proposed abnormal signaling cataract mechanisms. For example, in the absence of ephrin-A5, the receptor that normally interacts with ephrin-A5 (possibly EphA2) may bind a different ligand or self-activate through non-canonical autophosphorylation to trigger cell-cell adhesion dysfunction and EMT in the anterior epithelial cells (Fig. 2B). Similarly, it is possible that, in the absence of EphA2, the ligand that interacts with EphA2 (possibly ephrin-A5) disrupts equatorial cell organization by signaling through a different Eph receptor (Fig. 2C). Thus, to rigorously test whether aberrant bidirectional signaling via EphA2 causes ephrin-A5(−/−) anterior cataracts (abnormal signaling via interactions with another ligand or non-canonical signaling) or if atypical ephrin-A5 signaling leads to changes in EphA2(−/−) equatorial epithelial cells (abnormal signaling via interactions with another receptor), we generated DKO mice.
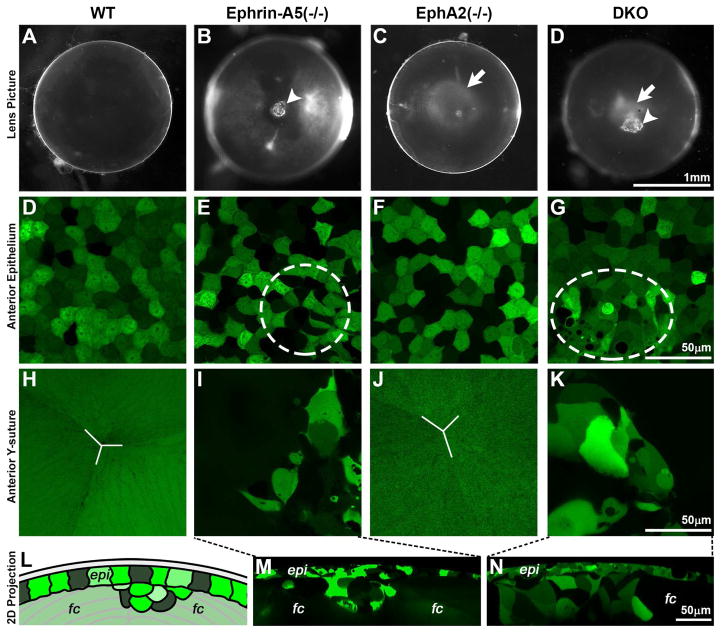
Our previous studies showed that EphA2(−/−) and ephrin-A5(−/−) lenses develop distinct congenital cataracts (Cheng et al., 2013; Cheng and Gong, 2011). In the C57BL6 strain background, ephrin-A5(−/−) lenses often display anterior cataracts due to EMT in anterior epithelial cells, while EphA2(−/−) lenses have mild nuclear opacities associated with disruptions in the organization and alignment of equatorial epithelial and fiber cells (Cheng et al., 2013; Cheng and Gong, 2011). At P21, DKO lenses displayed features present in the single knockout lenses, including anterior cataracts like the ephrin-A5(−/−) lens (Fig. 3B and 3D, arrowhead) and mild nuclear opacity, similar to the EphA2(−/−) lens (Fig. 3C and 3D, arrows). Examination of lenses containing a GFP-transgene showed the expected mosaic GFP pattern in anterior epithelial cells from both GFP+ WT and GFP+ EphA2(−/−) lenses (Fig. 3D and 3F). Anterior epithelial cells in these lenses were uniform in size and shape with a normal cobblestone-packing pattern. In contrast, ephrin-A5(−/−) and DKO lenses had altered anterior epithelial cells (Fig. 3E and 3G, dashed ellipses) with a cluster of aberrantly shaped cells invading the underlying fiber cell layer (Fig. 3I and 3K), which corresponds directly to anterior cataracts in ephrin-A5(−/−) and DKO lenses (Fig. 3B and 3D, arrowheads). WT and EphA2(−/−) lenses had normal anterior Y-sutures (Fig. 3H and 3J). In ephrin-A5(−/−) and DKO lenses without an anterior cataract, the anterior Y-suture also appears normal (data not shown). These results are consistent with our previous report (Cheng and Gong, 2011) and suggest that DKO anterior epithelial cells are probably undergoing EMT. Since the anterior epithelial cell defect is unchanged in the DKO lenses, we can conclude the EphA2 does not aberrantly signal in the absence of ephrin-A5 to cause EMT in these cells and that EphA2 and ephrin-A5 are not paired in anterior epithelial and fiber cells.
Fig. 3.
(A–D) Photos of P21 WT, ephrin-A5(−/−), EphA2(−/−) and double knockout (DKO) lenses. Ephrin-A5(−/−) and DKO lenses often have anterior cataracts (B and D, arrowheads). EphA2(−/−) and DKO lenses often display mild nuclear opacities (C and D, arrows). Scale bar, 1mm. (D–G) Confocal images of anterior epithelial cells in P21 GFP+ WT, ephrin-A5(−/−), EphA2(−/−) and DKO lenses. WT and EphA2(−/−) lenses have normal anterior epithelium with cobblestone shaped cells and mosaic GFP expression (D and F). Ephrin-A5(−/−) and DKO lenses display abnormal epithelial cell morphology (E and G, dashed ellipses). Scale bar, 50μm. (H–K) Images of the anterior Y-suture region just beneath the epithelium. WT and EphA2(−/−) lenses have normal Y-shaped sutures (H and J, white lines). The aberrant cluster of epithelial cells in ephrin-A5(−/−) and DKO lenses extends into the anterior suture region (I and K). Scale bar, 50μm. (L) A cartoon depicting a 2D projection of the 3D reconstruction of a Z-stack through the anterior epithelium and underlying fiber cells (not drawn to scale). (M–N) Aberrant clusters of epithelial cells invading the anterior suture and fiber cell layer can be seen in the ephrin-A5(−/−) and DKO lens. Scale bar, 50μm. H–J: epi, epithelial cells; fc, fiber cells.
We further examined equatorial epithelial and fiber cells in GFP+ lenses. In WT and ephrin-A5(−/−) lenses, equatorial epithelial cells are hexagon-shaped and organized into meridional rows (below white dashed line in Fig. 4A–4B), and newly differentiating fiber cells are straight, uniform in width and precisely aligned (Fig. 4E–4F). In contrast, similar to EphA2(−/−) cells, abnormally shaped DKO equatorial epithelial cells failed to form organized meridional rows (Fig. 4C–4D), leading to wavy and disorganized fiber cells (Fig. 4G–4F). We stained whole mount lenses to better visualize the defect in the meridional rows and determine whether there are changes at the lens fulcrum (Cheng et al., 2013; Sugiyama et al., 2009) or modiolus (Zampighi et al., 2000), where the apical tips of elongating equatorial epithelial cells constrict to form an anchor point before fiber cell elongation and migration. Whole mount lens staining with DAPI revealed that while the nuclei of WT and ephrin-A5(−/−) equatorial epithelial cell nuclei were well aligned into meridional rows (Fig. 4I–4J, arrowheads), there were many misaligned nuclei in EphA2(−/−) and DKO lenses (Fig. 4K–4L, arrows) in disrupted meridional rows. WGA staining of cell membranes show that WT and ephrin-A5(−/−) lenses have a distinct lens fulcrum (Fig. 4M–4N, arrows) while EphA2(−/−) and DKO equatorial epithelial cells do not form a fulcrum (Fig. 4O–4P). Our data indicates that DKO lenses have similar equatorial epithelial cell defects as we showed previously in EphA2(−/−) lenses (Cheng et al., 2013). These data indicate that ephrin-A5 is not involved in the organization of equatorial epithelial cells and that disorganization of EphA2(−/−) equatorial epithelial and fiber cells is not attributed to abnormal ephrin-A5 signaling.
Fig. 4.
(A–D) Confocal images of equatorial epithelial cells from P21 GFP+ WT, ephrin-A5(−/−), EphA2(−/−) and DKO lenses. WT and ephrin-A5(−/−) lenses display hexagonal equatorial epithelial cells (A and B) aligned into meridional rows (below the white dashed line). In contrast, equatorial epithelial cells in EphA2(−/−) and DKO lenses lack organized meridional rows (C and D). Scale bar, 50μm. (E–H) Confocal images of peripheral fiber cells in P21 GFP+ WT, ephrin-A5(−/−), EphA2(−/−) and DKO lenses. While WT and ephrin-A5(−/−) lenses have straight and organized peripheral differentiating fiber cells (E and F), fiber cells are wavy and disorganized in EphA2(−/−) and DKO lenses (G and H). Scale bar, 50μm. (I–L) Whole mount staining of P21 lenses with DAPI (nuclei) and images from the lens equator. WT and ephrin-A5(−/−) equatorial epithelial cells have organized and aligned rows of nuclei in meridional rows (I and J, arrowheads). However, loss of EphA2 in single KO and DKO lenses leads to disorganized cell nuclei (K and L, arrows) and disrupted meridional rows. Scale bar, 25μm. (M–P) WGA staining of cell membranes in whole mount P21 WT, ephrin-A5(−/−), EphA2(−/−) and DKO lenses. Imaging at the lens equator near the cortex reveals that the lens fulcrum is strongly stained by WGA in WT and ephrin-A5(−/−) lenses (M and N, arrows). In contrast, Epha2(−/−) and DKO lenses lack a well-defined lens fulcrum (O and P). Scale bar, 25μm.
Discussion
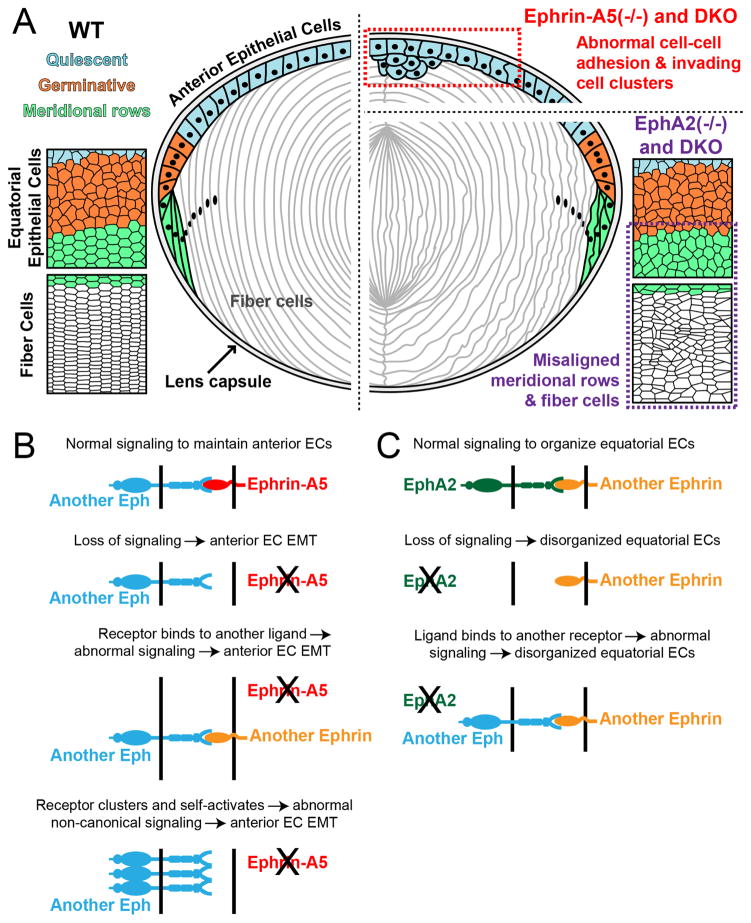
This work clearly demonstrates that DKO lenses have an additive phenotype, with anterior epithelial cell defects that resemble ephrin-A5(−/−) lenses, together with equatorial epithelial cell and fiber cell misalignment defects that resemble EphA2(−/−) lenses (Fig. 5A). This simple additive DKO phenotype that is not significantly different from single knockout phenotypes, indicates that EphA2 and ephrin-A5 do not function in similar pathways in the lens, and do not form a receptor-ligand pair in the lens. This clearly demonstrates that other Ephs and ephrins must interact with ephrin-A5 and EphA2, respectively. Our work also suggests that possible loss of function or abnormal signaling cataract mechanisms in EphA2(−/−) or ephrin-A5(−/−) lenses do not involve ephrin-A5 nor EphA2, respectively (Fig. 5B–5C). Based on these results, we hypothesize that ephrin-A5 interacts with another Eph receptor to regulate the integrity of anterior epithelial cells, while EphA2 interacts with another ephrin to control the alignment of lens epithelial cells as they differentiate and organize into rows of hexagon-shaped equatorial epithelial and fiber cells. Alternatively, EphA2 may operate via a ligand-independent non-canonical signaling mechanism in differentiating equatorial epithelial and fiber cells (Barquilla et al., 2016; Miao et al., 2009; Yang et al., 2011; Zhou et al., 2015).
Fig. 5.
(A) A summary of the ephrin-A5(−/−), EphA2(−/−) and DKO lens phenotypes. (Left panel) Normal mouse lenses consist of a monolayer of epithelial cells and bulk elongated fiber cells, wrapped by the lens capsule. Anterior epithelial cells (blue) are quiescent. Equatorial epithelial cells undergo proliferation (orange) and transform from a random cell packing organization into meridional rows of hexagonally packed cells (green). Hexagonal fiber cells retain organized rows. (Right panel) Loss of ephrin-A5 in single knockout and DKO lenses leads to abnormal cell-cell adhesion and clusters of anterior epithelial cells that invade into the underlying fiber cell layer (red box). In contrast, EphA2(−/−) and DKO lenses have disorganized meridional rows and fiber cells at the lens equator (purple box). (B) In the absence of ephrin-A5, dysfunction and EMT in anterior epithelial cells (EC) may result from loss of function or abnormal signaling mechanism through an unknown Eph receptor. (C) In EphA2(−/−) lenses, disorganization of equatorial epithelial cells (EC) may result from loss of function or abnormal ligand signaling.
Although it is clear that EphA2 and ephrin-A5 play important roles in the lens, the ephrin ligands and Eph receptors that interact with EphA2 and ephrin-A5, respectively, in the lens remain unknown. Based on interactions in other cells and tissues (Himanen et al., 2010; Li et al., 2009; Park et al., 2012; Shaw et al., 2014), others have theorized that EphA2-ephrin-A5 is a receptor-ligand pair in the lens (Cooper et al., 2008; Shi et al., 2012; Son et al., 2013). However, EphA2 is known to interact with other ligands, including ephrin-A1 (Hess et al., 2006; Miao et al., 2000; Miao et al., 2001; Ojima et al., 2006; Rodriguez et al., 2016; Sabet et al., 2015; Wiedemann et al., 2017; Yeddula et al., 2015; Youngblood et al., 2016; Zantek et al., 1999) and ephrin-B3 (Efazat et al., 2016). Ephrin-A5 is also known to pair up with other Eph receptors, including EphA3 (Carvalho et al., 2006; Forse et al., 2015), EphA4 (Orsulic and Kemler, 2000; Yumoto et al., 2008), EphA8 (Yoo et al., 2010) and EphB2 (Himanen et al., 2004).
The promiscuous nature of Eph receptors and ephrin ligands raises the complex possibility of orphaned receptors or ligands finding different partners in KO tissues, thereby initiating abnormal signaling or orphaned receptors that engage in aberrant non-canonical signaling. While abnormal signaling mechanisms with different binding partners have not been explicitly described in KO tissues, Ephs and ephrins are known to interact with multiple partners and change partners depending on the complement of ligands and receptors expressed in the neighboring cell type (Rohani et al., 2014). In addition, receptor-ligand pairs can produce competing signals in the same cell, and loss of the dominant signaling pathway may allow the competing pathway to change cell behavior (Astin et al., 2010). Eph receptor canonical signaling is induced by ephrin ligand binding leading to autophosphorylation on tyrosine residues (such as Y588 in EphA2) and increased kinase activity (Lisabeth et al., 2013). EphA2 can also signal in a non-canonical manner that depends on phosphorylation of S897 by several serine/threonine kinases (Barquilla et al., 2016; Miao et al., 2009; Yang et al., 2011; Zhou et al., 2015). Previous work in cancer cells has demonstrated that EphA2 non-canonical signaling leads to an invasive phenotype while canonical signaling can have the opposite effect (Gopal et al., 2011; Miao et al., 2009; Stahl et al., 2011). This suggests that the EMT phenotype in ephrin-A5(−/−) anterior epithelial cells could be explained by a loss of canonical signaling mediated by an Eph receptor coexpressed in these cells with ephrin-A5, abnormal non-canonical signaling by an Eph receptor, or a combination of both (Fig. 5B). It is interesting to note that in our KO lenses, loss of ephrin-A5 only affects a subset of anterior epithelial cells and does not affect the equatorial epithelial or fiber cells. Similar to other KO or transgenic lenses (Banh et al., 2006; Lovicu et al., 2004; Shin et al., 2012; Sugiyama et al., 2015), EMT in ephrin-A5(−/−) lenses occurs in a subpopulation of anterior epithelial cells. While anterior epithelial cells are often considered a homogenous monolayer, biological heterogeneity can occur through genetic and environmental factors that can create distinct cell subpopulations, similar to cancer cells within a tumor (Burrell et al., 2013; Easwaran et al., 2014). Ephrin-A5 is expressed in both anterior and equatorial epithelial cells, and this ligand may have different partners and, therefore, unique functions in these different cell populations. At present, it is not clear which other Ephs and ephrins are utilized in the lens and whether both canonical and non-canonical Eph signaling occurs in the lens. The complex nature of these possible signaling pathways require a more thorough investigation to reveal which other Ephs and ephrins are required for normal lens homeostasis, which are receptor-ligand pairs in the lens and the mechanisms for how canonical and/or non-canonical Eph and ephrin signaling establish and maintain lens transparency.
Highlights.
Eph-ephrin bidirectional signaling is required for normal lens epithelial cells.
Receptor EphA2 loss leads to disordered equatorial epithelial and fiber cells.
Ligand ephrin-A5 knockout causes anterior epithelial cells to undergo EMT.
Double knockout lenses display an additive lens cataract phenotype.
EphA2 and ephrin-A5 are not signaling partners in the lens.
Acknowledgments
We thank Dr. Eddie Wang for help with mouse line maintenance and Dr. Justin Parreno for critical reading of the manuscript. This work was supported by grants EY013849 (to X.G.) and EY017724 (to V.M.F.) from the National Eye Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arvanitis D, Davy A. Eph/ephrin signaling: networks. Genes Dev. 2008;22:416–429. doi: 10.1101/gad.1630408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asbell PA, Dualan I, Mindel J, Brocks D, Ahmad M, Epstein S. Age-related cataract. Lancet. 2005;365:599–609. doi: 10.1016/S0140-6736(05)17911-2. [DOI] [PubMed] [Google Scholar]
- Astin JW, Batson J, Kadir S, Charlet J, Persad RA, Gillatt D, Oxley JD, Nobes CD. Competition amongst Eph receptors regulates contact inhibition of locomotion and invasiveness in prostate cancer cells. Nature cell biology. 2010;12:1194–1204. doi: 10.1038/ncb2122. [DOI] [PubMed] [Google Scholar]
- Banh A, Deschamps PA, Gauldie J, Overbeek PA, Sivak JG, West-Mays JA. Lens-specific expression of TGF-beta induces anterior subcapsular cataract formation in the absence of Smad3. Investigative ophthalmology & visual science. 2006;47:3450–3460. doi: 10.1167/iovs.05-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barquilla A, Lamberto I, Noberini R, Heynen-Genel S, Brill LM, Pasquale EB. Protein kinase A can block EphA2 receptor-mediated cell repulsion by increasing EphA2 S897 phosphorylation. Mol Biol Cell. 2016;27:2757–2770. doi: 10.1091/mbc.E16-01-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barquilla A, Pasquale EB. Eph receptors and ephrins: therapeutic opportunities. Annual review of pharmacology and toxicology. 2015;55:465–487. doi: 10.1146/annurev-pharmtox-011112-140226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S, Son A, Yu Q, Zhou R, Lo WK. Breakdown of interlocking domains may contribute to formation of membranous globules and lens opacity in ephrin-A5(−/−) mice. Experimental eye research. 2016;145:130–139. doi: 10.1016/j.exer.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell RA, McGranahan N, Bartek J, Swanton C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature. 2013;501:338–345. doi: 10.1038/nature12625. [DOI] [PubMed] [Google Scholar]
- Carvalho RF, Beutler M, Marler KJ, Knoll B, Becker-Barroso E, Heintzmann R, Ng T, Drescher U. Silencing of EphA3 through a cis interaction with ephrinA5. Nat Neurosci. 2006;9:322–330. doi: 10.1038/nn1655. [DOI] [PubMed] [Google Scholar]
- Celojevic D, Abramsson A, Seibt Palmer M, Tasa G, Juronen E, Zetterberg H, Zetterberg M. EPHA2 polymorphisms in Estonian patients with age-related cataract. Ophthalmic genetics. 2015:1–5. doi: 10.3109/13816810.2014.902080. [DOI] [PubMed] [Google Scholar]
- Cheng C, Ansari MM, Cooper JA, Gong X. EphA2 and Src regulate equatorial cell morphogenesis during lens development. Development. 2013;140:4237–4245. doi: 10.1242/dev.100727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Gong X. Diverse roles of Eph/ephrin signaling in the mouse lens. PLoS One. 2011;6:e28147. doi: 10.1371/journal.pone.0028147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Xia CH, Li L, White TW, Niimi J, Gong X. Gap junction communication influences intercellular protein distribution in the lens. Experimental eye research. 2008;86:966–974. doi: 10.1016/j.exer.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, Son AI, Komlos D, Sun Y, Kleiman NJ, Zhou R. Loss of ephrin-A5 function disrupts lens fiber cell packing and leads to cataract. Proc Natl Acad Sci U S A. 2008;105:16620–16625. doi: 10.1073/pnas.0808987105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave A, Laurie K, Staffieri SE, Taranath D, Mackey DA, Mitchell P, Wang JJ, Craig JE, Burdon KP, Sharma S. Mutations in the EPHA2 gene are a major contributor to inherited cataracts in South-Eastern Australia. PLoS One. 2013;8:e72518. doi: 10.1371/journal.pone.0072518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy A, Gale NW, Murray EW, Klinghoffer RA, Soriano P, Feuerstein C, Robbins SM. Compartmentalized signaling by GPI-anchored ephrin-A5 requires the Fyn tyrosine kinase to regulate cellular adhesion. Genes Dev. 1999;13:3125–3135. doi: 10.1101/gad.13.23.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easwaran H, Tsai HC, Baylin SB. Cancer epigenetics: tumor heterogeneity, plasticity of stem-like states, and drug resistance. Molecular cell. 2014;54:716–727. doi: 10.1016/j.molcel.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efazat G, Novak M, Kaminskyy VO, De Petris L, Kanter L, Juntti T, Bergman P, Zhivotovsky B, Lewensohn R, Haag P, Viktorsson K. Ephrin B3 interacts with multiple EphA receptors and drives migration and invasion in non-small cell lung cancer. Oncotarget. 2016;7:60332–60347. doi: 10.18632/oncotarget.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forse GJ, Uson ML, Nasertorabi F, Kolatkar A, Lamberto I, Pasquale EB, Kuhn P. Distinctive Structure of the EphA3/Ephrin-A5 Complex Reveals a Dual Mode of Eph Receptor Interaction for Ephrin-A5. PLoS One. 2015;10:e0127081. doi: 10.1371/journal.pone.0127081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisen J, Yates PA, McLaughlin T, Friedman GC, O’Leary DD, Barbacid M. Ephrin-A5 (AL-1/RAGS) is essential for proper retinal axon guidance and topographic mapping in the mammalian visual system. Neuron. 1998;20:235–243. doi: 10.1016/s0896-6273(00)80452-3. [DOI] [PubMed] [Google Scholar]
- Gong X, Li E, Klier G, Huang Q, Wu Y, Lei H, Kumar NM, Horwitz J, Gilula NB. Disruption of alpha3 connexin gene leads to proteolysis and cataractogenesis in mice. Cell. 1997;91:833–843. doi: 10.1016/s0092-8674(00)80471-7. [DOI] [PubMed] [Google Scholar]
- Gopal U, Bohonowych JE, Lema-Tome C, Liu A, Garrett-Mayer E, Wang B, Isaacs JS. A novel extracellular Hsp90 mediated co-receptor function for LRP1 regulates EphA2 dependent glioblastoma cell invasion. PLoS One. 2011;6:e17649. doi: 10.1371/journal.pone.0017649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess AR, Seftor EA, Gruman LM, Kinch MS, Seftor RE, Hendrix MJ. VE-cadherin regulates EphA2 in aggressive melanoma cells through a novel signaling pathway: implications for vasculogenic mimicry. Cancer biology & therapy. 2006;5:228–233. doi: 10.4161/cbt.5.2.2510. [DOI] [PubMed] [Google Scholar]
- Himanen JP, Chumley MJ, Lackmann M, Li C, Barton WA, Jeffrey PD, Vearing C, Geleick D, Feldheim DA, Boyd AW, Henkemeyer M, Nikolov DB. Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling. Nat Neurosci. 2004;7:501–509. doi: 10.1038/nn1237. [DOI] [PubMed] [Google Scholar]
- Himanen JP, Saha N, Nikolov DB. Cell-cell signaling via Eph receptors and ephrins. Curr Opin Cell Biol. 2007;19:534–542. doi: 10.1016/j.ceb.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himanen JP, Yermekbayeva L, Janes PW, Walker JR, Xu K, Atapattu L, Rajashankar KR, Mensinga A, Lackmann M, Nikolov DB, Dhe-Paganon S. Architecture of Eph receptor clusters. Proc Natl Acad Sci U S A. 2010;107:10860–10865. doi: 10.1073/pnas.1004148107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland SJ, Gale NW, Mbamalu G, Yancopoulos GD, Henkemeyer M, Pawson T. Bidirectional signalling through the EPH-family receptor Nuk and its transmembrane ligands. Nature. 1996;383:722–725. doi: 10.1038/383722a0. [DOI] [PubMed] [Google Scholar]
- Jun G, Guo H, Klein BE, Klein R, Wang JJ, Mitchell P, Miao H, Lee KE, Joshi T, Buck M, Chugha P, Bardenstein D, Klein AP, Bailey-Wilson JE, Gong X, Spector TD, Andrew T, Hammond CJ, Elston RC, Iyengar SK, Wang B. EPHA2 is associated with age-related cortical cataract in mice and humans. PLoS Genet. 2009;5:e1000584. doi: 10.1371/journal.pgen.1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul H, Riazuddin SA, Shahid M, Kousar S, Butt NH, Zafar AU, Khan SN, Husnain T, Akram J, Hejtmancik JF, Riazuddin S. Autosomal recessive congenital cataract linked to EPHA2 in a consanguineous Pakistani family. Mol Vis. 2010;16:511–517. [PMC free article] [PubMed] [Google Scholar]
- Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol. 2002;3:475–486. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- Li D, Wang S, Ye H, Tang Y, Qiu X, Fan Q, Rong X, Liu X, Chen Y, Yang J, Lu Y. Distribution of gene mutations in sporadic congenital cataract in a Han Chinese population. Mol Vis. 2016;22:589–598. [PMC free article] [PubMed] [Google Scholar]
- Li JJ, Liu DP, Liu GT, Xie D. EphrinA5 acts as a tumor suppressor in glioma by negative regulation of epidermal growth factor receptor. Oncogene. 2009;28:1759–1768. doi: 10.1038/onc.2009.15. [DOI] [PubMed] [Google Scholar]
- Lin Q, Zhou N, Zhang N, Qi Y. Mutational screening of EFNA5 in Chinese age-related cataract patients. Ophthalmic research. 2014;52:124–129. doi: 10.1159/000363139. [DOI] [PubMed] [Google Scholar]
- Lisabeth EM, Falivelli G, Pasquale EB. Eph receptor signaling and ephrins. Cold Spring Harbor perspectives in biology. 2013;5 doi: 10.1101/cshperspect.a009159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovicu FJ, Steven P, Saika S, McAvoy JW. Aberrant lens fiber differentiation in anterior subcapsular cataract formation: a process dependent on reduced levels of Pax6. Investigative ophthalmology & visual science. 2004;45:1946–1953. doi: 10.1167/iovs.03-1206. [DOI] [PubMed] [Google Scholar]
- Masoodi TA, Shammari SA, Al-Muammar MN, Almubrad TM, Alhamdan AA. Screening and structural evaluation of deleterious Non-Synonymous SNPs of ePHA2 gene involved in susceptibility to cataract formation. Bioinformation. 2012;8:562–567. doi: 10.6026/97320630008562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H, Burnett E, Kinch M, Simon E, Wang B. Activation of EphA2 kinase suppresses integrin function and causes focal-adhesion-kinase dephosphorylation. Nature cell biology. 2000;2:62–69. doi: 10.1038/35000008. [DOI] [PubMed] [Google Scholar]
- Miao H, Li DQ, Mukherjee A, Guo H, Petty A, Cutter J, Basilion JP, Sedor J, Wu J, Danielpour D, Sloan AE, Cohen ML, Wang B. EphA2 mediates ligand-dependent inhibition and ligand-independent promotion of cell migration and invasion via a reciprocal regulatory loop with Akt. Cancer cell. 2009;16:9–20. doi: 10.1016/j.ccr.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H, Wei BR, Peehl DM, Li Q, Alexandrou T, Schelling JR, Rhim JS, Sedor JR, Burnett E, Wang B. Activation of EphA receptor tyrosine kinase inhibits the Ras/MAPK pathway. Nature cell biology. 2001;3:527–530. doi: 10.1038/35074604. [DOI] [PubMed] [Google Scholar]
- Ojima T, Takagi H, Suzuma K, Oh H, Suzuma I, Ohashi H, Watanabe D, Suganami E, Murakami T, Kurimoto M, Honda Y, Yoshimura N. EphrinA1 inhibits vascular endothelial growth factor-induced intracellular signaling and suppresses retinal neovascularization and blood-retinal barrier breakdown. The American journal of pathology. 2006;168:331–339. doi: 10.2353/ajpath.2006.050435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- Orsulic S, Kemler R. Expression of Eph receptors and ephrins is differentially regulated by E-cadherin. Journal of cell science. 2000;113(Pt 10):1793–1802. doi: 10.1242/jcs.113.10.1793. [DOI] [PubMed] [Google Scholar]
- Park JE, Son AI, Hua R, Wang L, Zhang X, Zhou R. Human cataract mutations in EPHA2 SAM domain alter receptor stability and function. PLoS One. 2012;7:e36564. doi: 10.1371/journal.pone.0036564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatigorsky J. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation. 1981;19:134–153. doi: 10.1111/j.1432-0436.1981.tb01141.x. [DOI] [PubMed] [Google Scholar]
- Poliakov A, Cotrina M, Wilkinson DG. Diverse roles of eph receptors and ephrins in the regulation of cell migration and tissue assembly. Dev Cell. 2004;7:465–480. doi: 10.1016/j.devcel.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Rodriguez S, Rudloff S, Koenig KF, Karthik S, Hoogewijs D, Huynh-Do U. Bidirectional signalling between EphA2 and ephrinA1 increases tubular cell attachment, laminin secretion and modulates erythropoietin expression after renal hypoxic injury. Pflugers Archiv: European journal of physiology. 2016;468:1433–1448. doi: 10.1007/s00424-016-1838-1. [DOI] [PubMed] [Google Scholar]
- Rohani N, Parmeggiani A, Winklbauer R, Fagotto F. Variable combinations of specific ephrin ligand/Eph receptor pairs control embryonic tissue separation. PLoS biology. 2014;12:e1001955. doi: 10.1371/journal.pbio.1001955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabet O, Stockert R, Xouri G, Bruggemann Y, Stanoev A, Bastiaens PI. Ubiquitination switches EphA2 vesicular traffic from a continuous safeguard to a finite signalling mode. Nature communications. 2015;6:8047. doi: 10.1038/ncomms9047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw A, Lundin V, Petrova E, Fordos F, Benson E, Al-Amin A, Herland A, Blokzijl A, Hogberg B, Teixeira AI. Spatial control of membrane receptor function using ligand nanocalipers. Nature methods. 2014;11:841–846. doi: 10.1038/nmeth.3025. [DOI] [PubMed] [Google Scholar]
- Shestopalov VI, Bassnett S. Development of a macromolecular diffusion pathway in the lens. Journal of cell science. 2003;116:4191–4199. doi: 10.1242/jcs.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, De Maria A, Bennett T, Shiels A, Bassnett S. A role for epha2 in cell migration and refractive organization of the ocular lens. Investigative ophthalmology & visual science. 2012;53:551–559. doi: 10.1167/iovs.11-8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels A, Bennett TM, Knopf HL, Maraini G, Li A, Jiao X, Hejtmancik JF. The EPHA2 gene is associated with cataracts linked to chromosome 1p. Mol Vis. 2008;14:2042–2055. [PMC free article] [PubMed] [Google Scholar]
- Shin EH, Basson MA, Robinson ML, McAvoy JW, Lovicu FJ. Sprouty is a negative regulator of transforming growth factor beta-induced epithelial-to-mesenchymal transition and cataract. Molecular medicine. 2012;18:861–873. doi: 10.2119/molmed.2012.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son AI, Cooper MA, Sheleg M, Sun Y, Kleiman NJ, Zhou R. Further analysis of the lens of ephrin-A5−/− mice: development of postnatal defects. Mol Vis. 2013;19:254–266. [PMC free article] [PubMed] [Google Scholar]
- Stahl S, Branca RM, Efazat G, Ruzzene M, Zhivotovsky B, Lewensohn R, Viktorsson K, Lehtio J. Phosphoproteomic profiling of NSCLC cells reveals that ephrin B3 regulates pro-survival signaling through Akt1-mediated phosphorylation of the EphA2 receptor. Journal of proteome research. 2011;10:2566–2578. doi: 10.1021/pr200037u. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y, Akimoto K, Robinson ML, Ohno S, Quinlan RA. A cell polarity protein aPKClambda is required for eye lens formation and growth. Developmental biology. 2009;336:246–256. doi: 10.1016/j.ydbio.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama Y, Shelley EJ, Badouel C, McNeill H, McAvoy JW. Atypical Cadherin Fat1 Is Required for Lens Epithelial Cell Polarity and Proliferation but Not for Fiber Differentiation. Investigative ophthalmology & visual science. 2015;56:4099–4107. doi: 10.1167/iovs.15-17008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan P, Ravindran RD, Vashist P, Shanker A, Nitsch D, Talwar B, Maraini G, Camparini M, Nonyane BA, Smeeth L, Chakravarthy U, Hejtmancik JF, Fletcher AE. EPHA2 polymorphisms and age-related cataract in India. PLoS One. 2012;7:e33001. doi: 10.1371/journal.pone.0033001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto M, Fukuda T, Sonoda R, Murakami F, Tanaka H, Yamamoto N. Ephrin-B3-EphA4 interactions regulate the growth of specific thalamocortical axon populations in vitro. Eur J Neurosci. 2002;16:1168–1172. doi: 10.1046/j.1460-9568.2002.02166.x. [DOI] [PubMed] [Google Scholar]
- Tan W, Hou S, Jiang Z, Hu Z, Yang P, Ye J. Association of EPHA2 polymorphisms and age-related cortical cataract in a Han Chinese population. Mol Vis. 2011;17:1553–1558. [PMC free article] [PubMed] [Google Scholar]
- Wiedemann E, Jellinghaus S, Ende G, Augstein A, Sczech R, Wielockx B, Weinert S, Strasser RH, Poitz DM. Regulation of endothelial migration and proliferation by ephrin-A1. Cellular signalling. 2017;29:84–95. doi: 10.1016/j.cellsig.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Yang NY, Fernandez C, Richter M, Xiao Z, Valencia F, Tice DA, Pasquale EB. Crosstalk of the EphA2 receptor with a serine/threonine phosphatase suppresses the Akt-mTORC1 pathway in cancer cells. Cellular signalling. 2011;23:201–212. doi: 10.1016/j.cellsig.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeddula N, Xia Y, Ke E, Beumer J, Verma IM. Screening for tumor suppressors: Loss of ephrin receptor A2 cooperates with oncogenic KRas in promoting lung adenocarcinoma. Proc Natl Acad Sci U S A. 2015;112:E6476–6485. doi: 10.1073/pnas.1520110112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S, Shin J, Park S. EphA8-ephrinA5 signaling and clathrin-mediated endocytosis is regulated by Tiam-1, a Rac-specific guanine nucleotide exchange factor. Molecules and cells. 2010;29:603–609. doi: 10.1007/s10059-010-0075-2. [DOI] [PubMed] [Google Scholar]
- Youngblood VM, Kim LC, Edwards DN, Hwang Y, Santapuram PR, Stirdivant SM, Lu P, Ye F, Brantley-Sieders DM, Chen J. The Ephrin-A1/EPHA2 Signaling Axis Regulates Glutamine Metabolism in HER2-Positive Breast Cancer. Cancer research. 2016;76:1825–1836. doi: 10.1158/0008-5472.CAN-15-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumoto N, Wakatsuki S, Kurisaki T, Hara Y, Osumi N, Frisen J, Sehara-Fujisawa A. Meltrin beta/ADAM19 interacting with EphA4 in developing neural cells participates in formation of the neuromuscular junction. PLoS One. 2008;3:e3322. doi: 10.1371/journal.pone.0003322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampighi GA, Eskandari S, Kreman M. Epithelial organization of the mammalian lens. Experimental eye research. 2000;71:415–435. doi: 10.1006/exer.2000.0895. [DOI] [PubMed] [Google Scholar]
- Zantek ND, Azimi M, Fedor-Chaiken M, Wang B, Brackenbury R, Kinch MS. E-cadherin regulates the function of the EphA2 receptor tyrosine kinase. Cell growth & differentiation: the molecular biology journal of the American Association for Cancer Research. 1999;10:629–638. [PubMed] [Google Scholar]
- Zhang T, Hua R, Xiao W, Burdon KP, Bhattacharya SS, Craig JE, Shang D, Zhao X, Mackey DA, Moore AT, Luo Y, Zhang J, Zhang X. Mutations of the EPHA2 receptor tyrosine kinase gene cause autosomal dominant congenital cataract. Hum Mutat. 2009;30:E603–611. doi: 10.1002/humu.20995. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Yamada N, Tanaka T, Hori T, Yokoyama S, Hayakawa Y, Yano S, Fukuoka J, Koizumi K, Saiki I, Sakurai H. Crucial roles of RSK in cell motility by catalysing serine phosphorylation of EphA2. Nature communications. 2015;6:7679. doi: 10.1038/ncomms8679. [DOI] [PMC free article] [PubMed] [Google Scholar]