Abstract
Cross-presentation is an MHC-I antigen processing pathway that results in the presentation of peptides from exogenous viral, bacterial, parasitic, and tumor antigens and ultimately leads to priming of naïve CD8+ T cells. This process involves several cellular compartments and multiple components. Successful generation of MHC-I-peptide complexes requires that these components act together in a coordinated fashion. We discuss recent findings on the source of MHC-I, the role of the TAP transporter, the importance of intracellular trafficking events, mechanisms of antigens access the cytosol, and how innate immune signals can affect presentation, with an emphasis on how these pathways compare to conventional antigen presentation and how they correlate with existing data.
Introduction
Processing of antigens and presentation of peptides on Major Histocompatibility Complex Class I (MHC-I) molecules is an important immunological event leading to CD8+ T cell recognition of tumor cells as well as cells infected with viruses, bacteria, and parasites. Most of the peptides bound by MHC-I are derived from cytosolic proteins that have been degraded by proteasomes, translocated by the Transporter associated with Antigen Processing (TAP) into the endoplasmic reticulum (ER) lumen, where they are loaded onto MHC-I with the aid of members of the peptide loading complex (PLC), which include TAP, tapasin, calreticulin, and ERp57 [1]. Although MHC-I processing and presentation of peptides from endogenous proteins is a highly coordinated and complex process requiring numerous accessory molecules, the general process is quite well characterized and understood. This is primarily because most of the players involved have been identified and their functions ascertained. In addition, a logical series of events culminates in peptide loading, beginning with digestion of cytosolic proteins by cytosolic proteasomes and ending with peptide loading in the ER and peptide editing facilitated by ER-localized PLC components.
In contrast, cross-presentation describes mechanisms by which antigens derived from extracellular sources are processed and loaded onto MHC-I for presentation to CD8+ T cells. It can occur in many cell types, but the most immunologically relevant is the dendritic cell (DC) and specialized subsets thereof [2,3]. Functionally, cross-presentation is the major mechanism by which naïve CD8+ T cells are primed, and it is essential for priming them to tumor antigens and antigens derived from pathogens that do not directly infect DC. To date, many of the proteins involved in cross-presentation remain unidentified and/or differ in professional APCs versus other cross-presenting cells. Furthermore, cross-presentation pathways can vary depending on the antigen, route of uptake, ligation of innate signaling pathways, and cell type. Overall, there does not appear to be one straightforward model for cross-presentation that incorporates all of the data published to date (Figure 1). In fact, recent advances have added complexity to an already ill-defined process. This review will focus on how findings in the last several years fit with existing data. We apologize in advance to readers whose contributions are not included due to length restrictions.
Figure 1. Cross-presentation of antigens depend on well-orchestrated delivery of factors to phagosomes.
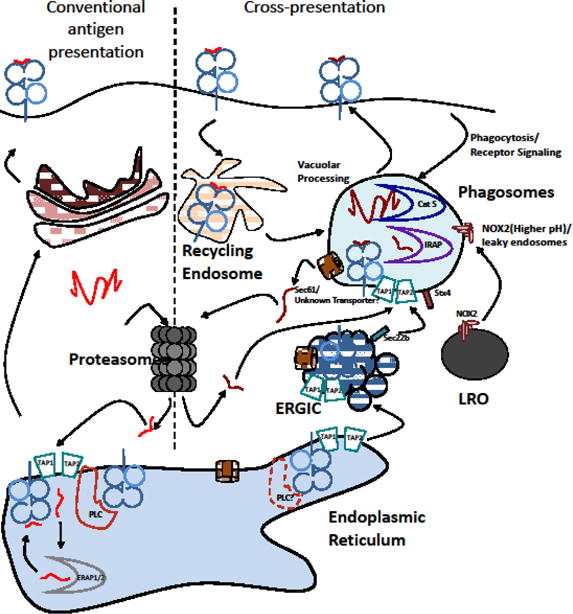
Conventional as well as cross-presentation of most antigens depend on proteasomal activity. In the model shown, phagocytosed antigens may be translocated into the cytosol via a transporter (possibly involving Sec61 and/or other processes), potentially delivered to the phagosome via the ER-Golgi Intermediate Compartment (ERGIC), and degraded by cytosolic proteasomes. Processed peptides are transported back into the phagosomes for loading via TAP or an unidentified transporter, also likely acquired from the ERGIC. Antigenic peptides may be further processed by IRAP and loaded onto MHC-I, acquired from recycling endosomes. Maintenance of a near neutral pH and reduced proteolysis is achieved by delivery of NOX2 machinery from Lysosome-Related Organelles (LRO), which may also facilitate antigen entry into the cytosol.
Formation of the antigen processing and loading compartment
In order to mount an effective immune response, the antigen cross-presentation pathway should mimic the conventional antigen presentation pathway in terms of the peptides generated and loaded onto MHC-I (Figure 2). This raises a fundamental question: for a given antigen how is the peptide-MHC-I complex generated by the cross-presentation machinery replicated by the conventional antigen presentation pathway? Two aspects of cross-presentation provide explanations for how cross-presentation and conventional antigen presentation pathways can overlap. First, the proteolytic machinery that generates the peptides, and second, the identity and functional properties of the intracellular compartment were the peptide is loaded.
Figure 2. To mount an effective immune response, peptide-MHC-I complexes generated by cross-presentation need to match those generated by the conventional MHC-I presentation pathway.
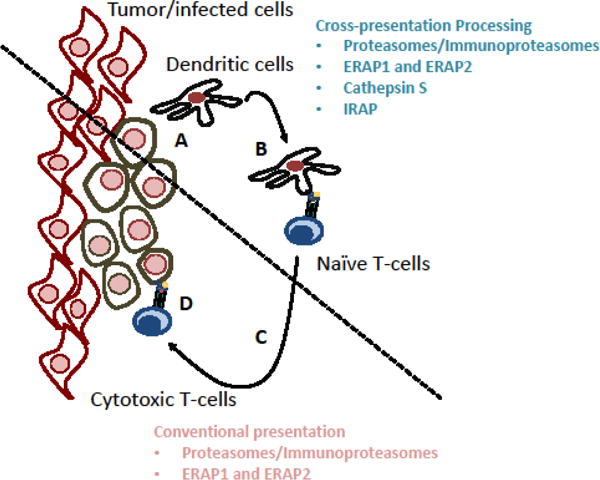
(A) Dendritic cells phagocytose/endocytose antigens from infected tissue. (B) These dendritic cells process and present antigenic peptides, generated by cross-presentation and loaded onto MHC-I, to naïve CD8-positive T-cells, priming them. (C) The primed T-cells migrate to the infected tissue and, (D), recognize peptide-MHC-I presented by infected cells and eliminate them. An effective response requires presentation of the same peptides by DCs (involving cross-presentation) and infected/tumor cells (involving conventional MHC-I antigen presentation). Processing of antigens by the conventional pathway depends on proteasomes and in some cases ER aminopeptidases. Along with proteasomes and ERAPs, cross-presentation can also depend on IRAP and cathepsin S.
Generation of Peptides for MHC-I Loading
Proteasomal processing plays a central role in MHC-I presentation. Constitutive proteasomes and immunoproteasomes are multi-catalytic and multi-subunit protein degradation machines that degrade cytosolic and nuclear proteins [4,5]. Antigen degradation by proteasomes generates peptides that are translocated into the ER and loaded onto MHC-I [1]. It has been shown that cross-presentation also depends on proteasomal activity for many antigens. Indeed, the finding that proteasomes are involved in cross-presentation was the first indication that cross-presented antigens can access the cytosol [6]. While most of the data supporting a proteasome requirement for cross-presentation has relied on inhibitors, it has also been shown that an antigenic peptide specifically generated by immunoproteasomes for conventional MHC-I presentation is also cross-presented in an immunoproteasome-dependent manner when delivered exogenously, supporting the role of cytosolic proteasomes in the cross-presentation pathway [7].
Apart from the cytosolic proteasomal machinery, the activity of cytosolic and ER localized peptidases impacts MHC-I presentation. Requirements for these enzymes are largely antigen dependent: presentation of some peptides requires the activity of certain peptidases, whereas the presentation of different epitopes is inhibited by the same peptidases. These findings have been reviewed elsewhere [8]. In addition to cytosolic peptidases, ER resident aminopeptidases have been identified, ERAP1, or ERAAP in mice, and ERAP2, that trim the amino termini of peptides generated by proteasomes post ER import to generate the appropriate peptide for MHC-I binding [9,10]. Interestingly, ERAAP knockout DCs efficiently cross-present a model soluble antigen (OVA), whereas they fail to cross-present OVA when given as immune complexes [11]. The variable requirement of ERAAP/ERAP1 adds complexity to the relationship of cross-presentation and conventional antigen presentation.
Lysosomal proteases can also play a role in the processing of antigens that are either completely processed/loaded in the endocytic system ([12–15], vacuolar pathway) or gain access to the cytosol for further proteasomal processing ([16] cytosolic pathway). A key lysosomal protease, cathepsin S, can process exogenous antigens to generate antigenic peptides [17]. Cell free experiments have shown that cathepsin S can process a model antigen (OVA) to generate antigenic peptides that can stimulate T-cells. However, it is not clear whether this is restricted to OVA or whether other antigenic peptides can be generated by cathepsin S. In addition to cathepsin S, endosome localized Insulin Regulated Aminopeptidase (IRAP), which is closely related to ERAP1 and ERAP2, has been show to play a role in cross-presentation. Interestingly, IRAP co-localizes with MHC-I in phagosomes [18], and it has been hypothesized that IRAP, in a manner analogous ERAP function in the ER, facilitates the formation of peptide-MHC-I complexes by trimming antigenic peptides generated within the endocytic compartment. Overall, the extent of overlap of peptides generated by lysosomal proteases and those generated by proteasomes is seriously in need of critical evaluation.
Intersection of Peptide-receptive MHC-I with Peptide and Loading Components
Four key functional attributes are required for effective MHC-I loading: an appropriate environment within the compartment, a mechanism to deliver peptide into the compartment, access of MHC-I molecules to the compartment, and the presence of critical accessory proteins. For conventional antigen presentation, the ER provides the proper environment. It contains newly synthesized MHC-I-β2m dimers, peptides (provided via TAP transport from the cytosol), and PLC components that interact with MHC-I and facilitate loading (reviewed in [1]). In the context of cross-presentation, antigen processed in the cytosol can be translocated into the ER and follow the conventional antigen presentation pathway [6]. However, multiple studies have shown that phagosomes and endosomes can form an MHC-I loading compartment for exogenous antigens [19–23]. In this situation, what is the origin of MHC-I and which components of the PLC, if any, participate in cross-presentation?
As phagosomes/endosomes mature in DCs after uptake of particulate or soluble antigens, they receive membrane from at least three distinct intracellular organelles that can influence cross-presentation, namely ER [19–26], lysosome related organelles [27] and recycling endosomes [28*,29**, 30]. Input from each of these organelles brings various functional attributes to phagosomes that make them competent for antigen survival and loading.
Lysosome-related organelles deliver the NADPH oxidase complex NOX2 to phagosomes [27]. The NOX2 complex generates free radicals within the phagosomal lumen resulting in an increased pH [31]. Thus the luminal environment of DC phagosomes remains close to neutral and less proteolytic compared to macrophage phagosomes [31,32]. This preserves internalized antigens and allows their delivery to the cytosol for proteasomal processing. It is also likely that the maintenance of neutrality within phagosomes contributes to an appropriate environment for peptide loading of MHC-I, although, to our knowledge, the precise pH range compatible with effective peptide loading has not been investigated.
Multiple studies have shown ER derived membranes along with ER proteins are recruited to the phagosomes and endosomes [19–26]. Perhaps the strongest functional evidence demonstrating ER-phagosome membrane fusion is that an appropriate peptide substrate undergoes N-linked glycosylation in phagosomes [25,33]. ER-derived membranes traffic through the ER-Golgi intermediate compartment (ERGIC), facilitated by an ER-Golgi SNARE Sec22b and possibly plasma membrane SNARE Stx4 [25]. The ER-derived membranes deliver TAP, other PLC components, and potentially MHC-I molecule to the phagosomes [25], creating an ER-phagosome hybrid compartment likely to have the proper environment for MHC-I loading.
A requirement for functional TAP has been identified in many cross-presentation systems. While TAP likely does play a major role in peptide transport during cross-presentation, old and recent data involving TAP deficient cells [14,34] should be interpreted with caution. Because MHC-I assembly is defective in the absence of TAP, post-ER MHC-I is drastically reduced. This means that any system requiring MHC-I from a post-ER source will have a limited pool available for loading. Therefore, decreased cross-presentation seen in TAP deficient cells could be due either to a requirement for TAP-dependent peptides or to a lack of available MHC-I. Furthermore, normal cross-presentation in TAP-deficient cells cannot necessarily be interpreted to mean a lack of antigen access to the cytosol. A proteasome-dependent, but TAP-independent pathway has been described recently, suggesting than cross-presented peptides generated in the cytosol may use an alternate peptide transporter [34]. Consistent with this, it was recently demonstrated that the SIINFEKL peptide, the classical Kb-restricted epitope derived from OVA, can be imported into purified phagosomes in an ATP-dependent but TAP-independent manner [35**]. A similar TAP-independent but proteasome-dependent pathway has also been characterized in our laboratory that functions under certain conditions (D. Sengupta, unpublished results).
Sec22b-mediated delivery of ER membranes can potentially deliver MHC-I molecules to phagosomes. However, endosomal recycling compartments regulated by, and containing, the small GTPases Rab11 and Rab22 may be a major source of MHC-I for cross-presentation [28*,29**]. When either of these Rab species is depleted by knockdown, both MHC-I trafficking to phagosomes and cross-presentation are decreased [28*,29**], suggesting that recycling MHC-I may acquire antigenic peptides in phagosomes/endosomes. Determining the source of MHC-I for cross-presentation is important for evaluating the role of accessory components in this form of peptide loading. Apart from the likely requirement for TAP-mediated peptide transport, there is little to no evidence supporting a role for the PLC during cross-presentation. Two PLC components directly interact with MHC-I, tapasin and calreticulin, the latter of which only interacts with MHC-I via its N-linked glycan, and then only when this is in the monoglucosylated form, characteristic of glycoproteins undergoing folding in the ER. One study demonstrated that most, if not all, of the phagosomal MHC-I pool contains glycans resistant to removal by endoglycosidase H, which therefore cannot be monoglucosylated [19]. Hence, it is unlikely that recycling MHC-I is able to functionally interact with the PLC in phagosomes as it does in the ER, leaving open the possibility that alternate accessory molecules may play a role. The tapasin homologue TAPBPR, which interacts with MHC-I independently of the PLC [36,37] and can mediate peptide exchange [38*,39*], is a strong candidate for an accessory role in cross-presentation.
How do antigens get into the cytosol?
Two pieces of evidence have been used to argue that a majority of cross-presented antigens need to access the cytosol, namely the requirements for both proteasomal processing and TAP transport (both discussed above). The process of antigen dislocation into the cytosol has been studied extensively. Indeed, many translocation assays have been developed using model proteins (OVA [23,40]), enzymes (cytochrome c [41], β-lactamase [25], luciferase [33,42]), and toxins (gelonin [6], exotoxin A [33], saporin [43]) that demonstrate the cytosolic appearance of exogenously added proteins. However, the proteins and mechanisms involved in cytosolic translocation remain ill defined.
Extensive studies on phagosomes have revealed that a specific set of ER components are recruited to endosomes/phagosomes during cross-presentation mediated by Sec22b and ER-phagosome fusion [19–26,33,44,45]. Importantly, knock down of Sec22b in DCs by shRNA results in less efficient antigen dislocation into the cytosol [25], suggesting that an ER-derived component functions in or regulates antigen translocation. These findings illuminated a important and intriguing question: does the ER-associated degradation (ERAD) machinery, which translocates ER-localized misfolded proteins to the cytosol, also translocate exogenous antigens for cross-presentation? Initial results pointed to yes, as a dominant negative version of the cytosolic ERAD factor p97, which facilitates extraction of misfolded proteins as they enter the cytosol, was shown to inhibit cross-presentation, while addition of recombinant p97 to purified phagosomes enhanced the export of trapped luciferase [33]. While others have reported a role of p97 in cross-presentation [46–48], there does not appear to be a role for additional ERAD factors tested, such as Hrd1, gp78, HERP, and Derlin-1 ([46,49**], reviewed in [50]). As these membrane proteins mediate ERAD, together, separately, or perhaps in cooperation with an as yet identified translocon, it appears unlikely that protein complexes that function in ERAD also mediate antigen translocation from phagosomes.
Secretory proteins are co-translationally translocated into the ER lumen by the Sec61 translocon, which consists of Sec61α, β, and γ, with Sec61α forming the core translocon channel [51]. Sec61 has long been proposed as a potential translocation channel for ERAD and cross-presented antigens. Because of its importance in translation from membrane-associated ribosomes as well as ER import, testing this hypothesis has been a technical nightmare. Recently, it was demonstrated that siRNA-mediated downregulation of Sec61 inhibited cross-presentation at a timepoint when conventional MHC-I and MHC-II processing was intact [49**]. Moreover, when Sec61 trafficking to endosomes was inhibited using an ER-targeted antibody (intrabody) designed to retain Sec61 in the ER, both cross-presentation and antigen dislocation were inhibited. While it remains possible that both methods of Sec61 inhibition could prevent proper translation or trafficking of a specific component of a translocation channel or a critical regulatory element, these findings are consistent with the hypothesis that Sec61 is the Sec22b-delivered ER protein responsible for antigen translocation to the cytosol.
Rather than a translocon-dependent mechanism for cytosolic access, recent evidence suggests that a different mechanism can release internalized antigens into the cytosol. NOX2-produced ROS can induce endosomal lipid peroxidation, membrane damage, and release of antigen from leaky endosomes into the cytosol of DCs [52**]. Inhibition of NOX2 activity by siRNA knockdown or scavenging free radicals significantly reduced release of endosomal antigen into the cytosol and cross-presentation [52**]. While endosomal leakiness or rupture may account for cytosolic delivery of antigens under certain conditions, there is substantial direct and indirect evidence suggesting that active transport is important in cross-presentation. The requirement of disulfide bond reduction by a lysosomal disulfide isomerase suggests unfolding is necessary for translocation [53], while a requirement for factors such as p97 [33] and Sec61 [49**] fit with a translocon model. Furthermore, antigens derived from bacteria or parasites can access the cytosol even at times when the membrane of the phagosome or parasitophorous vacuole is intact [22,26].
Regulation of cross-presentation pathways
Mouse bone marrow-derived DCs can cross-present in vitro in the absence of any additional stimuli, indicating that the ability can be constitutive. However, different DC subclasses exhibit different molecular requirements. For example, CD8α+ versus inflammatory DCs have different requirements for IRAP and Rab43 [54*,55]. Nevertheless, many studies have shown that, as well as regulating the migratory properties of DC, their phagocytic capacity, and their expression of co-stimulatory molecules, innate immune signaling alters their capacity for cross-presentation. LPS is the primary experimental stimulus used but variation in the quality of the LPS as well as in concentration and timing have left the literature somewhat confused (reviewed in [56,57]). A number of recent studies have provided some clarification, although different authors have attributed alterations in cross-presentation to different primary causes.
Prolonged LPS stimulation of DCs (24hrs) results in increased synthesis of the transcription factor TFEB and its translocation into the nucleus [58**]. TFEB is a component of the lysosomal nutrition sensing mechanism, and its upregulation and subsequent nuclear localization activates transcription of lysosomal proteases and increased lysosomal acidification [59]. Consistent with previous observations that increased lysosomal proteolysis inhibits cross-presentation, prolonged stimulation of bone marrow-derived DCs with LPS induces an increase in lysosomal proteolytic activity, which significantly decreases their capacity for cross-presentation [58**]. shRNA-mediated TFEB knock down reversed this decrease, as well as the concomitant enhancement of MHC-II-restricted antigen presentation observed [58**].
Addition of LPS to DCs concomitant with the addition of a particulate antigen causes endosomal compartments to undergo reorganization. A pool of MHC-I localized to a perinuclear recycling compartment is redistributed to phagosomes and the cross-presentation efficiency of the stimulated DC is enhanced, consistent with a role for recycling MHC-I in cross-presentation [29**]. Short term LPS stimulation also leads to NOX2 activation, lipid peroxidation, and leaky endosomes [52**], leading to increased access of internalized antigens to the cytosol and enhanced cross-presentation, as described above. DCs lacking TRIF signaling fail to recruit sec61 to endosomes and show reduced cytosolic translocation and cross-presentation [49**]. Others have found that cross-presentation by DCs is increased with longer (16hr) LPS treatment, but this was attributed to reduced phago-lysosomal fusion and antigen preservation in phagosomes with no effect on antigen translocation to the cytosol [60**]. Overall, while it is clear that innate signaling dramatically alters intracellular trafficking and the overall phagosomal/endosomal proteome, exactly how these processes coordinate to regulate cross-presentation in vivo remains obscure. Perhaps the variety of mechanisms proposed genuinely reflects a complex reality.
Conclusions
As discussed throughout this manuscript and illustrated in Figure 1, cross-presentation requires an elaborate series of steps that in the end result in MHC-I at the cell surface loaded with relevant peptides for stimulation of CD8+ T cells. Many different pathways have been identified to explain requisite cross-presentation events, such as reduced vacuolar proteolysis, cytosolic antigen access, peptide generation and transport, MHC-I trafficking/loading, and LPS-induced alterations. It may be that multiple pathways working concurrently give rise to the same or similar final result, and the relative importance of the different pathways varies with the antigen or cell type. Determining the relative importance of these pathways for cross-priming in vivo is an important next step.
Highlights.
Requirements for epitope matching in conventional MHC-I presentation and cross-presentation;
Vacuolar and cytosolic pathways;
Proteasomal versus lysosomal proteolysis;
Regulation of trafficking;
Source of the relevant MHC-I molecules
Acknowledgments
Work described from this laboratory was supported by the Howard Hughes Medical Institute and NIH Grant RO1-AI097206 awarded to P. Cresswell. J. Grotzke and D. Sengupta were supported by fellowships from the Cancer Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blum JS, Wearsch PA, Cresswell P. Pathways of antigen processing. Annu Rev Immunol. 2013;31:443–473. doi: 10.1146/annurev-immunol-032712-095910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gutierrez-Martinez E, Planes R, Anselmi G, Reynolds M, Menezes S, Adiko AC, Saveanu L, Guermonprez P. Cross-Presentation of Cell-Associated Antigens by MHC Class I in Dendritic Cell Subsets. Front Immunol. 2015;6:363. doi: 10.3389/fimmu.2015.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin ML, Zhan Y, Villadangos JA, Lew AM. The cell biology of cross-presentation and the role of dendritic cell subsets. Immunol Cell Biol. 2008;86:353–362. doi: 10.1038/icb.2008.3. [DOI] [PubMed] [Google Scholar]
- 4.Ferrington DA, Gregerson DS. Immunoproteasomes: structure, function, and antigen presentation. Prog Mol Biol Transl Sci. 2012;109:75–112. doi: 10.1016/B978-0-12-397863-9.00003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groettrup M, Kirk CJ, Basler M. Proteasomes in immune cells: more than peptide producers? Nat Rev Immunol. 2010;10:73–78. doi: 10.1038/nri2687. [DOI] [PubMed] [Google Scholar]
- 6.Kovacsovics-Bankowski M, Rock KL. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267:243–246. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 7.Palmowski MJ, Gileadi U, Salio M, Gallimore A, Millrain M, James E, Addey C, Scott D, Dyson J, Simpson E, et al. Role of immunoproteasomes in cross-presentation. J Immunol. 2006;177:983–990. doi: 10.4049/jimmunol.177.2.983. [DOI] [PubMed] [Google Scholar]
- 8.Lazaro S, Gamarra D, Del Val M. Proteolytic enzymes involved in MHC class I antigen processing: A guerrilla army that partners with the proteasome. Mol Immunol. 2015;68:72–76. doi: 10.1016/j.molimm.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Saric T, Chang SC, Hattori A, York IA, Markant S, Rock KL, Tsujimoto M, Goldberg AL. An IFN-gamma-induced aminopeptidase in the ER, ERAP1, trims precursors to MHC class I-presented peptides. Nat Immunol. 2002;3:1169–1176. doi: 10.1038/ni859. [DOI] [PubMed] [Google Scholar]
- 10.Serwold T, Gonzalez F, Kim J, Jacob R, Shastri N. ERAAP customizes peptides for MHC class I molecules in the endoplasmic reticulum. Nature. 2002;419:480–483. doi: 10.1038/nature01074. [DOI] [PubMed] [Google Scholar]
- 11.Firat E, Saveanu L, Aichele P, Staeheli P, Huai J, Gaedicke S, Nil A, Besin G, Kanzler B, van Endert P, et al. The role of endoplasmic reticulum-associated aminopeptidase 1 in immunity to infection and in cross-presentation. J Immunol. 2007;178:2241–2248. doi: 10.4049/jimmunol.178.4.2241. [DOI] [PubMed] [Google Scholar]
- 12.Bertholet S, Goldszmid R, Morrot A, Debrabant A, Afrin F, Collazo-Custodio C, Houde M, Desjardins M, Sher A, Sacks D. Leishmania antigens are presented to CD8+ T cells by a transporter associated with antigen processing-independent pathway in vitro and in vivo. J Immunol. 2006;177:3525–3533. doi: 10.4049/jimmunol.177.6.3525. [DOI] [PubMed] [Google Scholar]
- 13.Campbell DJ, Serwold T, Shastri N. Bacterial proteins can be processed by macrophages in a transporter associated with antigen processing-independent, cysteine protease-dependent manner for presentation by MHC class I molecules. J Immunol. 2000;164:168–175. doi: 10.4049/jimmunol.164.1.168. [DOI] [PubMed] [Google Scholar]
- 14.Song R, Harding CV. Roles of proteasomes, transporter for antigen presentation (TAP), and beta 2-microglobulin in the processing of bacterial or particulate antigens via an alternate class I MHC processing pathway. J Immunol. 1996;156:4182–4190. [PubMed] [Google Scholar]
- 15.Pfeifer JD, Wick MJ, Roberts RL, Findlay K, Normark SJ, Harding CV. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993;361:359–362. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- 16.Fonteneau JF, Kavanagh DG, Lirvall M, Sanders C, Cover TL, Bhardwaj N, Larsson M. Characterization of the MHC class I cross-presentation pathway for cell-associated antigens by human dendritic cells. Blood. 2003;102:4448–4455. doi: 10.1182/blood-2003-06-1801. [DOI] [PubMed] [Google Scholar]
- 17.Shen L, Sigal LJ, Boes M, Rock KL. Important role of cathepsin S in generating peptides for TAP-independent MHC class I crosspresentation in vivo. Immunity. 2004;21:155–165. doi: 10.1016/j.immuni.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Saveanu L, Carroll O, Weimershaus M, Guermonprez P, Firat E, Lindo V, Greer F, Davoust J, Kratzer R, Keller SR, et al. IRAP identifies an endosomal compartment required for MHC class I cross-presentation. Science. 2009;325:213–217. doi: 10.1126/science.1172845. [DOI] [PubMed] [Google Scholar]
- 19.Ackerman AL, Kyritsis C, Tampe R, Cresswell P. Early phagosomes in dendritic cells form a cellular compartment sufficient for cross presentation of exogenous antigens. Proc Natl Acad Sci U S A. 2003;100:12889–12894. doi: 10.1073/pnas.1735556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houde M, Bertholet S, Gagnon E, Brunet S, Goyette G, Laplante A, Princiotta MF, Thibault P, Sacks D, Desjardins M. Phagosomes are competent organelles for antigen cross-presentation. Nature. 2003;425:402–406. doi: 10.1038/nature01912. [DOI] [PubMed] [Google Scholar]
- 21.Guermonprez P, Saveanu L, Kleijmeer M, Davoust J, Van Endert P, Amigorena S. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature. 2003;425:397–402. doi: 10.1038/nature01911. [DOI] [PubMed] [Google Scholar]
- 22.Grotzke JE, Harriff MJ, Siler AC, Nolt D, Delepine J, Lewinsohn DA, Lewinsohn DM. The Mycobacterium tuberculosis phagosome is a HLA-I processing competent organelle. PLoS Pathog. 2009;5:e1000374. doi: 10.1371/journal.ppat.1000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burgdorf S, Scholz C, Kautz A, Tampe R, Kurts C. Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation. Nat Immunol. 2008;9:558–566. doi: 10.1038/ni.1601. [DOI] [PubMed] [Google Scholar]
- 24.Gagnon E, Duclos S, Rondeau C, Chevet E, Cameron PH, Steele-Mortimer O, Paiement J, Bergeron JJ, Desjardins M. Endoplasmic reticulum-mediated phagocytosis is a mechanism of entry into macrophages. Cell. 2002;110:119–131. doi: 10.1016/s0092-8674(02)00797-3. [DOI] [PubMed] [Google Scholar]
- 25.Cebrian I, Visentin G, Blanchard N, Jouve M, Bobard A, Moita C, Enninga J, Moita LF, Amigorena S, Savina A. Sec22b regulates phagosomal maturation and antigen crosspresentation by dendritic cells. Cell. 2011;147:1355–1368. doi: 10.1016/j.cell.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 26.Goldszmid RS, Coppens I, Lev A, Caspar P, Mellman I, Sher A. Host ER-parasitophorous vacuole interaction provides a route of entry for antigen cross-presentation in Toxoplasma gondii-infected dendritic cells. J Exp Med. 2009;206:399–410. doi: 10.1084/jem.20082108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jancic C, Savina A, Wasmeier C, Tolmachova T, El-Benna J, Dang PM, Pascolo S, Gougerot-Pocidalo MA, Raposo G, Seabra MC, et al. Rab27a regulates phagosomal pH and NADPH oxidase recruitment to dendritic cell phagosomes. Nat Cell Biol. 2007;9:367–378. doi: 10.1038/ncb1552. [DOI] [PubMed] [Google Scholar]
- 28.Cebrian I, Croce C, Guerrero NA, Blanchard N, Mayorga LS. Rab22a controls MHC-I intracellular trafficking and antigen cross-presentation by dendritic cells. EMBO Rep. 2016;17:1753–1765. doi: 10.15252/embr.201642358. In this study authors present evidence suggesting that a Rab-GTPase, Rab22a, which has been shown to play a role in MHC-I recycling, is recruited to the phagosomes. Rab22a is required for the maintenance of the perinuclear pool of MHC-I residing in the recycling endosome. Knock-down of Rab22a inhibits cross-presentation of soluble as well as particulate antigens. Both this study and reference 29 highly suggest that recycled MHC-I is loaded with peptides in the endocytic system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nair-Gupta P, Baccarini A, Tung N, Seyffer F, Florey O, Huang Y, Banerjee M, Overholtzer M, Roche PA, Tampe R, et al. TLR signals induce phagosomal MHC-I delivery from the endosomal recycling compartment to allow cross-presentation. Cell. 2014;158:506–521. doi: 10.1016/j.cell.2014.04.054. In this study authors demonstrate that engagement of TLR associated with MyD88 signaling on dendritic cells concomitantly with phagocytosis of particulate antigens (OVA beads) enhances cross-presentation of the antigen and redistributes MHC-I localization in the cells. Engagement of TLR4 results in the enrichment of MHC-I in the phagosomes from perinuclear recycling endosomes. Authors also show that phagosomal enrichment of MHC-I is regulated by the phosphorylation of an R-SNARE, SNAP23, by an innate signaling kinase IKK-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gromme M, Uytdehaag FG, Janssen H, Calafat J, van Binnendijk RS, Kenter MJ, Tulp A, Verwoerd D, Neefjes J. Recycling MHC class I molecules and endosomal peptide loading. Proc Natl Acad Sci U S A. 1999;96:10326–10331. doi: 10.1073/pnas.96.18.10326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savina A, Jancic C, Hugues S, Guermonprez P, Vargas P, Moura IC, Lennon-Dumenil AM, Seabra MC, Raposo G, Amigorena S. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell. 2006;126:205–218. doi: 10.1016/j.cell.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 32.Delamarre L, Pack M, Chang H, Mellman I, Trombetta ES. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science. 2005;307:1630–1634. doi: 10.1126/science.1108003. [DOI] [PubMed] [Google Scholar]
- 33.Ackerman AL, Giodini A, Cresswell P. A role for the endoplasmic reticulum protein retrotranslocation machinery during crosspresentation by dendritic cells. Immunity. 2006;25:607–617. doi: 10.1016/j.immuni.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 34.Merzougui N, Kratzer R, Saveanu L, van Endert P. A proteasome-dependent, TAP-independent pathway for cross-presentation of phagocytosed antigen. EMBO Rep. 2011;12:1257–1264. doi: 10.1038/embor.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawand M, Abramova A, Manceau V, Springer S, van Endert P. TAP-Dependent and -Independent Peptide Import into Dendritic Cell Phagosomes. J Immunol. 2016;197:3454–3463. doi: 10.4049/jimmunol.1501925. This is an important follow-up paper to [34], which described a TAP-independent but proteasome-dependent pathway of cross-presentation. The finding that an antigen required degradation in the cytosol but not TAP suggested that an alternate peptide transporter is used. This manuscript uses a phagosomal peptide import assay to assess peptide import in wild type versus TAP-deficient cells and shows that one of the two peptides tested, SIINFEKL, can be imported into phagosomes in a TAP-independent but ATP-dependent manner, confirming that an alternate mechanism can translocate peptides. The identity of this channel remains unknown. [DOI] [PubMed] [Google Scholar]
- 36.Hermann C, Strittmatter LM, Deane JE, Boyle LH. The binding of TAPBPR and Tapasin to MHC class I is mutually exclusive. J Immunol. 2013;191:5743–5750. doi: 10.4049/jimmunol.1300929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boyle LH, Hermann C, Boname JM, Porter KM, Patel PA, Burr ML, Duncan LM, Harbour ME, Rhodes DA, Skjodt K, et al. Tapasin-related protein TAPBPR is an additional component of the MHC class I presentation pathway. Proc Natl Acad Sci U S A. 2013;110:3465–3470. doi: 10.1073/pnas.1222342110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hermann C, van Hateren A, Trautwein N, Neerincx A, Duriez PJ, Stevanovic S, Trowsdale J, Deane JE, Elliott T, Boyle LH. TAPBPR alters MHC class I peptide presentation by functioning as a peptide exchange catalyst. Elife. 2015;4 doi: 10.7554/eLife.09617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morozov GI, Zhao H, Mage MG, Boyd LF, Jiang J, Dolan MA, Venna R, Norcross MA, McMurtrey CP, Hildebrand W, et al. Interaction of TAPBPR, a tapasin homolog, with MHC-I molecules promotes peptide editing. Proc Natl Acad Sci U S A. 2016;113:E1006–1015. doi: 10.1073/pnas.1519894113. These two papers demonstrate that the tapasin homologue, TAPBR can mediate removal of bound peptide from the MHC-I binding groove, stimulate peptide binding, and differentiate between peptide quality. This role in peptide editing may lead to loading of higher affinity peptides. Although speculative for cross-presentation, the peptide editing function and ability of TAPBPR to interact with MHC-I in the absence of other PLC members is provocative for a role in peptide loading in the endocytic pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hotta C, Fujimaki H, Yoshinari M, Nakazawa M, Minami M. The delivery of an antigen from the endocytic compartment into the cytosol for cross-presentation is restricted to early immature dendritic cells. Immunology. 2006;117:97–107. doi: 10.1111/j.1365-2567.2005.02270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin ML, Zhan Y, Proietto AI, Prato S, Wu L, Heath WR, Villadangos JA, Lew AM. Selective suicide of cross-presenting CD8+ dendritic cells by cytochrome c injection shows functional heterogeneity within this subset. Proc Natl Acad Sci U S A. 2008;105:3029–3034. doi: 10.1073/pnas.0712394105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giodini A, Cresswell P. Hsp90-mediated cytosolic refolding of exogenous proteins internalized by dendritic cells. EMBO J. 2008;27:201–211. doi: 10.1038/sj.emboj.7601941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia-Castillo MD, Tran T, Bobard A, Renard HF, Rathjen SJ, Dransart E, Stechmann B, Lamaze C, Lord M, Cintrat JC, et al. Retrograde transport is not required for cytosolic translocation of the B-subunit of Shiga toxin. J Cell Sci. 2015;128:2373–2387. doi: 10.1242/jcs.169383. [DOI] [PubMed] [Google Scholar]
- 44.Giodini A, Rahner C, Cresswell P. Receptor-mediated phagocytosis elicits cross-presentation in nonprofessional antigen-presenting cells. Proc Natl Acad Sci U S A. 2009;106:3324–3329. doi: 10.1073/pnas.0813305106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Imai J, Hasegawa H, Maruya M, Koyasu S, Yahara I. Exogenous antigens are processed through the endoplasmic reticulum-associated degradation (ERAD) in cross-presentation by dendritic cells. International Immunology. 2005;17:45–53. doi: 10.1093/intimm/dxh184. [DOI] [PubMed] [Google Scholar]
- 46.Menager J, Ebstein F, Oger R, Hulin P, Nedellec S, Duverger E, Lehmann A, Kloetzel PM, Jotereau F, Guilloux Y. Cross-presentation of synthetic long peptides by human dendritic cells: a process dependent on ERAD component p97/VCP but Not sec61 and/or Derlin-1. PLoS One. 2014;9:e89897. doi: 10.1371/journal.pone.0089897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zehner M, Chasan AI, Schuette V, Embgenbroich M, Quast T, Kolanus W, Burgdorf S. Mannose receptor polyubiquitination regulates endosomal recruitment of p97 and cytosolic antigen translocation for cross-presentation. Proc Natl Acad Sci U S A. 2011;108:9933–9938. doi: 10.1073/pnas.1102397108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Imai J, Hasegawa H, Maruya M, Koyasu S, Yahara I. Exogenous antigens are processed through the endoplasmic reticulum-associated degradation (ERAD) in cross-presentation by dendritic cells. Int Immunol. 2005;17:45–53. doi: 10.1093/intimm/dxh184. [DOI] [PubMed] [Google Scholar]
- 49.Zehner M, Marschall AL, Bos E, Schloetel JG, Kreer C, Fehrenschild D, Limmer A, Ossendorp F, Lang T, Koster AJ, et al. The translocon protein Sec61 mediates antigen transport from endosomes in the cytosol for cross-presentation to CD8(+) T cells. Immunity. 2015;42:850–863. doi: 10.1016/j.immuni.2015.04.008. While others have previously shown the presence of Sec61 in phagosomes and endosomes, this paper provides the first functional evidence that Sec61 may play a role in antigen translocation to the cytosol. Preventing trafficking of Sec61 to endosomes using an intrabody leads to both reduced cytosolic translocation and cross-presentation, suggesting that its presence in endosomes has a functional role. However, because of the many potential indirect effects of Sec61 knockdown or the Sec61 intrabody, further characterization and confirmation of this pathway is required. [DOI] [PubMed] [Google Scholar]
- 50.Grotzke JE, Cresswell P. Are ERAD components involved in cross-presentation? Mol Immunol. 2015;68:112–115. doi: 10.1016/j.molimm.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 51.Pfeffer S, Dudek J, Zimmermann R, Forster F. Organization of the native ribosome-translocon complex at the mammalian endoplasmic reticulum membrane. Biochim Biophys Acta. 2016;1860:2122–2129. doi: 10.1016/j.bbagen.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 52.Dingjan I, Verboogen DR, Paardekooper LM, Revelo NH, Sittig SP, Visser LJ, Mollard GF, Henriet SS, Figdor CG, Ter Beest M, et al. Lipid peroxidation causes endosomal antigen release for cross-presentation. Sci Rep. 2016;6:22064. doi: 10.1038/srep22064. This study demonstrates that an alternate mechanism might be responsible for cytosolic antigen access, at least from endosomes. LPS induces NOX2-dependent production of reactive oxygen species, which leads to lipid peroxidation and membrane damage, the final result being membrane leaks or rupture. Scavenging free radicals, knockdown of NOX2 activity, and using DC from Chronic Granulomatous Disease patients (which have defective NOX2 activity) all led to decreased antigen release and/or reduced membrane peroxidation and cross-presentation. Whether this pathway also functions in phagosomes remains undetermined. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh R, Cresswell P. Defective cross-presentation of viral antigens in GILT-free mice. Science. 2010;328:1394–1398. doi: 10.1126/science.1189176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Segura E, Albiston AL, Wicks IP, Chai SY, Villadangos JA. Different cross-presentation pathways in steady-state and inflammatory dendritic cells. Proc Natl Acad Sci U S A. 2009;106:20377–20381. doi: 10.1073/pnas.0910295106. Segura, et. al. analyzed expression of Rab-GTPases in different DC subsets by microarray, identifying Rab43 as highly enriched in the cross-presenting DC (CD8α+). Knockout of Rab43 did not alter DC development, but did lead to defects in cross-presentation of soluble and particulate antigens. Interestingly, cross-presentation defects were limited to CD8α+ DC suggesting that Rab43-mediated trafficking and/or fusion events only function in CD8α+ DC. The functional consequence of Rab43 in CD8α+ DC remains to be elucidated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kretzer NM, Theisen DJ, Tussiwand R, Briseno CG, Grajales-Reyes GE, Wu X, Durai V, Albring J, Bagadia P, Murphy TL, et al. RAB43 facilitates cross-presentation of cell-associated antigens by CD8alpha+ dendritic cells. J Exp Med. 2016;213:2871–2883. doi: 10.1084/jem.20160597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alloatti A, Kotsias F, Magalhaes JG, Amigorena S. Dendritic cell maturation and cross-presentation: timing matters! Immunol Rev. 2016;272:97–108. doi: 10.1111/imr.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wagner CS, Grotzke JE, Cresswell P. Intracellular events regulating cross-presentation. Front Immunol. 2012;3:138. doi: 10.3389/fimmu.2012.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Samie M, Cresswell P. The transcription factor TFEB acts as a molecular switch that regulates exogenous antigen-presentation pathways. Nat Immunol. 2015;16:729–736. doi: 10.1038/ni.3196. This study demonstrates that prolonged stimulation of DC with LPS leads to increased transcription levels of Transcription Factor, EB (TFEB) and translocation of protein to the nucleus. TFEB is a master regulator of lysosome biogenesis and function. TFEB-induced transcriptional and downstream events were shown to decrease cross-presentation and enhance MHC-II presentation, with phagosomes showing increased pH and proteolytic activity. Knockdown of TFEB partially rescued the LPS-induced decrease in cross-presentation, suggesting that TFEB-dependent phago-lysosomal remodeling is the cause of such decreases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA, Di Malta C, Donaudy F, Embrione V, Polishchuk RS, et al. A gene network regulating lysosomal biogenesis and function. Science. 2009;325:473–477. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- 60.Alloatti A, Kotsias F, Pauwels AM, Carpier JM, Jouve M, Timmerman E, Pace L, Vargas P, Maurin M, Gehrmann U, et al. Toll-like Receptor 4 Engagement on Dendritic Cells Restrains Phago-Lysosome Fusion and Promotes Cross-Presentation of Antigens. Immunity. 2015;43:1087–1100. doi: 10.1016/j.immuni.2015.11.006. Phenotyping phagosomes from resting and LPS-treated DC showed decreased lysosomal markers in stimulated cells. The authors demonstrate that this was due to decreased phago-lysosomal fusion. Retention of lysosomes to the perinuclear region, mediated by Rab34, was required for the LPS-induced decrease of lysosomal fusion and increase in cross-presentation. [DOI] [PubMed] [Google Scholar]


