Abstract
In most autoimmune diseases, a decade-long defect in self-tolerance eventually leads to clinically relevant, tissue-destructive inflammatory disease. The pathogenic potential of chronic persistent immune responses during the pre-clinical and clinical phase is ultimately linked to the bioenergetic fitness of innate and adaptive immune cells. Chronic immune cell stimulation, high cellular turn-over, structural damage to the host tissue and maladaptive wound healing, all require a reliable supply of nutrients, oxygen, and biosynthetic precursors. Here, we use the model system of rheumatoid arthritis (RA) to discuss immunometabolism from the vantage point of T-cells and macrophages that encounter fundamentally different metabolic stress scenarios in the RA host. We outline the general principle that both insufficient nutrient supply, as well as nutrient excess generate cellular stress responses and guide immune function. ATPlow, NADPHhigh, ROSlow T-cells hyperproliferate and are forced into premature senescence. ATPhigh, ROShigh macrophages dimerize the glycolytic enzyme pyruvate kinase to amplify STAT3-dependent inflammatory effector functions. A corollary of this model is that simple nutraceutical interventions will be insufficient to re-educate the immune system in RA. Instead, interference with cell-type-exclusive and differentiation-stage-dependent metabolic setpoints will be needed to reprogram arthritogenic pathways.
Introduction
Pathogenetic immunity leading to autoimmune disease follows the same principles as protective immunity: antigen recognition by CD4 T-cells triggers their rapid proliferation and differentiation into effector cells to aid the activation, expansion, and differentiation of CD8 T-cells. Similarly, such CD4 effector cells serve as helper cells for B-cells to undergo immunoglobulin class switching and differentiate into high-affinity antibody-producing plasma cells. Autoantigen-reactive CD4 T-cells support tissue-injurious cytotoxic T-cells and enable B-cells to produce autoantibodies. The transition into disease requires immune memory, which defines disease chronicity and the need for life-long immunosuppressive therapy. For most autoimmune diseases, the breakdown in self-tolerance precedes clinical disease by years to decades. In humans, the original encounter of self-antigens, the “original sin”, occurs in lymphoid tissue sites and for most autoimmune diseases the defect is now believed to relate to failing peripheral tolerance instead of central tolerance. Autoantigens are often widely distributed, yet autoimmune diseases display a fascinating tissue tropism, emphasizing the need for tissue-emanating factors in pathogenesis.
Rheumatoid arthritis (RA) patients present with symmetrical, painful and disabling joint inflammation years after the immune system has made fundamental mistakes [1,2]. Over time, the inflammatory lesion, the rheumatoid pannus, causes irreversible damage to tendons, cartilage, and bone. In a minority of patients, joint-infiltrating T- and B-cells form highly complex lymphoid structures mimicking germinal centers, in most patients, and immune cells form diffuse infiltrates [3]. The facilitators of tissue damage are highly activated macrophages, fibroblasts and osteoclasts, with several different types of tissue-intrusive synovial fibroblasts [4,5]. Recent work has revealed that posttranslationally modified self-proteins are the preferred target of autoantibodies [6] and mechanisms targeting tissue inflammation towards selected joints remain poorly understood [7,8]. Imaging studies with positron emission tomography/computed tomography (PET/CT), a method tracking the utilization of the glucose analogue (18)-F-fluorodeoxyglucose, document tracer enrichment in the inflamed joints, but also in central lymphoid organs [9,10], emphasizing the heterogeneity of tissue environments hosting pathogenic immune responses.
Despite the shared features of autoimmune diseases, considerable differences exist, imposed by restrictive and conditioning tissue milieus in which each of these diseases unfolds. Accordingly, disease-specific immunometabolic abnormalities have been described, exemplified by the very different conditions in two major autoimmune syndromes; RA and systemic lupus erythematosus (SLE) [11–13]. Here, we will focus on RA as one of the prototypic chronic autoimmune diseases. We will summarize recent data demonstrating that different disease-relevant cells, specifically T-cells and macrophages (Mø), deal with fundamentally different metabolic stressors. RA T-cells are ATPlow, NADPHhigh cells, shunting glucose into the pentose phosphate pathway (PPP) [14]. PPP shunting enables RA T-cells to ignore cell cycle checkpoints and become hyperproliferative. Proliferative pressure leads to premature T-cell aging and the acquisition of pro-inflammatory and tissue-invasive behavior [15]. In contrast, RA macrophages are overwhelmed by nutrient excess, become a victim of excessive glucose uptake and generate high levels of ATP and mitochondrial reactive oxygen species (ROS). Consequences include posttranslational modification of the cytoplasmic enzyme pyruvate kinase, which then “moonlights” as a nuclear kinase, phosphorylates STAT3 and drives aberrant cytokine production [16].
RA T-cells – energy-deprived T-cells with impaired redox signaling
Cell-fate decisions made by T-cells, including their activation, proliferation, differentiation, and polarization are tightly linked to metabolic conditions and vice versa [17–19]. Given the enormous cellular expansion of T-cells, particularly during primary immune responses, their need for nutrients and energy is explicitly high and they satisfy that need by utilizing glucose, glutamine, and fatty acids for ATP generation [20,21]. Also, clonal expansion creates high need for biosynthetic precursors (e.g. ribose for nucleic acid synthesis, lipids for membranes) to build new cells [22].
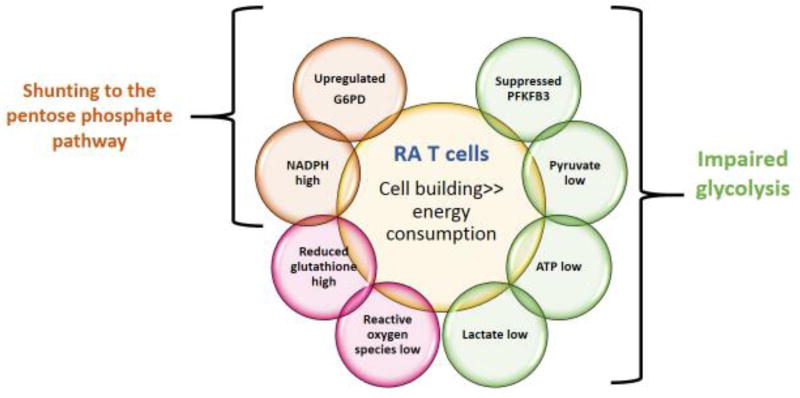
Glucose metabolism in CD4 T-cells from RA patients is fundamentally rewired (Fig. 1) [23–25]. Glycolytic flux is dampened by transcriptional repression of the regulatory glycolytic enzyme 6-phosphofructo-2-kinase (PFKFB3). Consequently, both ATP and pyruvate levels are low. Pyruvate deprivation slows down mitochondrial activity. In contrast to other cell types, T-cells can tolerate ATP deficiency and continue to proliferate and secrete cytokines [26], enabling energy-deprived T-cells to function as highly active effector cells. Studies in CD4 T-cells from RA patients have identified an interesting switch in glucose utilization. By upregulating the checkpoint enzyme Glucose-6-phosphate dehydrogenase (G6PD), patient-derived T-cells divert glucose into the PPP [13,24]. As a result, cellular NADPH levels increased, reduced glutathione is enriched and ROS are scavenged (Fig. 1). Although ROS have mostly been regarded as harmful, recent work has emphasized the essential contribution of oxidant signaling in keeping intracellular molecular communications fast and efficient [27,28]. Accordingly, ATPlow, NADPHhigh, ROSlow T-cells lose the ability to swiftly mobilize ROS-sensing mediators.
Figure 1. Metabolic Abnormalities in RA T cells.
Summary of the metabolic reprogramming that occurs in T-cells from patients with rheumatoid arthritis (RA). Due to repression of PFKFB3 and overexpression of G6PD, glucose utilization is diverted from ATP generation towards the pentose phosphate pathway. As a consequence, availability of intracellular metabolites shifts away from ATP towards excess NADPH and consumption of ROS.
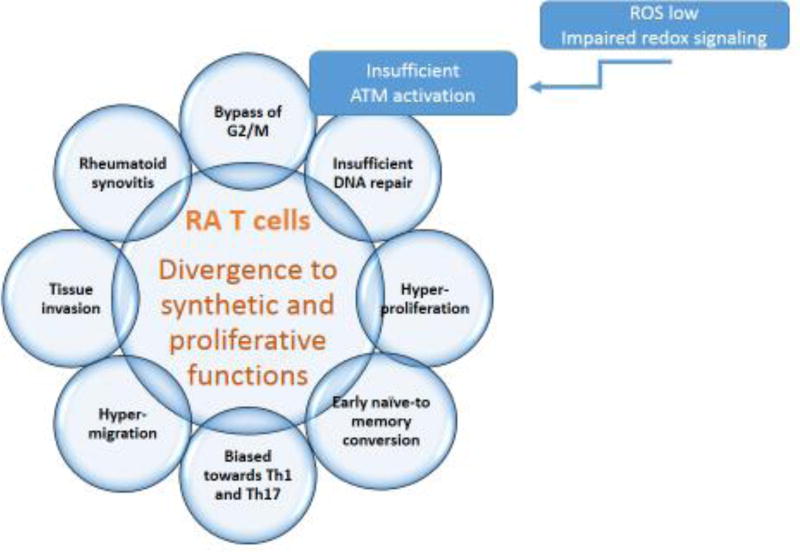
Rewiring of glucose utilization has major implications for RA patients. In fact, the ratio of PFKFB3/G6PD measured in activated CD4 T-cells is closely correlated with disease activity [24], suggesting that the specifics of glucose utilization have a disease relevant role. Functional consequences of re-routing glucose away from ATP production towards synthetic bioprograms have been investigated (Fig. 2). Reduced oxidant signaling affects functionality of the kinase Ataxia telangiectasia mutated (ATM), which regulates passage of dividing cells through the cell cycle. ATM is explicitly redox-sensitive [29] and ROSlow RA T-cells fail to dimerize and phosphorylate the enzyme. ATM detects DNA damage, stops cells during G2/M phase and initiates DNA repair, preferentially by homologous recombination of sister chromatids [30]. RA T-cells bypass the G2/M cell cycle checkpoint and fail to properly repair DNA breaks (Fig. 2) [31].
Figure 2. Functional Abnormalities in RA T cells.
RA T-cells divert energy use to a synthetic/proliferative phenotype (see Fig.1). Preferred glucose shunting to the PPP generates excess NADPH and consumes cellular ROS. Impaired redox signaling has profound impact on multiple functional domains of T-cells: insufficient activation of the cell cycle kinase ATM drives hyperproliferation and deviates lineage commitment towards the pro-inflammatory Th1 and Th17 subsets. The end result is a pool of T-cells with arthritogenic potential
Although CD4 T-cells are already highly proliferative, inadequacy of the cell cycle checkpoint in ATMlow CD4 T-cells accelerates their cell cycle passage and has profound implications for their functionality. They hyperproliferate and commit to early conversion from the naïve to the memory state. Cell cycle passage also changes differentiation and polarization of expanding T-cells, forcing hyperproliferative T-cells towards the Th1 and Th17 lineage (Fig. 2) [13,24]. Ultimately, redirecting glucose away from glycolytic breakdown towards the PPP diverts the RA T-cell from an energy-consuming to a synthetic/proliferative phenotype.
In vivo studies have defined the implications of a highly synthetic CD4 T-cell in RA (Fig. 2). In a humanized model system, where human synovium is engrafted into NSG mice, adoptively transferred patient-derived CD4 T-cells rapidly infiltrate into the synovial tissue site and induce an inflammatory reaction typical for rheumatoid synovium: high production of TNF-α, IL-1β and IL-6. Also, tissues occupied by PPP-biased T-cells accumulate RANKL+ T-cells, capable of supporting bone-destructive osteoclasts [24].
Glucose shunting away from ATP production towards synthetic capabilities affects naïve as well as memory CD4 T-cells [24], making it unlikely that metabolic abnormalities are solely caused by T-cells living in an inflamed tissue environment. Very early defects in the RA disease process, the breakdown of self-tolerance, is relevant for naïve cells and occur in lymphoid tissues. In contrast, the typical lesions of later disease, the inflammatory infiltrates in the synovial membranes, are composed of memory T-cells. Primary and secondary immune responses in RA patients thus rely on very different tissue environments and different fuel sources, yet the metabolic re-programming of T-cells is shared amongst naïve and memory populations, identifying it as a cell-intrinsic abnormality.
The counterintuitive scenario that sluggish instead of hyperactive glycolytic flux feeds inflammatory activity has been confirmed in animal models of arthritis [32]. Replenishing the product of PFKFB3, fructose 1,6-bisphosphate (FBP) provided strong anti-inflammatory effects in vivo, compatible with the concept that enhancing glycolytic breakdown improves T-cell function and prevents pro-inflammatory effector functions.
Another consequence of repressed glycolysis is the reduction of ATP, which has immediate impact for the energy fitness of the cell, but similarly reduces ATP’s ability to function as a signaling molecule [33–35]. Extracellular ATP is sensed by complex families of receptors and functions as a signaling molecule in cell-cell communications. Once outside of the cell, ATP is hydrolyzed by the ectonucleoside triphosphate diphosphohydrolase-1 (CD39) and the ecto-5'-nucleotidase (CD73). CD39 has been implicated in regulating survival of memory T-cells, thus shaping the durability of T-cell responses [36]. CD39high expression on effector memory T-cells is a marker of immunosenescence and facilitates increased apoptotic death of such T-cells, thus weakening the induction of durable memory responses. CD39’s role in memory formation was examined in anti-viral vaccine responses. Individuals with low CD39 induction on effector T-cells had significantly better anti-viral T-cell responses following vaccination [36].
While T-cells in RA patients have diverted from an energy-producing/consuming state to a biosynthetic mode, they are still exposed to environments characterized by high-glucose utilization. For more than 50 years, the inflamed joint has been recognized as a site of low glucose and high lactate [37,38], reflective of the intense cellular turn-over in the rheumatoid pannus. A major regulator of the glycolytic machinery is HIF-1a which sense falling oxygen levels typically encountered in expanding tissue masses [39,40]. The low glucose/high lactate ratio associated with rheumatoid joint disease suggests that glucose is actually incompletely metabolized and instead of producing pyruvate to feed the mitochondrial energy hub, cells synthesize lactate and release it into the extracellular space. Besides pro-inflammatory T-cells, mesenchymal stromal cells and macrophages dominate the growing, energy-absorbing lesion. Like in all cells, activation of synovial fibroblasts is associated with a switch to glycolytic consumption [41], further acidifying the entire tissue site. The glycolytic intermediate lactate is more than a waste product that suppresses extracellular pH [42]. Tissue-residing cells are equipped with acid-sensing ion channels through which they monitor conditions in their surroundings and read out the metabolic activity of their cell neighbors [43,44]. CD4 and CD8 T-cells sense lactate and lactic acid and reduce mobility in the acidic environment, possibly resulting in prolonged T-cell retention and amplified intensity of inflammation. Lactate biases commitment of CD4 T-cells to the Th17 lineage and disables cytotoxic function in CD8 T-cells [45]. Thus, glucose is not only a critical factor in defining the ratios of intracellular metabolites; it is equally important in shaping the extracellular ecosystem.
RA T-cells – energy-deprived and prematurely old
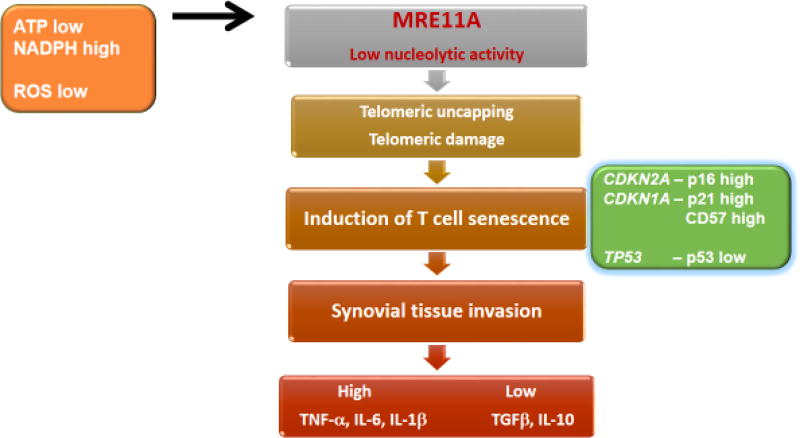
While metabolic conditions inside and outside of the cell impact T-cell fate decision, they are only part of a more complex network of pathway abnormalities that ultimately convert protective into host-damaging immunity. A typical example is the redox-sensitive cell cycle kinase ATM (Fig. 1), which is regulated by intracellular NADPH concentrations to profoundly alter cell cycle behavior and DNA damage repair. A hallmark characteristic of RA T-cells is their premature entry into cellular senescence [46,47]. Recent work has clarified the molecular underpinning of the accelerated aging phenotype of RA T-cells. Both, naïve and memory CD4 T-cells from RA patients have a defect in the activity of the nuclease MRE11A [48]. With evolutionarily highly conserved nuclease motifs, MRE11A has both exonuclease and endonuclease activity. The molecule has a nonredundant role in detecting DNA strand breaks, and directly participating in nonhomologous end-joining and homologous recombination [49]. In CD4 and CD8 T-cells, protein levels of MRE11A decline with progressive age [48]. This process is markedly accelerated in RA T-cells; RA T-cells from 20-year-old patients resemble T-cells from 70–80-year-old healthy elderly [48]. MRE11A deficiency is particularly relevant at the chromosomal ends. MRE11Alow RA telomeres accumulate damage proteins, typical for doubled-stranded DNA breakage. Telomeric erosion in RA T-cells had been reported [50], but had been attributed to high proliferative turn-over. Structural abnormalities of telomeric ends has profound consequences for RA T-cells (Fig. 3): they upregulate p16, a cyclin-dependent kinase inhibitor that decelerates progression from the G1 phase to the S phase. p16 acts as a tumor suppressor, in RA T-cells is signals prematurity of the aging program. MRE11A deficiency fails to upregulate p53, to the opposite, RA T-cells are p53poor, thus able to proliferate excessively [31,48] (Fig. 3). Lack of MRE11A’s nucleolytic activity alters the functional behavior of ATPlow RA T-cells: they acquire CD57 expression [51] and become tissue invasive. In human synovium-SCID chimeras, adoptively transferred MRE11Alow T-cells induce aggressive tissue infiltrates. Enforcing MRE11A protein expression in such T-cells corrects the arthritogenic potential and suppresses tissue inflammation. While not completely understood, available data suggest that MRE11A content, and thus telomeric intactness, shapes cell-cell communications in the tissue microenvironment (Fig. 3). MRE11A overexpression restores anti-inflammatory mechanisms, including tissue production of TGFβ and IL-10. While metabolic reprogramming and defective DNA repair in RA T-cells are closely correlated, and produce similar, arthritogenic phenotypes in vivo, the hierarchy and mechanistic connections between both defects requires further study.
Figure 3. Premature immune aging in energy-deprived RA T cells.
T-cells from RA patients have reduced expression of the nuclease MRE11A, a DNA repair molecule with endo- and exonuclease activity. Telomeres from RA T-cells are particularly low in MRE11A and accumulate damage proteins. Uncapping of MRE11 Alow telomeric ends initiates a T-cell senescence program, characterized by induction of p16, p21, CD57 and loss of p53. Prematurely aged T-cells rapidly invade the synovial tissue space, where they initiate and sustain inflammatory lesions.
RA macrophages – glucose-intoxicated, hyperinflammatory effector cells
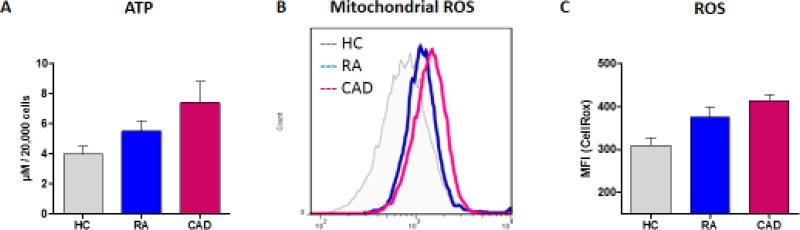
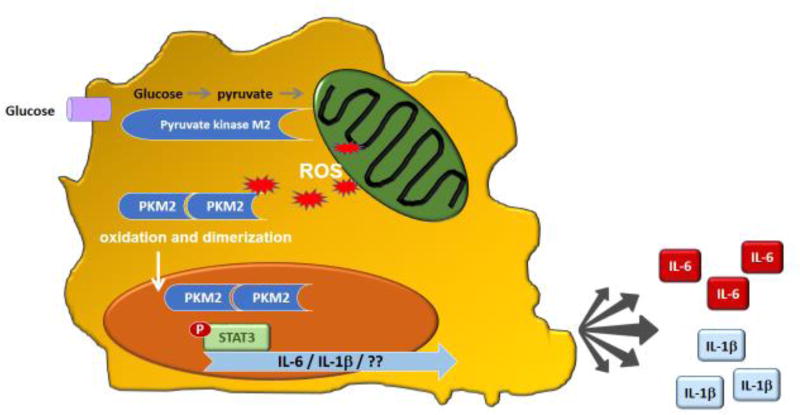
While CD4 helper T-cells may have a pinnacle role in the preclinical stage of autoimmunity as well as in the clinical stage of painful, swollen joints, they are not the sole cell type with disease-relevant functions. Innate immune cells are critically involved in shaping the tissue-damaging inflammatory lesions, often called the rheumatoid pannus. Macrophages (Mø) are a dominant cell population in the inflamed joint and are a major contributor to the cytokine-rich pro-inflammatory environment. Therapeutic targeting of macrophage-derived cytokines in RA has revolutionized the management of this autoimmune syndrome [52]. A clinically important dimension of RA is the increased risk for atherosclerotic disease (e.g. coronary artery disease (CAD)) now recognized as the cause of premature morbidity and mortality in RA patients [53]. Studies of metabolic abnormalities in Mø from patients with CAD have revealed a tight connection between glucose utilization and cytokine production [16,54]. Ex vivo generated Mø from patients with at least one myocardial infarction are in a state of hypermetabolism, and this is a shared phenotype with Mø derived from patients with RA (Fig. 4A). Patient-derived Mø produce higher levels of ATP; with an interesting hierarchy of RA Mø containing more ATP than healthy Mø and CAD Mø outpacing healthy and RA cells. Based on gene expression analysis, patient-derived Mø upregulate a glycolytic module, which includes the glucose transporters GLUT1 and GLUT3, as well as the major glycolytic enzymes PKM2, PFKFB3 and HK2 [16]. Glucose uptake in patient-derived Mø measured with the fluorescent glucose analog 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose is increased and correlates closely to mitochondrial activity. Accordingly, patient-derived Mø when compared to healthy cells have higher oxygen-consumption rates and push more glucose into glycolytic breakdown [16]. Even when resting, patient-derived Mø are in a hypermetabolic state, utilizing excess glucose and generating more ATP. However, the increased capacity of CAD and RA Mø to fuel their energy needs does not come without a price. As the cells ramp up oxidative mitochondrial metabolism, and as the breakdown of metabolites in the Krebs cycle exceeds the electron transport chain’s (ETC) capacity to assimilate the resulting electrons, leakage of ROS from ETC increases (Fig. 4A), mitochondrial and cellular ROS levels rise (Fig. 4A) and expose nearby, and even more distant proteins to a pro-oxidant environment. One of the redox-sensitive proteins in Mø is the glycolytic protein pyruvate kinase M2 (PKM2) (Fig. 4B). In its tetrameric formation, PKM2 is highly active as a pyruvate kinase, accelerating glycolytic flux. When oxidized, the dimeric form of PKM2 is less active in metabolizing glucose and instead acquires the ability to enter the nucleus. Nuclear, dimeric PKM2 “moonlights” as a protein kinase; phosphorylating the transcription factor STAT3. phosSTAT3 stimulates transcription of IL-1β and IL-6, enabling PKM2-rich-Mø to act as IL-1β and IL-6 hyperproducers (Fig. 4B). Both of these cytokines have been directly implicated as disease drivers, in RA as well as CAD [55,56]. The metabolic defect of excess glucose utilization translating into excess cytokine secretion is already present in Mø precursor cells and persists in tissue-residing Mø.
Figure 4.
A. Mitochondrial stress in RA macrophages. CD14+ monocytes from the peripheral blood of 5 healthy individuals (HC), 5 patients with rheumatoid arthritis (RA) and 5 patients with coronary artery disease (CAD) were differentiated into macrophages with M-CSF and activated with IFN-γ (100 U/ml) and LPS (100 ng/ml). (A) Cellular ATP in macrophage extracts was determined by a luminescent reaction. Data are mean+/− SEM. (B) Mitochondrial ROS were quantified by flow cytometric analysis of MitoSOX. Representative histograms are shown. (C) Cellular ROS were measured with CellROX Deep Red Reagent. Mean+/− SEM from 5 experiments.
B. Hyper-inflammatory Macrophages in Rheumatoid Arthritis
Schematic representation of functional abnormalities in RA macrophages which are sustained by excessive glycolytic activity. Due to upregulation of glucose transporters, RA macrophages binge on glucose. Pyruvate overload of the mitochondria increases ROS production, which induces posttranslational modifications of the cytoplasmic enzyme pyruvate kinase M2 (PKM2). Oxidized PKM2 enters the nucleus, where it functions as a protein kinase and phosphorylates STAT3. phosSTAT3 drives production of IL-1b and IL-6
By producing glycolytic and mitochondrial metabolites, RA Mø shape the tissue environment and regulate the functional activity of other cells with whom they share the tissue site. Glucose uptake and breakdown fuels production of lactate, acidifying the extracellular space [57]. Low glucose and high lacate has long been recognized as typical for the rheumatoid joint [58]. Extracellular pH sustains an important cell-cell communication network, as acid-sensing ion channels sense the metabolic activity of cellular partners in their direct surroundings [59]. Lactate has immediate impact on the functional behavior of T-cells [45], modifying their polarization, mobility and cytotoxic capacity. Metabolomic analysis of synovial fluids have drawn attention to the presence of tricarboxylic acid (TCA) cycle intermediates. Generated by the step-wise breakdown of pyruvate, succinate, citrate, glutamate, fumarate, and aspartate have all been implicated in regulating inflammatory activity. Reflective of the hypermetabolic status of RA Mø, glutamate is enriched in arthritic joints and stimulation of glutamate receptors regulates IL-6 release [60]. Similarly, succinate has been identified as a highly pro-inflammatory mediator [61,62]. GPR91-expressing Mø sense the succinate content in their environment, which triggers inflammasome activation and thus serves as an inflammatory amplification loop.
In essence, glucose fuels the inflammatory milieu of the rheumatoid joint through a multitude of mechanisms. Mø and stromal cells metabolize glucose to fulfill their energy needs, but also utilize metabolic intermediates to condition a tissue-injurious ecosystem.
Conclusions and outstanding questions
Protective and pathogenic immunity require high bioenergetic fitness of innate and adaptive immune cells, to enable swift and efficient responses to immune stimuli. Demand for massive clonal expansion, thus biomass generation, dictates the metabolic essentials of lymphocytes. Energy and biosynthetic precursors are equally needed to support the secretory activity of inflammatory cells, e.g. to fuel cytokine secretion. Relying on the model system of rheumatoid arthritis, we have summarized what is known about metabolic programs in this autoimmune disease, characterized by decades of relentless and tissue-damaging inflammation. Unexpectedly, disease-relevant cell populations adapt to quite opposing metabolic conditions: RA T-cells downregulate PFKFB3 and reduce glycolytic flux, whereas RA Mø binge on glucose to enable their transformation into cytokine superproducers.
Why T-cells and Mø would choose opposing metabolic adaptations is not understood. Indeed, upstream signals leading to divergent metabolic landscapes in and around T-cells and Mø in RA are likely complex, not as simple as the inflammatory milieu reprogramming immune cells. It is equally difficult to accuse systemic metabolic conditions in patients as the common driver. Instead, cell-type specific conditioning, possibly imposed by regional niches, is a much more promising hypothesis.
The divergent metabolic adaptations of T-cells and Mø provide useful insights into the cells’ pathogenic contributions. T-cells promote autoimmune disease through their regulatory functions, tightly linked to clonal expansion, memory induction, and decade-long survival. Macrophages are end-differentiated cells which in humans seize to proliferate and function exclusively as effector cells. Mø do not need to proliferate excessively, they can use their biosynthetic program to synthesize cytokines. T-cells need to build biomass, fabricate nucleotides, membranes, and organelles. The longevity of T-cells broadens the impact of dysregulated immunometabolism far beyond a local inflammatory lesion.
The coexistence of energy-derived T-cells and glucose-overloaded Mø in the same host has profound implications for the application of metabolic interference as a means of immunomodulation. Restricting or oversupplying glucose will only reach one of the arthritogenic cell types and may well aggravate the metabolic abnormalities encountered. A more sophisticated approach will be necessary to restore glycolytic flux in T-cells while stopping the glucose overindulgence of Mø.
Highlights.
In the autoimmune disease, rheumatoid arthritis (RA), abnormal metabolic programs have been identified in arthritogenic T-cells and in macrophages.
RA T-cells divert glucose from energy generation towards synthesis of biomass precursors and are ATPlow, NADPHhigh, and ROSlow. Functional consequences include hyperproliferation, G2/M bypass and deviated functional commitment.
Energy-deprived, hyperproliferative RA T-cells lose activity of the DNA repair nuclease MRE11A, accumulate telomeric damage and enter premature senescence.
RA macrophages overindulge on glucose and are ATPhigh and ROShigh. Oxidative modification of pyruvate kinase enables this cytoplasmic enzyme to enter the nucleus, phosphorylate STAT3 and promote IL-6 production.
The coexistence of energy-derived T-cells and glucose-overconsuming macrophages thwarts simple metabolic interferences (e.g. glucose restriction or oversupply), and necessitates cell-type-exclusive and microenvironment-specific metabolic interventions.
Acknowledgments
This work was supported by the National Institutes of Health (R01 AR042527, R01 HL 117913, R01 AI108906 and P01 HL129941 to CMW and R01 AI108891, R01 AG045779, U19 AI057266, and I01 BX001669 to JJG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mankia K, Emery P. Preclinical Rheumatoid Arthritis: Progress Toward Prevention. Arthritis Rheumatol. 2016;68:779–788. doi: 10.1002/art.39603. [DOI] [PubMed] [Google Scholar]
- 2.Dekkers JS, Schoones JW, Huizinga TW, et al. Possibilities for preventive treatment in rheumatoid arthritis? Lessons from experimental animal models of arthritis: a systematic literature review and meta-analysis. Ann Rheum Dis. 2016 doi: 10.1136/annrheumdis-2016-209830. [DOI] [PubMed] [Google Scholar]
- 3.Takemura S, Braun A, Crowson C, et al. Lymphoid neogenesis in rheumatoid synovitis. J Immunol. 2001;167:1072–1080. doi: 10.4049/jimmunol.167.2.1072. [DOI] [PubMed] [Google Scholar]
- 4.Shaw AT, Gravallese EM. Mediators of inflammation and bone remodeling in rheumatic disease. Semin Cell Dev Biol. 2016;49:2–10. doi: 10.1016/j.semcdb.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Croft AP, Naylor AJ, Marshall JL, et al. Rheumatoid synovial fibroblasts differentiate into distinct subsets in the presence of cytokines and cartilage. Arthritis Res Ther. 2016;18:270. doi: 10.1186/s13075-016-1156-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantagrel A, Degboe Y. New autoantibodies associated with rheumatoid arthritis recognize posttranslationally modified self-proteins. Joint Bone Spine. 2016;83:11–17. doi: 10.1016/j.jbspin.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 7.van Beers-Tas MH, Turk SA, van Schaardenburg D. How does established rheumatoid arthritis develop, and are there possibilities for prevention? Best Pract Res Clin Rheumatol. 2015;29:527–542. doi: 10.1016/j.berh.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Catrina AI, Joshua V, Klareskog L, et al. Mechanisms involved in triggering rheumatoid arthritis. Immunol Rev. 2016;269:162–174. doi: 10.1111/imr.12379. [DOI] [PubMed] [Google Scholar]
- 9.Bernelot Moens SJ, van der Valk FM, Strang AC, et al. Unexpected arterial wall and cellular inflammation in patients with rheumatoid arthritis in remission using biological therapy: a cross-sectional study. Arthritis Res Ther. 2016;18:115. doi: 10.1186/s13075-016-1008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu Z, Yin Y, Zheng K, et al. Evaluation of synovial angiogenesis in patients with rheumatoid arthritis using (6)(8)Ga-PRGD2 PET/CT: a prospective proof-of-concept cohort study. Ann Rheum Dis. 2014;73:1269–1272. doi: 10.1136/annrheumdis-2013-204820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsokos GC. Metabolic control of arthritis: Switch pathways to treat. Sci Transl Med. 2016;8:331fs338. doi: 10.1126/scitranslmed.aaf4953. [DOI] [PubMed] [Google Scholar]
- 12.Li W, Sivakumar R, Titov AA, et al. Metabolic Factors that Contribute to Lupus Pathogenesis. Crit Rev Immunol. 2016;36:75–98. doi: 10.1615/CritRevImmunol.2016017164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Z, Matteson EL, Goronzy JJ, et al. T-cell metabolism in autoimmune disease. Arthritis Res Ther. 2015;17:29. doi: 10.1186/s13075-015-0542-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weyand CM, Goronzy JJ. Immunometabolism in early and late stages of rheumatoid arthritis. Nat Rev Rheumatol. 2017 doi: 10.1038/nrrheum.2017.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goronzy JJ, Weyand CM. Successful and Maladaptive T Cell Aging. Immunity. 2017;46:364–378. doi: 10.1016/j.immuni.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16**.Shirai T, Nazarewicz RR, Wallis BB, et al. The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J Exp Med. 2016;213:337–354. doi: 10.1084/jem.20150900. This study identifies the molecular pathways through which metabolic activity controls macrophage effector functions, specifically the high production of IL-6 implicated in long-term complications of chronic-inflammatory disease. Excessive uptake and breakdown of glucose fuels mitochondrial ROS production and drives posttranslational modification of the enzyme puruvate kinase M2 (PKM2). Dimeric PKM2 transitions from the cytoplasm to the nucleus where it “moonlights” as a kinase, phosphorylates STAT3 and promotes IL-6 production. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson MO, Siska PJ, Contreras DC, et al. Nutrients and the microenvironment to feed a T cell army. Semin Immunol. 2016;28:505–513. doi: 10.1016/j.smim.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donnelly RP, Finlay DK. Glucose, glycolysis and lymphocyte responses. Mol Immunol. 2015;68:513–519. doi: 10.1016/j.molimm.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 19.Dimeloe S, Burgener AV, Grahlert J, et al. T-cell metabolism governing activation, proliferation and differentiation; a modular view. Immunology. 2017;150:35–44. doi: 10.1111/imm.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frauwirth KA, Thompson CB. Regulation of T lymphocyte metabolism. J Immunol. 2004;172:4661–4665. doi: 10.4049/jimmunol.172.8.4661. [DOI] [PubMed] [Google Scholar]
- 21.Frauwirth KA, Riley JL, Harris MH, et al. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 22.Waickman AT, Powell JD. mTOR, metabolism, and the regulation of T-cell differentiation and function. Immunol Rev. 2012;249:43–58. doi: 10.1111/j.1600-065X.2012.01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Z, Fujii H, Mohan SV, et al. Phosphofructokinase deficiency impairs ATP generation, autophagy, and redox balance in rheumatoid arthritis T cells. J Exp Med. 2013;210:2119–2134. doi: 10.1084/jem.20130252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24**.Yang Z, Shen Y, Oishi H, et al. Restoring oxidant signaling suppresses proarthritogenic T cell effector functions in rheumatoid arthritis. Sci Transl Med. 2016;8:331ra338. doi: 10.1126/scitranslmed.aad7151. This study identifies T-cells from RA patients as ATPlow, NADPHhigh, ROSlow cells; caused by disproportional shunting of glucose into the pentose phosphate pathway, while glycolytic flux is repressed. The resulting deficiency of intracellular reactive oxygen species alters the cell cycle behavior and the functional polarization of T-cells and is directly connected to their disease-inducing effector functions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Z, Goronzy JJ, Weyand CM. The glycolytic enzyme PFKFB3/phosphofructokinase regulates autophagy. Autophagy. 2014;10:382–383. doi: 10.4161/auto.27345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tripmacher R, Gaber T, Dziurla R, et al. Human CD4(+) T cells maintain specific functions even under conditions of extremely restricted ATP production. Eur J Immunol. 2008;38:1631–1642. doi: 10.1002/eji.200738047. [DOI] [PubMed] [Google Scholar]
- 27.Putker M, Vos HR, Dansen TB. Intermolecular disulfide-dependent redox signalling. Biochem Soc Trans. 2014;42:971–978. doi: 10.1042/BST20140097. [DOI] [PubMed] [Google Scholar]
- 28.O'Neill JS, Feeney KA. Circadian redox and metabolic oscillations in mammalian systems. Antioxid Redox Signal. 2014;20:2966–2981. doi: 10.1089/ars.2013.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ditch S, Paull TT. The ATM protein kinase and cellular redox signaling: beyond the DNA damage response. Trends Biochem Sci. 2012;37:15–22. doi: 10.1016/j.tibs.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lavin MF, Kozlov S, Gatei M, et al. ATM-Dependent Phosphorylation of All Three Members of the MRN Complex: From Sensor to Adaptor. Biomolecules. 2015;5:2877–2902. doi: 10.3390/biom5042877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shao L, Fujii H, Colmegna I, et al. Deficiency of the DNA repair enzyme ATM in rheumatoid arthritis. J Exp Med. 2009;206:1435–1449. doi: 10.1084/jem.20082251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32**.Veras FP, Peres RS, Saraiva AL, et al. Fructose 1,6-bisphosphate, a high-energy intermediate of glycolysis, attenuates experimental arthritis by activating anti-inflammatory adenosinergic pathway. Sci Rep. 2015;5:15171. doi: 10.1038/srep15171. This study demonstrates that metabolic intermediates are not just byproducts, but have functional activities in autoimmune disease. Specifically, the authors demonstrate the anti-inflammatory effects of high-energy intermediates of glycolysis in arthritis. This study dovetails with the identification of an energy-deprived state and suppressed glycolytic flux in key regulators of rheumatoid arthritis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howie D, Waldmann H, Cobbold S. Nutrient Sensing via mTOR in T Cells Maintains a Tolerogenic Microenvironment. Front Immunol. 2014;5:409. doi: 10.3389/fimmu.2014.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raker VK, Becker C, Steinbrink K. The cAMP Pathway as Therapeutic Target in Autoimmune and Inflammatory Diseases. Front Immunol. 2016;7:123. doi: 10.3389/fimmu.2016.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bono MR, Fernandez D, Flores-Santibanez F, et al. CD73 and CD39 ectonucleotidases in T cell differentiation: Beyond immunosuppression. FEBS Lett. 2015;589:3454–3460. doi: 10.1016/j.febslet.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 36.Fang F, Yu M, Cavanagh MM, et al. Expression of CD39 on Activated T Cells Impairs their Survival in Older Individuals. Cell Rep. 2016;14:1218–1231. doi: 10.1016/j.celrep.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goetzl EJ, Falchuk KH, Zeiger LS, et al. A physiological approach to the assessment of disease activity in rheumatoid arthritis. J Clin Invest. 1971;50:1167–1180. doi: 10.1172/JCI106594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Treuhaft PS, DJ MC. Synovial fluid pH, lactate, oxygen and carbon dioxide partial pressure in various joint diseases. Arthritis Rheum. 1971;14:475–484. doi: 10.1002/art.1780140407. [DOI] [PubMed] [Google Scholar]
- 39.Semenza GL, Artemov D, Bedi A, et al. 'The metabolism of tumours': 70 years later. Novartis Found Symp. 2001;240:251–260. discussion 260-254. [PubMed] [Google Scholar]
- 40.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, et al. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Garcia-Carbonell R, Divakaruni AS, Lodi A, et al. Critical Role of Glucose Metabolism in Rheumatoid Arthritis Fibroblast-like Synoviocytes. Arthritis Rheumatol. 2016;68:1614–1626. doi: 10.1002/art.39608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haas R, Cucchi D, Smith J, et al. Intermediates of Metabolism: From Bystanders to Signalling Molecules. Trends Biochem Sci. 2016;41:460–471. doi: 10.1016/j.tibs.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Osmakov DI, Andreev YA, Kozlov SA. Acid-sensing ion channels and their modulators. Biochemistry (Mosc) 2014;79:1528–1545. doi: 10.1134/S0006297914130069. [DOI] [PubMed] [Google Scholar]
- 44.Zhou RP, Wu XS, Wang ZS, et al. Novel Insights into Acid-Sensing Ion Channels: Implications for Degenerative Diseases. Aging Dis. 2016;7:491–501. doi: 10.14336/AD.2015.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45**.Haas R, Smith J, Rocher-Ros V, et al. Lactate Regulates Metabolic and Pro-inflammatory Circuits in Control of T Cell Migration and Effector Functions. PLoS Biol. 2015;13:e1002202. doi: 10.1371/journal.pbio.1002202. This study exemplifies the concept that metabolic intermediates have functions of their own. The authros analyzed the functional implications of lactate release into the tissue enviroment in chronic inflammatory tissue lesions, where it regulates T-cell migration and effector functions and thus serves as an inflammatory amplificator. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weyand CM, Yang Z, Goronzy JJ. T-cell aging in rheumatoid arthritis. Curr Opin Rheumatol. 2014;26:93–100. doi: 10.1097/BOR.0000000000000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weyand CM, Fujii H, Shao L, et al. Rejuvenating the immune system in rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:583–588. doi: 10.1038/nrrheum.2009.180. [DOI] [PubMed] [Google Scholar]
- 48**.Li Y, Shen Y, Hohensinner P, et al. Deficient Activity of the Nuclease MRE11A Induces T Cell Aging and Promotes Arthritogenic Effector Functions in Patients with Rheumatoid Arthritis. Immunity. 2016;45:903–916. doi: 10.1016/j.immuni.2016.09.013. This study identifies the DNA repair nuclease MRE11A as a key regulator of T-cell aging. Energy-derived T-cells from patients with rheumatoid arthritis loose the nucleolytic activity of MRE11A, fail to repair their telomeres, are forced into sensecene and reprogram their functional behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williams GJ, Lees-Miller SP, Tainer JA. Mre11-Rad50-Nbs1 conformations and the control of sensing, signaling, and effector responses at DNA double-strand breaks. DNA Repair (Amst) 2010;9:1299–1306. doi: 10.1016/j.dnarep.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schonland SO, Lopez C, Widmann T, et al. Premature telomeric loss in rheumatoid arthritis is genetically determined and involves both myeloid and lymphoid cell lineages. Proc Natl Acad Sci U S A. 2003;100:13471–13476. doi: 10.1073/pnas.2233561100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winchester R, Giles JT, Nativ S, et al. Association of Elevations of Specific T Cell and Monocyte Subpopulations in Rheumatoid Arthritis With Subclinical Coronary Artery Atherosclerosis. Arthritis Rheumatol. 2016;68:92–102. doi: 10.1002/art.39419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Onuora S. Rheumatoid arthritis: Anti-TNF agents go head-to-head. Nat Rev Rheumatol. 2017;13:2. doi: 10.1038/nrrheum.2016.206. [DOI] [PubMed] [Google Scholar]
- 53.Houri Levi E, Watad A, Whitby A, et al. Coexistence of ischemic heart disease and rheumatoid arthritis patients-A case control study. Autoimmun Rev. 2016;15:393–396. doi: 10.1016/j.autrev.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 54.Shirai T, Hilhorst M, Harrison DG, et al. Macrophages in vascular inflammation--From atherosclerosis to vasculitis. Autoimmunity. 2015;48:139–151. doi: 10.3109/08916934.2015.1027815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bradham WS, Ormseth MJ, Oeser A, et al. Insulin resistance is associated with increased concentrations of NT-proBNP in rheumatoid arthritis: IL-6 as a potential mediator. Inflammation. 2014;37:801–808. doi: 10.1007/s10753-013-9799-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rho YH, Chung CP, Oeser A, et al. Inflammatory mediators and premature coronary atherosclerosis in rheumatoid arthritis. Arthritis Rheum. 2009;61:1580–1585. doi: 10.1002/art.25009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fujii W, Kawahito Y, Nagahara H, et al. Monocarboxylate transporter 4, associated with the acidification of synovial fluid, is a novel therapeutic target for inflammatory arthritis. Arthritis Rheumatol. 2015;67:2888–2896. doi: 10.1002/art.39270. [DOI] [PubMed] [Google Scholar]
- 58.Thomas DP, Dingle JT. In vitro studies of rheumatoid synovium; preliminary metabolic comparison between synovial membrane and villi. Br J Exp Pathol. 1955;36:195–198. [PMC free article] [PubMed] [Google Scholar]
- 59.Pucino V, Bombardieri M, Pitzalis C, et al. Lactate at the crossroads of metabolism, inflammation, and autoimmunity. Eur J Immunol. 2016 doi: 10.1002/eji.201646477. [DOI] [PubMed] [Google Scholar]
- 60.Bonnet CS, Williams AS, Gilbert SJ, et al. AMPA/kainate glutamate receptors contribute to inflammation, degeneration and pain related behaviour in inflammatory stages of arthritis. Ann Rheum Dis. 2015;74:242–251. doi: 10.1136/annrheumdis-2013-203670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61**.Littlewood-Evans A, Sarret S, Apfel V, et al. GPR91 senses extracellular succinate released from inflammatory macrophages and exacerbates rheumatoid arthritis. J Exp Med. 2016;213:1655–1662. doi: 10.1084/jem.20160061. This study demonstrates the relevance of metabolic intermediates as regulators in inflammatory tissue lesions, including the rheumatoid joint. Specifically, succinate secreted from hypermetabolic macrophages is sensed by the GPR91 receptor and functions as an inflammatory amplificator. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim S, Hwang J, Xuan J, et al. Global metabolite profiling of synovial fluid for the specific diagnosis of rheumatoid arthritis from other inflammatory arthritis. PLoS One. 2014;9:e97501. doi: 10.1371/journal.pone.0097501. [DOI] [PMC free article] [PubMed] [Google Scholar]