Abstract
Herpes simplex virus type-1 (HSV-1) infection leads to impaired corneal sensation and, in severe cases, to corneal ulceration, melting and perforation. Here, we explore the potential therapeutic action of pigment epithelial-derived factor (PEDF) plus docosahexaenoic acid (DHA) on corneal inflammation and nerve regeneration following HSV-1 infection. Rabbits inoculated with 100,000 PFU/eye of HSV-1 strain 17Syn+ were treated with PEDF+DHA or vehicle. PEDF+DHA treatment resulted in a biphasic immune response with stronger infiltration of CD4+T cells, neutrophils and macrophages at 7-days post-treatment (p.t.) that was significantly decreased by 14 days, compared to the vehicle-treated group. Screening of 14 immune-related genes by q-PCR showed that treatment induced higher expression of IFN-γ and CCL20 and inhibition of IL-18 by 7 days in the cornea. PEDF+DHA-treated animals developed less dendritic corneal lesions, opacity and neovascularization. Corneal nerve density increased at 12-weeks p.t. with functional recovery of corneal sensation. Treatment with PEDF+DHA that was postponed by 3 weeks also showed increased nerve density when compared to vehicle. Our data demonstrate that PEDF+DHA promotes resolution of the inflammatory response to the virus and, most importantly, induces regeneration of damaged corneal nerves vital for maintaining ocular surface homeostasis.
Keywords: Herpes simplex virus type-1, cornea, inflammation, pigment epithelial derived factor, docosahexaenoic acid
1. Introduction
The cornea is densely innervated mainly by sensory nerves originating in the ophthalmic branch of the trigeminal ganglia (TG) (Muller et al., 2003). Previous studies, including work from our laboratory, have described the entire human cornea nerve distribution, showing that nerve trunks enter the cornea at the limbus radially at the level of the anterior stroma, and then subdivide into multiple, smaller branches that penetrate the epithelium, thus forming a nerve network that finishes in the epithelial surface as free nerve endings (He et al., 2010; Marfurt et al., 2010). The center of the cornea is more densely innervated than the periphery (He et al., 2010). Corneal nerves affect the overall function and homeostasis of the corneal surface by releasing soluble factors that contribute to wound healing and epithelial trophism. These sensory neurons also form the afferent arm of the blinking and tearing reflexes that provide protection and nutrition to the ocular surface (Muller et al., 2003; Marfurt et al., 2010; Shaheen et al, 2014).
Ocular disease caused by herpes simplex virus-1 (HSV-1) is a major cause of vision loss, with more than 300,000 new cases diagnosed yearly in the United States alone (Liesegang, 2001). After initial infection, the virus penetrates the nerve terminals and travels by retrograde axonal transport to sensory neurons of the TG, where it establishes latency (Miller et al., 1998). The disease course is characterized by periodic reactivation of the virus, in which new viral particles travel through the sensory axons to the epithelial surface, where the virus actively replicates. Although HSV-1 can affect any tissue in the eye, tissues of the anterior segment with rich sensory innervation as the cornea and iris are the most common tissue involved. Complications and sequelae of HSV-1 infection can often cause decreased vision and even blindness and are largely secondary to the decrease of corneal sensitivity as a consequence of nerve damage, resulting in neurotrophic keratitis (NK) and persistent recurrent inflammation, causing scarring, neovascularization and tissue loss (Carr et al., 2001; Davis and Dohlman, 2001; Liesegang, 2001; Nishida and Yanai, 2009).
Treatment of HSV-1 keratitis consists mostly of antivirals and modulation of the inflammatory response with topical steroids to reduce tissue damage. Unfortunately, there is no effective treatment available for restoring corneal sensation and reducing complications related to NK. As such, current therapy is directed toward alleviating symptoms as they appear. Some commonly used treatments include artificial tears, bandage contact lenses, punctual occlusion, autologous serum tears, tarsorrhaphy, and amniotic membrane grafting (Nishida and Yanai, 2009; Yanai et al., 2015). Local administration of neural factors such as substance-P (SP), particularly in combination with insulin-derived growth factor-1 (IGF-1), has been shown to promote epithelial cell migration and closure of persistent epithelial defects in the setting of NK (Yanai et al., 2015). Nerve growth factor (NGF) has also been shown to promote wound healing and induce nerve regeneration in neurotrophic corneas but local adverse effects have limited its use (Nishida and Yanai, 2009).
Previous studies from our laboratory using a rabbit model of lamellar keratectomy that damages both the epithelial and stromal nerves have shown that treatment with pigment epithelial-derived factor (PEDF) in combination with the omega-3 fatty acid docosahexaenoic acid (DHA) stimulates regeneration of corneal nerves, and accelerates wound healing and restoration of corneal sensitivity (Cortina et al., 2010; Cortina et al., 2012). HSV-1 corneal infection in the rabbit is a good model of stromal keratitis with similar characteristics to human disease. In the present study, we use this well-established rabbit model infected with HSV-1 to investigate the action of PEDF+DHA treatment on corneal inflammation, corneal sensitivity and nerve regeneration.
2. Materials and methods
2.1 Animals
New Zealand white rabbits of both sexes weighing between 2–3 kg were used. All the experimental procedures were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmology and Vision Research and were approved by the Institutional Animal Care and Use Committee, Louisiana State University Health New Orleans. All experiments described utilized the HSV-1 strain 17Syn+, a wild-type and virulent HSV-1 strain. The rabbits were anesthetized with an intramuscular injection of a mixture of ketamine/xilazine (50mg/kg/10mg/kg), and a 2-by-2 crosshatch pattern was made on the surface of the corneal epithelium using a sterile 27-gauge needle and very light scratching. Corneas were inoculated bilaterally with 100,000 pfu/eye of 17Syn+. The viral inoculum was placed directly on the surface of the eye, and the lid of the eye was gently massaged to ensure even distribution of the virus. All infections were confirmed through slit-lamp examination (SLE) (Topcon DC-1 SL-D7) on post-inoculation (p.i.) day 3.
2.2 Treatment
Twenty-four hours after infection, one group of rabbits were treated three times a day topically with 50μL of a solution of 2ng of PEDF (Bio Products, Middletown, MD) in PBS plus 400ng DHA (Cayman Chemical, Ann Arbor, MI), dissolved in 0.2% of human albumin free of fatty acids and the other group was treated with the vehicle consisting of 0.2% albumin in PBS. Treatment was continued for two weeks, and then the drugs and vehicle were delivered using a 72 h collagen shield (Oasis Medical, Glendora, CA) as previously described (Cortina et al., 2010; Cortina et al., 2012) for an additional 10 weeks. The collagen shields were changed twice a weeks. A third group of rabbits were treated with vehicle for three weeks and then switched to PEDF+DHA using the collagen shield for nine weeks. Figure 1 shows the time line of the treatments and samples taken. At one and two weeks p.t., three animals of each group were euthanized with an overdose of sodium pentobarbital via ear vein injection to process them for immunostaining of inflammatory cells. The experiment was repeated four times. In the last experiment, 10 additional rabbits from each group were infected and treated for one week to obtain corneas for q-PCR analysis (Figure 1). At the end of the 12 weeks, the rest of the rabbits were euthanized and corneas processed to determine nerve density as explained below.
Figure 1.
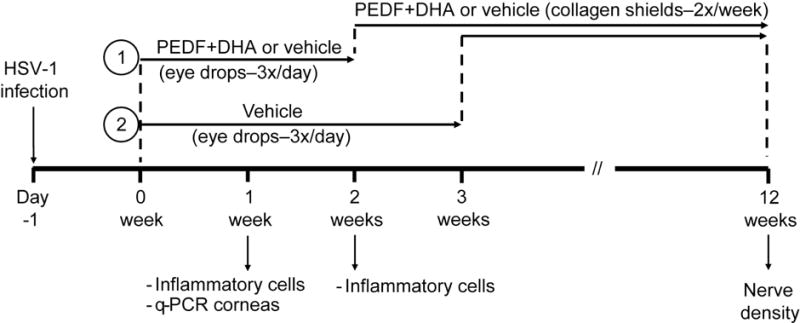
Time line of treatments and collection of samples after HSV-1 infection in rabbit corneas.
2.3 Slit-lamp examination and scoring
Corneal infection was monitored daily by an examiner masked to the condition of each rabbit with slit-lamp examination (SLE) from days 3 to 14 p.t., and then lesion size was recorded. After the first two weeks and resolution of the initial infection, SLE was performed to monitor corneal opacity and neovascularization. Corneal scoring criterion used for the assessment of lesion, opacity and neovascularization are described in Table 1.
Table 1.
Scoring criteria used for evaluation of corneal lesion size and corneal neovascularization.
| SLE scoring system for corneal lesion size | ||||||
|---|---|---|---|---|---|---|
| SLE score 0.0–0.5 | SLE score 0.6–0.9 | SLE score 1.0–1.9 | SLE score 2.0–2.9 | SLE score 3.0–3.5 | SLE score 3.6–4.0 | |
| Corneal Lesion Scoring | Normal or nonspecific corneal lesions, random superficial lesion | Punctate ulcerations | One or more dendritic ulcerations; Dendritic lesions involving <50% of cornea | Dendritic lesions involving >50% of the cornea | Geographic ulcerations of trophic erosion involving less than 50% of the cornea | Geographic ulcerations of trophic erosion involving more than 50% of the cornea |
| Grading of Corneal Neovascularization | ||||
|---|---|---|---|---|
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | |
| Corneal Neovascularization | No blood vessels visible | <25% of the corneal surface covered with developing network of blood vessels | 26–75% of the corneal surface covered with developing network of blood vessels | >75% of the corneal surface with developed network of blood vessels |
2.4 Corneal sensitivity
Sensitivity was measured once a week with a Cochet-Bonnet aesthesiometer (Laneau Ophthalmologie, Chartres Codex, France) as previously described (Chang-Lin et al., 1990; Cortina et al., 2012). Briefly, the length of the monofilament was changed from 6.0 to 0.5 cm in 0.5 cm fractions until the corneal touch threshold was found. The cornea was tested four times at each filament length. A negative response was considered when there was no blink response. A positive response was considered if the animal blinked 50% or more of the times stimulated. To improve accuracy and consistency, the number of times tested was increased from 4 to 10 when the filament length approached the threshold. If no blink response was obtained at monofilament length of 0.5 cm, corneal sensitivity was considered 0. The same examiner who was masked to the treatment conditions (JH) performed all the measurements.
2.5 Analysis of inflammatory cells
Rabbits were euthanized at 7 and 14 days p.t. Eyes were enucleated and corneas excised. The tissue was fixed with Zamboni’s fixative (American MasterTech Scientific, Lodi, CA) for 1 h at room temperature and then embedded in OCT compound (Sakura Finetek USA, Torrance, CA). Serial cryostat sections of 6μm were cut, mounted in microscope slides (Superfrost Plus, Fisher Scientific), air dried and stored at −20°C until use. For immunostaining, the sections were washed in PBS and blocked with 5% goat serum and 0.3% Triton X-100 in PBS for 30 min at room temperature, and then incubated overnight at 4°C with the following antibodies: anti-F4/80 rat monoclonal (Santa Cruz Biotechnology, Dallas, TX, sc-52664); anti-CD4 rat monoclonal (Santa Cruz Biotechnology, sc-13573); anti-CD8 mouse monoclonal (Santa Cruz Biotechnology, sc-1181); anti-CD11b rat monoclonal (Abcam, Cambridge, MA, ab-8878), all at a dilution of 1:100; and anti-neutrophil rat monoclonal (Abcam, ab-53457) at a dilution of 1:50. Afterwards, the sections were incubated with the corresponding secondary antibodies Alexa-Fluor 488 goat anti-rat or Alexa-Fluor 594 goat anti-mouse (Life Technologies, dilution 1:1000) for 1 h at room temperature. After each step, the sections were washed with PBS. DAPI (Sigma-Aldrich, St. Louis, MO) staining was performed to localize the nuclei. To exclude non-specific labeling, the primary antibodies were replaced by serum IgG of the same host origin, and no staining was shown. The specificity of the antibodies anti-CD4, anti-CD8 and anti-F4/80 was later confirmed by co-localization with anti-CD4 (BDPharmingen, USA), anti-CD8 (Kingfisher Biotech, Inc, MN) and anti-macrophage antibody [MAC387 from Abcam (Cambridge, MA)], all of which are known to be reactive in rabbits. The sections were examined with an Olympus IX71 fluorescent microscope equipped with a digital camera using the CellSens Dimension Imaging software (Olympus Corp, Tokyo, Japan) at ×200 magnification. Positive-stained cells were counted in a masked fashion (AK) from five to six randomly selected microscope fields per section of six sections/cornea and then averaged. Four to six corneas were analyzed per condition. The experiment was repeated four times.
2.6 Quantitative PCR
Seven days p.t., corneas from PEDF+DHA- and vehicle-treated rabbits (three rabbits each) were dissected, and total RNA was extracted using RNeasy Plus Mini kits (Qiagen). Purity and quantity of RNA was measured using a Nanodrop 1000 spectrophotometer (Thermo Scientific). Only samples with A260/A280 ratios higher than 1.8 were used for further analysis. One microgram of RNA was reverse transcribed using iScript Reverse Transcription Supermix (Bio Rad, Hercules, CA). For gene expression, cDNA was quantified using SsoAdvance Universal SYBER Green Supermix (Bio Rad). Details of the primers for 14 immune-related genes are described in Table 2 (Espino and Rivera, 2010; Schnupf and Sansonetti, 2012; Seol et al., 2011; Zucker et al., 2006). The oligonucleotide primers were synthetized by Eurofins MWG Operon LLC. Data was collected and analyzed using CFX Manager 3.0 software and the ∆∆Ct method. Values showing more than a 2-fold increase were considered significant.
Table 2.
Primer sequences for real-time PCR
| Target gene | Primers | Reference |
|---|---|---|
| CCL20 | 5′ – TATCGTGGGCTTCACACAGC – 3′ 5′-CCATTCCTTCTTCGGATCTGC-3′ |
[14] |
| IFN – γ | 5′ – TCCAACTATGGCACGGAAGTCT – 3′ 5′-TTCTGGAGCTGTTGTGGTTCCT-3′ |
[14] |
| IL – 18 | 5′ – ACCAAGGACAGCAACCTGTGTT – 3′ 5′-ACAGAGAGGCTTACAGCCATGC-3′ |
[14] |
| IL – 1β | 5′ – TTGAAGAAGAACCCGTCCTCTG – 3′ 5′-CTCATACGTGCCAGACAACACC-3′ |
[14] |
| IL – 2 | 5′ – TGCTGGATTTACAGGTGCTTTTGA – 3′ 5′-GGTATTTCCCCCATGAGAGTTTTTG-3′ |
[15] |
| IL – 4 | 5′ – AGAGCTCGGTGACCTCAGAC – 3′ 5′-CTTGCATGGCGGTCTTTAG-3′ |
[14] |
| IL – 6 | 5′ – CTACCGCTTTCCCCACTTCAG – 3′ 5′-TCCTCAGCTCCTTGATGGTCTC-3′ |
[14] |
| IL – 8 | 5′ – CCACACCTTTCCATCCCAAAT – 3′ 5′-CTTCTGCACCCACTTTTCCTTG-3′ |
[14] |
| IL – 10 | 5′ – CTTTGGCAGGGTGAAGACTTTC – 3′ 5′-AACTGGATCATCTCCGACAAGG-3′ |
[14] |
| IL – 12B | 5′ – CTCCGAAGAAGATGGCATTACC – 3′ 5′- TCTCCTTTGTGGCAGGTGTATTG-3′ |
[14] |
| IL – 17A | 5′ – CCAGCAAGAGATCCTGGTCCTA – 3′ 5′- ATGGATGATGGGGGTTACACAG-3′ |
[14] |
| IL – 17F | 5′ – AAAATCCCAAAGTGGAGGATGC – 3′ 5′- AGCGGTTCTGGAAGTCATGTGT-3′ |
[14] |
| PDGF | 5′ – GACTACCTGCACCGCAACA – 3′ 5′- CATGTAGCCGCCGTCACT-3′ |
[15] |
| TNF – α | 5′ – CTCCTACCCGAACAAGGTCA – 3′ 5′- CGGTCACCCTTCTCCAACT-3′ |
[16] |
| GAPDH | 5′ – GAATCCACTGGCGTCTTCAC – 3′ 5′-CGTTGCTGACAATCTTGAGAGA-3′ |
[16] |
| Ywhaz | 5′ – GGTCTGGCCCTTAACTTCTCTGTGTTCTA – 3′ 5′-GCGTGCTGTCTTTGTATGATTCTTCACTT-3′ |
[17] |
The genes in bold changed >2 with PEDF+DHA treatment. The last two genes (in italic) were used as house-keeping genes
2.7 Immunochemistry and imaging of corneal nerves
Rabbits were euthanized at 12 weeks p.t. and whole corneas were excised and fixed with 2% fresh paraformaldehyde in 0.01M phosphate buffer, pH 7.4 for 2 hours at room temperature or overnight at 4 °C. After gradient dehydration in 10%, 15%, and 20% sucrose in 0.01M phosphate-buffered saline (PBS) for 2 h each, the whole corneas were kept in a 24-well plate (one cornea per well) and incubated with 10% normal goat serum plus 0.3% Triton X-100 in PBS for 1 h at room temperature to block non-specific binding. This was followed by incubation with the mouse monoclonal anti-βIII-tubulin (Tuj1, MMS-435p, Covance Antibody Services Inc., Berkeley, CA; 1:1000) in PBS containing 1.5% normal goat serum plus 0.1% Triton X-100 for 72 h at 4°C. After thorough washing with PBS-BSA (4 × 15 minutes), the corneas were incubated with the secondary antibody Alexa fluor 488 goat anti-mouse Ig G (1: 1500) for 24 h at 4°C and washed again with PBS-BSA. To exclude non-specific labeling, the primary antibody was replaced by serum IgG of the same host species as the primary antibody. In controls without primary antibodies, there were no stains (data not shown). Images of the wholemount were acquired with the fluorescent microscope as previously described (Cortina et al., 2010; Cortina et al., 2012; He et al., 2010). The βIII-tubulin-positive tissue nerve area at the sub- epithelial level was calculated and compared with the total area using an image analysis program (Image Pro Plus 4.5; Cybernetics, Inc., Silver Spring, MD). Five images/cornea were recorded (one for each quadrant and one from the center), and the images from the two eyes of each rabbit were averaged.
2.8 Data analysis
Between 20 and 44 rabbits were used in each experiment. A power analysis with more than 90% confidence determined the number of animals needed in each experiment, taking into account that 40–50% of the rabbits would develop encephalitis and would need to be euthanized before the end of the experiment. Unless otherwise specified, there were two repetitions of each experiment. Investigators performing sensitivity measures, recording corneal lesions, opacity and neovascularization scores and analyzing images for nerve density and inflammatory cells were masked to the condition of each animal. Data were expressed as means ± SD. Statistical comparisons between PEDF+DHA- and vehicle-treated groups were performed using Student’s test or one or two way ANOVA as indicated in the figures legends. P values <0.05 were considered significant.
3. Results
3.1 Rabbits treated with PEDF+DHA develop less prominent epithelial keratitis and reduction in neovascularization
Rabbits were treated topically with PEDF+DHA or with vehicle three times a day starting at day 1 post infection (p.i.) with HSV-1 (see Figure 1). Corneal lesions were monitored by SLE daily from day 3 to day 14 and scored as detailed in Table 1. An initial dendritic corneal lesion is typical of HSV-1 primary infection in this rabbit model and is present up to 14–17 days p.i. The dendritic lesion is caused by disruption of epithelial cell junctions due to viral replication (Carr et al., 2001). At 14 days p.i. there was a modest but significant effect of PEDF+DHA in mitigating epithelial keratitis caused by acute HSV-1 corneal infection. The characteristic lesions formed in the PEDF+DHA group averaged a score of 0.9±0.1, consistent with mild keratitis in the form of punctuated epithelial ulceration, while the vehicle-treated group reached scores of 1.5±0.2, (p<0.05, n=22 rabbits in each group), corresponding to well-formed dendritic lesions affecting 50% of the cornea.
After 14 days, a 72-hour collagen shield soaked in PEDF+DHA or vehicle was applied. Collagen shields were changed twice a week for 10 weeks (see Figure 1). The degree of corneal neovascularization was graded as shown in Table 1 and recorded for each rabbit. There was a decrease of corneal neovascularization in rabbits treated with PEDF+DHA after 60 days p.t. 1±0.1 vs 1.5±0.3 in vehicle, p<0.05, n=8 rabbits. These differences can be observed in the slit lamp photo taken at 60 days shown in Figure 2.
Figure 2.
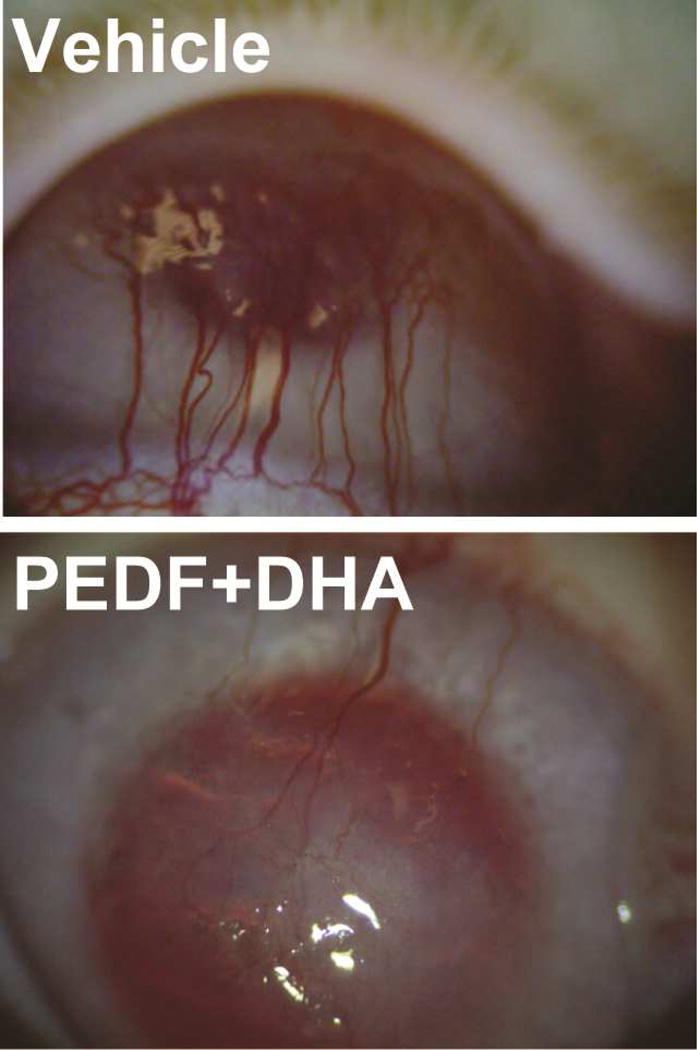
Representative images show the extent of the neovascularization in vehicle and treated rabbits at 60 days p.t. The vehicle-treated cornea depict aggressive neovascularization. The PEDF+DHA-treated cornea, show less than 25% of the corneal surface with blood vessels.
3.2 Effect of PEDF+DHA on the corneal inflammatory response to HSV infection
To study whether PEDF+DHA has an immunomodulatory effect, rabbit corneas infected with HSV-1 were processed for immunohistochemistry and evaluated for the presence of inflammatory cells at 7 and 14 days p.t. At 7 days p.t., there was significant infiltration of inflammatory cells in the cornea in both groups (Figure 3A). All markers tested, including anti-neutrophil, CD11b+, F4/80, CD4+ and CD8+, suggest the presence of a variety of cell types, such as neutrophils, macrophages and T-lymphocytes. Surprisingly, at 7 days p.t., cell infiltration was significantly increased in corneas treated with PEDF+DHA compared to the vehicle-treated group. The experiment was repeated four times with similar results. By 14 days the effect was reverted, with a significant decrease in the number of positive cells for all the markers tested in the group treated with PEDF+DHA compared to the vehicle-treated group, suggesting an accelerated resolution of the inflammatory response in treated animals (Figure 3B).
Figure 3.
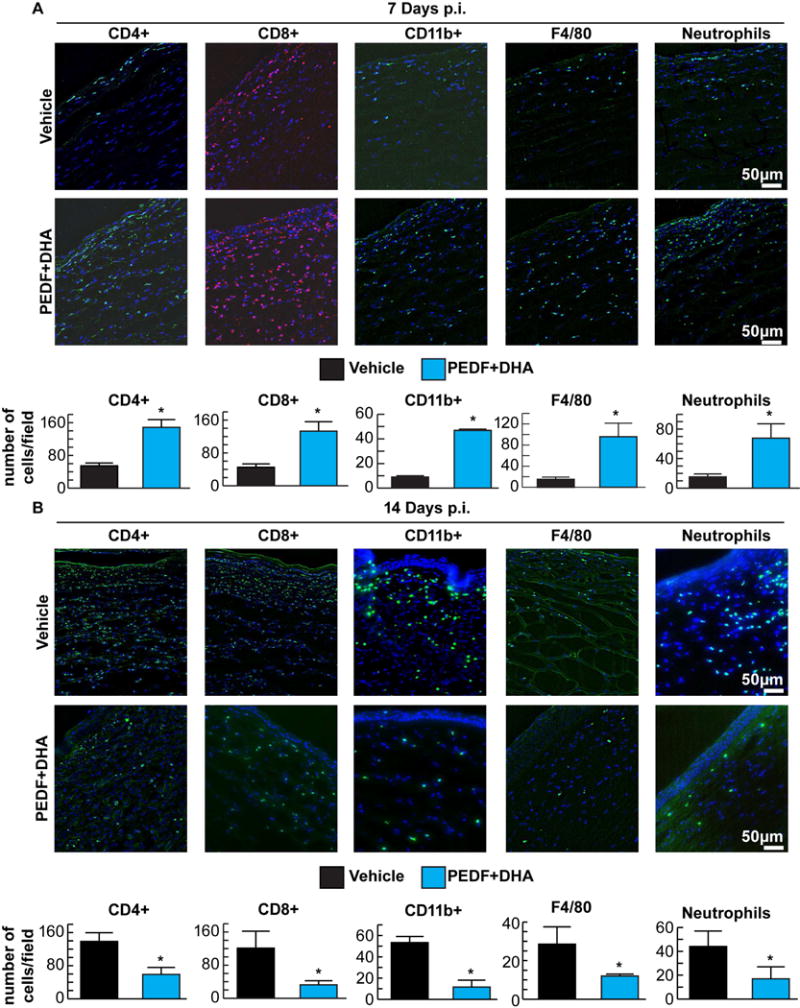
Rabbit corneas were stained with CD4+, CD8+, CD11b+, F4/80 and anti-neutrophil antibodies. The representative images show inflammatory cells in corneal sections at 7 (A) and 14 (B) days p.i. DAPI was used to stain the nuclei (blue). Positive cells from six corneal sections from each different cornea/condition were counted in five to six randomly selected fields in a blind fashion. Values are average of six corneas ± SD. The experiment was done four times with similar results. * Significant differences were analyzed by Student’s t-test (p<0.05).
3.3 PEDF+DHA modulates expression of cytokines and chemokines in the cornea
Due to the increase of the inflammatory-immune response in the cornea at 7 days p.t. with PEDF+DHA, we tested levels of 14 immune-related genes (using the primers described in Table 2) in corneas by real time PCR. Three genes (IFN-γ, IL-18 and CCL20) showed significant differences (≥2) between rabbits treated with PEDF+DHA and vehicle. One week after infection and treatment, the gene expression of IFN-γ and CCL20 were an average of 3.3- and 2.5-fold higher in the corneas treated with PEDF+DHA with respect to the vehicle-treated corneas. However, levels of IL-18 were completely inhibited by PEDF+DHA in comparison to vehicle-treated corneas (Figure 4).
Figure 4.
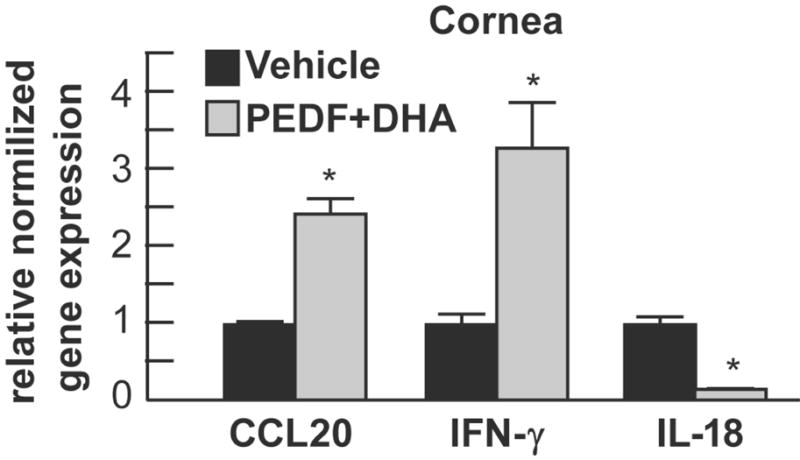
Levels of IFN-γ, CCL20 and IL-18 were assessed by real-time PCR in total RNA isolated from cornea of rabbits after 7 days p.t. Corneas from three rabbits treated with vehicle or with PEDF+DHA were used. The values are expressed as increase/decrease with respect to vehicle-treated rabbits and are average ± SD, * p<0.05. The experiment was repeated once with similar results.
3.5 PEDF+DHA treatment promotes recovery of corneal sensation and stimulates nerve regeneration
Corneal sensitivity measurements were performed weekly using the Cochet–Bonnet aesthesiometer. The three groups showed a complete absence of corneal sensation at 1 and 2 weeks p.i. (Figure 5A). The vehicle-treated group did not show evidence of corneal sensation in any of the time points measured from the beginning to the conclusion of the study. The group treated with PEDF+DHA began showing evidence of corneal sensation at the three-week mark with a slow but steady linear improvement in sensitivity throughout the duration of the study. At 12 weeks p.t., sensitivity was recorded as 1.8±0.3 (n=12 rabbits); this corresponds to a 48% recovery of the sensitivity in non-injured rabbits (3.7±0.3, n=8 rabbits) (Cortina et al., 2012). The group in which the treatment was delayed for 3 weeks did not show evidence of corneal sensation until 6 weeks p.i (3 weeks of treatment); thereafter, this group showed significant improvement in corneal sensation compared to the vehicle treated group (Fig 5A). At 12 weeks after HSV-1 infection and 9 weeks of treatment the sensitivity was 1.1±0.2 corresponding to 30% recovery of normal rabbit corneal sensation. Comparison between immediately and delayed PEDF+DHA treatment at 3 and 9 weeks (indicated by a and b in Fig 5A) shows there was higher sensitivity measurements at 3 weeks in corneas that were early treated, 0.8±0.2 vs 0.4±0.2, p<0.05. However, at 9 weeks, there was no significant difference between corneas treated immediately (1.2±0.5) or delayed (1.1±0.3).
Figure 5.
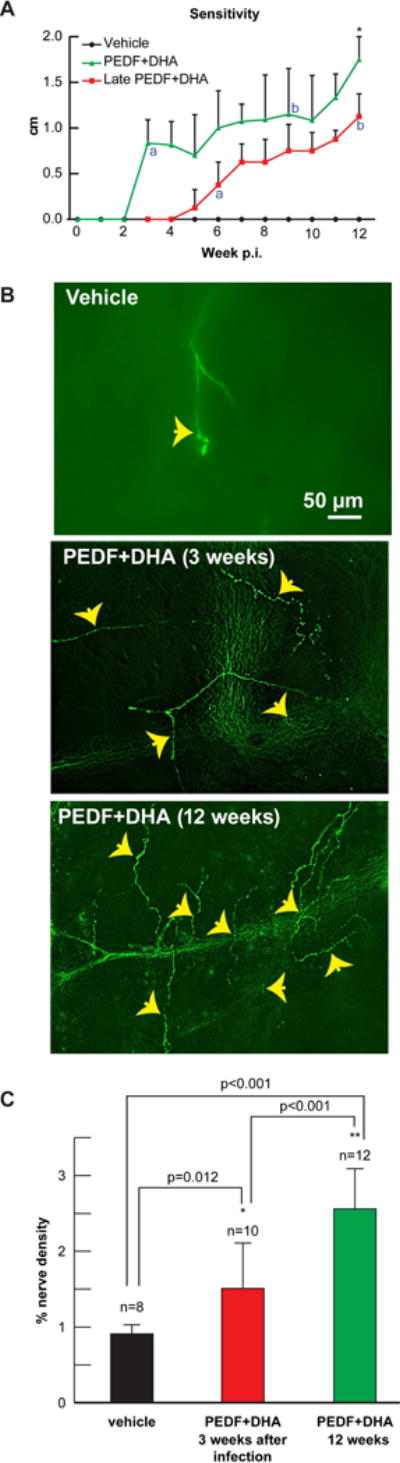
A) Central corneal sensitivity was measured with the Cochet-Bonnet aesthesiometer once a week starting at 1-week p.t. The measurements were performed by the same investigator (JH) masked to the treatment condition. Values correspond to 8–12 rabbits in each condition. Significant increase respect to vehicle was found in the PEDF+DHA-treated group from 3 to 12 weeks and in the 3 weeks p.i. PEDF+DHA treated group from 6 to 12 weeks, p<0.05 analyzed by two-way ANOVA and Tukey post hoc comparisons B) Immunocytochemistry of sub-basal nerve plexus of rabbit corneal wholemounts stained with anti-βIII tubulin antibody 12 weeks p.t. Representative images of each treatment group are shown. Top image of vehicle rabbit cornea shows a paucity of corneal nerves with very little evidence of regeneration activity. Central image depicts PEDF+DHA treated cornea in which treatment was administered from p.i. week 3 to 12. Bottom image shows rabbit cornea that received full 12 weeks of treatment with PEDF+DHA. Note the numerous newly regenerated sup-epithelial branches (arrows). C) Quantification of βIII tubulin sub-epithelial nerves in vehicle-and PEDF+DHA-treated groups. Five images/cornea were analyzed by one-way ANOVA and Tukey post hoc comparisons n = number of corneas analyzed. The experiment was repeated twice with similar results.
Corneal nerves stained with anti-βIII tubulin were quantified from rabbit corneal wholemounts at 12 weeks after initial infection with HSV-1. The vehicle-treated group showed severe damage to the sub-basal plexus (Figure 5B); a very small number of nerves were found, as well as little evidence of sprouting and regeneration. Recent studies have shown that the Syn+ virus produces serious nerve damage that lasts more than four months p.i. without reactivation (He et al., 2016). Compared to the vehicle-treated group, the two groups treated with PEDF+DHA showed a significant increase in nerve density. The group in which treatment was deferred until three weeks and carried out until week 12, however, showed reduced nerve density compared to the group treated for the full 12 weeks; 65% increase in the group treated for 9 weeks and 181% increase in the group treated for 12 weeks compared to the vehicle-treated group. The percentage of nerve density after 12 weeks with PEDF+DHA treatment was 2.6±0.5 (n=12 corneas corresponding to a 16% nerve recovery compare to nerve density in non-injured rabbit corneas (17±2.4, n=6 corneas) (Cortina et al., 2012).
4. Discussion
HSV-1 is one of the predominant viral infections in the human cornea that can cause vision loss (Liesegang, 2001). HSV-1 stromal keratitis represents an immunopathological process that involves damage to the cornea by inflammatory cells, causing tissue loss, scarring and neovascularization (Carr et al., 2001; Wickham and Carr, 2004). One of the most characteristic manifestations of the disease in humans is the alteration in corneal sensitivity, and clinical and experimental studies have reported decreased corneal innervation, aberrant nerve regeneration and persistent hypoesthesia after HSV-1 keratitis (Chucair-Elliott et al., 2015; Hamrah et al., 2010). Recently, we reported that rabbit corneas infected with the HSV-1 strain used in these experiments show a dramatic decrease in corneal nerve density that reaches an 88% loss of nerve density with respect to healthy rabbit corneas after four months of primary infection without reactivation (He et al., 2016). In the present study, we show a very significant increase in nerve regeneration by 12 weeks of PEDF+DHA treatment concomitant with a recovery of sensitivity equivalent to nearly 50% of the sensitivity observed in healthy rabbits (Cortina et al., 2012). Moreover, delaying the treatment for three weeks also stimulated corneal sensitivity and regeneration of nerves in a significant way suggesting that PEDF+DHA treatment could have a therapeutic use in patients that loose sensitivity and delay treatment. A recent study in mice has shown that in HSV-1-infected corneas there is retraction of sensory corneal nerves that are replaced by sympathetic nerves and increase in opacity is observed (Yun et al, 2016). The authors propose that sympathetic nerves will promote persistent inflammation. Although we have not studied this phenomenon in the rabbit, the increase in sensitivity after PEDF+DHA treatment and the increase in CGRP positive sensory nerves observed in our previous studies using a lamellar keratectomy model (Cortina et al, 2012, He et al, 2015) make us confident that sensory nerves are regenerated with the treatment.
Corneal nerves are important to maintain epithelial integrity, and their damage can impair wound healing, causing chronic epithelial defects and neurotrophic ulcers that are difficult to heal and can lead to permanent corneal opacity and even perforation (Beyer et al., 1990; Bonini et al., 2003; Davis and Dohlman, 2001; Lambiase et al., 1999; Pushker et al., 2001). When corneal transplantation is required in the setting of severe neurotrophic keratitis, the prognosis can be poor (Elbaz et al., 2014). In addition, available antiviral treatments for HSV keratitis do not stimulate the recovery of corneal sensation.
Mounting evidence suggests that there is a close interaction between neuroregenerative and inflammatory pathways in the cornea (Cruzat et al., 2011; Namavari et al., 2012a; Namavari et al., 2012b; Shaheen et al., 2014). In HSV-1 keratitis, it is thought that nerve damage is secondary to the virus’ neurotropism, but it is unclear yet whether it is directly caused by the virus or if it is a consequence of the immune response to the infection (Yun et al., 2014). Our study shows an interesting biphasic effect of the treatment in the immune-inflammatory response with increased infiltration of leukocytes, including monocytes, macrophages and neutrophils in the cornea at one week p.t. that was significantly decreased by two weeks. The results are in contrast to our previous studies using a rabbit model of refractive surgery in which PEDF+DHA reduce infiltration of neutrophils and CD11b+cells after two days of treatment (Cortina et al., 2013; He et al., 2015). The enhanced immune-inflammatory response observed in HSV-1-infected corneas at one week p.t. may aid with viral clearance, while resolution of inflammation at two weeks may result in less corneal damage.
While inflammation is essential for protection against pathogenic microorganisms, including viruses like HSV-1, an excessive and non-resolving inflammatory response is damaging to the host, as is the case in HSV-1 stromal keratitis. Out of all the responders to HSV-1 infection, monocytes have been associated with initial viral clearance, but neutrophils appear to be a surrogate for viral replication and tissue damage, suggesting a tight regulation of the inflammatory response is key in the treatment of this disease (Royer et al., 2015; Yun et al., 2014). In addition, Yun et al. (2014) has shown that corneal infiltration with CD4+ lymphocytes can negatively impact the process of nerve regeneration and recovery of sensitivity after HSV infection, supporting the concept that it is indeed persistent inflammation that may lead to permanent nerve damage. It also has been reported that in the absence of CD11b+ cells, axons failed to regenerate after spinal cord injury (Barrette et al., 2008). In our study, CD4+ and CD11b+ cells were increased at 7 days p.t. but were significantly decreased by 14 days in the PEDF+DHA-treated group, supporting our hypothesis that this biphasic response, which shows a faster resolution of inflammation in the PEDF+DHA-treated group, also may partially explain the increased nerve regeneration and recovery of sensitivity we observed. Recent work has shown that cornea-graft rejection in a mouse model of HSV-1 keratitis depends on the presence of CD4+ T cells (Kuffova et al., 2016).
Our previous studies using a model of refractive surgery that damages the nerves at the stromal level have shown that the docosanoid neuroprotection D1 (NPD1) is synthetized in the cornea after treatment with PEDF+DHA and topical treatment with NPD1 increases nerve regeneration (Cortina et al., 2010; Cortina and Bazan, 2011; Cortina et al., 2013; Kenchegowda et al., 2013). Treatment with PEDF or DHA alone does not have a significant effect in corneal nerve regeneration (Cortina et al., 2010), demonstrating that the combination is necessary to synthetize NPD1 as well as possible other docosanoids. NPD1 is produced in tissues, such as the cornea, on demand after injury as a pro-resolving component to reduce inflammation (Hong et al., 2014; Schwab et al., 2007; Serhan et al., 2015). In a mouse model of influenza, NPD1 was shown to decrease viral replication, while in a human model of severe influenza, levels were inversely correlated with disease severity (Morita et al., 2013). Moreover, NPD1 and another pro-resolving lipid mediator, the eicosapentaenoic acid (EPA)-derived resolvin E1 (RvE1), were shown to reduce the severity of stromal keratitis lesions in a mouse model of HSV (Rajasagi et al., 2011; Rajasagi et al., 2013). We believe that the pro-resolving activity of NPD1 and other lipid mediators generated from DHA (Serhan et al., 2015) can play a role in supporting nerve regeneration and reducing the severity of stromal keratitis in rabbits that receive treatment. Furthermore, both RvE1 and NPD1 have been shown to promote phagocyte removal during acute inflammation via regulating leukocyte infiltration and increasing macrophage ingestion of apoptotic PMNs in vivo and in vitro (Schwab et al., 2007).
PEDF is a 50-kDa multifaceted glycoprotein that has anti-angiogenic and neurotrophic properties and is widely expressed in the eye, including the cornea (Barnstable and Tombran-Tink, 2004; Becerra, 2008; Tombran-Tink and Barnstable, 2003). It appears to act through different receptors, including a PEDF receptor (PEDF-R), which has been shown to interact with the N-terminal portion of the PEDF molecule, mediating its neurotrophic effect and neuronal differentiating activity (Kenealey et al., 2015; Notari et al., 2006; Subramanian et al., 2010). Although the mechanism by which PEDF+DHA promotes nerve regeneration in the rabbit is not completely understood, we have previously found that corneal epithelial cells express a PEDF-R linked to phospholipase activity and that the peptide 44-mer-PEDF (which has neurotrophic properties), in conjunction with DHA, also increases corneal nerve regeneration (He et al., 2015).
In the present study, there was also a decrease in corneal neovascularization that could be due to the antiangiogenic portion of the glycoprotein, the 34-mer-PEDF (Barnstable and Tombran-Tink, 2004; Becerra, 2008; Kenealey et al., 2015). It has been shown that PEDF blocks the survival, proliferation and migration of vascular endothelial cells by disrupting the pro-angiogenic action of vascular endothelial factor (VEGF), suppressing the expression of metalloproteinases (MMPs)-2 and-9 and upregulating Fas/Fas ligand (Haurigot et al., 2012; Matsui et al., 2012, Volpert et al, 2002). Accordingly, the use of the entire PEDF molecule will be more suitable for treatment of HSV-1 infection.
To further investigate the early inflammatory response at one week p.t., we studied whether PEDF+DHA treatment may affect specific cytokines and chemokines in the cornea. Three cytokines previously reported to be involved in the responses after HSV-1 infection (IFN-γ, IL-18 and CCL20) changed their expression when animals were treated with PEDF+DHA. We found a significant increase in gene expression of IFN-γ in the cornea of rabbits treated for seven days. Early studies have shown that IFN-γ has a protective effect during infection (Austin et al., 2007; He et al., 1999; Minami et al., 2002). It has been reported that in mice, IFN-γ reduces replication of the HSV-1 virus in the TG by a mechanism that involves CD8+T cells and reduction of infected early protein 0 (ICP0) expression. We observed a significant increase of these cells in the cornea at 7 days p.t., and recent studies show that rabbits infected with a HSV-1 mutant with ICP0 deletion significantly attenuates the nerve damage induced by the virus (Decman et al., 2005; He et al., 2016). PEDF+DHA may prevent corneal viral proliferation through the upregulation of IFN-γ expression in vivo and contribute to its protective functions.
CCL20 was also increased after PEDF+DHA treatment. This chemokine is produced in the corneal epithelial cells and in stromal keratocytes after HSV-1 infection (Shirane et al., 2004), and it attracts immature dendritic cells and T cells that are important in promoting epithelial wound healing (Li et al., 2011). Our results suggest that this enhanced expression of CCL20 resulted in the higher infiltration of immune cells in the PEDF+DHA-treated corneas and the decrease in corneal lesions at p.t. day 7. On the other hand, PEDF+DHA inhibited the expression of IL-18 in the cornea, a pro-inflammatory cytokine that has been reported to increase HSV-1 production in infected T cells (Shapiro et al., 1998). In addition, IL-18 is one of the key pro-inflammatory factors in the inflammasome (Strowing et al., 2012).
In summary, we believe that modulation of the immune-inflammatory response by PEDF+DHA treatment is likely to play an important role in promoting recovery of corneal sensation after HSV-1 infection. Targeting the pro-resolving pathways seems like a logical approach to treating infections like HSV keratitis, in which host inflammation plays a key role in tissue damage. In addition, the anti-angiogenic action of PEDF reduces corneal vascularization. Our findings suggest that PEDF in combination with DHA may constitute an effective adjunctive treatment to antivirals with the potential to reduce long-term sequelae of HSV-1 keratitis.
PEDF+DHA treatment to rabbit corneas with acute HSV-1 infection restores sensitivity
The treatment regenerates corneal nerves highly damage by HSV-1 infection
There is a biphasic immune-inflammatory reaction
As a result there is decreased corneal lesions, opacity and neovascularization in treated rabbits
Acknowledgments
Funding
We acknowledge support from NIH/NEI grant R01 EY019465 (to HEPB) and a grant from the Research to Prevent Blindness (to the LSU Eye Center). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. The content of this manuscript is new and solely the responsibility of the authors and do not necessarily represent the official views of the granting agencies.
Supported by NIH/NEI grant R01 EY019465 (to HEPB) and a grant from the Research to Prevent Blindness (to the LSU Eye Center).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
AK and TLP contributed to acquisition and analysis of data and reviewed the manuscript. FM helped with the examination of the animals using the slit lamp and analysis of data. MSC contributed to the clinical view of HSV-1 infection, analysis of data and drafting the manuscript. HEPB designed and supervised the study, wrote and reviewed the manuscript, and is responsible for the integrity of the present work.
Disclosure: J. He, None; D. Neumann, None; A. Kakazu, None; T.L. Pham, None; F. Musarrat, None; M.S. Cortina, None; H.E.P. Bazan, None
References
- Austin BA, Halford WP, William BRG, Carr DJJ. Oligoadenylate synthetase/protein kinase R pathways and αβ TCR+Tcells are required for adenovirus Vector:IFN-γ inhibition of herpes simplex virus -1 in cornea. J Immunol. 2007;178:516. doi: 10.4049/jimmunol.178.8.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnstable CJ, Tombran-Tink J. Neuroprotective and antiangiogenic actions of PEDF in the eye: molecular targets and therapeutic potential. Prog Retin Eye Res. 2004;23:561–577. doi: 10.1016/j.preteyeres.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Barrette B, Hébert MA, Filali M, Lafortune K, Vallières N, Gowing G, Julien JP, Lacroix S. Requirement of myeloid cells for axon regeneration. J Neurosci. 2008;28:9363–9376. doi: 10.1523/JNEUROSCI.1447-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra SP. Focus on Molecules: Pigment epithelium-derived factor (PEDF) Exp Eye Res. 2008;82:739–740. doi: 10.1016/j.exer.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Beyer CF, Hill JM, Reidy JJ, Beuerman RW. Corneal nerve disruption reactivates virus in rabbits latently infected with HSV-1. Invest Ophthalmol Vis Sci. 1990;31:925–932. [PubMed] [Google Scholar]
- Bonini S, Rama P, Olzi D, Lambiase A. Neurotrophic keratitis Eye. 2003;17:989–995. doi: 10.1038/sj.eye.6700616. [DOI] [PubMed] [Google Scholar]
- Carr DJ, Harle P, Gebhardt BM. The immune response to ocular herpes simplex virus type 1 infection. Exp Biol Med (Maywood) 2001;226:353–366. doi: 10.1177/153537020122600501. [DOI] [PubMed] [Google Scholar]
- Chang-Lin T, Vannas A, Holden BA, O’Leary DJ. Incision depth affects the recovery of corneal sensitivity and neural regeneration in the cat. Invest Ophthalmol Vis Sci. 1990;31:1533–1541. [PubMed] [Google Scholar]
- Chucair-Elliott AJ, Zheng M, Carr DJ. Degeneration and regeneration of corneal nerves in response to HSV-1 infection. Invest Ophthalmol Vis Sci. 2015;56:1097–1107. doi: 10.1167/iovs.14-15596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortina MS, He J, Li N, Bazan NG, Bazan HEP. Neuroprotectin D1 synthesis and corneal nerve regeneration after experimental surgery and treatment with PEDF plus DHA. Invest Ophthalmol Vis Sci. 2010;51:804–810. doi: 10.1167/iovs.09-3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortina MS, Bazan HE. Docosahexaenoic acid, protectins and dry eye. Curr Opin Clin Nutr Metab Care. 2011;14:132–137. doi: 10.1097/MCO.0b013e328342bb1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortina MS, He J, Li N, Bazan NG, Bazan HEP. Recovery of corneal sensitivity, calcitonin gene related peptide-positive nerves and increased wound healing induced by PEDF plus DHA after experimental surgery. Arch Ophthalmol. 2012;130:76–83. doi: 10.1001/archophthalmol.2011.287. [DOI] [PubMed] [Google Scholar]
- Cortina MS, He J, Russ T, Bazan NG, Bazan HE. Neuroprotectin D1 restores corneal nerve integrity and function after damage from experimental surgery. Invest Ophthalmol Vis Sci. 2013;54:4109–4116. doi: 10.1167/iovs.13-12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruzat A, Witkin D, Baniasadi N, Zheng L, Ciolino JB, Jurkunas UV, Chodosh J, Pavan-Langston D, Dana R, Hamrah P. Inflammation and the nervous system: the connection in the cornea in patients with infectious keratitis. Invest Ophthalmol Vis Sci. 2011;52:5136–5143. doi: 10.1167/iovs.10-7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EA, Dohlman CH. Neurotrophic keratitis. Int Ophthalmol Clin. 2001;41:1–11. doi: 10.1097/00004397-200101000-00003. [DOI] [PubMed] [Google Scholar]
- Decman V, Kinchington PR, Harvey SA, Hendricks RL. Gamma interferon can block herpes simplex virus reactivation from latency, even in the presence of late gene expression. J Virol. 2005;79:10339–10347. doi: 10.1128/JVI.79.16.10339-10347.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaz U, Bains R, Zuker RM, Borschel GH, Ali A. Restoration of corneal sensation with regional nerve transfers and nerve grafts: a new approach to a difficult problem. JAMA Ophthalmol. 2014;132:1289–1295. doi: 10.1001/jamaophthalmol.2014.2316. [DOI] [PubMed] [Google Scholar]
- Espino AM, Rivera F. Quantitation of cytokine mRNA by real time RT-PCR during a vaccination trial in a rabbit model of fascioliasis. Vet Parasitol. 2010;169:82–92. doi: 10.1016/j.vetpar.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamrah P, Cruzat A, Dastjerdi MH, Zheng L, Shahatit BM, Bayhan HA, Dana R, Pavan-Langston D. Corneal sensation and subbasal nerve alterations in patients with herpes simplex keratitis: an in vivo confocal microscopy study. Ophthalmology. 2010;117:1930–1936. doi: 10.1016/j.ophtha.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haurigot V, Villacampa P, Ribera A, Bosch A, Ramos D, Ruberte J, Bosch F. Long term PEDF overexpression prevents neovascularization in a murine adult model of retinopathy. PLoS One. 2012;7:e41511. doi: 10.1371/journal.pone.0041511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Ichimura H, Iida T, Minami M, Kobayashi K, Kita M, Sotozono C, Tagawa YI, Iwakura Y, Imanishi J. Kinetics of cytokine production in the cornea and trigeminal ganglion of C57BL/6 mice after corneal HSV-1 infection. J Interf Cytokine Res. 1999;19:609–615. doi: 10.1089/107999099313749. [DOI] [PubMed] [Google Scholar]
- He J, Bazan NG, Bazan HE. Mapping the entire human corneal nerve architecture. Exp Eye Res. 2010;91:513–523. doi: 10.1016/j.exer.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Cortina MS, Kakazu A, Bazan HE. The PEDF neuroprotective domain plus DHA induces corneal nerve regeneration after experimental surgery. Invest Ophthalmol Vis Sci. 2015;56:3505–3513. doi: 10.1167/iovs.15-16755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Cosby R, Hill JM, Bazan HEP. Changes in corneal innervation after HSV-1 latency established with different reactivation phenotypes. Curr Eye Res. 2016;17:1–6. doi: 10.3109/02713683.2016.1167919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Tian H, Lu Y, Laborde JM, Muhale FA, Wang Q, Alapure BV, Serhan CN, Bazan NG. Neuroprotectin/protectin D1: endogenous biosynthesis and actions on diabetic macrophages in promoting wound healing and innervation impaired by diabetes. Am J Physiol Cell Physiol. 2014;307:C1058–C1067. doi: 10.1152/ajpcell.00270.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenchegowda S, He J, Bazan HE. Involvement of pigment epithelium-derived factor, docosahexaenoic acid and neuroprotectin D1 in corneal inflammation and nerve integrity after refractive surgery. Prostaglandins Leukot Essent Fatty Acids. 2013;88:27–31. doi: 10.1016/j.plefa.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenealey J, Subramanian P, Comitato A, Bullock J, Keehan L, Polato F, Hoover D, Marigo V, Becerra SP. Small retinoprotective peptides reveal a receptor-binding region on pigment epithelium-derived factor. J Biol Chem. 2015;290:25241–25253. doi: 10.1074/jbc.M115.645846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffova L, Knickelbein JE, Yu T, Medina C, Amescua G, Rowe AM, Hendricks RL, Forrester JV. High-risk corneal graft rejection in the setting of previous corneal herpes simplex virus (HSV)-1 infection. Invest Ophthalmol Vis Sci. 2016;57:1578–1587. doi: 10.1167/iovs.15-17894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambiase A, Rama P, Aloe L, Bonini S. Management of neurotrophic keratopathy. Curr Opin Ophthalmol. 1999;10:270–276. doi: 10.1097/00055735-199908000-00009. [DOI] [PubMed] [Google Scholar]
- Li Z, Burns AR, Miller SB, Smith CW. CCL20, γδ Tcells, and IL-22 in corneal epithelial healing. FASEB J. 2011;25:2659–2668. doi: 10.1096/fj.11-184804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001;20:1–13. doi: 10.1097/00003226-200101000-00001. [DOI] [PubMed] [Google Scholar]
- Marfurt CF, Cox J, Deek S, Dvorscak L. Anatomy of the human corneal innervation. Exp Eye Res. 2010;90:478–492. doi: 10.1016/j.exer.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Matsui T, Nishino Y, Maeda S, Yamagishi S. PEDF-derived peptide inhibits corneal angiogenesis by suppressing VEGF expression. Microvasc Res. 2012;84:105–108. doi: 10.1016/j.mvr.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Miller CS, Danaher RJ, Jacob RJ. Molecular aspects of herpes simplex virus I latency, reactivation, and recurrence. Crit Rev Oral Biol Med. 1998;9:541–562. doi: 10.1177/10454411980090040901. [DOI] [PubMed] [Google Scholar]
- Minami M, Kita M, Yan XQ, Yamamoto T, Iida T, Sekikawa K, Iwakura Y, Imanishi J. Role of IFN-γ and tumor necrosis factor-α in herpes simplex virus type 1 infection. J Interf Cytok Res. 22:671–676. doi: 10.1089/10799900260100150. [DOI] [PubMed] [Google Scholar]
- Morita M, Kuba K, Ichikawa A, Nakayama M, Katahira J, Iwamoto R, Watanebe T, Sakabe S, Daidoji T, Nakamura S, Kadowaki A, Ohto T, Nakanishi H, Taguchi R, Nakaya T, Murakami M, Yoneda Y, Arai H, Kawaoka Y, Penninger JM, Arita M, Imai Y. The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell. 2013;153:112–125. doi: 10.1016/j.cell.2013.02.027. [DOI] [PubMed] [Google Scholar]
- Muller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76:521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- Namavari A, Chaudhary S, Chang JH, Yco L, Sonawane S, Khanolkar V, Yue BY, Sarkar J, Jain S. Cyclosporine immunomodulation retards regeneration of surgically transected corneal nerves. Invest Ophthalmol Vis Sci. 2012a;53:732–740. doi: 10.1167/iovs.11-8445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namavari A, Chaudhary S, Ozturk O, Chang JH, Yco L, Sonawane S, Katam N, Khanolkar V, Hallak J, Sarkar J, Jain S. Semaphorin 7a links nerve regeneration and inflammation in the cornea. Invest Ophthalmol Vis Sci. 2012b;53:4575–4585. doi: 10.1167/iovs.12-9760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida T, Yanai R. Advances in treatment for neurotrophic keratopathy. Curr Opin Ophthalmol. 2009;20:276–281. doi: 10.1097/icu.0b013e32832b758f. [DOI] [PubMed] [Google Scholar]
- Notari L, Baladron V, Aroca-Aguilar JD, Balko N, Heredia R, Meyer C, Notario PM, Saravanamuthu S, Nueda ML, Sanchez-Sanchez F, Escribano J, Laborda J, Becerra SP. Identification of a lipase-linked cell membrane receptor for pigment epithelium-derived factor. J Biol Chem. 2006;281:38022–38037. doi: 10.1074/jbc.M600353200. [DOI] [PubMed] [Google Scholar]
- Pushker N, Dada T, Vajpayee RB, Gupta V, Aggrawal T, Titiyal JS. Neurotrophic keratopathy. CLAO J. 2001;27:100–107. [PubMed] [Google Scholar]
- Rajasagi NK, Reddy PB, Suryawanshi A, Mulik S, Gjorstrup P, Rouse BT. Controlling herpes simplex virus-induced ocular inflammatory lesions with the lipid-derived mediator resolvin E1. J Immunol. 2011;186:1735–1746. doi: 10.4049/jimmunol.1003456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasagi NK, Reddt PB, Mulik S, Gjorstrup P, Rouse BT. Neuroprotectin D1 reduces the severity of herpes simplex virus-induced corneal immunopathology. Invest Ophthalmol Vis Sci. 2013;54:6269–6279. doi: 10.1167/iovs.13-12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer DJ, Zheng M, Conrady CD, Carr DJ. Granulocytes in Ocular HSV-1 Infection: Opposing Roles of Mast Cells and Neutrophils. Invest Ophthalmol Vis Sci. 2015;56:3763–3775. doi: 10.1167/iovs.15-16900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnupf P, Sansonetti PJ. Quantitative RT-PCR profiling of the rabbit immune response: assessment of acute Shigella flexneri infection. PLoS One. 2012;7:e36446. doi: 10.1371/journal.pone.0036446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Dalli J, Colas RA, Winkler JW, Chiang N. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim Biophys Acta. 2015;1851:397–413. doi: 10.1016/j.bbalip.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen BS, Bakir M, Jain S. Corneal nerves in health and disease. Surv Ophthalmol. 2014;59:263–285. doi: 10.1016/j.survophthal.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L, Puren AJ, Barton HA, Novick D, Peskind RL, Shenkar R, Gu Y, Su MS, Dinarello CA. Interlukin 18 stimulates HIV type 1 in monocytic cells. Proc Natl Acad Sci, USA. 1998;95:12550–12555. doi: 10.1073/pnas.95.21.12550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirane J, Nakayama T, Nagakubo D, Izawa D, Hieshima K, Shimomura Y, Yoshie O. Corneal epithelial cells and stromal keratocytes efficiently produce CC chemokine-ligand 20 (CCL20) and attract cells expressing its receptor CCR6 in mouse herpetic stromal keratitis. Curr Eye Res. 2004;28:297–306. doi: 10.1076/ceyr.28.5.297.28682. [DOI] [PubMed] [Google Scholar]
- Seol D, Choe H, Zheng H, Jang K, Ramakrishnan PS, Lim TH, Martin JA. Selection of reference genes for normalization of quantitative real-time PCR in organ culture of the rat and rabbit intervertebral disc. BMC Res Notes. 2011;4:162. doi: 10.1186/1756-0500-4-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strowing T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- Subramanian P, Notario PM, Becerra SP. Pigment epithelium-derived factor receptor (PEDF-R): a plasma membrane-linked phospholipase with PEDF binding affinity. Adv Exp Med Biol. 2010;664:29–37. doi: 10.1007/978-1-4419-1399-9_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombran-Tink J, Barnstable CJ. PEDF: a multifaceted neurotrophic factor. Nat Rev Neurosci. 2003;4:628–636. doi: 10.1038/nrn1176. [DOI] [PubMed] [Google Scholar]
- Volpert OV, Zaichuk T, Zhou W, Reiher F, Ferguson TA, Stuart PM, Amin M, Bouck NP. Nat Med. 2002;8:349–357. doi: 10.1038/nm0402-349. [DOI] [PubMed] [Google Scholar]
- Wickham S, Carr DJ. Molecular mimicry versus bystander activation: herpetic stromal keratitis. Autoimmunity. 2004;37:393–397. doi: 10.1080/08916930410001713106. [DOI] [PubMed] [Google Scholar]
- Yanai R, Nishida T, Chikama T, Morishige N, Yamada N, Sonoda KH. Potential new modes of treatment of neurotrophic keratopathy. Cornea. 2015;34(Suppl 11):S121–S127. doi: 10.1097/ICO.0000000000000587. [DOI] [PubMed] [Google Scholar]
- Yun H, Rowe AM, Lathrop KL, Harvey SA, Hendricks RL. Reversible nerve damage and corneal pathology in murine herpes simplex stromal keratitis. J Virol. 2014;88:7870–7880. doi: 10.1128/JVI.01146-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun H, Lathrop KL, Hendricks RL. A central role for sympathetic nerves in herpes stromal keratitis in mice. Invest Ophthalmol Vis Sci. 2016;57:1749–1756. doi: 10.1167/iovs.16-19183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker KE, Kamberi P, Sobel RA, Cloud G, Meli DN, Clemons KV, Stevens DA, Williams PL, Leib SL. Temporal expression of inflammatory mediators in brain basilar artery vasculitis and cerebrospinal fluid of rabbits with coccidioidal meningitis. Clin Exp Immunol. 2006;143:458–466. doi: 10.1111/j.1365-2249.2006.03011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


