Abstract
Exercise associated muscle cramping (EAMC) is a poorly understood problem that is neuromuscular in origin. Ingestion of TRP channel agonists has been efficacious in attenuating electrically-induced muscle cramps.
Purpose
To examine the effect of TRP agonist ingestion on voluntarily-induced EAMC and motor function.
Methods
Study 1: 39 subjects completed 2 trials after ingesting TRP agonist-containing active treatment (A), or vehicle (V) control. Cramping in the triceps surae was induced via voluntary isometric contraction. Study 2: After ingesting A or V, 31 subjects performed kinematic and psychomotor tests of manual dexterity.
Results
A increased pre-cramp contraction duration (A:36.9±4.1, V:27.8±3.1 s), decreased cramp EMG area under the curve (A:37.3±7.7, V:77.2±17.7 %EMGmax•s), increased contraction force to produce the cramp (A:13.8±1.8, V:9.9±1.6 kg), and decreased post-cramp soreness (A:4.1±0.3, V:4.7±0.3 a.u.). Kinematic and psychomotor tests were not affected.
Discussion
TRP agonist ingestion attenuated EAMC characteristics without affecting motor function.
Keywords: exercise associated muscle cramp, α-motor neuron hyperexcitability, TRPV1, TRPA1, EMG
Introduction
Transient receptor potential (TRP) channels are involved in sensory transduction of temperature and chemicals1,2. Within the TRP superfamily, activation of different subtypes produces diverse physiological responses. TRP vanilloid 1 (TRPV1) opens in response to high temperature (>42°C) and capsaicin3, a compound that elicits hot/spicy sensations. TRPV1 is expressed in C and A delta (Aδ)-sensory nerve fibers in most tissues3. TRP Ankyrin 1 (TRPA1) opens in response to cinnamaldehyde4, which evokes the flavor of cinnamon. TRPA1 is primarily co-located on C and Aδ-nerve fibers that express TRPV15. Both TRPV1 and TRPA1 channels are expressed in the mucosal linings of the mouth, oropharynx, esophagus, and stomach6-8.
Strong excitatory sensory stimuli can elicit generalized depression of efferent neural output9. For example, stimulation of oropharyngeal chemoreceptors with citric acid decreased gastric myoelectrical activity10. Given the role of TRP channels in sensory transduction, potent TRP channel stimulation has the potential to decrease efferent neural function, including α-motor neurons innervating skeletal muscle, as demonstrated by Mandadi et al., who showed that treatment of spinal afferents with capsaicin decreased spinal motor nerve output11. Given the widespread expression of TRP channels in the mouth and digestive tract, orally administered TRP channel agonists may decrease excitability of α-motor neurons.
Though exercise associated muscle cramps (EAMC) are common, their etiology is not fully understood. Common theories are that EAMCs are caused by dehydration, altered plasma electrolyte concentrations, or α-motor neuron hyperexcitability12. Neither hydration nor serum electrolyte status varies between crampers and non-crampers during endurance events13,14. Hypohydration does not change cramp threshold frequency in electrically-induced muscle cramps15. These findings suggest that dehydration does not initiate cramping, but does not rule out a role in muscle cramp triggering or susceptibility. Dehydration and electrolyte depletion may increase the rate of muscle fatigue, causing cramping to develop more quickly during exercise12. Stretching relieves muscle cramps without altering hydration or electrolyte status16-18, demonstrating that restoring electrolyte and fluid balance is not requisite for alleviating EAMC. It has been proposed that a neural mechanism, linked to α-motor neuron hyperexcitability, mediates EAMC and almost certainly comprises the final common pathway.
Given that EAMC is likely neural in nature, stimulation of oral sensory nerves with TRP agonists has the potential to decrease α-motor neuron hyperexcitability and delay or attenuate EAMC. Short et al. showed a 3-fold reduction in electrically-induced muscle cramp intensity in the flexor halluces brevis after TRP agonist ingestion19 and Rosen et al. found TRP agonists attenuated electrically-induced muscle cramps in the abductor hallucis brevis20. While electrically-induced cramps are highly reproducible, an exercise-based model of EAMC better reflects real-world conditions.
The goals of the current studies were to (1) assess the effect of oral TRP channel activators on voluntarily-induced muscle cramps, and (2) assess whether potential TRP channel mediated alterations in neural output impair performance of fine psychomotor skills.
Materials and Methods
All protocols complied with the Declaration of Helsinki and were approved by the Institutional Review Board at The Pennsylvania State University. Participants provided voluntary written and verbal consent prior to participation in the study.
Study 1: Voluntarily-induced muscle cramps
Subjects
Subject characteristics are presented in table 1. Subjects were young, recreationally active, non-smokers, and free of neuromuscular disease. All subjects reported at least one instance of EAMC during the previous year.
Table 1.
Subject characteristics.
| Sex (M,F) | Age (y) | BMI (kg•m-2) | |
|---|---|---|---|
| Study 1 | 15, 24 | 22 ± 1 | 24 ± 1 |
| Study 2 | 12, 19 | 23 ± 3 | 23 ± 1 |
Subject characteristics are shown for both study one (muscle cramp) and study two (motor function).
Data are mean ± SE.
Study Design
This was a double blind, randomized, cross-over study. Subjects visited the lab for three familiarization trials during which they ingested a placebo beverage (flavored water) and underwent a protocol to induce muscle cramping (described below). To be included in the randomized study phase, subjects had to cramp during the first familiarization trial and during one of the subsequent two trials. These initial three trials served to identify subjects with ability to voluntarily reproduce cramps and to minimize learning effects.
Repeat crampers visited the lab on two additional occasions during which they received either the active treatment containing naturally occurring TRP channel activators (A) or a strongly flavored vehicle control (drink base minus the TRP agonists; V) in a randomized, counter-balanced order. All trials, including the familiarization trials, were separated by at least one, but not more than two, weeks.
To ensure blinding of the researchers, drinks were provided in coded bottles. Researchers involved in data analysis left the room while a research assistant delivered the test beverage to prevent researchers from inadvertently smelling the substances or observing involuntary verbal or physical responses from the subjects. Subjects were instructed not to divulge information about the taste profile or other characteristics of the beverages to the researchers. Beverage A had a potent spicy profile while beverage V had a strong fruit taste profile; subjects reported no preconception about which contained active ingredients (see Discussion).
Voluntary Cramp Model
Subjects refrained from vigorous exercise for 24 hours, and from consuming food, caffeine, or alcohol for 12 hours prior to each experiment. Subjects were instructed to drink at least six 8-oz glasses of water the day before each experiment.
Upon arrival, subjects consumed 50 mL of beverage. After finishing the beverage, the lower leg of the subject's choice was prepared for EMG electrode placement. Electromyography (EMG) signals were recorded using a wired amplifier system (iWorx Systems, Inc. IX-BIO8, Dover NH). After skin preparation, 50-mm bipolar, silver-silver chloride, surface electrodes, (SKINTACT, Inverness, FL) were placed over the muscle centers of the medial gastrocnemius, lateral gastrocnemius, tibialis anterior, and soleus with an inter-electrode distance of 2 cm. A single ground was secured to the tibia. After electrode placement, participants were positioned supine on a padded table with a comfortable bend in their hip and knee. The upper leg was supported in the self-selected position with foam padding and the foot was strapped to an adjustable pedal set to the subject's angle of maximal plantar flexion. The pedal was connected to a force transducer to measure force production throughout the study.
Fifteen min after beverage consumption, subjects performed a 5 sec maximum voluntary contraction (MVC) of the triceps surae against the foot pedal. The EAMC protocol involved performing a sustained maximum isometric contraction of the triceps surae against the foot pedal until muscle cramp onset. Immediately upon cramp onset, subjects ceased voluntary contraction and remained motionless until the muscle cramp subsided. If 90 sec of isometric contraction did not produce a cramp, contraction was halted and after 10 min rest the subject again attempted to produce a cramp. This pattern of 90 sec contraction/10 min rest was repeated either until a muscle cramp occurred or the subject was unsuccessful at producing a cramp for 5 attempts.
Immediately after the cramp subsided, subjects rated muscle soreness on a 1 (no soreness) to 10 (extreme soreness) scale. The subject then performed a post-cramp MVC. After 20 min rest, a rating of residual soreness was obtained on the same 1 to 10 scale. Joint angles of the subject's lower limb were measured with a goniometer and recorded for identical repositioning on subsequent visits.
Treatments
The active treatment was a proprietary blend of TRPV1 and TRPA1 agonists including one or more of the following: capsicum (up to 38 mg), cinnamon (up to 500 mg), and ginger (up to 750 mg) dissolved in a fruit flavored base. V comprised the same base solution, free of the TRP channel agonists. Because TRP channel activation is involved in flavor transduction, it is impossible to create a placebo with the same flavor profile as the active treatment. Importantly, both A and V possessed strong, albeit different, flavor profiles. Subjects were not pre-informed about the flavor profiles.
Data Analysis
EMG data were measured on an IX-RA-834 recorder with attached iWire BIO8 and IX-BIO4 biopotential amplifiers (iWorx) and recorded, stored, and analyzed offline using LabScribe3 software (iWorx). Data were sampled at 1000 samples•s-1, bandpass filtered at 100Hz-10kHz, rectified, and integrated with a time constant of 0.001s to calculate area under the curve (AUC) during the precramp isometric contraction and muscle cramp. There was no between-treatment difference in maximum EMG signal (A: 0.59 ± 0.04, V: 0.55 ± 0.04 μV; p=0.39); to account for slight differences in electrode placement between visits altering EMG signal intensity all data were normalized to a percentage of maximum EMG signal (%EMGmax; greatest 1% of EMG values during MVC). Cramp onset was noted when subjects first reported a cramp and was confirmed by visual inspection of the EMG signal, identified by quieting of the EMG signal in non-cramping musculature, but continued EMG activity in the cramping musculature21.
Statistics
Results are reported as mean ± standard error (SE). The Student's t-test for related samples (1-tailed) was used to assess differences between A and V. Effect size (ES, Cohen's d), adjusted for correlation, is reported for mean differences. Interpretation of ES follows the convention of Cohen: 0.2, 0.5, 0.8 correspond to ES of “small”, “medium”, and “large”, respectively. IBM SPSS (version 21) was used for statistical analyses with a 5% level of significance for all statistical tests22.
Study 2: Motor Function Study
Subjects
Subject characteristics are shown in table 1. Subjects were young, healthy, recreationally active, non-smokers, free of neurological disease. Subjects in study 2 represented a unique subject sample from study 1 and were not required to have a history of EAMC.
Study Design
Subjects came to the lab on three separate occasions. Participants refrained from vigorous exercise and from consuming caffeine or alcohol for 12 hours. The first visit served as a familiarization trial to orient subjects to the experimental set up and minimize learning effects. Subjects then completed two experimental trials, each 15 min after ingesting either A or V in a randomized, counterbalanced order. Rigorous blinding of subjects and investigators was performed as described for study 1. Experimental trials were separated by at least 24 hours to allow for treatment washout.
Tests
Subjects completed a simulated kinematic reaching task to evaluate upper extremity coordination using an experimental virtual reality based apparatus described previously23,24. Briefly, subjects sat in a chair with arms supported against the effects of gravity and friction by air sleds. Subjects faced an interactive 2D virtual workspace in which stimuli displayed on a 52″ HDTV (Sony Electronic Inc.) were reflected onto a mirror that obscured vision of the arms. The distance between the monitor and the parallel mirror was adjusted to give the illusion that the stimuli appeared in the plane of the fingertips.
A six-degree-of-freedom (6-DOF) Flock of Birds tracking system (Ascension Technology) recorded the position and orientation of the limb segments at 116 Hz sampling rate. 6-DOF sensors were attached to the middle of each wrist and the middle of each upper arm. The position of the tip of the index finger was recorded relative to the wrist sensor, and positions of the elbow and shoulder joints were recorded relative to the upper arm sensor. The 2D position of the tip of the index finger was used to project the cursor onto the mirror. Data were low-pass filtered using a 12 Hz zero-lag Butterworth filter, prior to differentiating to yield velocity profiles. Outcome measures of interest were maximum velocity, final position error, and deviation from linearity.
Each subject was instructed to adjust display cursor location by moving the cursor from a starting point to a target as quickly and accurately as possible. Each target was individually determined based on subject limb segment length, so all subjects reached for targets that required the same shoulder and elbow angles. Subjects completed 60 trials (30 per arm) then the same protocol was repeated without air sleds and subjects holding their arms up off the table to introduce the effects of gravity.
Subjects then completed a series of standardized neuropsychological tests to measure upper limb motor function and manual dexterity. Table 2 provides descriptions of the psychomotor tests and outcome measures, as well as descriptions of the three outcome measures of interest for kinematics. All psychomotor tests used are reliable, have been repeatedly validated, and are commonly used in neuropsychological research25-28.
Table 2. Description of motor function tests and measures performed during study 2.
| Measure | Description |
|---|---|
| Hand Grip Dynamometer (kg) | Average of maximum force produced across three trials. |
| Finger Tapping (taps) | Average of number of taps achieved across five 10-second trials. Measures distal motor speed and lateralized coordination. |
| JTHFT (s) | Total time to complete battery of simple tests: writing, page turning, lifting small objects, simulated feeding, stacking checkers, lifting light objects, and lifting heavy objects. Measures daily function of upper extremities. |
| Grooved Pegboard (s) | Time to fill a board of 25 holes with keys. Measured upper extremity motor function. |
| Vmax in 2D (m·s-1) | Maximum velocity achieved during path movement from start to target with air sleds (2 Dimensions). |
| Final Potion Error in 2D (cm) | Distance between final cursor position and target with air sleds (2 Dimensions). |
| Linearity Deviation in 2D | Ratio of cumulative hand path length to the straight-line distance between the starting point and target with air sleds (2 Dimensions). |
| Vmax in 3D (m·s-1) | Maximum velocity achieved during path movement from start to finish without air sleds (3 Dimensions). |
| Final Position Error in 3D (cm) | Distance between final cursor position and target without air sleds (3 Dimensions). |
| Linearity Deviation in 3D | Ratio of cumulative hand path length to the straight-line distance between the starting point and target without air sleds (3 Dimensions). |
Statistics
Results are reported as mean ± SE. Student's t-test for related samples (2-tailed) was used to assess differences between treatment and vehicle control conditions. IBM SPSS (version 21) was used for statistical analyses with a 5% level of significance for all statistical tests.
Results
Study 1
Sixty-four potential subjects with a self-reported history of muscle cramps were initially screened. Nineteen were unsuccessful in producing a muscle cramp on visit 1 and did not participate further. Two others failed to cramp on visits 2 and 3, while 1 failed to cramp during visit 4. Three other participants withdrew from the study due to time constraints, resulting in 39 participants completing the entire 5-visit protocol. The 64% success rate (excluding dropouts) is similar to previous studies of self-induced muscle cramps21,29,30.
Figure 1 shows a representative original EMG record during isometric muscle contraction and subsequent muscle cramp in two EMG leads. Both leads exhibit activity during voluntary muscle contraction, but activity continues only in the lead over the cramping musculature once voluntary contraction ceases.
Figure 1.
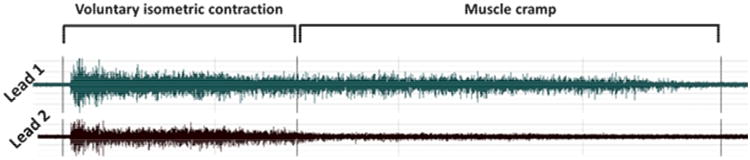
Representative tracing of EMG signal showing only 2 leads. EMG activity is apparent in both leads during voluntary isometric contraction. When voluntary contraction is halted at cramp onset, EMG activity in lead 1 persists, but lead 2 becomes quite, indicating that cramping is occurring under lead 1. Muscle cramp duration continues until EMG activity in lead 1 becomes quiescent.
Results from Study 1 are shown in Figure 2 as both the mean ± SE and individual responses. Panel A is the time to cramp onset, i.e., the time subjects held the isometric contraction before a cramp occurred. There was a significant difference between treatments, with a significantly longer time to cramp onset for A (A: 36.9 ± 4.1, V: 27.8 ± 3.1 s; p=0.003). The effect size for this variable was large (ES=0.70). Panel B depicts the AUC for the EMG signal during pre-cramp contraction, showing no difference between treatments (A: 299 ± 48, V: 277 ± 46 %EMGmax•s; p=0.334) and the effect size was small (ES=0.10). Panel C shows the average force produced during the muscle contraction prior to cramp onset. There was a significant difference between treatments (A: 13.8 ± 1.8, V: 9.9 ± 1.6 kg; p=0.002), with subjects producing more force before cramping after consuming A (large effect size; ES=0.72).
Figure 2.
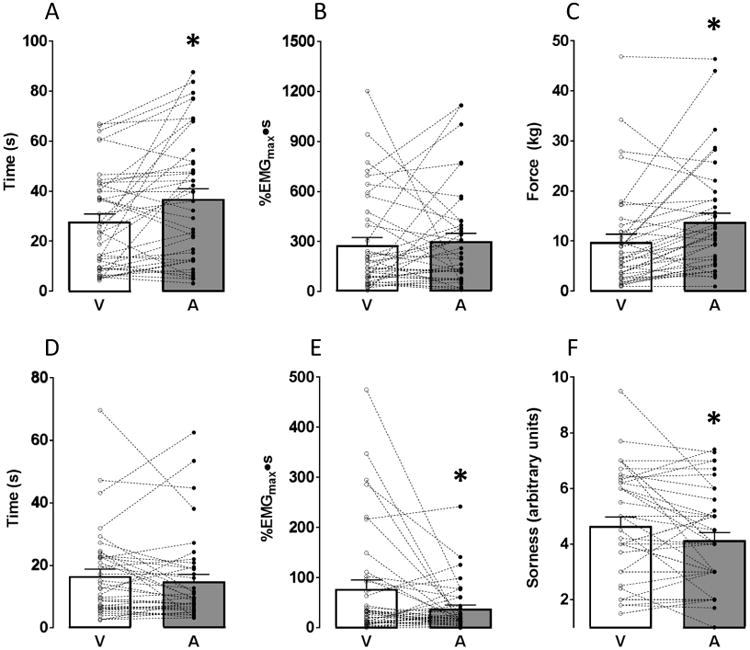
Study 1 results. (A) time to cramp onset, i.e., the duration that subjects held isometric contractions before the onset of a muscle cramp. Time to cramp onset was greater when subjects consumed A compared to V; (B) EMG intensity-duration area under the curve (AUC) during the pre-cramp isometric muscle contraction (no difference between treatments); (C) mean force produced during pre-cramp isometric contraction. Subjects produced significantly more force when they consumed A compared to V; (D) duration of muscle cramp (no difference between treatments); (E) EMG intensity-duration AUC during EIMC. AUC was significantly smaller when subjects consumed A compared to V; (F) subjective rating of cramp soreness immediately post-cramp. Subjects reported significantly less soreness after consuming A compared to V. * implies treatment difference at p<0.05.
Panel D shows the duration that the cramp persisted. There was no difference between treatments (A: 14.8 ± 2.2, V: 16.4 ± 2.2 s; p=0.144) and the effect size was small (ES=0.24). Panel E depicts the EMG AUC during the muscle cramp, showing a significant difference between treatments (A: 37.3 ± 7.2, V: 77.2 ± 17.7 %EMGmax•s; p=0.010) with a smaller cramp intensity-duration profile after subjects consumed A (medium effect size; ES=0.63).
Panel F shows the subjective rating of muscle soreness immediately post-cramp. There was a significant difference between treatments (A: 4.1 ± 0.3, V: 4.7 ± 0.3 a.u.; p=0.011), with reported soreness significantly lower after subjects consumed A (medium effect size; ES=0.55). There was no difference in the ratings of residual muscle soreness 20 minutes post-cramp (A: 2.6 ± 0.2, V: 2.8 ± 0.3 a.u; p=0.092; data not shown). There was no difference in the MVC force either before cramping (A: 13.5 ± 1.5, V: 13.2 ± 1.6 kg; p=0.378) or after cramping (A: 12.9 ± 1.6, V: 12.6 ± 1.3 kg; p=0.299; data not shown).
Study 2
Thirty-one subjects were enrolled in and completed the motor function tests. Results for study 2 are presented in table 3. There was no treatment effect on any of the kinematic or psychomotor tests.
Table 3.
Study 2 results.
| Measure | A | V | p-value |
|---|---|---|---|
| Hand Grip Dynamometer (kg) | 35.22 ± 1.78 | 35.01 ± 1.82 | 0.95 |
| Finger Tapping (taps) | 41.98 ± 0.98 | 41.11 ± 1.31 | 0.58 |
| JTHFT (s) | 103.08 ± 3.18 | 102.74 ± 3.20 | 0.86 |
| Pegboard (s) | 123.56 ± 2.25 | 123.08 ± 2.60 | 0.94 |
| Vmax in 2D (m·s-1) | 0.83 ± 0.01 | 0.83 ± 0.02 | 0.89 |
| Final Potion Error in 2D (cm) | 0.031 ± 0.002 | 0.031 ± 0.002 | 0.85 |
| Linearity Deviation in 2D | 0.091 ± 0.004 | 0.090 ± 0.005 | 0.95 |
| Vmax in 3D (m·s-1) | 0.87 ± 0.019 | 0.86 ± 0.019 | 0.76 |
| Final Position Error in 3D (cm) | 0.027 ± 0.001 | 0.027 ± 0.001 | 0.91 |
| Linearity Deviation in 3D | 0.088 ± 0.005 | 0.090 ± 0.005 | 0.78 |
Subjects completed a battery of motor function tests after consuming either the active treatment (A) or vehicle control (V). There was no difference between treatments for any of the tests. (JTHFT: Jebsen-Taylor hand function test). Values are Mean ± SE.
Discussion
Because activation of oral and mucosal TRPV1 and TRPA1 channels has shown efficacy in reducing the intensity of electrically-induced muscle cramps19,20, we sought to determine whether similar treatment could mitigate voluntarily-induced muscle cramps. Since depression of α-motor neuron excitability could potentially inhibit motor performance, we also sought to determine whether TRP channel activation might decrease performance on fine motor function tests (ostensibly reflecting alterations in voluntary neuromuscular activity). We found that ingesting TRP channel activators positively improved several cramp-related features in our isometric cramp model, increasing the contraction time and force production prior to cramp initiation, decreasing the EMG time-intensity relation during cramping, and decreasing subjective ratings of muscle soreness immediately after cramp cessation. Conversely, we observed no effect of oral TRP channel activators on fine motor performance. Therefore, we conclude that ingestion of known oral and upper gastrointestinal mucosal TRP channel agonists had positive benefits on EAMCs without altering fine motor performance.
Sustained isometric contraction of the triceps surae in a maximally plantar-flexed position has previously been used to examine self-induced muscle cramps. Utilizing this model, Khan & Burne elicited cramping in 8 of 13 subjects21, while Ross & Thomas had success in 4 of 8 participants30. Roeleveld et al. induced cramping in 8 of 8 participants (chosen from a previous cohort of 104) with a clearly demonstrated ability to self-induce muscle cramps31. Goodman and Zwetsloot had a low success rate (31%), but demonstrated the repeatability of the model by successfully inducing cramps in two follow-up experiments in 97% of those who cramped on visit one32. We demonstrated a 70% success rate in inducing cramps during visit one and a 64% success rate in inducing cramps over the entire 5-experiment study, in general agreement with previous reports.
Although increasing evidence points to hyperactive motor neurons as the final mediator of EAMCs, putative triggers that may provoke that hyperactivity are many and varied. Dehydration, hyperthermia, low muscle glycogen, fatigue, muscle damage, and alterations in electrolyte concentration are among the putative triggers for EAMCs. In theory, sufficient perturbations in neuromuscular function resulting from one or more of these triggers results in sustained excitatory input to α-motor neurons. While the present data do not rule out the aforementioned influences on EAMC, our subjects were presumably euhydrated and in normal electrolyte balance, suggesting that disturbances in these parameters are not requisite for voluntarily-induced cramp generation. Ingesting a solution of TRP channel agonists positively altered muscle cramp profiles, supporting the hypothesis that muscle cramps are neuromuscular in etiology regardless of the initiating environment or local milieu.
TRP channels are highly expressed in sensory nerves and other tissues3,5. TRP channel activation has many effects, including decreasing muscle tension. Direct application of the TRPV1 agonist capsaicin into motor neurons decreased muscle twitch tension in a dose-dependent manner33. In an isolated in vivo spinal cord preparation, treatment of C and Aδ afferents with capsaicin decreased output from spinal motor nerves11. Our data provide yet another example of TRP channel activation affecting motor nerve output. Though TRP channel activation was not measured, TRP channel activation is required for transduction of spicy sensations and Short et al. have empirically demonstrated that a blend of similar TRP agonists opens TRP channels19.
TRPV1 and TRPA1 channels are involved in the transduction of spicy/hot tastes3 and likely play a role in the mechanism(s) through which muscle cramps were mitigated in this study. It was therefore not possible to formulate a placebo with the same flavor profile as A. Consequently, we used the fluid base and flavoring without the TRP channel agonists for our blinded comparison. The use of a vehicle control beverage opposed to another placebo ensured that the only difference between trials was the presence or absence of TRP channel agonists. To circumvent the taste difference, subjects were not informed about the active treatment flavor profile. Further, both beverages possessed strong flavor profiles (sweet/fruit and spicy, respectively) leading subjects to assume that either (or both) beverages were designed to treat muscle cramping. In a post-study survey of a subset of subjects (n=19), no subject reported prior knowledge that spicy/hot-tasting products might mitigate muscle cramps, suggesting that the spicy flavor profile was unlikely to bias the subjects. Only 5 subjects “suspected” that they knew which beverage was the active treatment, while 14 subjects reported no suspicion as to beverage identity. Importantly, any placebo effect would necessitate that subjects be able to willfully and selectively control the activation of an isolated area of muscle in the midst of a painful muscle cramp, a highly unlikely scenario.
Consumption of a blend of known TRP agonists altered several characteristics of muscle cramp generation and quality. Ingestion of A (relative to V) increased the static muscle contraction time and force required to produce a cramp. That orally ingested TRP agonists may increase both the time and intensity required for muscle cramp initiation has relevance for sport-related activities. Despite the changes in duration and force, EMG activity remained the same between treatments, potentially due to reduced α-motor neuron excitability via TRP channel activation.
Cramp duration was not different between A and V; however, the EMG time-intensity relation (i.e., the AUC) during cramping was significantly reduced by A. This reduction in EMG activity could be due to either decreased average motor unit electrical activity or a decreased number of motor units active during cramping. Though there is expected high inter-individual variability, subjects with the highest EMG AUC with V experienced the greatest reduction in this variable with A. This would suggest a greater efficacy for TRP agonists treating more intense muscle cramps compared to relatively benign cramps. The reason for this difference is not readily apparent but could be due to a different balance of local and neural factors contributing to the generation of cramps of varying intensities. Finally, post-cramp muscle soreness was reduced with TRP agonist ingestion, likely due to the reduction in muscle cramp intensity producing less residual discomfort.
Our muscle cramp data suggest that TRP channel activation may have dampened α-motor neuron hyperexcitability to mitigate cramping. However, inhibition of α-motor neurons could potentially adversely affect fine motor function important for sport-related movements. TRP agonist-mediated decreases in α-motor neuron excitability did not alter psychomotor function. These data illustrate that small motor units were not affected by TRP channel agonists, in contrast to the larger motor units that apparently were muted by the consumption of A.
Limitations
We did not directly measure TRP channel activation. However, Short et al. tested individual TRP channel agonists derived from biological extracts on ex vivo human dorsal root ganglia and found that these TRP channel agonists are capable of opening TRP channels19. Our subjects perceived the spicy flavor, sensory perception that only occurs with TRP channel activation.
Subjects were young and recreationally active. These results cannot be extrapolated to elite athletes or groups with pathology-associated muscle cramps (MS, ALS). EAMCs were initiated via isometric muscle contraction. At this point it is unknown whether dynamic exercises would yield similar results. However, there is no reason to assume that we would have dissimilar findings with cramps induced with different methods.
Our voluntary cramp model inherently possessed greater variability than electrically-induced models. We sought to decrease within subject variability by having participants complete three familiarization trials. Because this voluntary isometric muscle contraction model was only used in rested, presumably euhydrated subjects, the impact of varying levels of hydration and fatigue are unknown.
Conclusion
We hypothesized that activation of oral and upper GI TRPV1 and TRPA1 channels would attenuate muscle cramps in a self-induced isometric cramp model, likely by decreasing α-motor neuron hyperexcitability. In a group of young healthy subjects with a prior cramp history, ingestion of TRP channel agonists 15 min before testing positively altered EAMC characteristics. Conversely, there was no effect of consuming TRP channel activators on any aspect of kinematic or neuropsychological measures of motor function mediated by smaller motor units. We conclude that consuming a beverage containing documented TRPV1 and TRPA1 channel activators mitigates self-induced muscle cramps, but does not affect fine motor function.
Acknowledgments
Kyle Volz, Megan Clarke, Ashlee Snyder
Funding for this project was provided by Flex Pharma, Inc. (Boston, MA). Sport Science Insights (Bob Murray) serves as an independent consultant to Flex Pharma, Inc.
Abbreviations
- A
active treatment
- AUC
area under the curve
- EAMC
exercise associated muscle cramps
- EMG
electromyography
- ES
effect size
- LG
lateral gastocnemius
- MG
medial gastrocnemius
- MVC
maximum voluntary contraction
- SE
standard error
- SL
soleus
- TA
tibialis anterior
- TRP
transient receptor potential
- TRPA1
transient receptor potential Ankyrin subtype 1
- TRPV1
transient receptor potential vanilloid receptor 1
- V
vehicle control
Footnotes
Ethical Publication Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Conflicts of Interest: W. Larry Kenney currently serves on the Scientific Advisory Board of Flex Pharma; however no financial or other potentially conflicting relationship was in place at the time of the experiments reported herein. All results in this study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
References
- 1.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426(6966):517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 2.Venkatachalam K, Montell C. TRP channels. Annual review of biochemistry. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bevan S, Quallo T, Andersson DA. Trpv1. Handbook of experimental pharmacology. 2014;222:207–245. doi: 10.1007/978-3-642-54215-2_9. [DOI] [PubMed] [Google Scholar]
- 4.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41(6):849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 5.Zygmunt PM, Hogestatt ED. Trpa1. Handbook of experimental pharmacology. 2014;222:583–630. doi: 10.1007/978-3-642-54215-2_23. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez-Berdugo D, Rofes L, Farre R, Casamitjana JF, Enrique A, Chamizo J, Padron A, Navarro X, Clave P. Localization and expression of TRPV1 and TRPA1 in the human oropharynx and larynx. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2016;28(1):91–100. doi: 10.1111/nmo.12701. [DOI] [PubMed] [Google Scholar]
- 7.Yu X, Yu M, Liu Y, Yu S. TRP channel functions in the gastrointestinal tract. Seminars in immunopathology. 2016;38(3):385–396. doi: 10.1007/s00281-015-0528-y. [DOI] [PubMed] [Google Scholar]
- 8.Sobhan U, Sato M, Shinomiya T, Okubo M, Tsumura M, Muramatsu T, Kawaguchi M, Tazaki M, Shibukawa Y. Immunolocalization and distribution of functional temperature-sensitive TRP channels in salivary glands. Cell and tissue research. 2013;354(2):507–519. doi: 10.1007/s00441-013-1691-x. [DOI] [PubMed] [Google Scholar]
- 9.Okun M, Lampl I. Instantaneous correlation of excitation and inhibition during ongoing and sensory-evoked activities. Nature neuroscience. 2008;11(5):535–537. doi: 10.1038/nn.2105. [DOI] [PubMed] [Google Scholar]
- 10.Ashida C, Kojima A, Kobashi M, Koga T. Oro-pharyngeal chemoreceptor activation induces gastric motor response in healthy volunteer subjects. Journal of smooth muscle research = Nihon Heikatsukin Gakkai kikanshi. 2004;40(4-5):211–217. doi: 10.1540/jsmr.40.211. [DOI] [PubMed] [Google Scholar]
- 11.Mandadi S, Nakanishi ST, Takashima Y, Dhaka A, Patapoutian A, McKemy DD, Whelan PJ. Locomotor networks are targets of modulation by sensory transient receptor potential vanilloid 1 and transient receptor potential melastatin 8 channels. Neuroscience. 2009;162(4):1377–1397. doi: 10.1016/j.neuroscience.2009.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwellnus MP. Cause of exercise associated muscle cramps (EAMC)--altered neuromuscular control, dehydration or electrolyte depletion? British journal of sports medicine. 2009;43(6):401–408. doi: 10.1136/bjsm.2008.050401. [DOI] [PubMed] [Google Scholar]
- 13.Schwellnus MP, Nicol J, Laubscher R, Noakes TD. Serum electrolyte concentrations and hydration status are not associated with exercise associated muscle cramping (EAMC) in distance runners. British journal of sports medicine. 2004;38(4):488–492. doi: 10.1136/bjsm.2003.007021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maughan RJ. Exercise-induced muscle cramp: a prospective biochemical study in marathon runners. Journal of sports sciences. 1986;4(1):31–34. doi: 10.1080/02640418608732095. [DOI] [PubMed] [Google Scholar]
- 15.Miller KC, Mack GW, Knight KL, Hopkins JT, Draper DO, Fields PJ, Hunter I. Three percent hypohydration does not affect threshold frequency of electrically induced cramps. Medicine and science in sports and exercise. 2010;42(11):2056–2063. doi: 10.1249/MSS.0b013e3181dd5e3a. [DOI] [PubMed] [Google Scholar]
- 16.Rowland LP. Cramps, spasms and muscle stiffness. Revue neurologique. 1985;141(4):261–273. [PubMed] [Google Scholar]
- 17.Fowler AW. Relief of cramp. Lancet. 1973;1(7794):99. doi: 10.1016/s0140-6736(73)90492-3. [DOI] [PubMed] [Google Scholar]
- 18.Daniell HW. Simple cure for nocturnal leg cramps. The New England journal of medicine. 1979;301(4):216. [PubMed] [Google Scholar]
- 19.Short G, Hegarty B, Mackinnon R, Bean B, Westphal C, Cermak J. Orally-administered TRPV1 and TRPA1 activators inhibit electrically-induced muscle cramps in normal healthy volunteers. Neurology. 2015;84(14 Supplement S17.003) [Google Scholar]
- 20.Rosen L, Sutherland R, Solberg E, Tornblom A, Hegarty B, Wessel T, Westphal C, Cermak J. Synthetic TRP activators demonstrate efficacy in preventing human muscle cramping: potential new drug treatment for muscle cramps and spasticity. Neurology. 2015;86(16 Supplement P2.275) [Google Scholar]
- 21.Khan SI, Burne JA. Reflex inhibition of normal cramp following electrical stimulation of the muscle tendon. Journal of neurophysiology. 2007;98(3):1102–1107. doi: 10.1152/jn.00371.2007. [DOI] [PubMed] [Google Scholar]
- 22.Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, N.J.: L. Erlbaum Associates; 1988. pp. xxi–567. [Google Scholar]
- 23.Mutha PK, Stapp LH, Sainburg RL, Haaland KY. Frontal and parietal cortex contributions to action modification. Cortex; a journal devoted to the study of the nervous system and behavior. 2014;57:38–50. doi: 10.1016/j.cortex.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yadav V, Sainburg RL. Handedness can be explained by a serial hybrid control scheme. Neuroscience. 2014;278:385–396. doi: 10.1016/j.neuroscience.2014.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujisaki W. Effects of delayed visual feedback on grooved pegboard test performance. Frontiers in psychology. 2012;3:61. doi: 10.3389/fpsyg.2012.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jebsen RH, Taylor N, Trieschmann RB, Trotter MJ, Howard LA. An objective and standardized test of hand function. Archives of physical medicine and rehabilitation. 1969;50(6):311–319. [PubMed] [Google Scholar]
- 27.Liu CJ, Marie D, Fredrick A, Bertram J, Utley K, Fess EE. Predicting hand function in older adults: evaluations of grip strength, arm curl strength, and manual dexterity. Aging clinical and experimental research. 2016 doi: 10.1007/s40520-016-0628-0. [DOI] [PubMed] [Google Scholar]
- 28.Schmitt L. Finger-Tapping Test. In: Volkmar F, editor. Encyclopedia of Autism Spectrum Disorders. New York, NY: Springer New York; 2013. pp. 1296–1296. [Google Scholar]
- 29.Bertolasi L, De Grandis D, Bongiovanni LG, Zanette GP, Gasperini M. The influence of muscular lengthening on cramps. Annals of neurology. 1993;33(2):176–180. doi: 10.1002/ana.410330207. [DOI] [PubMed] [Google Scholar]
- 30.Ross BH, Thomas CK. Human motor unit activity during induced muscle cramp. Brain : a journal of neurology. 1995;118(Pt 4):983–993. doi: 10.1093/brain/118.4.983. [DOI] [PubMed] [Google Scholar]
- 31.Roeleveld K, van Engelen BG, Stegeman DF. Possible mechanisms of muscle cramp from temporal and spatial surface EMG characteristics. Journal of applied physiology. 2000;88(5):1698–1706. doi: 10.1152/jappl.2000.88.5.1698. [DOI] [PubMed] [Google Scholar]
- 32.Goodman A, Zwetsloot KA. Voluntary inducement of triceps surae muscle cramping. International Journal of Athletic Therapy & Training. 2013;18(6):40–43. [Google Scholar]
- 33.Thyagarajan B, Potian JG, Baskaran P, McArdle JJ. Capsaicin modulates acetylcholine release at the myoneural junction. European journal of pharmacology. 2014;744:211–219. doi: 10.1016/j.ejphar.2014.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]


