Abstract
Alzheimer’s disease (AD) is the most common neurodegenerative disease in humans and will pose a considerable challenge to healthcare systems in the coming years. Aggregation of the β-amyloid (Aβ) peptide within the brain is thought to be an initiating event in AD pathogenesis. Many recent studies in transgenic mice have provided evidence that Aβ aggregates become self-propagating during disease, leading to a cascade of protein aggregation in the brain, which may underlie the progressive nature of AD. The ability to self-propagate and the existence of distinct “strains” reveals that Aβ aggregates exhibit many properties indistinguishable from those of prions composed of PrPSc proteins. Here, we review the evidence that Aβ can become a prion during disease and discuss how Aβ prions may be important for understanding the pathobiology of AD.
Alzheimer’s disease (AD) is the most common cause of dementia in humans and is most frequently diagnosed in individuals 65 yr of age or older. The incidence of AD varies with age: about one in nine people age 65 or older will develop AD, whereas about one in three individuals age 85 or older will be diagnosed with the disease (Hebert et al. 2013). Early-onset cases of AD, which occur in individuals younger than age 65, are comparatively rare and include familial cases caused by mutations in genes linked to the production of the β-amyloid (Aβ) peptide. The predominant clinical symptoms of AD are cognitive deficits and behavioral changes. AD patients typically first present with difficulties in remembering recent occurrences, but with intact memories of older events. The earliest, predementia stages of the disease are often classified clinically as mild cognitive impairment, which describes the presence of memory problems greater than those expected from normal aging. As the disease progresses, additional symptoms become apparent, which may include problems with language, confusion, mood swings, long-term memory loss, and withdrawal from society. Eventually, AD patients become completely dependent on caregiver assistance. There are currently no cures or effective treatments for AD. Although available treatments may temporarily improve behavioral problems, they do not address the root cause of the disease or its progression.
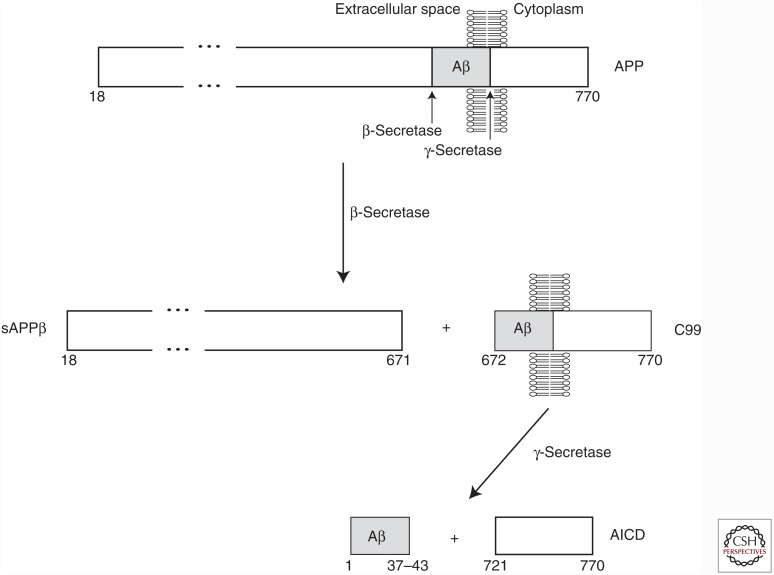
AD is a progressive neurodegenerative disease of aging. The brains of AD patients exhibit a remarkable loss of synapses and neurons, which results in an overall shrinkage of the brain, particularly in the temporal and parietal lobes as well as in the frontal cortex. At the microscopic level, two principal pathologies are observed: amyloid (senile) plaques and neurofibrillary tangles (NFTs). The dense, extracellular amyloid plaques are composed of aggregated Aβ peptide, whereas the intracellular NFTs are made up of paired helical filaments of hyperphosphorylated tau protein. Aβ is generated by the sequential endoproteolytic cleavage of the amyloid precursor protein (APP), a Type-1 transmembrane protein that exists as three different isoforms with lengths of 695, 751, and 770 amino acids. In the amyloidogenic processing pathway, APP is cleaved by two different proteases: β-secretase and γ-secretase (Fig. 1). β-Secretase first cleaves the extracellular juxtamembrane region of APP, resulting in a membrane-embedded fragment termed C99. The transmembrane portion of C99 is then cleaved by γ-secretase, a multiprotein complex that includes the enzymatically active presenilin proteins (De Strooper et al. 2012), freeing the Aβ peptide. Cleavage of C99 by γ-secretase is heterogeneous in nature and results in the generation of Aβ peptides varying in length from 37 to 43 amino acids (Benilova et al. 2012). If APP is first cleaved by α-secretase instead of β-secretase, the generation of Aβ is prevented.
Figure 1.
Generation of β-amyloid (Aβ) peptide via endoproteolytic cleavage of amyloid precursor protein (APP). The mature form of the longest isoform of human APP (following removal of an N-terminal signal peptide) spans residues 18–770. In the amyloidogenic pathway, cleavage of the APP extracellular domain by β-secretase produces a secreted fragment termed sAPPβ and a membrane-embedded C-terminal fragment called C99. Cleavage of C99 by γ-secretase produces the APP intracellular domain (AICD) and the Aβ peptide. Cleavage of APP by γ-secretase is heterogeneous, resulting in the generation of Aβ peptides that vary in length between 37 and 43 residues.
The amyloid cascade hypothesis, which is the most widely accepted theory for the molecular sequence of events in AD, postulates that the initiating event in AD is the aggregation and subsequent deposition of Aβ peptide in the brain (Hardy and Selkoe 2002). This results in the hyperphosphorylation and polymerization of tau into NFTs and, ultimately, degeneration of neurons. This hypothesis is supported by the following observations: (1) Mutations in the gene encoding APP cause early-onset forms of AD and increase the production of Aβ or enhance its aggregation potential (Chartier-Harlin et al. 1991; Citron et al. 1992); (2) duplication of the APP coding locus results in early-onset AD (Rovelet-Lecrux et al. 2006); (3) in Down’s syndrome patients, an extra copy of chromosome 21, which contains the APP gene, invariably causes AD-like pathological changes in the brain (Wisniewski et al. 1985); (4) mutations in the APP gene that decrease Aβ production are protective against AD (Jonsson et al. 2012); and (5) mutations in the genes encoding the presenilin proteins increase the relative abundance of pro-amyloidogenic Aβ isoforms and cause early-onset AD (Sherrington et al. 1995). Thus, increased Aβ production and deposition in the brain is sufficient to cause AD, suggesting that Aβ aggregation is central to AD pathogenesis. In contrast, mutations in the gene encoding tau result in the production of NFTs in the brain (Hutton et al. 1998; Spillantini et al. 1998), but not amyloid plaques. Such patients develop a tauopathy termed FTDP-17 that is clinically and pathologically distinct from AD, revealing that tau mutations are insufficient to elicit the full neuropathological spectrum observed in AD.
MOUSE MODELS OF ALZHEIMER’S DISEASE
Transgenic (Tg) mice that overexpress mutant human APP containing one or more mutations that cause early-onset, familial forms of AD develop Aβ-containing amyloid plaques with aging (Morrissette et al. 2009). These mice, referred to hereafter as Tg(AD) mice, thus recapitulate one of the key neuropathological hallmarks of AD. The kinetics of Aβ deposition in Tg(AD) mice is governed by the relative level of APP overexpression as well as the specific mutations present. For example, Tg2576 mice expressing human APP containing the Swedish mutation (K670N/M671L) (Citron et al. 1992; Mullan et al. 1992), which causes enhanced cleavage of APP by β-secretase (Haass et al. 1995) and, consequently, increased Aβ production, develop Aβ plaques beginning at ∼12 mo of age (Hsiao et al. 1996). In contrast, TgCRND8 mice expressing a mutant APP allele containing the Swedish mutation and the Indiana mutation (V717F) (Murrell et al. 1991), which increases the relative abundance of the more aggregation-prone Aβ42 isoform (Suzuki et al. 1994), develop amyloid plaques at ∼3–4 mo of age (Chishti et al. 2001).
Some lines of Tg(AD) mice exhibit age-dependent memory impairment, mimicking the cognitive deficits observed in AD patients, and exhibit some tau hyperphosphorylation (for review, see Morrissette et al. 2009). Moreover, the cerebrospinal fluid (CSF) of Tg(AD) mice contains increased levels of tau and decreased levels of Aβ42 peptide, mirroring the biomarker signature of individuals with AD (Maia et al. 2013). These data support the translational utility of Tg(AD) mice. Indeed, these animals have been used to show that reducing levels of Aβ aggregates in the brain, either by vaccination or passive immunotherapy, can rescue cognitive deficits (Schenk et al. 1999; Bard et al. 2000; Janus et al. 2000). However, current Tg(AD) mice do not constitute an ideal animal model of AD because, unlike AD patients, Tg(AD) mice do not develop NFTs in response to Aβ aggregation and do not display frank neuronal loss (Jucker 2010).
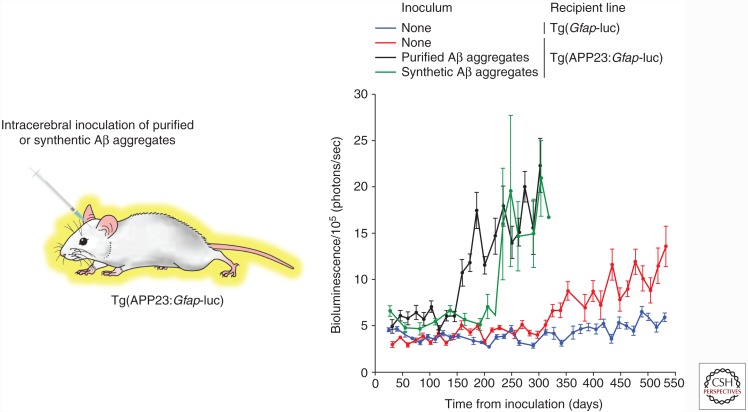
Tg(AD) mice do not exhibit overt progressive clinical signs of neurological illness with aging, despite the presence of abundant Aβ plaques. Thus, the only way of assessing the kinetics of Aβ deposition in the brain is to perform postmortem analysis of fixed tissue using neuropathological techniques. This is not ideal for several reasons. First, this greatly increases the number of animals needed for a study, as separate cohorts are required for each time point under investigation. Second, it necessitates “guessing” when Aβ deposition is expected to occur in the brain. Finally, it hinders the development of therapeutics because of the lack of easily and rapidly quantifiable measures of disease. One solution to this problem is the use of bioluminescence imaging (BLI) to monitor the kinetics of Aβ deposition in living mice. Tg mice that express firefly luciferase (luc) under the control of the glial fibrillary acidic protein (GFAP) promoter (Zhu et al. 2004) emit increased levels of light from their brains in response to stimuli that cause astrocytic gliosis and a concomitant upregulation of GFAP protein in astrocytes. Because GFAP-positive astrocytes are frequently found around the perimeters of Aβ amyloid deposits in the brain, we hypothesized that Tg(Gfap-luc) mice would be useful for tracking the kinetics of Aβ deposition in vivo. Indeed, bigenic mice expressing a mutant APP transgene and the Gfap-luc reporter exhibited age-dependent increases in the brain bioluminescence signal (Fig. 2), providing a quantitative assessment of the kinetics of Aβ deposition in living mice (Watts et al. 2011).
Figure 2.
Monitoring spontaneous and induced Aβ deposition in bigenic mice using in vivo bioluminescence imaging (BLI). The brain BLI signal in bigenic Tg(APP23:Gfap-luc) mice (red) exhibits an age-dependent increase, which is indicative of cerebral Aβ deposition, compared with Tg mice expressing just the Gfap-luc reporter (blue). Intracerebral inoculation of Tg(APP23:Gfap-luc) mice with either Aβ aggregates purified from the brain of an aged APP23 mouse (black) or Aβ aggregates formed from synthetic Aβ(1-40) peptide (green) accelerate the onset of the brain BLI signal increase compared with uninoculated mice, indicating that both inocula contain Aβ prions. (This figure has been adapted from Stöhr et al. 2012, with permission from the National Academy of Sciences © 2012.)
Two recently described Tg(AD) models have provided significant advances to the field. Knock-in mice in which the mouse APP gene was modified so that it contains a humanized Aβ sequence and the Swedish and Iberian (I716F) (Guardia-Laguarta et al. 2010) mutations develop typical age-dependent Aβ pathology and memory deficits (Saito et al. 2014). These mice are noteworthy in that APP is expressed at physiological levels and with correct spatiotemporal patterning. A promising Tg(AD) rat model has also been developed recently. TgF344-AD rats, which express human APP containing the Swedish mutation and a mutant presenilin 1 transgene, develop Aβ pathology and cognitive deficits and some associated tau pathology and neuronal loss (Cohen et al. 2013).
EVIDENCE FOR THE EXISTENCE OF Aβ PRIONS IN AD
The pioneering work of Gajdusek, Gibbs, and Alpers revealed that Creutzfeldt–Jakob disease (CJD) and kuru could be transmitted to nonhuman primates, demonstrating for the first time that these human spongiform encephalopathies are transmissible illnesses (Gajdusek et al. 1966; Gibbs et al. 1968). It is now known that prions composed of misfolded and aggregated prion protein (PrP) are the infectious agent in these diseases (Colby and Prusiner 2011). PrP prions were originally defined as proteinaceous infectious particles that cause rare, transmissible neurodegenerative diseases such as scrapie and CJD (Prusiner 1982) but are now more generally defined as alternative protein conformations that exhibit self-propagation (Prusiner 2012). There are two key features of prions: (1) At least two distinct stable conformational states of the protein exist in the absence of any posttranslational modifications, one of which exhibits a propensity for forming multimeric structures and/or aggregates; and (2) the multimeric state is self-propagating, meaning that it can catalyze the conversion of the “normal,” monomeric conformation into an additional copy of the prion conformation.
Evidence that Aβ becomes a prion during AD pathogenesis is growing. In AD patients, the pattern of Aβ deposition in the brain is not random in nature. Instead, Aβ deposition follows a stereotypical progression through five distinct phases. Phases 1–3 are associated with Aβ deposits occurring in nondemented individuals, whereas phases 3–5 are associated with AD patients (Thal et al. 2002). In phase 1, Aβ deposits are exclusively found in the neocortex and then spread to the hippocampus in phase 2. In phase 3, Aβ deposits can additionally be found in the amygdala, thalamus, and striatum. In phase 4, certain areas of the brainstem and the substantia nigra become laden with Aβ deposits; finally, in phase 5, Aβ deposits can also be found in additional brainstem nuclei and the cerebellum. This progressive, nonrandom spreading of cerebral Aβ deposition is reminiscent of the spread of PrP prions throughout the brain by templated conformational conversion. Unlike in AD, amyloid plaques are only present in the brain in ∼10% of CJD cases (DeArmond et al. 2004). However, upon purification, PrP prions aggregate to form rod-like structures that exhibit all of the properties of amyloid, including Congo red birefringence (Prusiner et al. 1983). The molecular and pathological similarities between AD and the PrP prion diseases led to speculation that prions may feature in the pathogenesis of AD, and that like CJD and kuru, AD may be transmissible to nonhuman primates (Prusiner 1984).
Attempts to transmit AD to monkeys produced discordant results (Table 1). Gajdusek and colleagues inoculated 52 different cases of sporadic and familial AD into a variety of primate species and found that only two of the cases transmitted disease (Goudsmit et al. 1980). However, the resultant pathology in the monkeys inoculated with these two cases did not resemble AD; instead, it was identical to that observed in monkeys inoculated with CJD and kuru. Moreover, new preparations of inocula from these two cases failed to transmit disease, suggesting that contamination or other laboratory errors are a more likely explanation for the initial positive transmissions. Similar issues may explain the inability to reproduce an initial finding that buffy coat fractions from the blood of AD patients induce a spongiform encephalopathy in inoculated hamsters (Manuelidis et al. 1988; Godec et al. 1994).
Table 1.
Attempts to transmit Alzheimer’s disease to primates
| Number of cases inoculated | Host primate(s) | Incubation period examined | Findings | Reference(s) |
|---|---|---|---|---|
| 34 (sporadic AD) | Chimpanzees, capuchins, squirrel monkeys, spider monkeys, African green monkey, stumptail monkeys | 13 cases examined for >50 mo; 21 cases examined for <50 mo | No clinical signs of neurological disease in any of the inoculated animals; Aβ pathology analysis not performed | Goudsmit et al. 1980 |
| 18 (familial AD) | Chimpanzees, capuchins, mangabeys, squirrel monkeys, spider monkeys, African green monkey, stumptail monkeys, rhesus monkeys, cynomolgus monkeys | 4 cases examined for >50 mo; 14 cases examined for <50 mo | 16/18 cases produced no clinical signs of neurological disease in the inoculated animals; Aβ pathology analysis not performed; 2/18 cases produced a spongiform encephalopathy at 23–40 mo postinoculationa | Goudsmit et al. 1980 |
| 3 (sporadic AD) | Marmosets | Up to 95 mo | No clinical signs of neurological disease in any of the inoculated animals; 8/9 inoculated animals exhibited Aβ pathology (plaques and cerebral amyloid angiopathy) | Baker et al. 1994; Ridley et al. 2006 |
| 1 (familial AD) | Marmosets | Up to 72 mo | No clinical signs of neurological disease in any of the inoculated animals; 4/5 inoculated animals exhibited Aβ pathology (plaques and cerebral amyloid angiopathy) | Ridley et al. 2006 |
aThese two positive transmissions were not reproducible.
Later, Ridley, Baker, and colleagues showed that marmosets inoculated with brain homogenate from an AD patient develop some Aβ-containing senile plaques and cerebral amyloid angiopathy 6–7 yr postinjection (Baker et al. 1993, 1994). A total of 24 out of 27 marmosets injected with brain homogenate containing Aβ aggregates exhibited modest numbers of Aβ deposits after incubation periods of up to ∼8 yr (Ridley et al. 2006). All monkeys that survived >3.5 yr postinoculation exhibited Aβ deposits. Only five of 29 uninjected marmosets aged >10 yr and none of the uninjected marmosets aged 5–10 yr exhibited spontaneous Aβ deposits. These studies also found that brain homogenate from non-AD patients, synthetic Aβ aggregates, and CSF from AD patients were poor inducers of Aβ deposition in marmosets. Importantly, no clinical signs of AD or NFTs were observed in any of the Aβ-positive inoculated animals, suggesting that although cerebral β-amyloidosis can be initiated or accelerated by inoculation of preformed Aβ aggregates, the full clinicopathologic spectrum of AD cannot be recapitulated by inoculation, at least within the time frame studied.
USING TRANSGENIC MICE TO STUDY Aβ PRIONS
The most convincing evidence for the existence of self-propagating Aβ aggregates (or, simply, Aβ prions) in AD has been shown by in vivo seeding studies in Tg(AD) mice. In many lines of Tg(AD) mice, such as the widely used Tg2576 and APP23 (Sturchler-Pierrat et al. 1997) models, spontaneous Aβ deposits are not observed until the animals are older, providing a window during which the induction of Aβ deposition by inoculation of exogenous Aβ aggregates can be assessed. In a typical in vivo seeding experiment, young (2- to 3-mo-old) Tg mice (that have not yet developed deposits) are intracerebrally injected with brain extract containing abundant Aβ aggregates, and, subsequently, the induction of Aβ deposition is examined following an incubation period of 3–5 mo. Two inoculation techniques, stereotaxic and non-stereotaxic (“standard”) injections, have been used to introduce exogenous Aβ aggregates into the brains of Tg(AD) mice. Stereotaxic inoculations commonly involve the injection of 2–3 µL of brain extract directly into the hippocampus and overlying cortex using a Hamilton syringe. Although this technique is time-consuming, it is advantageous because Aβ aggregates can be introduced into highly defined brain regions (Eisele et al. 2009). The standard procedure involves the inoculation of ∼30 µL of brain extract into the right cerebral hemisphere using a standard syringe and a 27-gauge needle (Stöhr et al. 2012). This technique is less precise (roughly injecting the inoculum into the thalamus and the overlying hippocampal and cortical regions) but is much more rapid.
In 2000, Lary Walker and colleagues showed that intracerebral inoculation of brain extracts from AD patients into Tg2576 mice induced small amounts of cerebral Aβ deposition following an incubation period of 5 mo (Kane et al. 2000). No Aβ deposition was observed in mice injected with brain extract from a young individual or in age-matched uninjected animals, and only minor amounts of induced Aβ deposition were seen in mice inoculated with brain extract from an aged patient without AD. Later, Walker, working with Mathias Jucker and colleagues, revealed that Aβ aggregates present in the brain extracts were necessary to induce Aβ deposition (Meyer-Luehmann et al. 2006). Stereotactic inoculation of APP23 mice into the hippocampus and overlying cortex with brain extract from aged Tg(AD) mice resulted in the induction of Aβ deposition after an incubation period of 4 mo. The induction of cerebral Aβ deposition could be blocked or significantly attenuated by either (1) immunodepleting the brain extracts of Aβ aggregates before inoculation, (2) passively immunizing the mice with anti-Aβ antibodies, or (3) treating the brain extract with formic acid before inoculation. Depletion of Aβ aggregates from brain extracts using a small molecule also suppressed seeding activity (Duran-Aniotz et al. 2014). These experiments indicated that, like PrP prions, brain-derived Aβ aggregates are self-propagating. However, no induced Aβ deposition was observed in mice inoculated with a variety of synthetic Aβ aggregate preparations, implying that not all Aβ aggregates are capable of self-propagation (Meyer-Luehmann et al. 2006).
Although earlier efforts failed to show any seeding of cerebral Aβ deposition following peripheral or systemic application of Aβ seeds (Eisele et al. 2009), examining later time points following intraperitoneal inoculation of APP23 mice with Tg(AD) brain extract revealed that cerebral Aβ deposition can be initiated by peripheral inoculation, suggesting that Aβ prions, like PrP prions, are neuroinvasive (Eisele et al. 2010). This is not an artifact of ectopic APP expression in the periphery owing to the use of the Thy-1 promoter to drive mutant APP expression in APP23 mice, as cerebral Aβ deposition can also be induced by peripheral Aβ inoculation in R1.40 Tg mice, which express mutant APP under the control of its endogenous promoter (Eisele et al. 2014). Similarly, cerebral Aβ deposition can also be induced by intracerebral inoculation of R1.40 mice with Aβ-containing brain extract (Hamaguchi et al. 2012), arguing that cerebral Aβ induction cannot be explained as an artifact arising from improper patterns of APP expression. Although a majority of studies have shown that cerebral Aβ deposition can be induced in Tg(AD) mice or rats (Rosen et al. 2012) that express mutant APP, it is also possible to induce Aβ deposition in Tg mice that overexpress wild-type human APP and do not develop spontaneous cerebral Aβ deposition within their normal lifespan. Intracerebral inoculation of HuAPPwt mice with AD patient brain extract resulted in the induction of Aβ pathology at ∼10 mo postinoculation (Morales et al. 2012). It is not yet known whether Aβ induction can be induced in non-Tg mice or rats.
The specific assemblies of Aβ responsible for inducing cerebral Aβ deposition are not currently known. Aβ aggregates purified from the brains of Tg(AD) mice are potent inducers of Aβ deposition in the brain (Fig. 2) (Stöhr et al. 2012), suggesting that Aβ aggregates themselves are the infectious prion species. This was confirmed by demonstrating that Aβ aggregates composed exclusively of synthetic Aβ peptides can also initiate cerebral Aβ deposition in APP23 mice (Fig. 2) (Stöhr et al. 2012, 2014). Treatment of Aβ prion preparations with proteinase K (PK) did not abolish their infectivity (Langer et al. 2011; Stöhr et al. 2012), implying that self-propagating Aβ aggregates are densely packed and likely large in size. This is supported by the observation that formaldehyde treatment does not inactivate Aβ prion activity present in brain extracts (Fritschi et al. 2014a) and the remarkable finding that Aβ prions can persist for up to 6 mo postinoculation in Aβ-inoculated APP knockout mice (Ye et al. 2015a). However, soluble Aβ species present in Tg(AD) or AD patient brain extracts that are sensitive to PK digestion can also induce cerebral Aβ deposition in APP23 mice (Langer et al. 2011; Fritschi et al. 2014b), suggesting that multiple distinct Aβ prion strains are capable of exhibiting self-propagation.
Like PrP prions, Aβ prions are capable of inducing cerebral Aβ deposition even when present at low levels in the inoculum. For example, induced Aβ deposition was still apparent in Tg2576 mice when injected with 105- to 106-fold dilutions of aged Tg2576 brain extract (Morales et al. 2015). Moreover, brain extracts from patients with mild cognitive impairment or nondemented individuals with AD neuropathology were also capable of inducing cerebral Aβ deposition in Tg(AD) mice (Duran-Aniotz et al. 2013). Induced Aβ prions also appear to “spread” away from the original site of inoculation (Eisele et al. 2009; Ye et al. 2015b), providing a potential molecular explanation for the progression of Aβ pathology observed in AD patients (Thal et al. 2002).
STRAINS OF Aβ PRIONS
In the PrP prion diseases, distinct strains of prions can be classified according to their incubation periods upon inoculation into rodents, the resultant clinical signs of disease and neuropathological features, and their biochemical characteristics, such as their relative resistance to protease digestion and their stability upon exposure to chemical denaturants (Collinge and Clarke 2007). A myriad of evidence argues that prion strain-specific properties are encoded within unique conformations of PrP aggregates (Bessen and Marsh 1994; Telling et al. 1996). AD is a clinically and pathologically heterogeneous disorder, with variability present in the age of onset, the rate of cognitive decline, and the morphology and neuroanatomical location of Aβ pathology (i.e., parenchymal vs. vascular). It is conceivable that some of this heterogeneity may arise because of the presence of distinct strains of Aβ prions in the brain.
The first evidence for conformational heterogeneity of Aβ aggregates was observed in Aβ fibrils generated from synthetic Aβ using either quiescent or agitated conditions (Petkova et al. 2005). The two types of fibrils exhibited different morphologies when examined using electron microscopy and solid-state nuclear magnetic resonance (NMR) spectroscopy. Moreover, the distinct morphologies were maintained upon serial seeding, indicating that these unique structural states are self-propagating. Variations in the morphology of fibrils formed from synthetic Aβ have also been obtained by varying the buffer conditions and/or the formation temperature (Meinhardt et al. 2009; Kodali et al. 2010). At the molecular level, structural polymorphism may arise as a result of differences in symmetry and the conformations of Aβ residues not involved in the core parallel in-register β-sheet structure (Paravastu et al. 2008). Interestingly, Aβ fibrils formed from synthetic Aβ can also exhibit strain-like behavior in vivo. Synthetic Aβ42 polymerized in the presence of 0.1% (wt/vol) sodium dodecyl sulfate (SDS) resulted in shorter fibrils than when Aβ42 was aggregated in the absence of SDS (Stöhr et al. 2014). When inoculated into APP23 mice, Aβ42 fibrils generated in the absence of SDS induced a greater number of Aβ plaques, which contained a higher ratio of Aβ42/Aβ40 peptides than the plaques in mice inoculated with Aβ42 fibrils produced in the presence of SDS.
Distinct strains (or “morphotypes”) of Aβ prions have also been observed between two different lines of Tg(AD) mice, APP23 and APPPS1 (Radde et al. 2006). Using luminescent conjugated polymers (Sigurdson et al. 2007), it was shown that the Aβ deposits present in the brains of the two Tg(AD) lines emit distinct fluorescent spectra, which is suggestive of conformationally unique aggregates (Heilbronner et al. 2013). When APP23 Aβ aggregates were inoculated into APP23 mice, the induced Aβ prions were spectrally similar to those present in aged APP23 mice. In contrast, when APPPS1 Aβ prions were inoculated into APP23 mice, the induced Aβ aggregates produced spectra similar to those present in aged APPPS1 mice, indicating that the brain extracts from aged APP23 and APPPS1 mice contain distinct strains of self-propagating Aβ aggregates.
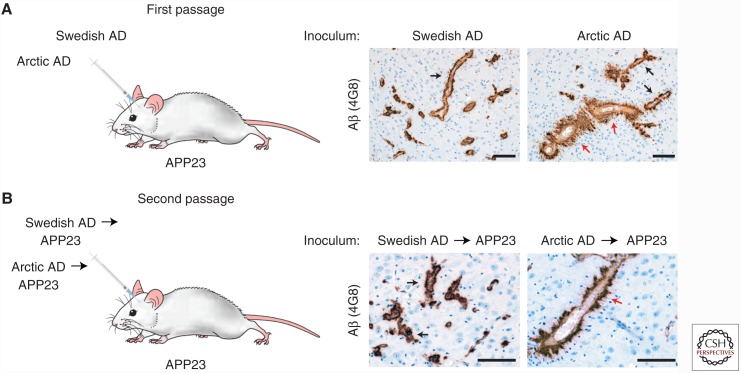
By mixing synthetic Aβ with fibrils isolated from an AD patient sample, it was shown that the Aβ aggregates present in AD patients are structurally distinct from those formed spontaneously from synthetic Aβ (Paravastu et al. 2009). Using a similar approach, it was recently determined that Aβ aggregates isolated from two different sporadic AD patients produce seeded Aβ aggregates with unique structural properties, arguing that distinct strains of Aβ aggregates may exist among AD patients (Lu et al. 2013). Indeed, the Aβ42 aggregates in patients with a more rapidly progressive version of AD are conformationally distinct from those in AD patients with a stereotypical disease course, as determined by a conformation-dependent immunoassay (Cohen et al. 2015). The existence of distinct Aβ strains among AD patients has also been demonstrated using brain extracts from two patients with early-onset familial versions of the disease. Using a conformational stability assay that measures the relative resistance of Aβ aggregates to chemical denaturation, it was shown that the Aβ aggregates from an AD patient with the Swedish mutation in APP can be distinguished from those in an AD patient with the Arctic mutation (E693G) (Nilsberth et al. 2001) in APP (Watts et al. 2014). When brain extracts from these two patients were inoculated into APP23 mice, distinct vascular Aβ pathologies were observed (Fig. 3), and these differences were maintained upon second passage in APP23 mice. These results reveal that, like PrP prions, strain-specific properties of Aβ prions are serially transmissible in mice.
Figure 3.
Arctic and Swedish Alzheimer’s disease (AD) brain extracts induce distinct pathologies in APP23 mice. (A) Intracerebral inoculation of APP23 mice with brain extract from an AD patient with the Swedish APP mutation, which generates wild-type Aβ, or brain extract from an AD patient with the Arctic APP mutation, which generates E22G-mutant Aβ. At 11 mo postinoculation, induced Aβ cerebral amyloid angiopathy is present in the thalamus (4G8 immunostaining). Whereas mice inoculated with Swedish AD extract exhibited a thin layer of Aβ deposition surrounding blood vessels (black arrows), many of the blood vessels in mice inoculated with Arctic AD extract were surrounded by thick, “furry” Aβ deposits (red arrows). (B) Similar morphological differences in Aβ cerebral amyloid angiopathy were observed at 11 mo postinoculation of APP23 mice with APP23-passaged Swedish and Arctic AD extract, indicating that Aβ strain-specified properties are serially transmissible. Scale bars, 100 µm. (This figure has been adapted from Watts et al. 2014, with permission from the National Academy of Sciences © 2014.)
CONCLUDING REMARKS
It is becoming increasingly clear that Aβ prions exhibit many similarities to prions composed of PrP (Table 2), making it more difficult to refute the notion that Aβ can become a prion during AD. However, there is currently minimal evidence to suggest that Aβ prions can be transmitted from human to human. Many individuals that received human cadaveric growth hormone injections ultimately died of CJD because the pituitary extracts were contaminated with PrP prions (Brown et al. 2000). Although no increased incidence of AD has been observed in living individuals who received growth hormone injections (Irwin et al. 2013), it has recently been shown that the brains of growth hormone recipients who died of CJD contain much higher levels of Aβ deposition than would be expected (Jaunmuktane et al. 2015). One possible interpretation is that Aβ prions were present in the growth hormone extracts, which initiated cerebral Aβ deposition following peripheral injection. Increased amounts of Aβ deposition have also been observed in patients that developed iatrogenic CJD following dura mater grafts (Frontzek et al. 2016). Notably, tau deposition was not observed in either of these instances, potentially indicating an early stage of AD pathogenesis. Although iatrogenic AD transmission is only one potential explanation for these observations, sterilization methods for surgical tools may need to be reconsidered, especially because Aβ prions remain active once bound to stainless steel (Eisele et al. 2009).
Table 2.
Similarities and differences between PrP and Aβ prions
| Characteristic | PrP prions | Aβ prions | Reference(s) for Aβ |
|---|---|---|---|
| Induction of protein aggregation in Tg mice expressing mutant precursor protein | Yes | Yes | Kane et al. 2000; Meyer-Luehmann et al. 2006 |
| Induction of protein aggregation in Tg mice expressing wild-type precursor protein | Yes | Yes | Morales et al. 2012 |
| Induction of protein aggregation in non-Tg mice | Yes | No | Meyer-Luehmann et al. 2006 |
| Induction of protein aggregation in primates | Yes | Yes | Ridley et al. 2006 |
| Induction of a lethal disease in mice | Yes | No | Stöhr et al. 2012 |
| Induction of neuronal loss in mice | Yes | No | Ye et al. 2015b |
| Induction of astrocytic gliosis in mice | Yes | Yes | Watts et al. 2011 |
| Progressive spreading of protein aggregation | Yes | Yes | |
| Neuroinvasion following peripheral inoculation | Yes | Yes | Eisele et al. 2014 |
| Horizontal or iatrogenic transmission | Yes | Minimal evidence | Frontzek et al. 2016 |
| Zoonotic transmission | Yes | No | |
| Serially transmissible | Yes | Yes | Watts et al. 2014 |
| Existence of distinct strains | Yes | Yes | Stöhr et al. 2014 |
| Titratable levels of infectivity | Yes | Yes | Morales et al. 2015 |
| Resistance to digestion with proteinase K | Yes | Yes | Stöhr et al. 2012 |
| Resistance to formaldehyde inactivation | Yes | Yes | Fritschi et al. 2014a |
| Adherence to stainless steel wires | Yes | Yes | Eisele et al. 2009 |
Although AD is unlikely to be transmissible among people under physiological circumstances, the realization that Aβ peptides can become prions during disease has many potential implications. First, the existence of self-propagating Aβ prions may provide a molecular explanation for why AD is a progressive disorder. Spreading of Aβ prions along neuroanatomical pathways in conjunction with a potential stimulation of tau prion formation may cause a progressive worsening of disease symptoms. Moreover, like CJD, ∼90% of AD cases are sporadic, suggesting that the initiating event may be the rare, age-related stochastic formation of a self-propagating Aβ aggregate seed. In early-onset familial AD, the necessity for Aβ prion formation may explain why disease most commonly manifests in the fifth or sixth decade of life, despite the fact that elevated levels of pathogenic Aβ are produced from birth. Second, the formation of self-propagating Aβ species may represent an early event in disease progression that can be targeted pharmacologically. Third, the existence of distinct strains of Aβ prions poses a considerable challenge for the development of Aβ-directed therapeutics for AD. Heterogeneity in the conformation of Aβ aggregates may partially explain the disappointing results obtained thus far in clinical trials of Aβ monoclonal antibodies (Delrieu et al. 2012) because antibody efficacy may be highly strain specific. Although studies of the biology of Aβ prions are still in their infancy, they hold great promise for understanding basic disease mechanisms and for the development of effective therapeutics for AD.
ACKNOWLEDGMENTS
The authors acknowledge support from the Canadian Institutes of Health Research and the Alzheimer Society Canada (J.C.W.), as well as the National Institutes of Health (AG002132 and AG031220), Dana Foundation, Glenn Foundation, Sherman Fairchild Foundation, and a gift from the Rainwater Charitable Foundation (S.B.P.).
REFERENCES
- Baker HF, Ridley RM, Duchen LW, Crow TJ, Bruton CJ. 1993. Evidence for the experimental transmission of cerebral β-amyloidosis to primates. Int J Exp Pathol 74: 441–454. [PMC free article] [PubMed] [Google Scholar]
- Baker HF, Ridley RM, Duchen LW, Crow TJ, Bruton CJ. 1994. Induction of β(A4)-amyloid in primates by injection of Alzheimer’s disease brain homogenate. Mol Neurobiol 8: 25–39. [DOI] [PubMed] [Google Scholar]
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, et al. 2000. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med 6: 916–919. [DOI] [PubMed] [Google Scholar]
- Benilova I, Karran E, De Strooper B. 2012. The toxic Aβ oligomer and Alzheimer’s disease: An emperor in need of clothes. Nat Neurosci 15: 349–357. [DOI] [PubMed] [Google Scholar]
- Bessen RA, Marsh RF. 1994. Distinct PrP properties suggest the molecular basis of strain variation in transmissible mink encephalopathy. J Virol 68: 7859–7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P, Preece M, Brandel JP, Sato T, McShane L, Zerr I, Fletcher A, Will RG, Pocchiari M, Cashman NR, et al. 2000. Iatrogenic Creutzfeldt–Jakob disease at the millennium. Neurology 55: 1075–1081. [DOI] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Crawford F, Houlden H, Warren A, Hughes D, Fidani L, Goate A, Rossor M, Roques P, Hardy J, et al. 1991. Early-onset Alzheimer’s disease caused by mutations at codon 717 of the β-amyloid precursor protein gene. Nature 353: 844–846. [DOI] [PubMed] [Google Scholar]
- Chishti MA, Yang DS, Janus C, Phinney AL, Horne P, Pearson J, Strome R, Zuker N, Loukides J, French J, et al. 2001. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J Biol Chem 276: 21562–21570. [DOI] [PubMed] [Google Scholar]
- Citron M, Oltersdorf T, Haass C, McConlogue L, Hung AY, Seubert P, Vigo-Pelfrey C, Lieberburg I, Selkoe DJ. 1992. Mutation of the β-amyloid precursor protein in familial Alzheimer’s disease increases β-protein production. Nature 360: 672–674. [DOI] [PubMed] [Google Scholar]
- Cohen RM, Rezai-Zadeh K, Weitz TM, Rentsendorj A, Gate D, Spivak I, Bholat Y, Vasilevko V, Glabe CG, Breunig JJ, et al. 2013. A transgenic Alzheimer rat with plaques, tau pathology, behavioral impairment, oligomeric Aβ, and frank neuronal loss. J Neurosci 33: 6245–6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen ML, Kim C, Haldiman T, ElHag M, Mehndiratta P, Pichet T, Lissemore F, Shea M, Cohen Y, Chen W, et al. 2015. Rapidly progressive Alzheimer’s disease features distinct structures of amyloid-β. Brain 138: 1009–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby DW, Prusiner SB. 2011. Prions. Cold Spring Harb Perspect Biol 3: a006833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinge J, Clarke AR. 2007. A general model of prion strains and their pathogenicity. Science 318: 930–936. [DOI] [PubMed] [Google Scholar]
- DeArmond SJ, Ironside JW, Bouzamondo-Bernstein E, Peretz D, Fraser JR. 2004. Neuropathology of prion diseases. In Prion biology and diseases (ed. Prusiner SB), pp. 777–856. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Delrieu J, Ousset PJ, Caillaud C, Vellas B. 2012. “Clinical trials in Alzheimer’s disease”: Immunotherapy approaches. J Neurochem 120: 186–193. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Iwatsubo T, Wolfe MS. 2012. Presenilins and γ-secretase: Structure, function, and role in Alzheimer Disease. Cold Spring Harb Perspect Med 2: a006304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran-Aniotz C, Morales R, Moreno-Gonzalez I, Hu PP, Soto C. 2013. Brains from non-Alzheimer’s individuals containing amyloid deposits accelerate Aβ deposition in vivo. Acta Neuropathol Commun 1: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran-Aniotz C, Morales R, Moreno-Gonzalez I, Hu PP, Fedynyshyn J, Soto C. 2014. Aggregate-depleted brain fails to induce Aβ deposition in a mouse model of Alzheimer’s disease. PLoS ONE 9: e89014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele YS, Bolmont T, Heikenwalder M, Langer F, Jacobson LH, Yan ZX, Roth K, Aguzzi A, Staufenbiel M, Walker LC, et al. 2009. Induction of cerebral β-amyloidosis: Intracerebral versus systemic Aβ inoculation. Proc Natl Acad Sci 106: 12926–12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele YS, Obermüller U, Heilbronner G, Baumann F, Kaeser SA, Wolburg H, Walker LC, Staufenbiel M, Heikenwalder M, Jucker M. 2010. Peripherally applied Aβ-containing inoculates induce cerebral β-amyloidosis. Science 330: 980–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele YS, Fritschi SK, Hamaguchi T, Obermüller U, Füger P, Skodras A, Schäfer C, Odenthal J, Heikenwalder M, Staufenbiel M, et al. 2014. Multiple factors contribute to the peripheral induction of cerebral β-amyloidosis. J Neurosci 34: 10264–10273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschi SK, Cintron A, Ye L, Mahler J, Bühler A, Baumann F, Neumann M, Nilsson KP, Hammarström P, Walker LC, et al. 2014a. Aβ seeds resist inactivation by formaldehyde. Acta Neuropathol 128: 477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschi SK, Langer F, Kaeser SA, Maia LF, Portelius E, Pinotsi D, Kaminski CF, Winkler DT, Maetzler W, Keyvani K, et al. 2014b. Highly potent soluble amyloid-β seeds in human Alzheimer brain but not cerebrospinal fluid. Brain 137: 2909–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontzek K, Lutz MI, Aguzzi A, Kovacs GG, Budka H. 2016. Amyloid-β pathology and cerebral amyloid angiopathy are frequent in iatrogenic Creutzfeldt–Jakob disease after dural grafting. Swiss Med Wkly 146: w14287. [DOI] [PubMed] [Google Scholar]
- Gajdusek DC, Gibbs CJ Jr, Alpers M. 1966. Experimental transmission of a kuru-like syndrome to chimpanzees. Nature 209: 794–796. [DOI] [PubMed] [Google Scholar]
- Gibbs CJ Jr, Gajdusek DC, Asher DM, Alpers MP, Beck E, Daniel PM, Matthews WB. 1968. Creutzfeldt–Jakob disease (spongiform encephalopathy): Transmission to the chimpanzee. Science 161: 388–389. [DOI] [PubMed] [Google Scholar]
- Godec MS, Asher DM, Kozachuk WE, Masters CL, Rubi JU, Payne JA, Rubi-Villa DJ, Wagner EE, Rapoport SI, Schapiro MB. 1994. Blood buffy coat from Alzheimer’s disease patients and their relatives does not transmit spongiform encephalopathy to hamsters. Neurology 44: 1111–1115. [DOI] [PubMed] [Google Scholar]
- Goudsmit J, Morrow CH, Asher DM, Yanagihara RT, Masters CL, Gibbs CJ Jr, Gajdusek DC. 1980. Evidence for and against the transmissibility of Alzheimer’s disease. Neurology 30: 945–950. [DOI] [PubMed] [Google Scholar]
- Guardia-Laguarta C, Pera M, Clarimón J, Molinuevo JL, Sánchez-Valle R, Lladó A, Coma M, Gómez-Isla T, Blesa R, Ferrer I, et al. 2010. Clinical, neuropathologic, and biochemical profile of the amyloid precursor protein I716F mutation. J Neuropathol Exp Neurol 69: 53–59. [DOI] [PubMed] [Google Scholar]
- Haass C, Lemere CA, Capell A, Citron M, Seubert P, Schenk D, Lannfelt L, Selkoe DJ. 1995. The Swedish mutation causes early-onset Alzheimer’s disease by β-secretase cleavage within the secretory pathway. Nat Med 1: 1291–1296. [DOI] [PubMed] [Google Scholar]
- Hamaguchi T, Eisele YS, Varvel NH, Lamb BT, Walker LC, Jucker M. 2012. The presence of Aβ seeds, and not age per se, is critical to the initiation of Aβ deposition in the brain. Acta Neuropathol 123: 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. 2002. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 297: 353–356. [DOI] [PubMed] [Google Scholar]
- Hebert LE, Weuve J, Scherr PA, Evans DA. 2013. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 80: 1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronner G, Eisele YS, Langer F, Kaeser SA, Novotny R, Nagarathinam A, Åslund A, Hammarström P, Nilsson KP, Jucker M. 2013. Seeded strain-like transmission of β-amyloid morphotypes in APP transgenic mice. EMBO Rep 14: 1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole GJ. 1996. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science 274: 99–102. [DOI] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, et al. 1998. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393: 702–705. [DOI] [PubMed] [Google Scholar]
- Irwin DJ, Abrams JY, Schonberger LB, Leschek EW, Mills JL, Lee VM, Trojanowski JQ. 2013. Evaluation of potential infectivity of Alzheimer and Parkinson disease proteins in recipients of cadaver-derived human growth hormone. JAMA Neurol 70: 462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, Chishti MA, Horne P, Heslin D, French J, et al. 2000. Aβ peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer’s disease. Nature 408: 979–982. [DOI] [PubMed] [Google Scholar]
- Jaunmuktane Z, Mead S, Ellis M, Wadsworth JDF, Nicoll AJ, Kenny J, Launchbury F, Linehan J, Richard-Loendt A, Walker AS, et al. 2015. Evidence for human transmission of amyloid-β pathology and cerebral amyloid angiopathy. Nature 525: 247–250. [DOI] [PubMed] [Google Scholar]
- Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, Stefansson H, Sulem P, Gudbjartsson D, Maloney J, et al. 2012. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature 488: 96–99. [DOI] [PubMed] [Google Scholar]
- Jucker M. 2010. The benefits and limitations of animal models for translational research in neurodegenerative diseases. Nat Med 16: 1210–1214. [DOI] [PubMed] [Google Scholar]
- Kane MD, Lipinski WJ, Callahan MJ, Bian F, Durham RA, Schwarz RD, Roher AE, Walker LC. 2000. Evidence for seeding of β-amyloid by intracerebral infusion of Alzheimer brain extracts in β-amyloid precursor protein-transgenic mice. J Neurosci 20: 3606–3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodali R, Williams AD, Chemuru S, Wetzel R. 2010. Aβ(1–40) forms five distinct amyloid structures whose β-sheet contents and fibril stabilities are correlated. J Mol Biol 401: 503–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer F, Eisele YS, Fritschi SK, Staufenbiel M, Walker LC, Jucker M. 2011. Soluble Aβ seeds are potent inducers of cerebral β-amyloid deposition. J Neurosci 31: 14488–14495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu JX, Qiang W, Yau WM, Schwieters CD, Meredith SC, Tycko R. 2013. Molecular structure of β-amyloid fibrils in Alzheimer’s disease brain tissue. Cell 154: 1257–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia LF, Kaeser SA, Reichwald J, Hruscha M, Martus P, Staufenbiel M, Jucker M. 2013. Changes in amyloid-β and tau in the cerebrospinal fluid of transgenic mice overexpressing amyloid precursor protein. Sci Transl Med 5: 194re2. [DOI] [PubMed] [Google Scholar]
- Manuelidis EE, de Figueiredo JM, Kim JH, Fritch WW, Manuelidis L. 1988. Transmission studies from blood of Alzheimer disease patients and healthy relatives. Proc Natl Acad Sci 85: 4898–4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt J, Sachse C, Hortschansky P, Grigorieff N, Fändrich M. 2009. Aβ(1–40) fibril polymorphism implies diverse interaction patterns in amyloid fibrils. J Mol Biol 386: 869–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Coomaraswamy J, Bolmont T, Kaeser S, Schaefer C, Kilger E, Neuenschwander A, Abramowski D, Frey P, Jaton AL, et al. 2006. Exogenous induction of cerebral β-amyloidogenesis is governed by agent and host. Science 313: 1781–1784. [DOI] [PubMed] [Google Scholar]
- Morales R, Duran-Aniotz C, Castilla J, Estrada LD, Soto C. 2012. De novo induction of amyloid-β deposition in vivo. Mol Psychiatry 17: 1347–1353. [DOI] [PubMed] [Google Scholar]
- Morales R, Bravo-Alegria J, Duran-Aniotz C, Soto C. 2015. Titration of biologically active amyloid-β seeds in a transgenic mouse model of Alzheimer’s disease. Sci Rep 5: 9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissette DA, Parachikova A, Green KN, LaFerla FM. 2009. Relevance of transgenic mouse models to human Alzheimer disease. J Biol Chem 284: 6033–6037. [DOI] [PubMed] [Google Scholar]
- Mullan M, Crawford F, Axelman K, Houlden H, Lilius L, Winblad B, Lannfelt L. 1992. A pathogenic mutation for probable Alzheimer’s disease in APP gene at the N-terminus of β-amyloid. Nat Genet 1: 345–347. [DOI] [PubMed] [Google Scholar]
- Murrell J, Farlow M, Ghetti B, Benson MD. 1991. A mutation in the amyloid precursor protein associated with hereditary Alzheimer’s disease. Science 254: 97–99. [DOI] [PubMed] [Google Scholar]
- Nilsberth C, Westlind-Danielsson A, Eckman CB, Condron MM, Axelman K, Forsell C, Stenh C, Luthman J, Teplow DB, Younkin SG, et al. 2001. The ‘Arctic’ APP mutation (E693G) causes Alzheimer’s disease by enhanced Aβ protofibril formation. Nat Neurosci 4: 887–893. [DOI] [PubMed] [Google Scholar]
- Paravastu AK, Leapman RD, Yau WM, Tycko R. 2008. Molecular structural basis for polymorphism in Alzheimer’s β-amyloid fibrils. Proc Natl Acad Sci 105: 18349–18354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paravastu AK, Qahwash I, Leapman RD, Meredith SC, Tycko R. 2009. Seeded growth of β-amyloid fibrils from Alzheimer’s brain-derived fibrils produces a distinct fibril structure. Proc Natl Acad Sci 106: 7443–7448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkova AT, Leapman RD, Guo Z, Yau WM, Mattson MP, Tycko R. 2005. Self-propagating, molecular-level polymorphism in Alzheimer’s β-amyloid fibrils. Science 307: 262–265. [DOI] [PubMed] [Google Scholar]
- Prusiner SB. 1982. Novel proteinaceous infectious particles cause scrapie. Science 216: 136–144. [DOI] [PubMed] [Google Scholar]
- Prusiner SB. 1984. Some speculations about prions, amyloid, and Alzheimer’s disease. N Engl J Med 310: 661–663. [DOI] [PubMed] [Google Scholar]
- Prusiner SB. 2012. A unifying role for prions in neurodegenerative diseases. Science 336: 1511–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner SB, McKinley MP, Bowman KA, Bolton DC, Bendheim PE, Groth DF, Glenner GG. 1983. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell 35: 349–358. [DOI] [PubMed] [Google Scholar]
- Radde R, Bolmont T, Kaeser SA, Coomaraswamy J, Lindau D, Stoltze L, Calhoun ME, Jäggi F, Wolburg H, Gengler S, et al. 2006. Aβ42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep 7: 940–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley RM, Baker HF, Windle CP, Cummings RM. 2006. Very long term studies of the seeding of β-amyloidosis in primates. J Neural Transm 113: 1243–1251. [DOI] [PubMed] [Google Scholar]
- Rosen RF, Fritz JJ, Dooyema J, Cintron AF, Hamaguchi T, Lah JJ, Levine H 3rd, Jucker M, Walker LC. 2012. Exogenous seeding of cerebral β-amyloid deposition in βAPP-transgenic rats. J Neurochem 120: 660–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovelet-Lecrux A, Hannequin D, Raux G, Le Meur N, Laquerriere A, Vital A, Dumanchin C, Feuillette S, Brice A, Vercelletto M, et al. 2006. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat Genet 38: 24–26. [DOI] [PubMed] [Google Scholar]
- Saito T, Matsuba Y, Mihira N, Takano J, Nilsson P, Itohara S, Iwata N, Saido TC. 2014. Single App knock-in mouse models of Alzheimer’s disease. Nat Neurosci 17: 661–663. [DOI] [PubMed] [Google Scholar]
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, et al. 1999. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400: 173–177. [DOI] [PubMed] [Google Scholar]
- Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, et al. 1995. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature 375: 754–760. [DOI] [PubMed] [Google Scholar]
- Sigurdson CJ, Nilsson KP, Hornemann S, Manco G, Polymenidou M, Schwarz P, Leclerc M, Hammarström P, Wüthrich K, Aguzzi A. 2007. Prion strain discrimination using luminescent conjugated polymers. Nat Methods 4: 1023–1030. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Murrell JR, Goedert M, Farlow MR, Klug A, Ghetti B. 1998. Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci 95: 7737–7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöhr J, Watts JC, Mensinger ZL, Oehler A, Grillo SK, DeArmond SJ, Prusiner SB, Giles K. 2012. Purified and synthetic Alzheimer’s amyloid β (Aβ) prions. Proc Natl Acad Sci 109: 11025–11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöhr J, Condello C, Watts JC, Bloch L, Oehler A, Nick M, DeArmond SJ, Giles K, DeGrado WF, Prusiner SB. 2014. Distinct synthetic Aβ prion strains producing different amyloid deposits in bigenic mice. Proc Natl Acad Sci 111: 10329–10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturchler-Pierrat C, Abramowski D, Duke M, Wiederhold KH, Mistl C, Rothacher S, Ledermann B, Burki K, Frey P, Paganetti PA, et al. 1997. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci 94: 13287–13292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Cheung TT, Cai XD, Odaka A, Otvos L Jr, Eckman C, Golde TE, Younkin SG. 1994. An increased percentage of long amyloid β protein secreted by familial amyloid β protein precursor (β APP717) mutants. Science 264: 1336–1340. [DOI] [PubMed] [Google Scholar]
- Telling GC, Parchi P, DeArmond SJ, Cortelli P, Montagna P, Gabizon R, Mastrianni J, Lugaresi E, Gambetti P, Prusiner SB. 1996. Evidence for the conformation of the pathologic isoform of the prion protein enciphering and propagating prion diversity. Science 274: 2079–2082. [DOI] [PubMed] [Google Scholar]
- Thal DR, Rüb U, Orantes M, Braak H. 2002. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology 58: 1791–1800. [DOI] [PubMed] [Google Scholar]
- Watts JC, Giles K, Grillo SK, Lemus A, DeArmond SJ, Prusiner SB. 2011. Bioluminescence imaging of Aβ deposition in bigenic mouse models of Alzheimer’s disease. Proc Natl Acad Sci 108: 2528–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts JC, Condello C, Stöhr J, Oehler A, Lee J, DeArmond SJ, Lannfelt L, Ingelsson M, Giles K, Prusiner SB. 2014. Serial propagation of distinct strains of Aβ prions from Alzheimer’s disease patients. Proc Natl Acad Sci 111: 10323–10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski KE, Wisniewski HM, Wen GY. 1985. Occurrence of neuropathological changes and dementia of Alzheimer’s disease in Down’s syndrome. Ann Neurol 17: 278–282. [DOI] [PubMed] [Google Scholar]
- Ye L, Hamaguchi T, Fritschi SK, Eisele YS, Obermüller U, Jucker M, Walker LC. 2015a. Progression of seed-induced Aβ deposition within the limbic connectome. Brain Pathol 25: 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Fritschi SK, Schelle J, Obermüller U, Degenhardt K, Kaeser SA, Eisele YS, Walker LC, Baumann F, Staufenbiel M, et al. 2015b. Persistence of Aβ seeds in APP null mouse brain. Nat Neurosci 18: 1559–1561. [DOI] [PubMed] [Google Scholar]
- Zhu L, Ramboz S, Hewitt D, Boring L, Grass DS, Purchio AF. 2004. Non-invasive imaging of GFAP expression after neuronal damage in mice. Neurosci Lett 367: 210–212. [DOI] [PubMed] [Google Scholar]